Effects of a Novel Waterlogging-Tolerant Growth-Promoting Pelletizing Agent on the Growth of Brassica napus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Seed Pelleting Method
2.2.2. Agronomic Trait Investigation
2.2.3. Quality Determination Method
2.2.4. Resistance Investigation Method
- Grade 0 (No freezing injury)
- Grade 1 (1/3 leaves wilted)
- Grade 2 (1/2 leaves browned)
- Grade 3 (Apical meristem necrosis)
- Grade 4 (Whole plant death)
- Grade 0 (Normal growth, no symptoms)
- Grade 1 (1/3 outer leaves reddening, normal heart leaves)
- Grade 2 (1/3–2/3 outer leaves red/yellow, or <1/3 shriveled leaves)
- Grade 3 (2/3 leaves yellowing, 1/3–2/3 shriveled leaves)
- Grade 4 (>2/3 leaves necrotic, plant death)
- Disease incidence rate (%) = (Number of infected plants/Total plants) × 100%
- Disease severity index (0–4 scale by stem lesion area):
- −
- Grade 0: No disease
- −
- Grade 1: ≤5%
- −
- Grade 2: 6–15%
- −
- Grade 3: 16–25%
- −
- Grade 4: >25%
2.2.5. Physiological Index Determination Method
2.2.6. Benefit Analysis
2.3. Data Analysis Methods
3. Results and Analysis
3.1. Agronomic Traits
3.1.1. Investigation of Seedling Traits at 3–4 Leaf Stage
3.1.2. Pre-Winter Agronomic Traits Survey
3.1.3. Harvest Trait Survey
3.1.4. Quality Analysis at Harvest Stage
3.2. Resistance Investigation
3.2.1. Frost Damage Survey
3.2.2. Waterlogging Damage Survey
3.2.3. Disease Survey
3.3. Physiological Indicators Measurement
3.3.1. POD Activity Measurement
3.3.2. SOD Activity Determination
3.3.3. MDA Content Measurement
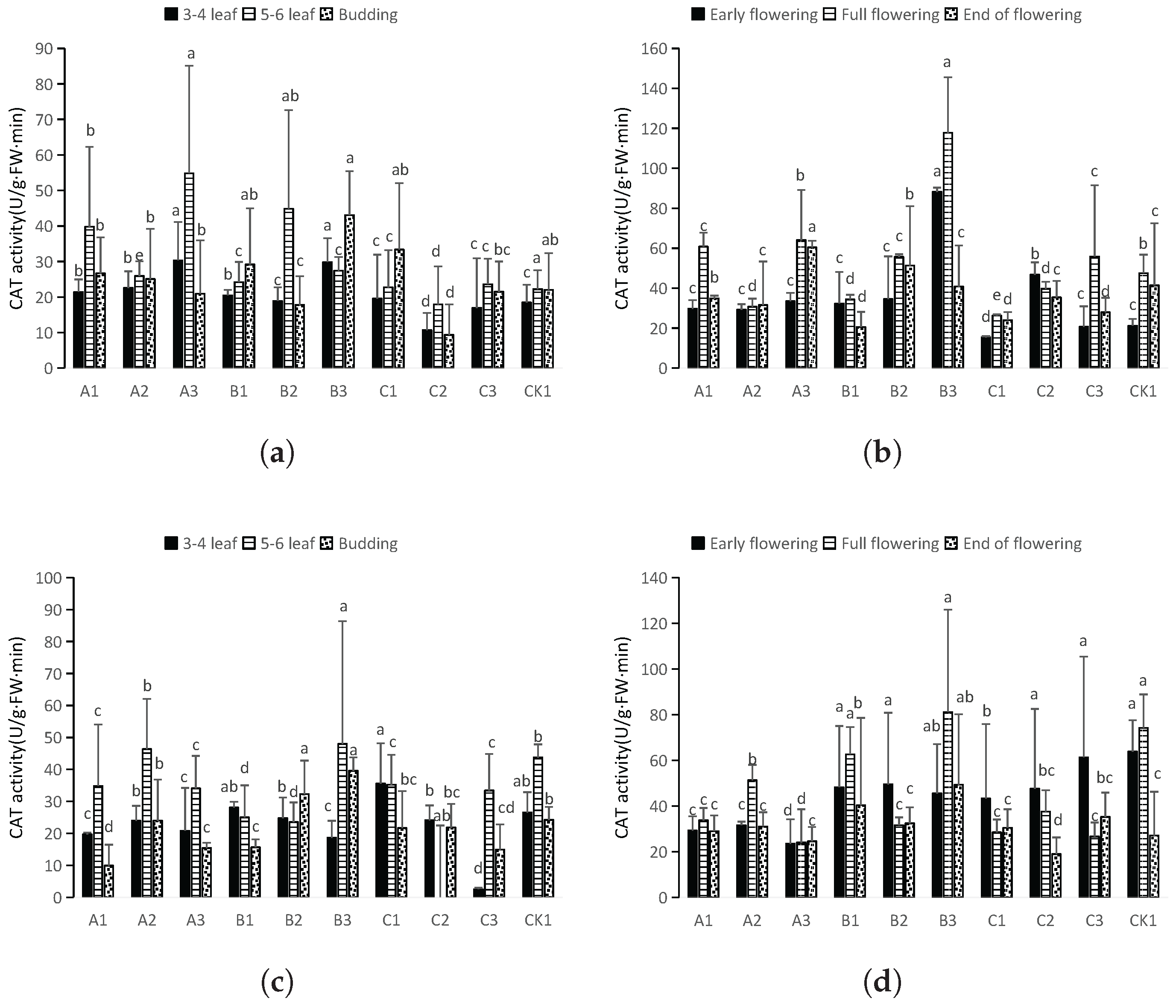
3.3.4. CAT Activity Assay
3.3.5. Soluble Protein Content Measurement
3.3.6. Relative Electrical Conductivity Measurement
3.4. Benefit Analysis
4. Discussion and Conclusions
4.1. Discussion
4.2. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, L.; Zhang, T.; Chen, Y. Identification of Rapeseed Seedling Number Based on YC-YOLO v7 Model. Trans. Chin. Soc. Agric. Mach. 2024, 55, 322–332. [Google Scholar]
- Yan, M.; Ge, W.; Zhang, X.; Huang, Y.; Zhang, Z.; Zhang, Y. Situation analysis and development countermeasures of China’s oilseed industry. China Oils Fats 2023, 48, 8–18. [Google Scholar] [CrossRef]
- Yu, Z.; Yuelin, J. Influence of Forced Water Logging During Florescence on the Growth and Output of Winter Rape. J. Agric. 2014, 4, 24–27. [Google Scholar]
- Liu, R. Identification of Brassica napus Anthocyanin-susceptibleMutant as and Its Response to Adverse Stress. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2019. [Google Scholar]
- Zhou, X.; Xu, J.; Xie, L.; Xu, B.; Zhang, X. Physiological Mechanisms in Response to Waterlogging During Seedling Stage of Brassica napus L. Crop J. 2024, 50, 1015–1029. [Google Scholar]
- Zhang, X.; Fan, Q.; Chen, J.; Li, J.; Wang, H. Physiological Reaction Differences of Different WaterloggingTolerant Genotype Rapeseed (Brassica napus L.) to Anoxia. Sci. Agric. Sin. 2007, 40, 485–491. [Google Scholar]
- Zhou, X. Effects of Waterlogging Stress on Physiological and Biochemical Indexes of Brassica napus L. Master’s Thesis, Yangtze University, Jingzhou, Chins, 2024. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, L.; Chen, S.; Cai, Y.; Du, X.; Yang, L.; Huang, X.; Xue, J. Research progress of seed pelleting technology at home and abroad. Crops 2024, 6, 18–25. [Google Scholar] [CrossRef]
- Kaufman, G. Seed Coating: A Tool for Stand Establishment; a Stimulus to Seed Quality. HortTechnology 1991, 1, 98–102. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H. New Generation Seed Coating Agent 63% F·K·W Corn Dry Powder Seed Coating Agent. China Seed Ind. 2005, 45–46. [Google Scholar]
- Otani, T.; Nakata, H.; Akao, T.; Maeda, Y. Coated Seed and Process for Producing the Same. U.S. Patent US5918413A, 6 July 1999. [Google Scholar]
- Hirano, S.; Hayashi, M.; Okuno, S. Soybean Seeds Surface-Coated with Depolymerised Chitins: Chitinase Activity as a Predictive Index for the Harvest of Beans in Field Culture. J. Sci. Food Agric. 2001, 81, 205–209. [Google Scholar] [CrossRef]
- Chandrika, K.P.; Prasad, R.; Godbole, V. Development of Chitosan-PEG Blended Films Using Trichoderma: Enhancement of Antimicrobial Activity and Seed Quality. Int. J. Biol. Macromol. 2019, 126, 282–290. [Google Scholar] [CrossRef]
- Sanchez, P.L.; Chen, M.k.; Pessarakli, M.; Hill, H.J.; Gore, M.A.; Jenks, M.A. Effects of Temperature and Salinity on Germination of Non-Pelleted and Pelleted Guayule (Parthenium Argentatum A. Gray) Seeds. Ind. Crops Prod. 2014, 55, 90–96. [Google Scholar] [CrossRef]
- Wang, H.; Gao, F.; Li, D. Drought-Resistant Seed Composite Coating Agent and Preparation Method Thereof. China CN1108878A, 30 September 1995. [Google Scholar]
- Xiong, H.; Zhang, X.; Lin, H.; Li, X.; Xiong, Y.; Zou, Y. Effects of Seed-coating Agent on Seedling Growth of No-tillage and Direct-seeding Rape. Chin. Agric. Sci. Bull. 2011, 27, 274–278. [Google Scholar]
- Yu, J.; Zhang, G.; Zhang, X.; Zhu, A.; Zhang, H.; Wang, X.; Miao, K.; Zhang, J.; Shu, Z.; Yao, K. The efficacy of rice seed pelleting agent on the control of key pests and yield under wet direct seeding mode. Plant Prot. 2025, 51, 339–345, 351. [Google Scholar] [CrossRef]
- Bai, W.; Huang, J.; Wei, Y.; Liu, W.; Chai, J.; Yang, F.; Li, X. Screening of quinoa seed pelleting formula and effect verification. Crops 2025, 6–14, 1–8. [Google Scholar]
- She, H.; Sun, M.; Li, S.; Wang, D.; Cheng, T.; Jiang, B.; Chen, A.; Wang, J.; Zhao, J.; Wang, B.; et al. Effect of coating with fulvic acid and alginate oligosaccharide on emergence and yield of late-sown rapeseed. Crop J. 2025, 51, 1022–1036. [Google Scholar]
- Li, L.; Chen, Z.; Zhou, Y.; Ding, F.; Wang, G.; Zhang, Z. Effects of Different Seed Coating Formulations on Growth of Rapeseed. J. Agric. 2024, 14, 1–5. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zhang, Y.; Li, X.; Zhai, C.; Li, Q.; Chen, L. Effects of Cotton Seed Pelletizing on the Cold-resistance and PhysiologicalCharacteristics in Germinating Process. J. Cotton Res. 2008, 20, 73–75. [Google Scholar]
- Chen, Y. Optimization of Pellet Granulation Technology for Allium mongolicum seeds and Analysis of Drought and Salinity Resistance Effects. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2024. [Google Scholar] [CrossRef]
- Xie, J.; Han, L. Current status and prospects of seed pelleting research in China. Chin. J.-Eco-Agric. 2024, 32, 605–615. [Google Scholar]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin. Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef]
- Mandal, A.B.; Mondal, R.; Dutta, P.M.S. Seed enhancement through priming, coating and pelleting for uniform crop stand and increased productivity. J. Andaman Sci. Assoc. 2015, 20, 26–33. [Google Scholar]
- Chen, D.; Zhou, L.; Chen, Q.; Yang, J.; Ren, J. Study on the Technology of Pelletizing and Coating Rapeseed. Seed 2004, 23, 85–86. [Google Scholar] [CrossRef]
- Li, J. Dynamic Process and Genetic Basis of Rapeseed in Response to Waterlogging Occurred at Seedling Stage. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2022. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Lei, Y.; Xiao, S.; She, H.; Duan, S.; Huang, M.; Kuai, J.; Wang, B.; Wang, J.; et al. Effects of seed soaking with exogenous substances on late-seeded rapeseed cold resistance of during overwintering period and yield. Crop J. 2024, 50, 1271–1286. [Google Scholar]
- Ma, R. Identification of Early Flowering Mutants in Brassica napus L. and Study on Its Molecular Mechanism. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2022. [Google Scholar] [CrossRef]
- Jiang, L.; Ren, J.; Zhang, Z.; Miao, P. Effects of Harvest Time on the Yield and Quality of Different Maturing Stage Rapeseed Varieties. China Seed Ind. 2023, 9, 100–104. [Google Scholar] [CrossRef]
- Gao, S.; Huang, J.; Gao, F.; Yao, X. Analysis on the impact of frost damage level and index on rapeseed production. Rural. Econ.-Sci.-Technol. 2014, 25, 36–38. [Google Scholar]
- Wang, X.; Chen, H.; Zhang, Z.; Guan, C. Analysis of the Relationship Between Two Disease-Resistant Related Genes and Diseases in High Oleic Acid Rapeseed. J. Nucl. Agric. Sci. 2019, 33, 894–901. [Google Scholar]
- Li, H. Principles and Techniques of Plant Physiological and Biochemical Experiments, 3rd ed.; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Chen, A.; Han, R.; Li, D.; Ling, L.; Luo, H.; Tang, S. Comparative study on the determination methods of relative electrical conductivity of plant leaves. J. Guangdong Educ. Inst. 2010, 30, 88–91. [Google Scholar]
- Qiu, Q.; Xiao, Y.; Yan, M.; Liao, L. Large-Area Demonstration and Plot Comparison Experiment of a New Rape Variety Xiangzayou 787. Hunan Agric. Sci. 2020, 10, 13–16. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Zhang, S.; Li, G. Effects of waterlogging on growth and physiological changes ofwinter rapeseed seedling (Brassica napus L.). Oil Crop Sci. 2011, 33, 247–252. [Google Scholar]
- Peng, D.; Dai, Y.; Zhang, Q.; Chen, H.; Yang, L.; Zhang, Z. Study on Difference of Seedling Growth and Physiological Characteristics Between Three-way Cross Rapeseed and Its Parents Under Different Water Stress. Chin. Agric. Sci. Bull. 2024, 40, 20–26. [Google Scholar]
- Nie, L. Identification and Related Mechanisms of Waterlogging Tolerance at the Seedling Emergence of Oilseed Rape. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2024. [Google Scholar] [CrossRef]
- Li, C. The current status of seed coating technology and its impact on crop yield. Seed Sci. Technol. 2025, 43, 198–200. [Google Scholar] [CrossRef]
- Huang, B. Effects of Melatonin on the Structure and Function of Photosystem II Under High Light Stress. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2020. [Google Scholar]
- Guan, W. Overexpression of BnaNAC100 Regulated Salttolerance and Plant Type in Brassica napus. Master’s Thesis, Northwest A & F University, Yangling, China, 2024. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, W.; Yang, S.; Sun, Y.; Zhang, L. Study on high-yield and high-efficiency cultivation technology of rapeseed in Anhui Yangtze River area by direct seeding and mechanical harvesting. Nongcun Shiyong Jishu 2023, 11, 119–121. [Google Scholar]
- Fang, D. Effects of Waterlogging on Winter Wheat Rootgrowth and Development, and Its Alleviationby Zinc Application in the Huai River Region, Anhui Province, China. Ph.D. Thesis, Anhui Agricultural University, Hefei, China, 2023. [Google Scholar]
- Liu, C.; Liang, S.; Wu, J.; Gu, J.; Duan, H. Responses of growth and physiological and biochemical characteristics of Pinus yunnanensis seedlings to drought and rewatering. J. Northwest A&F Univ. 2025, 54, 99–108. [Google Scholar] [CrossRef]





| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1mg/mL BSA (mL) | 0 | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 | 1.0 |
| Distilled water (mL) | 1.0 | 0.9 | 0.7 | 0.5 | 0.3 | 0.1 | 0 |
| Coomassie brilliant blue G-250 (mL) | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Protein content (mg) | 0 | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 | 1.0 |
| Va. | Tr. | FWag (g) | DWag (g) | DFag | FWrt (g) | DWrt (g) | DFrt | Lag (cm) | Rlen (cm) | Llen (cm) | Lwid (cm) | Diam (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xiangzayou 787 | A1 | 14.15 ± 0.42 a | 1.93 ± 0.31 a | 0.14 ± 0.02 | 0.98 ± 0.21 ab | 0.33 ± 0.04 ab | 0.07 ± 0.02 a | 3.85 ± 0.75 a | 7.82 ± 1.35 a | 9.28 ± 1.52 a | 7.95 ± 0.19 ab | 1.62 ± 0.03 a |
| A2 | 6.31 ± 1.22 d | 0.97 ± 0.19 b | 0.15 ± 0.04 | 0.65 ± 0.20 bc | 0.36 ± 0.34 a | 0.10 ± 0.04 b | 3.58 ± 0.48 a | 5.77 ± 0.18 a | 7.05 ± 0.85 cd | 6.01 ± 0.18 cd | 0.64 ± 0.09 a | |
| A3 | 5.12 ± 0.95 e | 0.85 ± 0.16 bc | 0.17 ± 0.05 | 0.38 ± 0.18 c | 0.07 ± 0.05 b | 0.07 ± 0.03 c | 3.61 ± 1.05 a | 6.69 ± 2.89 a | 6.73 ± 0.68 abc | 5.04 ± 0.23 ef | 0.34 ± 0.19 a | |
| B1 | 9.32 ± 0.58 c | 1.16 ± 0.05 b | 0.12 ± 0.01 | 1.27 ± 0.26 a | 0.16 ± 0.03 ab | 0.14 ± 0.03 c | 2.65 ± 0.36 ab | 7.29 ± 1.52 a | 8.35 ± 0.27 abc | 6.66 ± 0.34 c | 0.49 ± 0.03 a | |
| B2 | 8.55 ± 0.85 c | 1.22 ± 0.22 b | 0.14 ± 0.03 | 0.63 ± 1.03 bc | 0.10 ± 0.02 b | 0.07 ± 0.11 c | 3.32 ± 1.04 ab | 6.91 ± 2.22 a | 7.83 ± 0.90 bc | 6.73 ± 0.73 c | 0.53 ± 0.10 a | |
| B3 | 6.75 ± 1.17 d | 1.07 ± 0.19 b | 0.16 ± 0.04 | 0.98 ± 0.07 ab | 0.19 ± 0.03 ab | 0.15 ± 0.02 c | 3.63 ± 0.11 a | 5.70 ± 0.09 a | 7.46 ± 0.57 cd | 6.17 ± 0.36 cd | 0.48 ± 0.04 a | |
| C1 | 7.10 ± 0.26 d | 1.12 ± 0.16 b | 0.16 ± 0.02 | 0.52 ± 0.21 bc | 0.10 ± 0.05 b | 0.07 ± 0.03 c | 3.81 ± 1.28 a | 6.11 ± 2.20 a | 6.63 ± 0.21 ab | 5.56 ± 0.53 de | 0.55 ± 0.05 a | |
| C2 | 12.08 ± 0.13 b | 1.71 ± 0.23 a | 0.14 ± 0.02 | 1.11 ± 0.46 a | 0.24 ± 0.11 ab | 0.09 ± 0.04 b | 4.22 ± 0.99 a | 8.13 ± 1.74 a | 9.00 ± 0.71 | 8.60 ± 0.39 a | 1.50 ± 0.10 a | |
| C3 | 3.46 ± 0.17f | 0.53 ± 0.04 c | 0.15 ± 0.01 | 0.48 ± 0.09 c | 0.07 ± 0.01 b | 0.14 ± 0.03 c | 1.86 ± 0.09 b | 6.12 ± 0.55 a | 5.49 ± 0.01 d | 4.58 ± 0.17 f | 1.50 ± 1.76 a | |
| CK1 | 12.10 ± 0.95 b | 1.75 ± 0.19 a | 0.14 ± 0.02 | 0.97 ± 0.04 ab | 0.20 ± 0.01 ab | 0.08 ± 0.01 b | 3.70 ± 0.48 a | 7.00 ± 1.02 a | 8.90 ± 1.37 ab | 7.57 ± 0.61 b | 1.24 ± 2.62 a | |
| Fanmingyoutai | A1 | 18.25 ± 0.80 bc | 1.97 ± 0.09 bc | 0.11 ± 0.01 | 1.05 ± 0.31 abc | 0.24 ± 0.05 bcd | 0.06 ± 0.02 b | 6.00 ± 1.65 ab | 9.60 ± 1.73 a | 11.10 ± 0.97 bc | 8.46 ± 0.77 a | 0.83 ± 0.02 bc |
| A2 | 18.38 ± 0.52 de | 1.13 ± 0.12 cd | 0.06 ± 0.01 | 1.04 ± 0.21 ab | 0.25 ± 0.05 ab | 0.06 ± 0.01 b | 5.28 ± 0.63 de | 9.47 ± 0.85 a | 11.30 ± 0.27 d | 8.58 ± 0.28 b | 0.75 ± 0.04 b | |
| A3 | 17.79 ± 3.46 a | 2.12 ± 0.34 a | 0.12 ± 0.03 | 0.76 ± 0.29bcd | 0.17 ± 0.05abc | 0.04 ± 0.02 b | 7.13 ± 0.51 a | 5.32 ± 1.12a | 10.71 ± 1.80 abc | 9.08 ± 0.61 a | 0.54 ± 0.05 bc | |
| B1 | 17.62 ± 2.66 ab | 1.63 ± 0.55 abc | 0.09 ± 0.03 | 1.34 ± 0.61 a | 0.29 ± 0.15 a | 0.07 ± 0.04b | 5.61 ± 0.26 bcd | 9.05 ± 1.64 a | 12.27 ± 0.27 a | 9.46 ± 1.36 a | 0.76 ± 0.15 b | |
| B2 | 9.85 ± 0.41cd | 1.19 ± 0.24 cd | 0.12 ± 0.03 | 0.61 ± 0.09 bcd | 0.12 ± 0.02 cd | 0.06 ± 0.01 b | 4.17 ± 0.34 cd | 9.64 ± 1.28 a | 9.56 ± 0.83 c | 8.66 ± 0.52 a | 0.40 ± 0.04 cd | |
| B3 | 13.11 ± 0.84 abc | 1.43 ± 0.27 bc | 0.11 ± 0.02 | 0.80 ± 0.23 abc | 0.17 ± 0.04 abc | 0.06 ± 0.02 b | 5.75 ± 0.73 ab | 7.74 ± 1.29 a | 11.78 ± 1.17 ab | 9.36 ± 0.68 a | 0.46 ± 0.03 bcd | |
| C1 | 10.05 ± 1.35 cd | 1.26 ± 0.32 c | 0.13 ± 0.04 | 0.68 ± 0.15 bcd | 0.12 ± 0.04 cd | 0.07 ± 0.02 c | 3.42 ± 0.68 de | 8.67 ± 2.41 a | 10.20 ± 0.36 bc | 8.53 ± 0.83 a | 0.42 ± 0.02 bcd | |
| C2 | 3.89 ± 0.57 e | 0.60 ± 0.09 d | 0.15 ± 0.03 | 0.27 ± 0.08 cd | 0.03 ± 0.02 d | 0.07 ± 0.03 c | 2.46 ± 0.36 e | 5.51 ± 1.00 a | 5.77 ± 0.42 d | 5.07 ± 0.99 b | 0.32 ± 0.08 d | |
| C3 | 3.57 ± 0.46 e | 0.62 ± 0.13 d | 0.17 ± 0.04 | 0.20 ± 0.06 d | 0.02 ± 0.01 d | 0.06 ± 0.02 c | 2.43 ± 0.44 e | 7.38 ± 1.62 a | 5.77 ± 0.68 d | 4.93 ± 0.61 b | 0.34 ± 0.01 d | |
| CK2 | 17.61 ± 3.84 a | 1.98 ± 0.44 ab | 0.11 ± 0.03 | 1.00 ± 0.20 ab | 0.18 ± 0.03 abc | 0.06 ± 0.02 c | 5.43 ± 0.35 bc | 9.38 ± 4.90 a | 10.85 ± 0.85 abc | 8.28 ± 0.64 a | 0.71 ± 0.11 a |
| Va. | Tr. | TL | GL | Llen (cm) | Lwid (cm) | Diam (cm) | Lag (cm) |
|---|---|---|---|---|---|---|---|
| Xiangzayou 787 | A1 | 7.8 ± 1.3 a | 8.6 ± 1.1 a | 14.6 ± 2.3 a | 8.4 ± 1.1 a | 1.80 ± 0.30 a | 11.4 ± 1.5 a |
| A2 | 7.2 ± 1.5 ab | 7.8 ± 1.3 ab | 12.1 ± 2.6 b | 7.9 ± 0.9 ab | 1.68 ± 0.28 ab | 9.4 ± 1.3 b | |
| A3 | 7.6 ± 0.9 a | 8.4 ± 0.5 a | 13.3 ± 2.9 ab | 8.6 ± 1.3 a | 1.12 ± 0.38 c | 9.9 ± 1.8 ab | |
| B1 | 6.8 ± 1.8 b | 7.6 ± 1.5 b | 13.4 ± 2.0 ab | 8.1 ± 1.7 ab | 1.56 ± 0.25 b | 10.6 ± 1.4 a | |
| B2 | 6.2 ± 0.8 c | 7.0 ± 0.7 c | 11.8 ± 2.7 b | 7.3 ± 1.1 b | 1.40 ± 0.34 bc | 8.6 ± 1.1 c | |
| B3 | 6.4 ± 1.1 bc | 7.2 ± 1.0 bc | 14.1 ± 3.1 a | 8.9 ± 1.9 a | 1.22 ± 0.43 cd | 9.8 ± 1.6 ab | |
| C1 | 5.8 ± 0.4 d | 6.6 ± 1.0 d | 12.6 ± 2.2 ab | 7.8 ± 1.5 ab | 1.48 ± 0.31 b | 8.9 ± 1.2 bc | |
| C2 | 6.0 ± 1.2 cd | 6.8 ± 1.2 cd | 13.8 ± 2.7 a | 8.3 ± 1.3 a | 1.64 ± 0.29 ab | 10.2 ± 1.7 a | |
| C3 | 5.6 ± 1.4 d | 6.4 ± 1.6 d | 11.9 ± 2.8 b | 7.1 ± 1.0 b | 1.32 ± 0.36 c | 8.1 ± 1.0c | |
| CK1 | 5.2 ± 1.0 e | 5.8 ± 1.2 e | 10.3 ± 2.5 c | 6.7 ± 1.2 c | 0.98 ± 0.18 d | 7.6 ± 0.9d | |
| Fanmingyoutai | A1 | 7.4 ± 1.1 a | 8.0 ± 0.8 a | 13.2 ± 2.1 a | 8.2 ± 1.0 a | 1.54 ± 0.27 a | 9.8 ± 1.3 a |
| A2 | 6.8 ± 1.3 b | 7.6 ± 1.4 ab | 12.8 ± 2.6 a | 7.9 ± 1.3 ab | 1.42 ± 0.31 ab | 8.9 ± 1.5 b | |
| A3 | 7.0 ± 1.0 ab | 7.8 ± 0.9 a | 11.9 ± 2.3 b | 7.6 ± 1.1 b | 1.18 ± 0.35 c | 8.6 ± 1.2 bc | |
| B1 | 6.6 ± 1.2 bc | 7.2 ± 1.1 b | 12.4 ± 2.8 ab | 7.8 ± 1.5 ab | 1.34 ± 0.29 b | 9.2 ± 1.4 ab | |
| B2 | 6.0 ± 0.9 c | 6.8 ± 0.7 c | 13.6 ± 2.5 a | 8.4 ± 1.2 a | 1.08 ± 0.39 d | 8.4 ± 1.0 c | |
| B3 | 6.4 ± 1.4 ab | 7.0 ± 1.3 bc | 12.1 ± 2.7 b | 7.7 ± 1.4 ab | 1.26 ± 0.33 bc | 8.1 ± 1.1 d | |
| C1 | 5.8 ± 1.5 d | 6.6 ± 1.2 d | 10.7 ± 2.0 c | 7.0 ± 1.0 c | 1.46 ± 0.30 ab | 8.7 ± 1.3 bc | |
| C2 | 6.2 ± 1.7 cd | 6.4 ± 1.6 d | 12.3 ± 2.9 ab | 7.5 ± 1.3 b | 1.22 ± 0.36 c | 7.9 ± 1.0 d | |
| C3 | 5.4 ± 1.8 e | 6.0 ± 1.4 e | 11.4 ± 2.4 b | 6.9 ± 0.9 c | 1.10 ± 0.28 d | 7.3 ± 0.8 e | |
| CK2 | 4.8 ± 1.2 f | 5.6 ± 1.0 f | 9.8 ± 2.1 d | 6.3 ± 0.8 d | 0.92 ± 0.15 e | 6.9 ± 0.7 f |
| Va. | Tr. | PH (cm) | EBH (cm) | MIEL (cm) | PEB (no.) | ESMI (no.) | TS (no.) | SL (cm) | SPS (no.) | YP (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Xiangzayou 787 | A1 | 122 ± 9.4 b | 54.3 ± 5.8 ab | 45.0 ± 4.7 ab | 5.0 ± 0.8 ab | 21.0 ± 2.3 d | 97.3 ± 22.1 b | 6.01 ± 0.25 ab | 24.3 ± 0.50 ab | 4.74 ± 1.22a |
| A2 | 126.3 ± 11.8 a | 57.3 ± 7.0 ab | 47.7 ± 7.6 a | 4.3 ± 0.5 ab | 24.3 ± 1.8 c | 69.7 ± 8.3 c | 6.16 ± 0.16 ab | 22.5 ± 0.55 a | 3.92 ± 0.56 ab | |
| A3 | 117.3 ± 1.9 b | 51.3 ± 6.4 ab | 33.7 ± 1.6 d | 5.0 ± 0.8 ab | 31.7 ± 4.7 b | 81.0 ± 4.0 c | 5.76 ± 0.22 c | 22.6 ± 0.56 a | 3.15 ± 0.69 ab | |
| B1 | 127.7 ± 5.3 a | 55.0 ± 3.5 ab | 43.7 ± 3.7 ab | 5.3 ± 1.2 ab | 28.0 ± 4.3 b | 83.7 ± 5.0 c | 6.09 ± 0.07 ab | 21.4 ± 1.76 ab | 3.96 ± 0.09 ab | |
| B2 | 123.0 ± 3.4 a | 50.0 ± 10.5 b | 42.3 ± 0.9 ab | 6.7 ± 2.0 a | 18.7 ± 1.6 d | 120.3 ± 5.8 a | 6.42 ± 0.23 a | 22.6 ± 0.16 a | 3.99 ± 1.00 ab | |
| B3 | 118.6 ± 3.9 b | 54.0 ± 3.1 ab | 36.0 ± 1.3 c | 6.3 ± 2.0 a | 19.0 ± 0.8 d | 84.3 ± 21.7 c | 6.53 ± 0.50 a | 20.9 ± 1.16 ab | 3.77 ± 1.04 ab | |
| C1 | 108.0 ± 3.4 c | 50.3 ± 0.9 b | 37.0 ± 1.3 bc | 5.3 ± 0.5 ab | 18.7 ± 1.2 d | 50.7 ± 1.8 d | 5.93 ± 0.09 b | 20.2 ± 0.82 b | 2.24 ± 0.22 b | |
| C2 | 109.3 ± 5.0 c | 65.0 ± 4.7 a | 33.7 ± 2.7 d | 3.0 ± 0.8 b | 21.3 ± 1.6 d | 39.7 ± 2.9 e | 6.08 ± 0.14 ab | 21.3 ± 0.55 ab | 2.26 ± 0.19 b | |
| C3 | 116.7 ± 4.7 b | 58.3 ± 0.5 ab | 40.7 ± 2.4 ab | 4.0 ± 0.8 ab | 29.7 ± 2.0 b | 84.3 ± 6.8 c | 6.13 ± 0.09 ab | 21.5 ± 1.16 ab | 4.40 ± 1.03 a | |
| CK1 | 132.0 ± 4.1 a | 56.7 ± 6.6 ab | 48.0 ± 2.3 a | 6.3 ± 1.2 a | 38.3 ± 4.0 a | 140.7 ± 12.4 a | 6.03 ± 0.13 ab | 21.3 ± 0.50 ab | 4.30 ± 0.73 a | |
| Fanmingyoutai | A1 | 112.3 ± 5.1 b | 42.3 ± 7.2 c | 58.0 ± 9.6 ab | 3.2 ± 1.2 a | 32.3 ± 3.5 c | 88.7 ± 9.3 b | 7.81 ± 0.72 c | 21.7 ± 0.85 ab | 3.81 ± 0.31 b |
| A2 | 100.0 ± 10.1d | 50.7 ± 9.7 ab | 50.0 ± 10.9 ab | 2.3 ± 0.5 d | 26.3 ± 2.7 d | 37.3 ± 8.2 e | 5.56 ± 0.04 d | 21.6 ± 1.52 ab | 1.26 ± 0.08 d | |
| A3 | 119.7 ± 3.1 b | 65.0 ± 5.8 a | 64.0 ± 6.3 a | 3.3 ± 1.2 c | 37.7 ± 5.0 b | 68.0 ± 17.1 d | 7.36 ± 0.24 c | 19.8 ± 0.55 b | 2.35 ± 0.59 c | |
| B1 | 126.3 ± 5.0 a | 49.0 ± 2.3 ab | 54.0 ± 6.1 ab | 5.3 ± 0.5 ab | 52.3 ± 0.9 a | 114.0 ± 29.3 a | 6.98 ± 0.44 c | 18.8 ± 1.57 b | 3.50 ± 0.87 b | |
| B2 | 122.0 ± 8.6 b | 51.6 ± 11.1 ab | 57.7 ± 3.5 ab | 5.3 ± 0.9 ab | 49.0 ± 2.1 a | 107.0 ± 14.0 a | 7.36 ± 0.03 c | 23.1 ± 1.14 a | 4.33 ± 0.21 b | |
| B3 | 137.3 ± 4.0 a | 61.3 ± 13.9 a | 65.6 ± 1.6 a | 6.3 ± 1.2 a | 46.3 ± 3.2 a | 134.0 ± 23.5 a | 6.89 ± 0.41 c | 19.2 ± 2.42 b | 6.59 ± 1.18 a | |
| C1 | 122.0 ± 4.1 b | 48.3 ± 10.3 ab | 64.0 ± 4.8 a | 6.3 ± 1.8 a | 50.7 ± 7.4 a | 128.0 ± 13.9 a | 7.44 ± 0.16 c | 21.2 ± 1.12 ab | 6.15 ± 0.93 a | |
| C2 | 106.0 ± 4.8 c | 40.0 ± 1.3 c | 56.0 ± 3.5 ab | 5.3 ± 1.2 ab | 33.0 ± 10.1 c | 115.0 ± 22.6 a | 11.80 ± 1.04 b | 11.7 ± 0.91 d | 2.54 ± 0.37 c | |
| C3 | 101.0 ± 0.8 d | 46.6 ± 0.4 ab | 48.7 ± 5.8 b | 4.0 ± 0.8 b | 40.3 ± 4.5 b | 70.7 ± 14.3 d | 7.05 ± 0.21 c | 20.0 ± 1.79 ab | 1.54 ± 0.06 d | |
| CK2 | 116.3 ± 7.8 b | 44.3 ± 3.8 b | 56.3 ± 3.9 ab | 4.3 ± 0.5 b | 41.0 ± 2.1 b | 76.3 ± 12.1 c | 14.47 ± 0.95 a | 16.6 ± 2.34 c | 2.81 ± 1.05 c |
| Va. | Tr. | Oleic Acid (%) | Erucic Acid (%) | Glucosinolate (µmol/g) | Protein (%) | Oil Content (%) |
|---|---|---|---|---|---|---|
| Xiangzayou 787 | A1 | 70.07 | 0.98 | 17.78 | 19.17 | 51.75 |
| A2 | 67.19 | 1.21 | 21.39 | 18.71 | 50.36 | |
| A3 | 66.59 | 1.46 | 19.24 | 17.96 | 50.33 | |
| B1 | 65.30 | 1.51 | 19.61 | 18.94 | 50.27 | |
| B2 | 68.54 | 1.40 | 19.05 | 17.89 | 50.87 | |
| B3 | 67.18 | 1.43 | 17.43 | 18.01 | 50.68 | |
| C1 | 62.32 | 1.36 | 19.26 | 19.43 | 48.49 | |
| C2 | 63.27 | 1.41 | 26.67 | 19.66 | 47.98 | |
| C3 | 70.38 | 1.39 | 18.58 | 17.03 | 52.33 | |
| CK1 | 71.61 | 1.25 | 17.92 | 18.79 | 50.24 | |
| Fanmingyoutai | A1 | 72.85 | 0.61 | 29.72 | 20.37 | 45.59 |
| A2 | 73.62 | 1.04 | 29.20 | 20.04 | 43.73 | |
| A3 | 71.73 | 0.98 | 23.57 | 19.67 | 47.08 | |
| B1 | 68.46 | 1.38 | 46.06 | 19.43 | 47.59 | |
| B2 | 74.90 | 0.83 | 25.89 | 18.05 | 49.17 | |
| B3 | 69.19 | 1.01 | 31.47 | 20.59 | 44.47 | |
| C1 | 66.79 | 0.88 | 33.37 | 19.13 | 48.17 | |
| C2 | 66.79 | 0.82 | 32.54 | 20.83 | 43.59 | |
| C3 | 70.48 | 0.77 | 25.59 | 19.79 | 45.94 | |
| CK2 | 67.89 | 1.02 | 30.78 | 18.59 | 47.17 |
| Va. | Tr. | TP (no.) | Freezing Damage | Waterlogging Damage | Disease | |||
|---|---|---|---|---|---|---|---|---|
| Rate (%) | Index | Rate (%) | Index | Rate (%) | Index | |||
| Xiangzayou 787 | A1 | 79 | 3.8 | 0.01 | 45.6 | 0.13 | 27.8 | 0.08 |
| A2 | 94 | 12.8 | 0.01 | 25.5 | 0.09 | 32.9 | 0.10 | |
| A3 | 70 | 4.3 | 0.01 | 32.9 | 0.13 | 34.2 | 0.10 | |
| B1 | 78 | 5.1 | 0.02 | 34.6 | 0.13 | 20.5 | 0.08 | |
| B2 | 68 | 17.6 | 0.06 | 32.4 | 0.12 | 23.5 | 0.07 | |
| B3 | 65 | 21.5 | 0.06 | 29.2 | 0.11 | 30.7 | 0.09 | |
| C1 | 57 | 15.8 | 0.05 | 43.9 | 0.15 | 42.1 | 0.14 | |
| C2 | 54 | 20.4 | 0.06 | 40.7 | 0.14 | 48.1 | 0.14 | |
| C3 | 53 | 17.0 | 0.04 | 37.7 | 0.11 | 37.7 | 0.10 | |
| CK1 | 42 | 28.6 | 0.11 | 64.3 | 0.23 | 59.5 | 0.19 | |
| Fanmingyoutai | A1 | 44 | 43.2 | 0.06 | 51.1 | 0.16 | 40.0 | 0.14 |
| A2 | 45 | 35.6 | 0.14 | 68.2 | 0.24 | 51.1 | 0.16 | |
| A3 | 52 | 30.8 | 0.10 | 53.8 | 0.19 | 42.3 | 0.14 | |
| B1 | 64 | 28.1 | 0.08 | 39.1 | 0.12 | 28.1 | 0.08 | |
| B2 | 52 | 26.9 | 0.10 | 50.0 | 0.16 | 38.5 | 0.10 | |
| B3 | 60 | 35.0 | 0.17 | 48.3 | 0.16 | 23.3 | 0.06 | |
| C1 | 40 | 42.5 | 0.13 | 75.0 | 0.24 | 40.0 | 0.11 | |
| C2 | 39 | 30.8 | 0.12 | 66.7 | 0.20 | 38.5 | 0.12 | |
| C3 | 51 | 27.5 | 0.10 | 54.9 | 0.19 | 31.3 | 0.10 | |
| CK2 | 52 | 34.6 | 0.12 | 67.3 | 0.23 | 46.1 | 0.14 | |
| Parameter | Xiangzayou 787 | Fanmingyoutai |
|---|---|---|
| Yield per plant (g) Treatment A1 | 4.74 | 3.81 |
| Yield per plant (g) Control (CK) | 4.30 | 2.81 |
| Yield increase per plant (%) | 10.20 | 35.60 |
| Planting density (plants/ha) | 300,000 | 300,000 |
| Control yield (kg/ha) | 1290 | 843 |
| Treatment yield (kg/ha) | 1422 | 1143 |
| Yield increase (kg/ha) | 132 | 300 |
| Pelleting agent cost (CNY ¥/ha) | 8.10 | 8.10 |
| Rapeseed price (CNY ¥/kg) | 6.00 | 6.00 |
| Revenue increase (CNY ¥/ha) | 792 | 1800 |
| Input–output ratio | 1:97.8 | 1:222.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Xiao, G.; Jin, H.; Wang, Y.; Xie, C.; Zhang, Z. Effects of a Novel Waterlogging-Tolerant Growth-Promoting Pelletizing Agent on the Growth of Brassica napus. Horticulturae 2025, 11, 946. https://doi.org/10.3390/horticulturae11080946
Li L, Xiao G, Jin H, Wang Y, Xie C, Zhang Z. Effects of a Novel Waterlogging-Tolerant Growth-Promoting Pelletizing Agent on the Growth of Brassica napus. Horticulturae. 2025; 11(8):946. https://doi.org/10.3390/horticulturae11080946
Chicago/Turabian StyleLi, Lingyu, Gang Xiao, Hao Jin, Yue Wang, Chunfeng Xie, and Zhenqian Zhang. 2025. "Effects of a Novel Waterlogging-Tolerant Growth-Promoting Pelletizing Agent on the Growth of Brassica napus" Horticulturae 11, no. 8: 946. https://doi.org/10.3390/horticulturae11080946
APA StyleLi, L., Xiao, G., Jin, H., Wang, Y., Xie, C., & Zhang, Z. (2025). Effects of a Novel Waterlogging-Tolerant Growth-Promoting Pelletizing Agent on the Growth of Brassica napus. Horticulturae, 11(8), 946. https://doi.org/10.3390/horticulturae11080946





