Stimulation of Abscisic Acid Biosynthesis by Ethylene with Suppressive Action of MiR396 in Postharvest Strawberry Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material and Treatments
2.2. Fruit Quality Measurements
2.3. Ethylene Production
2.4. ABA Extraction and Analysis
2.5. RNA Extraction, cDNA Synthesis, and RT-qPCR
2.6. Plasmid Construction and Agro-Infiltration
2.7. Dual-Luciferase Reporter
2.8. Statistical Analysis
3. Results
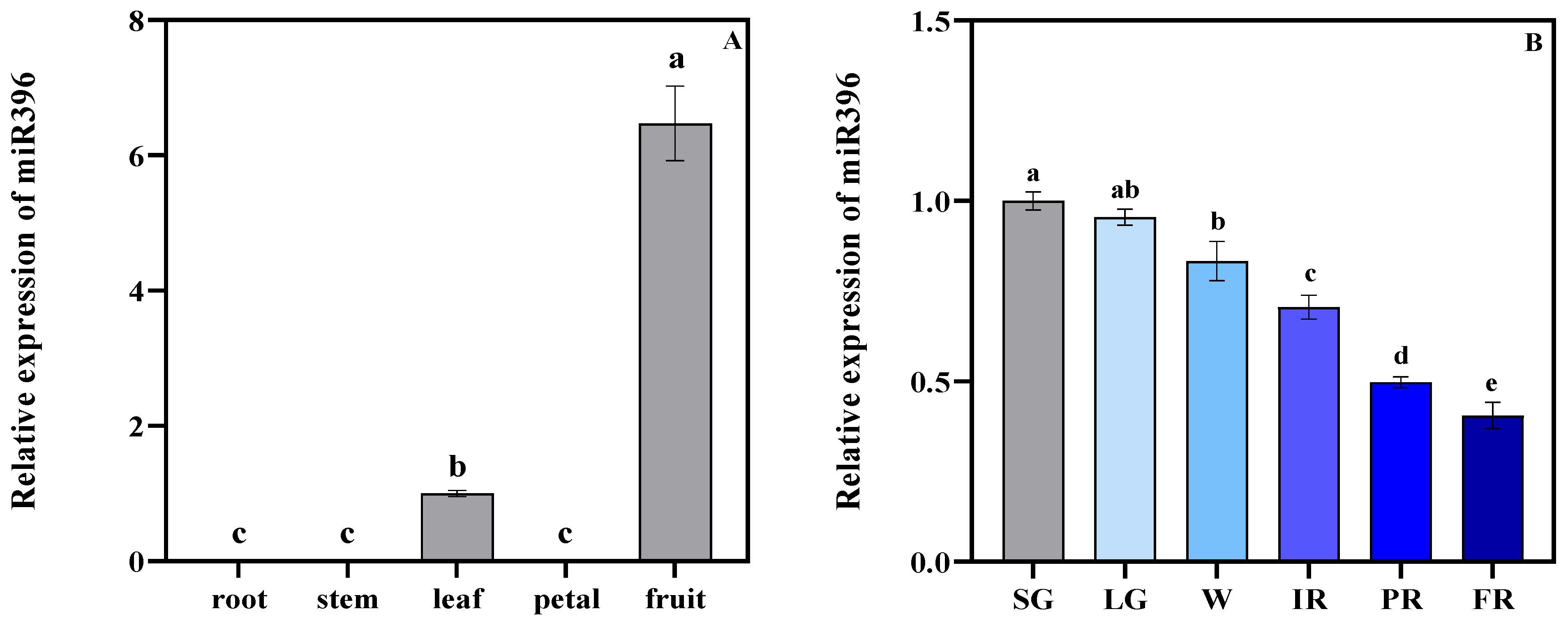
3.1. Temporospatial Expression of MiR396
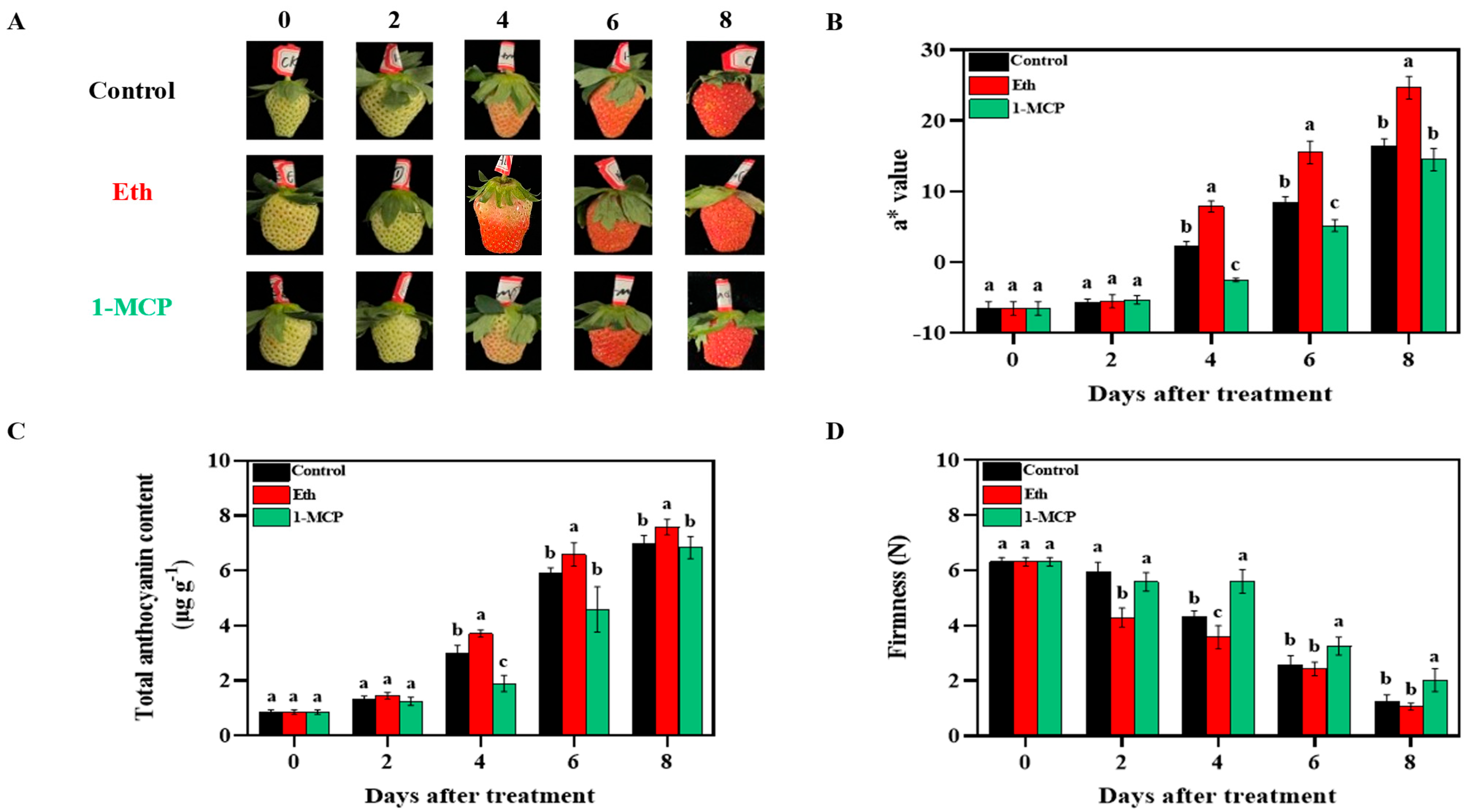
3.2. Ethylene Promoted Anthocyanin Accumulation and Softening of Strawberry Fruit
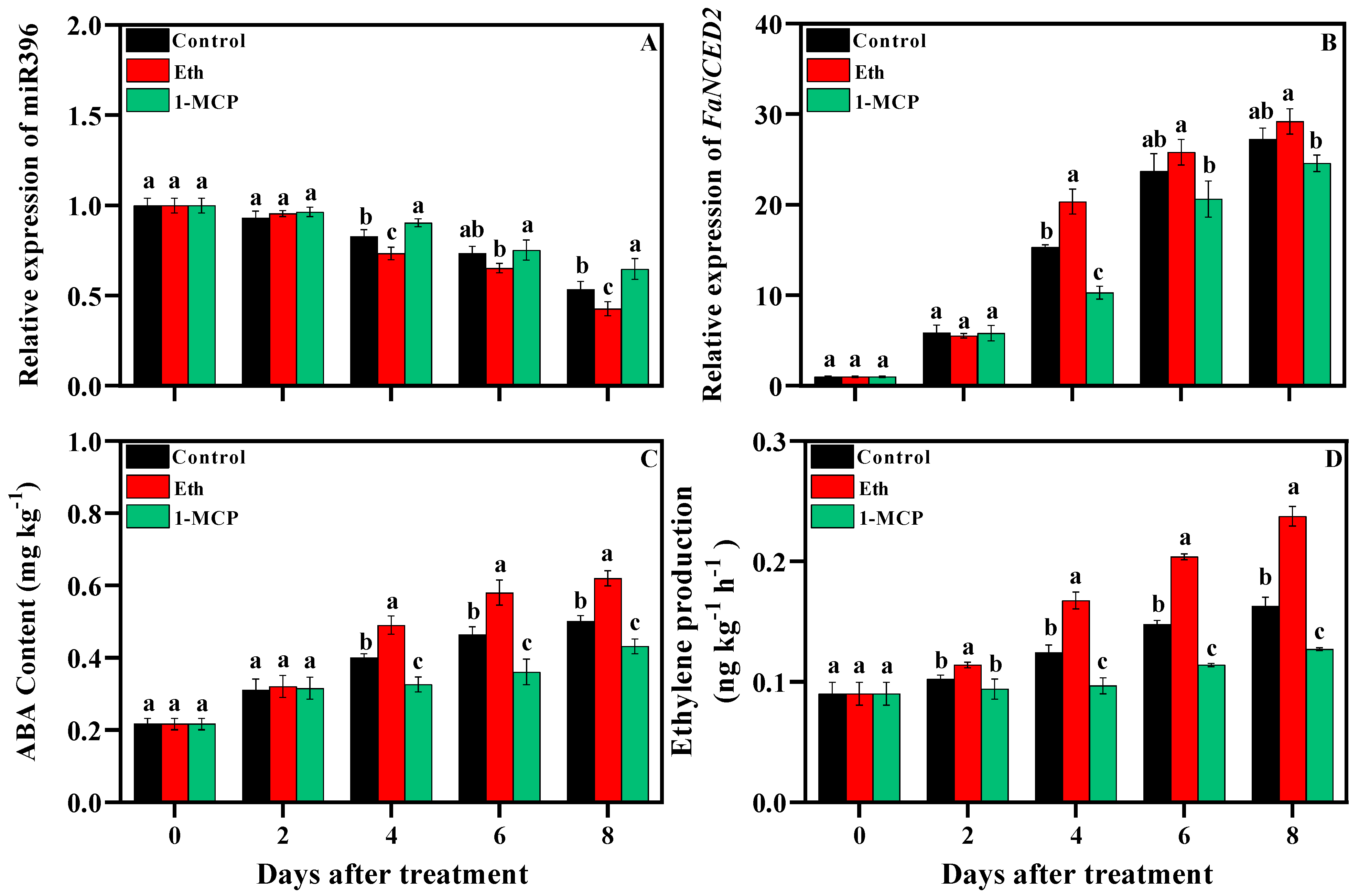
3.3. Change in Mir396, and FaNCED2 Expression and ABA Content After Treatments
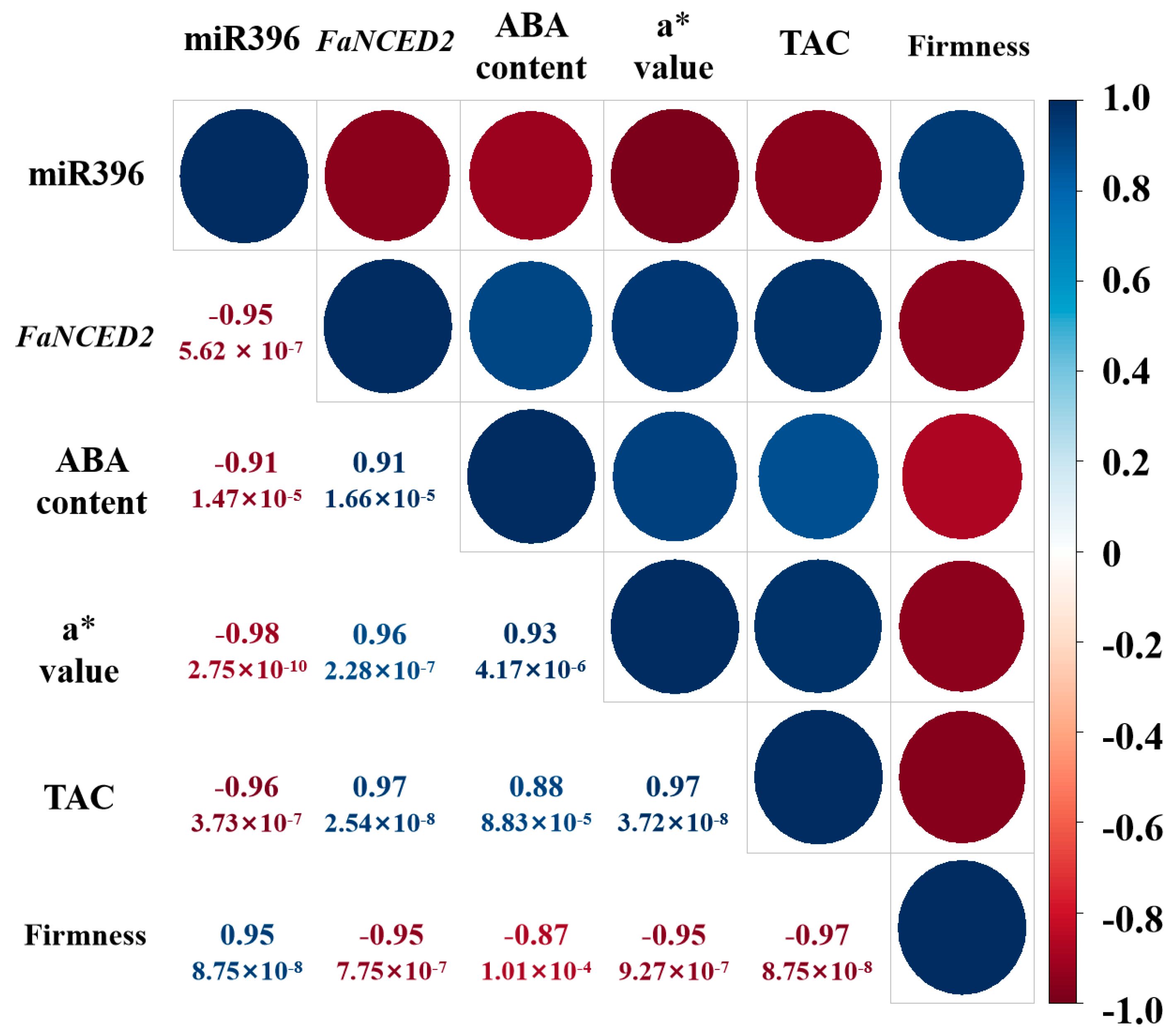
3.4. Correlation Analysis
3.5. Silencing of MiR396
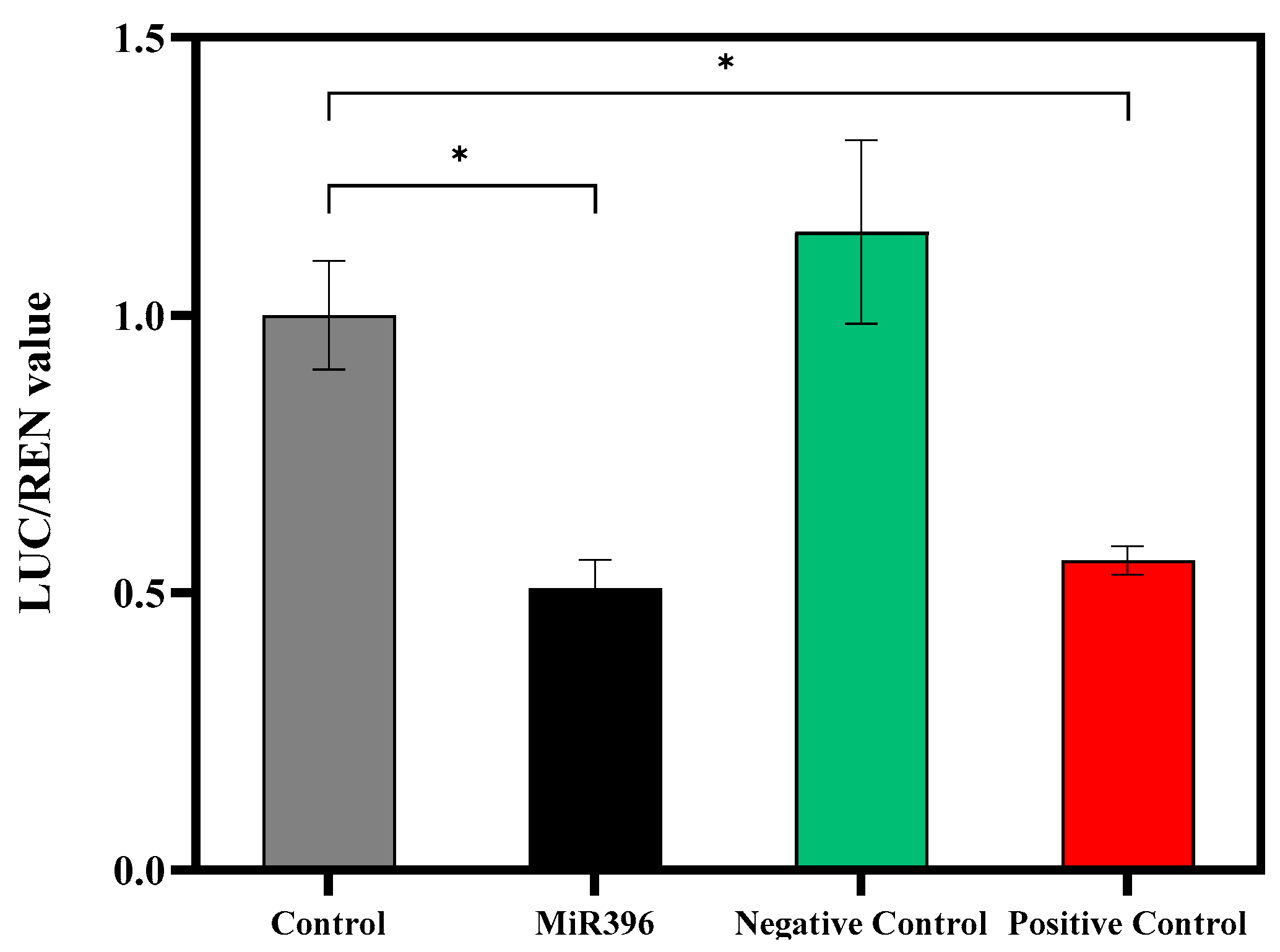
3.6. Dual-Luciferase Reporter of MiR396 and FaNCED2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, W.; Liu, Z.; Pan, X.; Yang, T.; An, C.; Wang, Y.; Li, L.; Liao, W.; Wang, C. Effects of reactive oxygen species on fruit ripening and postharvest fruit quality. Plant Sci. 2025, 352, 112391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Z.; Su, X.; Hou, H.; Jiang, Y.; Duan, X.; Qu, H.; Jiang, G. Histone deacetylase SlHDA7 impacts fruit ripening and shelf life in tomato. Hortic. Res. 2024, 11, uhae234. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, C.; Wei, W.; Shan, W.; Kuang, J.; Chen, J.; Zhou, E.; Lu, W.; Yang, Y. MaWRKY147-MaMADS68 transcriptional cascade module regulates low-temperature-affected banana fruit ripening. Postharvest Biol. Technol. 2024, 207, 112625. [Google Scholar] [CrossRef]
- Perotti, M.F.; Posé, D.; Martín-Pizarro, C. Non-climacteric fruit development and ripening regulation: ‘the phytohormones show’. J. Exp. Bot. 2023, 74, 6237–6253. [Google Scholar] [CrossRef]
- Wang, P.; Yu, A.; Ji, X.; Mu, Q.; Salman, H.M.; Wei, R.; Leng, X.; Fang, J. Transcriptome and metabolite integrated analysis reveals that exogenous ethylene controls berry ripening processes in grapevine. Food Res. Int. 2022, 155, 111084. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, D.; Hao, Q.; Jia, W. Signal transduction in non-climacteric fruit ripening. Hortic. Res. 2022, 9, uhac190. [Google Scholar] [CrossRef]
- Xiao, Z.; Tyerman, S.D.; Stait-Gardner, T.; Price, W.S.; Pagay, V.; Schmidtke, L.M.; Rogiers, S.Y. Characterisation of internal oxygen concentration of strawberry (Fragaria × ananassa) and blueberry (Vaccinium corymbosum). Funct. Plant Biol. 2022, 50, 256–265. [Google Scholar] [CrossRef]
- Guo, F.; Lv, M.; Zhang, J.; Li, J. Crosstalk between brassinosteroids and other phytohormones during plant development and stress adaptation. Plant Cell Physiol. 2024, 65, 1530–1543. [Google Scholar] [CrossRef]
- Bai, Q.; Huang, Y.; Shen, Y. The physiological and molecular mechanism of abscisic acid in regulation of fleshy fruit ripening. Front. Plant Sci. 2021, 11, 619953. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhan, Y. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M. Recent advances in hormonal regulation and cross-talk during non-climacteric fruit development and ripening. Horticulturae 2019, 5, 45. [Google Scholar] [CrossRef]
- Li, B.; Grierson, D.; Shi, Y.; Chen, K. Roles of abscisic acid in regulating ripening and quality of strawberry, a model non-climacteric fruit. Hortic. Res. 2022, 9, uhac089. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Molina-Hidalgo, F.; Boersma, M.; Schuurink, R.; López-Vidriero, I.; Solano, R.; Franco-Zorrilla, J.; Caballero, J.; Blanco-Portales, R.; Muñoz-Blanco, J. An R2R3-MYB transcription factor regulates eugenol production in ripe strawberry fruit receptacles. Plant Physiol. 2015, 168, 598–614. [Google Scholar] [CrossRef]
- Jia, H.; Chai, Y.; Li, C.; Lu, D.; Luo, J.; Qin, L. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cao, S.; Shi, L.; Chen, W.; Yin, X.; Yang, Z. Abscisic acid biosynthesis, metabolism and signaling in ripening fruit. Front. Plant Sci. 2023, 14, 1279031. [Google Scholar] [CrossRef]
- Tian, M.; Prakash, S.; Elgar, H.; Young, H.; Burmeister, D.; Ross, G. Responses of strawberry fruit to 1-methylcyclopropene (1-MCP) and ethylene. Plant Growth Regul. 2000, 32, 83–90. [Google Scholar] [CrossRef]
- Villarreal, N.; Bustamante, C.; Civello, P.; MartÃnez, G. Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J. Sci. Food Agric. 2000, 90, 683–689. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Mi, H.; Zhao, Y.; Ying, T.; Luo, Z. Detachment-accelerated ripening and senescence of strawberry (Fragaria × ananassa Duch. cv. Akihime) fruit and the regulation role of multiple phytohormones. Acta Physiol. Plant 2014, 36, 2441–2451. [Google Scholar] [CrossRef]
- Botondi, R.; Lodola, L.; Mencarelli, F. Postharvest ethylene treatment affects berry dehydration, polyphenol and anthocyanin content by increasing the activity of cell wall enzymes in Aleatico wine grape. Eur. Food Res. Technol. 2010, 232, 679–685. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, M.; Gu, X.; Guo, C.; Chen, Y.; Lin, Y.; Chen, Q.; Wang, Y.; Zhang, Y.; Luo, Y.; et al. Ethylene signaling pathway genes in strawberry and their expression patterns during fruit ripening. Agronomy 2023, 13, 1930. [Google Scholar] [CrossRef]
- Elmi, F.; Pradas, I.; Tosetti, R.; Cools, K.; Terry, L. Effect of ethylene on postharvest strawberry fruit tissue biochemistry. Acta Hortic. 2017, 1156, 667–672. [Google Scholar] [CrossRef]
- Tosetti, R.; Elmi, F.; Pradas, I.; Cools, K.; Terry, L. Continuous exposure to ethylene differentially affects senescence in receptacle and achene tissues in strawberry fruit. Front. Plant Sci. 2020, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lai, T.; Song, H.; Xu, X. MiR164 is involved in delaying senescence of strawberry (Fragaria ananassa) fruit by negatively regulating NAC transcription factor genes under low temperature. Russ. J. Plant Physiol. 2017, 64, 251–259. [Google Scholar] [CrossRef]
- Li, D.; Mou, M.; Luo, Z.; Li, L.; Limwachiranon, J.; Mao, L.; Ying, T. Developmental and stress regulation on expression of a novel miRNA, Fan-miR73, and its target ABI5 in strawberry. Sci. Rep. 2016, 6, 28385. [Google Scholar] [CrossRef]
- Chen, R.; Wu, Y.; Wei, X.; Huang, Z.; Mao, L. Ethylene promotes ABA biosynthesis by repressing the expression of miR161 in postharvest strawberry fruit. Postharvest Biol. Technol. 2023, 199, 112302. [Google Scholar] [CrossRef]
- Lu, W.; Chen, J.; Ren, X.; Yuan, J.; Han, X.; Mao, L.; Ying, T.; Luo, Z. One novel strawberry MADS-box transcription factor FaMADS1a acts as a negative regulator in fruit ripening. Sci. Hortic. 2018, 227, 124–131. [Google Scholar] [CrossRef]
- Chen, R.; Mao, L.; Guan, W.; Wei, X.; Huang, Z.; Wu, Y. ABA-mediated miR5290 promotes anthocyanin biosynthesis by inhibiting the expression of FaMADS1 in postharvest strawberry fruit. Postharvest Biol. Technol. 2022, 189, 111934. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Wang, L.; Lin, X.; Wang, Y.; Luo, Z. Exogenous ATP attenuated fermentative metabolism in postharvest strawberry fruit under elevated CO2 atmosphere by maintaining energy status. Postharvest Biol. Technol. 2021, 182, 111701. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Xu, Y.; Li, L.; Aghdam, M.; Luo, Z. Effect of exogenous sucrose on anthocyanin synthesis in postharvest strawberry fruit. Food Chem. 2019, 289, 112–120. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.; Luo, Z.; Roussel, D.; Rolland, D. Potential link between fruit yield, quality parameters and phytohormonal changes in preharvest UV-C treated strawberry. Plant Physiol. Biochem. 2017, 116, 80–90. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Yang, M.; Liang, Z.; Qi, M.; Dong, Y.; Xu, Y.; Lin, X.; Li, L. The action of RED light: Specific elevation of pelargonidin-based anthocyanin through ABA-related pathway in strawberry. Postharvest Biol. Technol. 2022, 186, 111835. [Google Scholar] [CrossRef]
- Wei, X.; Guan, W.; Yang, Y.; Shao, Y.; Mao, L. Methyl jasmonate promotes wound healing by activation of phenylpropanoid metabolism in harvested kiwifruit. Postharvest Biol. Technol. 2021, 175, 111472. [Google Scholar] [CrossRef]
- Sha, A.; Zhao, J.; Yin, K.; Tang, Y.; Wang, Y.; Wei, X.; Hong, Y.; Liu, Y. Virus-based microRNA silencing in plants. Plant Physiol. 2014, 164, 36–47. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Wu, Y.; Li, D.; Allan, A.C.; Yin, X. Genome-wide analysis of coding and non-coding RNA reveals a conserved miR164-NAC regulatory pathway for fruit ripening. New Phytol. 2020, 225, 1618–1634. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Figueroa, C.; Nair, H. ‘Movers and shakers’ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003, 39, 171–174. [Google Scholar] [CrossRef]
- Pramoolkit, P.; Lertpanyasampatha, M.; Viboonjun, U.; Kongsawadworakul, P.; Chrestin, H.; Narangajavana, J. Involvement of ethylene-responsive microRNAs and their targets in increased latex yield in the rubber tree in response to ethylene treatment. Plant Physiol. Biochem. 2014, 84, 203–212. [Google Scholar] [CrossRef]
- Brodersen, P.; Sakvarelidze-Achard, L.; Bruun-Rasmussen, M.; Dunoyer, P.; Yamamoto, Y.; Sieburth, L.; Voinnet, O. Widespread translational inhibition by plant miRNAs and siRNAs. Science 2008, 320, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Sakai, H.; Nourizadeh, S.; Chen, Q.; Bleecker, A.; Ecker, J.; Meyerowitz, E. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 1998, 10, 1321–1332. [Google Scholar] [CrossRef]
- Ju, C.; Yoon, G.; Shemansky, J.; Lin, D.; Ying, Z.; Chang, J.; Garrett, W.; Kessenbrock, M.; Groth, G.; Tucker, M.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef]
- Gaddam, S.; Bhatia, C.; Gautama, H.; Pathak, P.; Sharma, A.; Saxena, G.; Trivedi, P. Ethylene regulates miRNA-mediated lignin biosynthesis and leaf serration in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2022, 605, 51–55. [Google Scholar] [CrossRef]
- Guo, Y.; Xiong, H.; Fan, Q.; Duanmu, D. Heterologous gene expression in Chlamydomonas reinhardtii chloroplast by heterologous promoters and terminators, intercistronic expression elements and minichromosome. Microb. Biotechnol. 2024, 17, e70069. [Google Scholar] [CrossRef]
- Wang, X.; Prokhnevsky, A.; Skarjinskaia, M.; Razzak, M.A.; Streatfield, S.J.; Lee, J. Facilitating viral vector movement enhances heterologous protein production in an established plant system. Plant Biotechnol. J. 2023, 21, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Mei, M.; Wei, R.; Hou, W.; Tang, X.; Zhao, Y.; Liao, X.; Zhou, R. Molecular cloning and heterologous expression of the mitochondrial ATP6 gene from kenaf (Hibiscus cannabinus) in tobacco (Nicotiana tabacum). Genes 2025, 16, 479. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Millar, A.A. TRUEE; a bioinformatic pipeline to define the functional microRNA targetome of Arabidopsis. Plant J. 2022, 110, 1476–1492. [Google Scholar] [CrossRef]
- Dong, Q.; Hu, B.; Zhang, C. microRNAs and their roles in plant development. Front. Plant Sci. 2022, 13, 824240. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Chen, Y.; He, C.; Pan, X.; Zhang, Y.; Xu, J.; Zheng, L.; Guan, H.; Wu, M.; et al. A plant bunyaviral protein disrupts SERRATE phase separation to modulate microRNA biogenesis during viral pathogenesis. Nat. Commun. 2025, 16, 6197. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Yan, Y.; Guan, W.; Huang, Z. Stimulation of Abscisic Acid Biosynthesis by Ethylene with Suppressive Action of MiR396 in Postharvest Strawberry Fruits. Horticulturae 2025, 11, 1280. https://doi.org/10.3390/horticulturae11111280
Chen R, Yan Y, Guan W, Huang Z. Stimulation of Abscisic Acid Biosynthesis by Ethylene with Suppressive Action of MiR396 in Postharvest Strawberry Fruits. Horticulturae. 2025; 11(11):1280. https://doi.org/10.3390/horticulturae11111280
Chicago/Turabian StyleChen, Renchi, Yuhua Yan, Weiliang Guan, and Zhihai Huang. 2025. "Stimulation of Abscisic Acid Biosynthesis by Ethylene with Suppressive Action of MiR396 in Postharvest Strawberry Fruits" Horticulturae 11, no. 11: 1280. https://doi.org/10.3390/horticulturae11111280
APA StyleChen, R., Yan, Y., Guan, W., & Huang, Z. (2025). Stimulation of Abscisic Acid Biosynthesis by Ethylene with Suppressive Action of MiR396 in Postharvest Strawberry Fruits. Horticulturae, 11(11), 1280. https://doi.org/10.3390/horticulturae11111280




