Integrating Transcriptomics and Metabolomics Analyses to Reveal the Potential Molecular Mechanism of Citrus junos Aroma Enhancement by Protected Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Electronic Nose (E-Nose) Analysis
2.3. Extraction of Volatile Organic Compounds (VOCs)
2.4. Metabolomic Analysis of VOCs
2.5. RNA-Sequencing and Data Analysis
3. Results
3.1. Effect of Protected Cultivation on Key Quality Traits of Yuzu
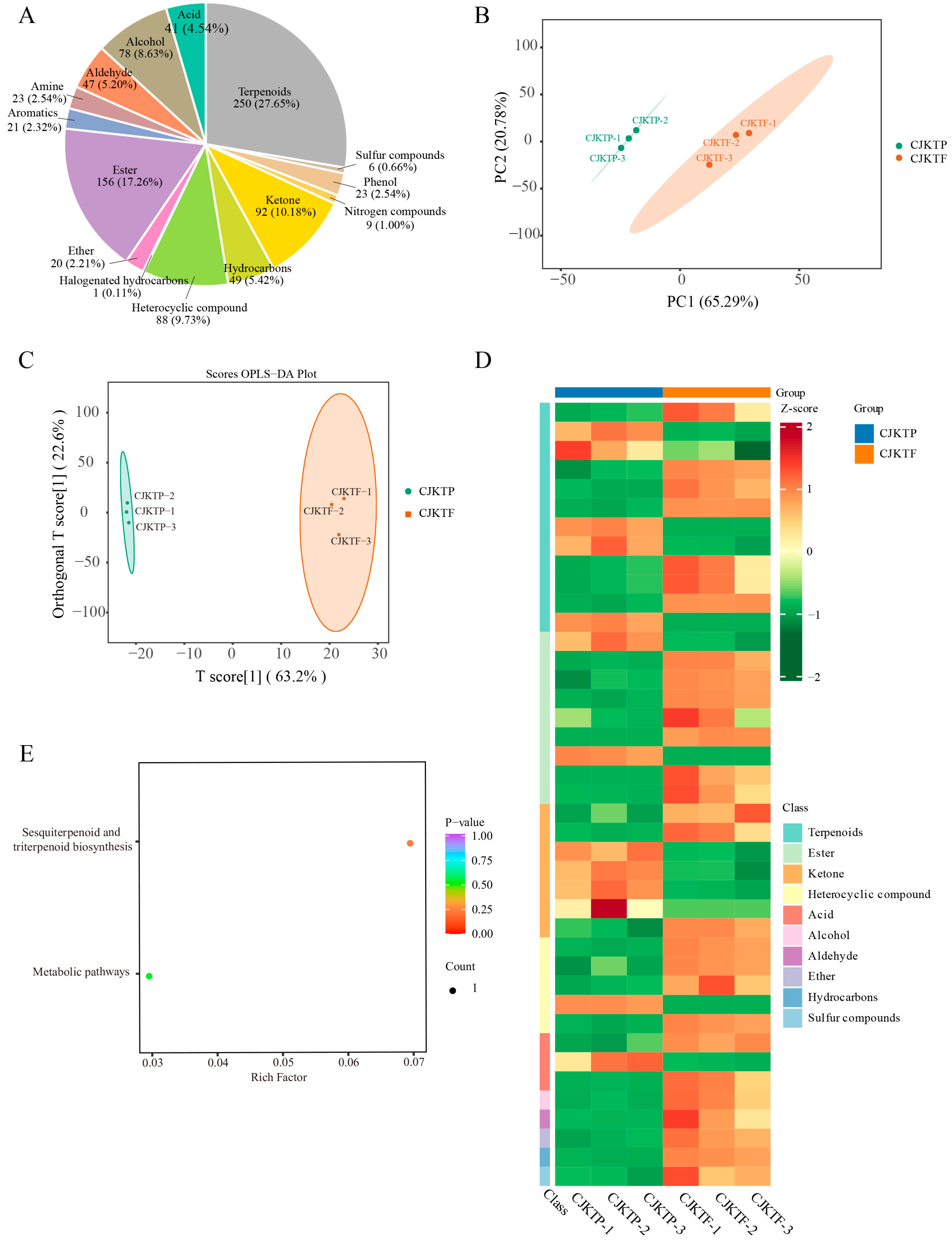
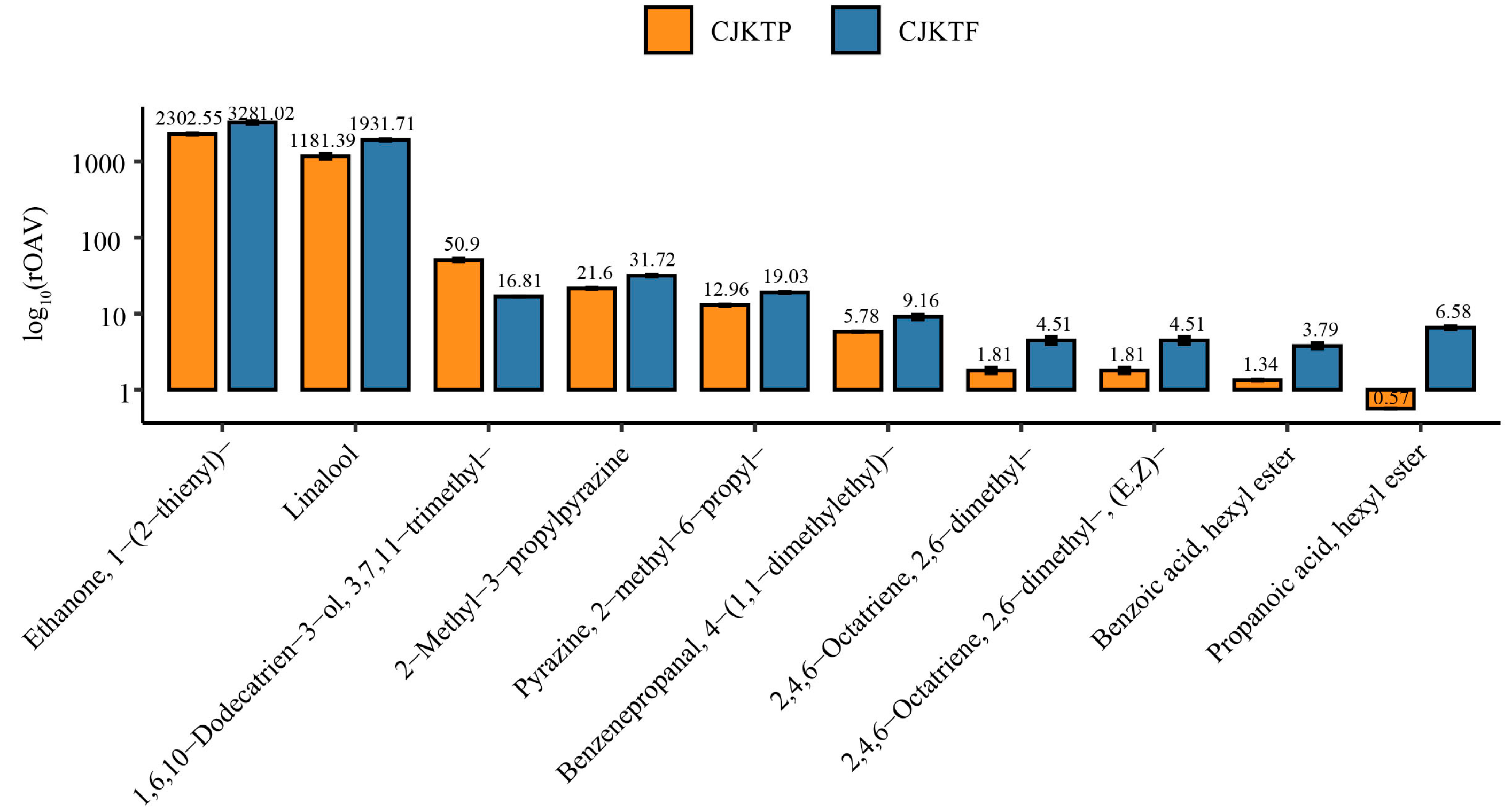
3.2. Comparison of VOCs Between CJKTP and CJKTF
3.3. Differential Accumulation of VOCs Between CJKTP and CJKTF
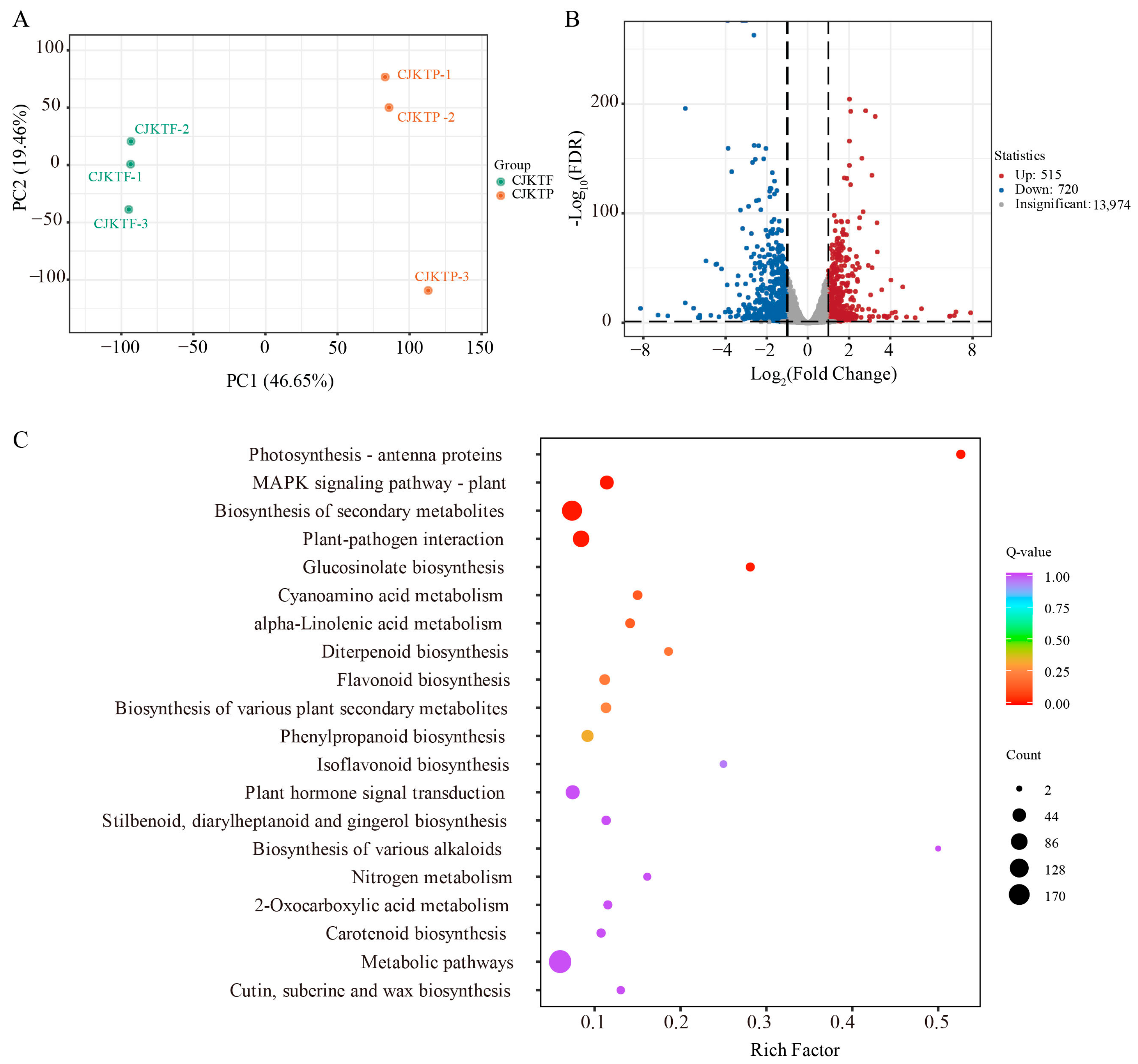
3.4. Transcriptomic Comparison Between CJKTP and CJKTF
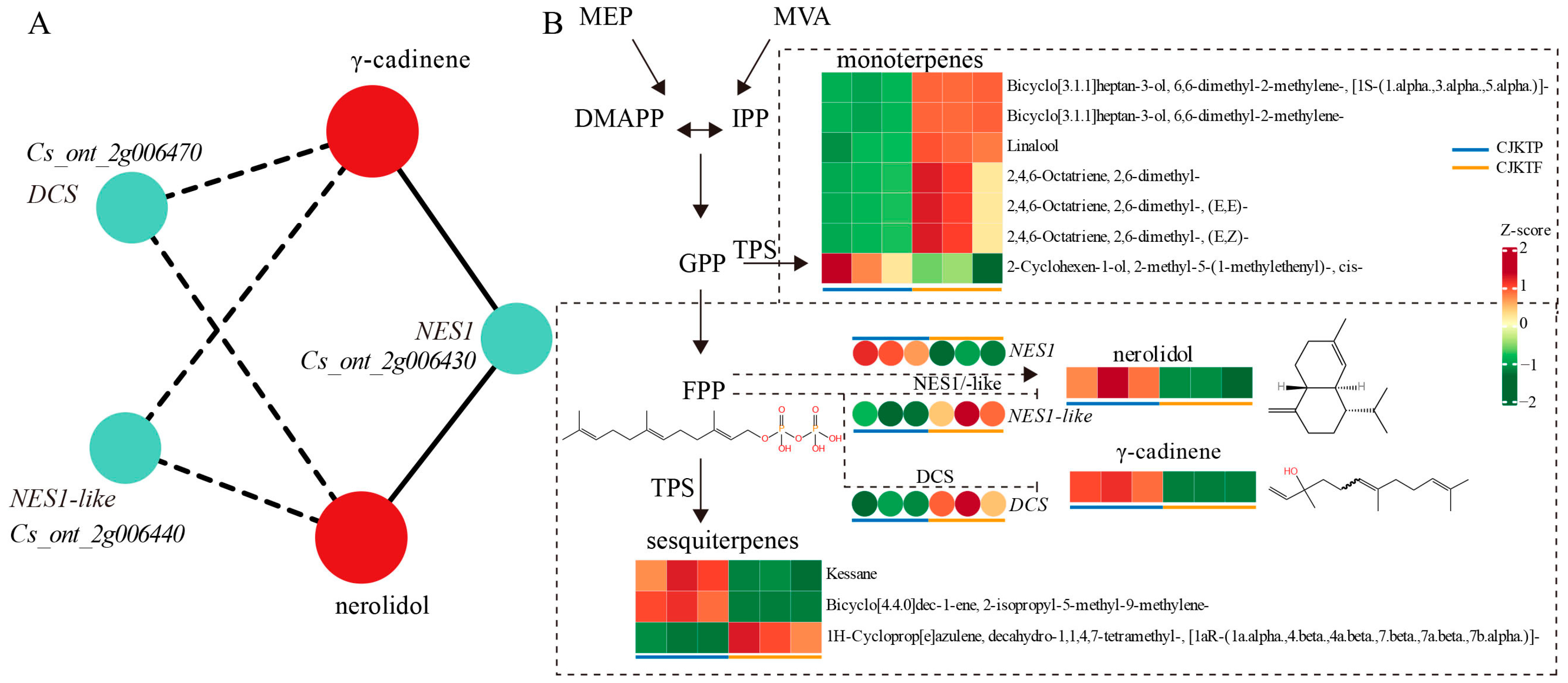
3.5. Combined Analyses of Differential Metabolites and DEGs
4. Discussion
4.1. Effect of Plastic Greenhouse Cultivation on Yuzu Quality
4.2. Effect of Plastic Greenhouse Cultivation on Metabolic Pathways of Yuzu
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOCs | Volatile organic compounds |
| TSS | Soluble solids content |
| TA | Titratable acid |
| VIP | Variable importance in projection |
| rOAV | Relative odor activity value |
References
- Park, M.K.; Cha, J.Y.; Kang, M.C.; Jang, H.W.; Choi, Y.S. The effects of different extraction methods on essential oils from orange and tangor: From the peel to the essential oil. Food Sci. Nutr. 2024, 12, 804–814. [Google Scholar] [CrossRef]
- Lan Phi, N.T.; Sawamura, M. Characteristic aroma composition profile of mature stage Citrus junos (Yuzu) peel oil from different origins. Food Sci. Technol. Res. 2008, 14, 359–366. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, T.; Asakura, H.; Hayashi, T. Effects of olfactory stimulation from the fragrance of the Japanese citrus fruit Yuzu (Citrus junos Sieb. ex Tanaka) on mood states and salivary chromogranin a as an endocrinologic stress marker. J. Altern. Complem. Med. 2014, 20, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, H.; Hashimoto, S.; Hayashi, S. First identification of the odour-active unsaturated aliphatic acid (E)-4-methyl-3-hexenoic acid in yuzu (Citrus junos Sieb. ex Tanaka). Flavour Fragr. J. 2013, 28, 62–69. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kimura, T.; Hayashi, T. Aromatic effects of a Japanese citrus fruit—Yuzu (Citrus junos Sieb. ex Tanaka)—On psychoemotional states and autonomic nervous system activity during the menstrual cycle: A single-blind randomized controlled crossover study. BioPsychoSoc. Med. 2016, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Cho, H.S.; Jeong, H.; Lee, B.B.; Cho, Y.S.; Rameeza, F.; Eun, J.B. Physiochemical properties, dietary fibers, and functional characterization of three yuzu cultivars at five harvesting times. Food Sci. Biotechnol. 2021, 30, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, J.; Zhang, J.; Cheng, H.; Zhu, C.; Zheng, X. Changes and differential analysis of aroma composition of Citrus junos and Citrus ichangensis at different developmental periods. China Fruits 2023, 8, 58–65. (In Chinese) [Google Scholar]
- Liu, C.; Cheng, Y.; Zhang, H.; Deng, X.; Chen, F.; Xu, J. Volatile constituents of wild citrus Mangshanyegan (Citrus nobilis Lauriro) peel oil. J. Agric. Food Chem. 2012, 60, 2617–2628. [Google Scholar] [CrossRef]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef]
- Asikin, Y.; Taira, I.; Inafuku-Teramoto, S.; Sumi, H.; Ohta, H.; Takara, K.; Wada, K. The composition of volatile aroma components, flavanones, and polymethoxylated flavones in Shiikuwasha (Citrus depressa Hayata) peels of different cultivation lines. J. Agric. Food Chem. 2012, 60, 7973–7980. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhao, H.; Zhong, T.; Chen, D.; Wu, Y.; Xie, Z. Molecular regulatory mechanisms affecting fruit aroma. Foods 2024, 13, 1870. [Google Scholar] [CrossRef]
- Ke, F.; Nie, Z.; Huang, X.; Cui, C.; Yang, Y.; Xu, J.; Wang, L.; Sun, L. Investigating the Effect of Two Interstocks, Changshanhuyou and Ponkan, on the fruit quality and volatile flavor of cocktail grapefruit (Citrus paradisi Macf. cv. Cocktail). Horticulturae 2025, 11, 403. [Google Scholar] [CrossRef]
- Noda, T.; Daiou, K.; Mihara, T.; Murakami, H.; Nagano, Y. Efficient method for generating citrus hybrids with polyembryonic Satsuma mandarin as the female parent. Mol. Breed. 2022, 42, 51. [Google Scholar] [CrossRef]
- Pachiyappan, P.; Kumar, P.; Reddy, K.V.; Kumar, K.N.R.; Konduru, S.; Paramesh, V.; Rajanna, G.A.; Shankarappa, S.K.; Jaganathan, D.; Immanuel, S.; et al. Protected cultivation of horticultural crops as a livelihood opportunity in western India: An economic assessment. Sustainability 2022, 14, 7430. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Rahimi Devin, S.; Salazar, J.A.; López-Alcolea, J.; Rubio, M.; Martínez-García, P.J. Principles and prospects of prunus cultivation in greenhouse. Agronomy 2021, 11, 474. [Google Scholar] [CrossRef]
- Stanghellini, C.; Montero, J.I. Resource use efficiency in protected cultivation: Towards the greenhouse with zero emissions. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927, Lisbon, Portugal, 22–27 August 2010; pp. 91–100. [Google Scholar]
- Zhao, X.; Peng, J.; Zhang, L.; Yang, X.; Qiu, Y.; Cai, C.; Hu, J.; Huang, T.; Liang, Y.; Li, Z.; et al. Optimizing the quality of horticultural crop: Insights into pre-harvest practices in controlled environment agriculture. Front. Plant Sci. 2024, 15, 1427471. [Google Scholar] [CrossRef]
- Li, W.; Zheng, T.; Zhang, J.; Li, W.; Chen, K.; Zhang, K.; Fang, Y. Supplementary light with different wavelengths improved the monoterpenes aroma and quality traits of ‘Shine Muscat’ grape berries under facility cultivation. Food Chem. 2025, 474, 143255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Tian, X.; Yu, H.; Yu, Y. Optimization of sensor array and detection of stored duration of wheat by electronic nose. J. Food Eng. 2007, 82, 403–408. [Google Scholar] [CrossRef]
- Zhai, Y.; Guo, M.; Meng, Q.; Song, H. Characterization of key odor-active compounds in high quality high-salt liquid-state soy sauce. J. Food Compos. Anal. 2023, 117, 105148. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Xiao, J.; Liu, J.; Tang, N.; Zhou, A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, L.; Viau, C.; Lu, Y.; Salavati, R.; Basu, N.; Xia, J. MetaboAnalystR 4.0: A unified LC-MS workflow for global metabolomics. Nat. Commun. 2024, 15, 3675. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, P.; Yin, J.; Wang, W.; Zhang, J.; Wang, W.; Le, T.; Ni, D.; Jiang, H. Dynamic changes in volatile compounds of shaken black tea during its manufacture by GC × GC–TOFMS and multivariate data analysis. Foods 2022, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Quan, S.; Xiao, H. Towards efficient terpenoid biosynthesis: Manipulating IPP and DMAPP supply. Bioresour. Bioprocess. 2019, 6, 6. [Google Scholar] [CrossRef]
- Choudhury, S.; Islam, N.; Mustaki, S.; Uddain, J.; Azad, M.O.K.; Choi, K.Y.; Naznin, M.T. Evaluation of the different low-tech protective cultivation approaches to improve yield and phytochemical accumulation of papaya (Carica papaya L.) in bangladesh. Horticulturae 2022, 8, 210. [Google Scholar] [CrossRef]
- DurgaÇ, C.; Polat, A.A.; KamİLoĞLu, Ö. Comparison of open field and protected cultivation of five early table grape cultivars under Mediterranean conditions. Turk. J. Agric. For. 2011, 35, 491–499. [Google Scholar] [CrossRef]
- Adeeko, A.; Yudelevich, F.; Raphael, G.; Avraham, L.; Alon, H.; Zaaroor Presman, M.; Alkalai-Tuvia, S.; Fallik, E.; Paris, H.S.; Ziv, C. Trellising is advantageous over ground culture for out-of-season, protected production and storage of sweet acorn squash. Front. Hortic. 2024, 3, 1365147. [Google Scholar] [CrossRef]
- Brunele Caliman, F.R.; Henriques da Silva, D.J.; Stringheta, P.C.; Rezende Fontes, P.C.; Rodrigues Moreira, G.; Chartuni Mantovani, E. Quality of tomatoes grown under a protected environment and field conditions. Idesia 2010, 28, 75–82. [Google Scholar] [CrossRef]
- Ahemd, H.A.; Al-Faraj, A.A.; Abdel-Ghany, A.M. Shading greenhouses to improve the microclimate, energy and water saving in hot regions: A review. Sci. Hortic. 2016, 201, 36–45. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Jens, R.; Rolf, N.; Johannes, A.F.; Tor Henning, I. Influence of rain cover cultivation on taste and aroma quality of strawberries (Fragaria ananassa Duch.). J. Food Agric. Environ. 2004, 2, 74–82. [Google Scholar]
- Selli, S.; Gubbuk, H.; Kafkas, E.; Gunes, E. Comparison of aroma compounds in Dwarf Cavendish banana (Musa spp. AAA) grown from open-field and protected cultivation area. Sci. Hortic. 2012, 141, 76–82. [Google Scholar] [CrossRef]
- Hendges, M.V.; Moreira, M.A.; Steffens, C.A.; Talamini do Amarante, C.V. Aromatic profile of Feijoa (Feijoa sellowiana) fruit in protected cultivation, at harvest and after cold storage. Sci. Hortic. 2022, 293, 110691. [Google Scholar] [CrossRef]
- Liu, M.; Xie, F.; Cao, R.; Qi, X.; Chen, X. Effect of different cover cultivations in later summer on aroma constituents of autumn tea (Camellia sinensis L.). J. Agric. Chem. Environ. 2014, 03, 1–6. [Google Scholar] [CrossRef]
- Feng, J.; Ye, S.; Wang, J.; Wu, J.; Zhao, J.; Tian, W.; Pan, G.; Yu, B.; Qiu, D.; Lin, H.; et al. From water migration to aroma development: Revealing the influence of environmental airflow on the aroma of white tea during withering. Food Chem. 2025, 479, 143797. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A Review. New Zeal. J.Crop Hort Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Xue, W.; Liu, N.; Lu, P.; Yang, Y.; Chen, S. Photosynthesis and fatty acid metabolism reveals the effects of shading treatment on the fruit aroma quality of cucumber (Cucumis sativus L.). Sci. Hortic. 2024, 337, 113508. [Google Scholar] [CrossRef]
- Gouinguené, S.P.; Turlings, T.C.J. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef]
- Kang, Z.; Liu, F.; Zhang, Z.; Tian, H.; Liu, T. Volatile β-ocimene can regulate developmental performance of peach aphid myzus persicae through activation of defense responses in Chinese cabbage Brassica pekinensis. Front. Plant Sci. 2018, 9, 708. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Q.; Wang, L.; Lv, M.; Hou, Y.; Li, S. Linalool: A ubiquitous floral volatile mediating the communication between plants and insects. J. Syst. Evol. 2023, 61, 538–549. [Google Scholar] [CrossRef]
- Wiraswati, H.L.; Fauziah, N.; Pradini, G.W.; Kurnia, D.; Kodir, R.A.; Berbudi, A.; Arimdayu, A.R.; Laelalugina, A.; Supandi; Ma’ruf, I.F. Breynia cernua: Chemical profiling of volatile compounds in the stem extract and its antioxidant, antibacterial, antiplasmodial and anticancer activity in vitro and in silico. Metabolites 2023, 13, 281. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xu, Y.; Chen, T.; Li, B.; Zhang, Z.; Tian, S. Application and mechanism of benzyl-isothiocyanate, a natural antimicrobial agent from cruciferous vegetables, in controlling postharvest decay of strawberry. Postharvest Biol. Technol. 2021, 180, 111604. [Google Scholar] [CrossRef]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Dong, N.; Lin, H. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef]
- Wang, W.; Shen, X.; Zhang, S.; Lv, R.; Liu, M.; Xu, W.; Chen, Y.; Wang, H. Research on very volatile organic compounds and odors from veneered medium density fiberboard coated with water-based lacquers. Molecules 2022, 27, 3626. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yan, W.; Fan, Z.; Yang, Y.; Yu, A. Free volatile terpenoids and their interconversions in the pulp of Newhall navel orange during shelf life. J. Food Compos. Anal. 2025, 145, 107772. [Google Scholar] [CrossRef]
- Green, S.A.; Chen, X.; Nieuwenhuizen, N.J.; Matich, A.J.; Wang, M.Y.; Bunn, B.J.; Yauk, Y.K.; Atkinson, R.G. Identification, functional characterization, and regulation of the enzyme responsible for floral (E)-nerolidol biosynthesis in kiwifruit (Actinidia chinensis). J. Exp. Bot. 2012, 63, 1951–1967. [Google Scholar] [CrossRef]
- Aharoni, A.; Giri, A.P.; Deuerlein, S.; Griepink, F.; de Kogel, W.-J.; Verstappen, F.W.A.; Verhoeven, H.A.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Terpenoid metabolism in wild-type and transgenic arabidopsis plants. Plant Cell 2003, 15, 2866–2884. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, C.; Abodi, M.; D’Oria, V.; Milani, G.P.; Agostoni, C.; Mazzocchi, A. Alpha-linolenic acid and cardiovascular events: A narrative review. Int. J. Mol. Sci. 2023, 24, 14319. [Google Scholar] [CrossRef]
- Gürbüz, M.; Aktaç, Ş. Understanding the role of vitamin A and its precursors in the immune system. Nutr. Clin. Et Métab. 2022, 36, 89–98. [Google Scholar] [CrossRef]
- Bufka, J.; Vaňková, L.; Sýkora, J.; Křížková, V. Exploring carotenoids: Metabolism, antioxidants, and impacts on human health. J. Func. Foods 2024, 118, 106284. [Google Scholar] [CrossRef]

| Class | Compound Name | Log2FC | VIP |
|---|---|---|---|
| Ester | Benzeneacetic acid, methyl ester | −6.59 | 1.26 |
| Ester | Propanoic acid, hexyl ester | −4.03 | 1.26 |
| Acid | Benzoic acid, 2-nitro- | −2.43 | 1.25 |
| Ester | cis-3-Hexenyl iso-butyrate | −2.33 | 1.04 |
| Ester | Butanoic acid, 5-hexenyl ester | −2.22 | 1.25 |
| Ester | Benzoic acid, hexyl ester | −2.00 | 1.24 |
| Terpenoid | 2,4,6-Octatriene, 2,6-dimethyl-, (E,E)- | −1.82 | 1.21 |
| Terpenoid | 2,4,6-Octatriene, 2,6-dimethyl-, (E,Z)- | −1.82 | 1.21 |
| Terpenoid | 2,4,6-Octatriene, 2,6-dimethyl- | −1.82 | 1.21 |
| Sulfur compound | Benzene, isothiocyanato- | −1.30 | 1.23 |
| Ether | 2,3-Dimethylanisole | −1.30 | 1.25 |
| Terpenoid | Linalool | −1.20 | 1.24 |
| Aldehyde | Benzenepropanal, 4-(1,1-dimethylethyl)- | −1.16 | 1.22 |
| Heterocyclic compound | 1,3-Benzodioxole, 2-acetyl-4-methoxy-2-methyl- | −1.16 | 1.23 |
| Heterocyclic compound | 3-Acetyl-2,5-dimethyl furan | −1.14 | 1.22 |
| Ester | Butanoic acid, 2-pentenyl ester, (Z)- | −1.14 | 1.24 |
| Hydrocarbon | Cyclohexene, 2-ethenyl-1,3,3-trimethyl- | −1.12 | 1.26 |
| Alcohol | 2-Naphthalenethiol | −1.07 | 1.24 |
| Heterocyclic compound | 2-Methyl-3-propylpyrazine | −1.04 | 1.26 |
| Heterocyclic compound | Pyrazine, 2-methyl-6-propyl- | −1.04 | 1.26 |
| Ester | 3-(Methylthio)propanoic acid ethyl ester | −1.04 | 1.26 |
| Terpenoid | Bicyclo[3.1.1]heptan-3-ol, 6,6-dimethyl-2-methylene-, [1S-(1.alpha.,3.alpha.,5.alpha.)]- | −1.03 | 1.26 |
| Terpenoid | Bicyclo[3.1.1]heptan-3-ol, 6,6-dimethyl-2-methylene- | −1.03 | 1.26 |
| Terpenoid | 1H-Cycloprop[e]azulene, decahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha.,4.beta.,4a.beta.,7.beta.,7a.beta.,7b.alpha.)]- | −1.02 | 1.25 |
| Ketone | Ketone, methyl 2,4,5-trimethylpyrrol-3-yl | −1.02 | 1.23 |
| Acid | Benzoic acid, 2-amino-4-methyl- | −1.01 | 1.24 |
| Ketone | Ethanone, 1-(2-thienyl)- | −1.01 | 1.23 |
| Ketone | Ethanone, 1-(2-methyl-1-cyclopenten-1-yl)- | −1.00 | 1.22 |
| Terpenoid | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl- | 1.11 | 1.24 |
| Ketone | 1(2H)-Naphthalenone, octahydro-4-hydroxy-, trans- | 1.11 | 1.24 |
| Ketone | 3,5,9-Undecatrien-2-one, 6,10-dimethyl-, (E, Z)- | 1.14 | 1.24 |
| Ester | Citronellyl butyrate | 1.17 | 1.24 |
| Ketone | 2(3H)-Furanone, 5-hexyldihydro-4-methyl-, trans- | 1.27 | 1.22 |
| Acid | 2,3,4-Trihydroxybenzoic acid | 1.35 | 1.22 |
| Terpenoid | 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, cis- | 1.39 | 1.03 |
| Terpenoid | Kessane | 1.40 | 1.25 |
| Ester | Benzoic acid, 4-ethoxy-, ethyl ester | 2.60 | 1.26 |
| Ketone | Cyclohexanone, 2-methyl-5-(1-methylethyl)- | 3.13 | 1.19 |
| Terpenoid | Bicyclo[4.4.0]dec-1-ene, 2-isopropyl-5-methyl-9-methylene- | 3.73 | 1.26 |
| Terpenoid | Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1.alpha.,4a.beta.,8a.alpha.)- | 3.73 | 1.26 |
| Heterocyclic compound | Pyrazine, 2-methyl-5-(1-methylethyl)- | 8.23 | 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, F.; Huang, X.; Sun, L.; Wang, L.; Nie, Z.; Yang, Y.; Cui, C. Integrating Transcriptomics and Metabolomics Analyses to Reveal the Potential Molecular Mechanism of Citrus junos Aroma Enhancement by Protected Cultivation. Horticulturae 2025, 11, 945. https://doi.org/10.3390/horticulturae11080945
Ke F, Huang X, Sun L, Wang L, Nie Z, Yang Y, Cui C. Integrating Transcriptomics and Metabolomics Analyses to Reveal the Potential Molecular Mechanism of Citrus junos Aroma Enhancement by Protected Cultivation. Horticulturae. 2025; 11(8):945. https://doi.org/10.3390/horticulturae11080945
Chicago/Turabian StyleKe, Fuzhi, Xiu Huang, Lifang Sun, Luoyun Wang, Zhenpeng Nie, Yi Yang, and Changjiang Cui. 2025. "Integrating Transcriptomics and Metabolomics Analyses to Reveal the Potential Molecular Mechanism of Citrus junos Aroma Enhancement by Protected Cultivation" Horticulturae 11, no. 8: 945. https://doi.org/10.3390/horticulturae11080945
APA StyleKe, F., Huang, X., Sun, L., Wang, L., Nie, Z., Yang, Y., & Cui, C. (2025). Integrating Transcriptomics and Metabolomics Analyses to Reveal the Potential Molecular Mechanism of Citrus junos Aroma Enhancement by Protected Cultivation. Horticulturae, 11(8), 945. https://doi.org/10.3390/horticulturae11080945






