Abstract
Protected cultivation is a cultivation practice that plays an important role in improving crop quality. Aroma is an important flavour that assesses the quality of yuzu. In this study, C. junos cv. ‘Kitou’ grown in open fields (CJKTF) and plastic greenhouses (CJKTP) were selected as the study material. Significant differences in aroma performance between CJKTF and CJKTP were found by the olfactory senses of the members of this research group and an electronic nose, with CJKTP having a stronger aroma. Regarding VOCs, GC-MS analyses revealed 13 VOCs up-regulated and 28 VOCs down-regulated in CJKTP compared to CJKTF. Transcriptome analysis revealed that 515 genes were up-regulated and 720 genes were down-regulated in CJKTP compared to CJKTF. The differential VOCs nerolidol and γ-cadinene, and the differential genes nerolidol synthase 1 (NES1), nerolidol synthase 1-like (NES1-like), and cadinene synthase (DCS), were in the sesquiterpene synthesis pathway and showed significant correlation. NES1, NES1-like, and DCS encode terpene synthases, which may be involved in the biosynthetic pathway of nerolidol and γ-cadinene. In conclusion, the use of plastic greenhouses for cultivation may improve the quality and aroma intensity of yuzu, as well as alter the expression of related genes, compared to field cultivation. These results suggest that protected cultivation is a suitable cultivation practice to enhance the aroma of yuzu.
1. Introduction
Citrus fruits are widely utilized across various industrial sectors, including the food, cosmetic, and pharmaceutical industries, due to their distinctive and appealing aroma profiles. Citrus, a genus within the Rutaceae family, encompasses a broad spectrum of species such as mandarin (Citrus reticulata), pomelo (Citrus maxima), sweet orange (Citrus sinensis), and yuzu (Citrus junos) [1]. Among citrus, yuzu is particularly distinguished by its potent and uniquely nuanced aroma, which differs markedly from that of other citrus species in terms of olfactory perception [2]. Originally native to the upper reaches of the Yangtze River in China—where it is locally known as xiangcheng—yuzu is now predominantly cultivated in East Asia, including China, Japan, and Korea [3]. Its refreshing and characteristic aroma has led to widespread usage in culinary applications, condiments, traditional medicine, and wellness products [4,5]. In addition, yuzu has been used as an aromatic stomachic and sweating agent in traditional Chinese medicine. Hot yuzu baths increase blood circulation and prevent colds. Yuzu has anti-inflammatory, antioxidant, and anticancer properties, thus effectively preventing certain diseases. Therefore, yuzu has important nutritional and utilization values [6]. In recent years, the yuzu industry in China has witnessed significant expansion, underscoring both its economic potential and research value [7].
The aromatic profile of citrus fruits is largely governed by the composition of volatile organic compounds (VOCs) present in the essential oils of the peel, which are the principal contributors to citrus aroma [1]. VOCs can be perceived through the human olfactory system and are critical to defining the sensory quality of aroma. However, not all VOCs are odor-active; their olfactory impact depends on factors such as molecular structure, physicochemical properties, and their relative odor activity value (rOAV), which considers both concentration and olfactory threshold [8,9]. To date, over 200 aroma-related VOCs have been identified in citrus species. These compounds are typically classified into several major groups, including terpenes, esters, aldehydes, alcohols, ketones, oxides, and ethers [10]. Among these, terpenes are the most abundant and structurally diverse, contributing significantly to citrus aroma [11,12].
The composition and concentration of VOCs in citrus are influenced by multiple factors, including genetic background (germplasm), environmental conditions, and agronomic practices [13]. While varietal improvement has traditionally been a focus for enhancing fruit quality, progress in citrus breeding has been constrained by biological challenges such as prolonged juvenile phases and polyembryony [14,15]. In this context, cultivation practices—particularly those capable of modulating environmental variables—have emerged as a practical strategy for improving fruit quality traits, including aroma. Protected cultivation refers to the practice of growing crops in controlled environments using structures such as plastic greenhouses, net houses, or tunnels, and incorporating protective measures like windbreaks or targeted irrigation [16]. Compared with conventional open-field cultivation, protected cultivation offers improved sustainability by mitigating climatic risks and promoting more efficient utilization of resources such as fertilizers, pesticides, and water. It also facilitates enhancements in crop yield and quality [17,18]. This approach has been successfully applied in various crops to enhance desirable traits [19]; for example, supplemental lighting under protected conditions has been shown to improve the aroma and quality of grape berries [20]. Therefore, the use of protected cultivation may be promising for improving the quality of yuzu aroma. However, research on how protected cultivation affects citrus aroma and its underlying molecular mechanisms remains limited.
Therefore, in this study, we investigated the effect of cultivation practices on the aroma profile of yuzu (C. junos cv. ‘Kitou’) by comparing fruits grown in open fields and plastic greenhouses. We evaluated key quality traits, with a particular focus on aromatic attributes, under different cultivation conditions. To elucidate the molecular basis of these differences, integrated metabolomic and transcriptomic analyses were performed. This approach enabled the identification of key differential VOCs and associated candidate genes. The findings offer valuable insights into the regulatory mechanisms by which protected cultivation influences aroma formation in yuzu and provide a foundation for future functional studies and molecular breeding efforts aimed at improving citrus aroma quality.
2. Materials and Methods
2.1. Plant Materials
Yuzu (Citrus junos) cultivar ‘Kitou’ was used in this study. The plants were cultivated under two conditions: a naturally ventilated polyhouse (plastic greenhouse) and open-field environments. To minimize errors, fruit trees from both treatments were planted in the same area at the Zhejiang Citrus Research Institute (E: 121.9, N: 28.38). Peel samples were collected from randomly selected fruits with the same level of maturity in both environments on 1 December. Each treatment consisted of three biological replicates. Immediately after harvest, the peels were snap-frozen in liquid nitrogen and stored at −80 °C until RNA and volatile compound extraction.
2.2. Electronic Nose (E-Nose) Analysis
Yuzu peel aroma profiles were analyzed using an PNE3 electronic nose system (Airsense Analytics GmbH, Schwerin, Germany) equipped with 10 metal oxide semiconductor sensors (W1C, primarily sensitive to aromatic components and benzene compounds; W5S, primarily sensitive to nitrous oxides compounds; W3C, primarily sensitive to ammonia and aromatic components; W6S, primarily sensitive to hydrocarbons; W5C, primarily sensitive to alkanes, aromatics, and small polar compounds; W1S, primarily sensitive to a broad range of methane compounds; W1W, primarily sensitive to inorganic sulfides and many terpenes; W2S, primarily sensitive to most alcohols, aldehydes, and ketones; W2W, primarily sensitive to aromatics and organic sulfides; W3S, primarily sensitive to long-chain alkanes) [21,22]. Approximately 3.0 g of peel tissue was ground, placed into a sealed vial, and equilibrated at room temperature for 30 min before analysis. Measurement parameters were based on the methodology described by Chen et al. [23]. The response value for each sensor is G/G0, where G and G0 are the resistance of the sensor when exposed to sample volatiles and clean air, respectively. Data were collected from 57 to 60 s steady-state response values for each test and analyzed using WinMuster (version 1.6.2) software.
2.3. Extraction of Volatile Organic Compounds (VOCs)
For volatile compound extraction, 500 mg of powdered peel sample was transferred into a 20 mL headspace vial (Agilent Technologies, Palo Alto, CA, USA) containing a saturated sodium chloride solution. The vials were sealed with TFE-silicone septa and crimp caps. For SPME (solid-phase microextraction), samples were pre-incubated at 60 °C for 5 min. A 120 µm DVB/CWR/PDMS fiber was then exposed to the vial headspace at 60 °C for 15 min.
2.4. Metabolomic Analysis of VOCs
The VOCs adsorbed on the SPME fiber were thermally desorbed in the injection port of a gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) operating in splitless mode at 250 °C for 5 min. Separation and identification were performed using an Agilent 8890 GC (Agilent Technologies, Palo Alto, CA, USA) coupled to a 7000D triple quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) and equipped with a DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm, 5% phenyl-polymethylsiloxane) (Agilent Technologies, Palo Alto, CA, USA). Helium served as the carrier gas at a linear flow rate of 1.2 mL/min. The GC oven was programmed as follows: initial temperature 40 °C (held for 3.5 min), ramped to 100 °C at 10 °C/min, then to 180 °C at 7 °C/min, and finally to 280 °C at 25 °C/min (held for 5 min). The MS operated under 70 eV electron impact (EI) ionization. Quadrupole, ion source, and transfer line temperatures were set at 150 °C, 230 °C, and 280 °C, respectively. VOCs were detected using selected ion monitoring (SIM) mode.
Differential metabolite analysis was based on Variable Importance in Projection (VIP) values (>1.0) and absolute log2 fold change (|log2FC| ≥ 1.0). VIP scores were obtained from Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA), including score plots and 200-permutation validation plots, performed using the MetaboAnalystR package in R (version 4.5.1) [24]. Before analysis, data were log2-transformed and mean-centered. Identified metabolites were annotated using the KEGG Compound database at https://www.kegg.jp/kegg/compound/ (accessed on 10 August 2024) and mapped to KEGG Pathways at https://www.kegg.jp/kegg/pathway.html (accessed on 10 August 2024).
2.5. RNA-Sequencing and Data Analysis
Total RNA was extracted from CJKTP and CJKTF peel tissues, and cDNA library construction and 150-bp paired-end sequencing were conducted using the NovaSeq X Plus platform (Illumina, Inc., San Diego, CA, USA) by Metware Biotechnology Co., Ltd. (Wuhan, China). Raw reads were processed with fastp (parameters: -n_base_limit 15, -qualified_quality_phred 20) to generate high-quality clean reads [25]. Reads were mapped to the Citrus sinensis reference genome using Hisat2 [26], and read counts were calculated using featureCounts [27]. Gene expression levels were normalized to FPKM (Fragments Per Kilobase of transcript per Million mapped reads).
Differential expression gene analysis was performed using the DESeq2 package. Statistical significance was determined using the Benjamini–Hochberg procedure to control the false discovery rate (FDR ≤ 0.05). Genes were defined as differentially expressed (DEGs) with |log2FC| ≥ 1 and q-value ≤ 0.05.
3. Results
3.1. Effect of Protected Cultivation on Key Quality Traits of Yuzu
To evaluate the impact of protected cultivation on fruit characteristics, we compared C. junos cv. ‘Kitou’ (CJKT) yuzu fruits are grown in open-field conditions (denoted CJKTF) and in plastic greenhouse conditions (denoted CJKTP). Morphological assessment revealed no observable differences in external fruit characteristics between the two groups, including fruit size, shape index, and peel coloration (Figure 1A). The total soluble solids (TSS) content in CJKTF and CJKTP was 9.83 ± 0.84 °Brix and 8.50 ± 0.01 °Brix, respectively. Similarly, titratable acidity (TA) levels were 3.00 ± 0.19% in CJKTF and 1.26 ± 0.22% in CJKTP. These results indicate that plastic greenhouse cultivation somewhat reduced both the sugar content and acidity of the yuzu fruits compared to open-field cultivation (Figure 1B).
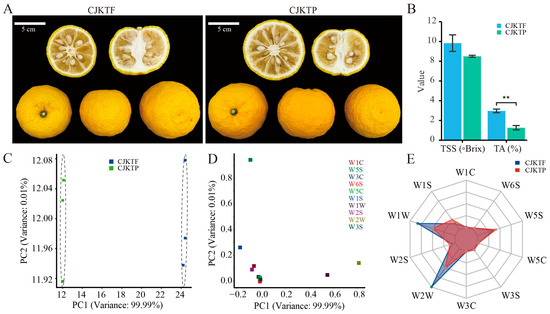
Figure 1.
Traits of C. junos cv. ‘Kitou’ grown in the fields (CJKTF) and plastic greenhouses (CJKTP). (A) Morphological characteristics of CJKTF and CJKTP fruits in different perspectives and sections. (B) Bar plot of TSS and TA contents of CJKTF and CJKTP pulp, and asterisks indicate the presence of significance (** p < 0.01, t-tests). (C) Principal component analysis (PCA) of the aroma intensity in different CJKTF and CJKTP samples using E-nose. (D) Loading analysis (LDA) of E-nose for CJKTF and CJKTP samples. (E) Radargram of sensor response value of CJKTF and CJKTP.
To assess differences in aroma, a sensory evaluation was conducted by nine panelists (four male, five female) under blinded conditions. All panelists independently confirmed a perceptible difference in aroma between CJKTF and CJKTP, consistently describing CJKTP as having a more intense and appealing fragrance. To further quantify these differences, an electronic nose (e-nose) system was employed. Principal Component Analysis (PCA) of the e-nose data demonstrated clear discrimination between CJKTF and CJKTP samples. PC1 and PC2 accounted for 99.9% and 0.01% of the variance, respectively (Figure 1C). Sensor response analysis revealed that W2W and W1W contributed most to PC1, while W5S and W1S were major contributors to PC2 (Figure 1D). Specifically, W2W is responsive to aromatic and sulfur-containing compounds, W1W to sulfur compounds and many terpenes, W5S to nitrogen oxides, and W1S to methane [21].
3.2. Comparison of VOCs Between CJKTP and CJKTF
Aroma is one of the most defining and attractive traits of yuzu. To determine the effect of plastic greenhouse cultivation on the fruit’s volatile profile, we performed headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography–mass spectrometry (GC-MS) on both CJKTF and CJKTP samples. The results showed that, in total, 904 volatiles were detected across both cultivation conditions. These VOCs were classified into 15 chemical groups, including 250 terpenes, 156 esters, 92 ketones, 88 heterocyclic compounds, 78 alcohols, 49 hydrocarbons, 47 aldehydes, 41 organic acids, 23 amines, 23 phenols, 21 aromatics, 20 ethers, 9 nitrogen-containing compounds, 6 sulfur compounds, and 1 halogenated hydrocarbon (Figure 2A). Among these compounds, several were uniquely present in one condition but absent in the other. Specifically, CJKTF lacked one aromatic compound, one ester, two heterocyclic compounds, two ketones, and two terpenoids that were present in CJKTP. Conversely, CJKTP lacked one alcohol, four esters, one heterocyclic compound, one hydrocarbon, one phenol, and one terpenoid found in CJKTF.
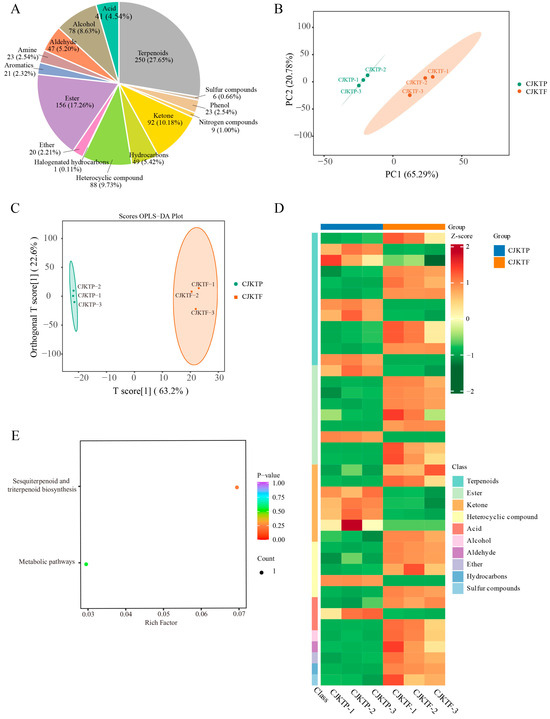
Figure 2.
Analysis of VOCs comparing CJKTP and CJKTF. (A) Classes and proportions of VOCs detected by GC-MS in total in CJKTF and CJKTP. (B) PCA analysis based on CJKTF and CJKTP VOC contents. (C) OPLS-DA analysis based on CJKTF and CJKTP VOC contents. (D) Heat map of the content of differential VOCs between CSCXF and CJKTP in each sample. (E) KEGG analysis of differential VOCs between CJKTF and CJKTP.
Multivariate statistical analyses were performed to further evaluate differences in the volatile profiles. PCA revealed a clear separation between CJKTF and CJKTP, with PC1 and PC2 accounting for 65.29% and 20.78% of the total variance, respectively (Figure 2B). Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) also demonstrated complete separation between the two groups, corroborating the PCA results (Figure 2C). The above results indicate that greenhouse cultivation significantly affects the quality of CJKT, especially the aroma of CJKT from the sensory and VOC profiles.
3.3. Differential Accumulation of VOCs Between CJKTP and CJKTF
To further elucidate the metabolic basis underlying the aroma differences between CJKTP and CJKTF, we conducted a differential analysis of VOCs with CJKTF as the control. Using the OPLS-DA model, 359 VOCs with significant variation between the two cultivation conditions were identified, each having a variable importance in projection (VIP) score > 1. Given the high dimensionality and complexity of metabolomics data, further filtering was applied using fold-change thresholds (≥2 or ≤0.5) to refine the identification of significant differential metabolites. This yielded a total of 41 differential VOCs, of which 13 were upregulated and 28 were downregulated in CJKTP compared to CJKTF. These differentially accumulated VOCs comprised twelve terpenoids, nine esters, seven ketones, five heterocyclic compounds, three organic acids, and one each of alcohol, sulfur compound, ether, aldehyde, and hydrocarbon, of which 16 of these belong to the aromatic compounds (Figure 2D; Table 1). There were more aromatic and terpene compound differentiators between CJKTP and CJKTF, and consistent with the results of the electronic nose analyses, the W2W and W1W sensors, which are primarily sensitive to aromatic and terpene compounds, were the main contributors to the organoleptic differences in the aromas of CJKTP and CJKTF (Figure 1D). This composition highlights a clear metabolic shift in aroma-related compounds under protected cultivation.

Table 1.
Differential VOCs between CJKTP and CJKTF.
To explore the underlying metabolic pathways associated with these changes, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was conducted on the 41 differential VOCs. The most significantly enriched pathway was sesquiterpenoid and triterpenoid biosynthesis, suggesting that plastic greenhouse cultivation may enhance or suppress specific branches of the terpenoid biosynthetic network (Figure 2E). The relative odor activity value (rOAV) is a method for determining the key flavour compounds of a foodstuff established in conjunction with the sensory thresholds of the compounds, and is used to elucidate the contribution of each aroma compound to the overall aroma profile of the sample [28]. Based on some of the available sensory thresholds of the compounds, we calculated the corresponding rOAV, where the total rOAVs were 5.1 × 107 for CJKTP and 4.8 × 107 for CJKTF (Table S1). We selected the top 10 leading key differential VOC substances, namely: ethanone, 1-(2-thienyl)-; linalool; 2-methyl-3-propylpyrazine; pyrazine, 2-methyl-6-propyl-; 1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl-; benzenepropanal, 4-(1,1-dimethylethyl)-; propanoic acid, hexyl ester; 2,4,6-octatriene, 2,6-dimethyl-; 2,4,6-octatriene, 2,6-dimethyl-, (E, Z)-; benzoic acid, hexyl ester. These VOCs are likely key contributors to the distinct aromatic profiles between CJKTF and CJKTP (Figure 3).
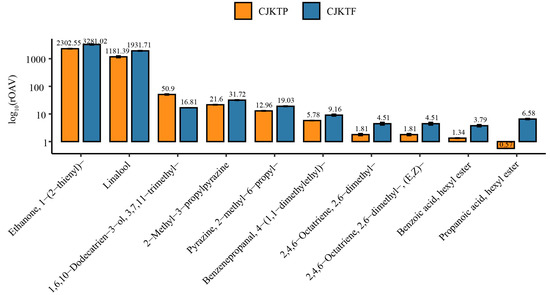
Figure 3.
Bar plot of log rOAVs for the top-ranked key VOCs.
3.4. Transcriptomic Comparison Between CJKTP and CJKTF
To gain insight into the molecular mechanisms regulating VOC biosynthesis under different cultivation conditions, we performed transcriptome analysis of CJKTP and CJKTF. Clean reads passing quality control were mapped to the C. sinensis (sweet orange) reference genome, resulting in the detection of 15,209 expressed genes across all samples. PCA revealed clear transcriptomic differentiation between CJKTP and CJKTF. PC1 and PC2 accounted for 46.65% and 19.46% of the total variance, respectively (Figure 4A), indicating a strong effect of cultivation environment on global gene expression patterns. Differential expression gene (DEG) analysis identified 1235 genes with significant changes in expression: 515 were upregulated and 720 were downregulated in CJKTP relative to CJKTF (control group) (Figure 4B). KEGG analysis of the DEGs resulted in significant enrichment of these genes in photosynthesis, the MAPK signalling pathway, plant–pathogen interactions, plant secondary metabolic pathways, and glucosinolate biosynthetic pathways (Figure 4C).
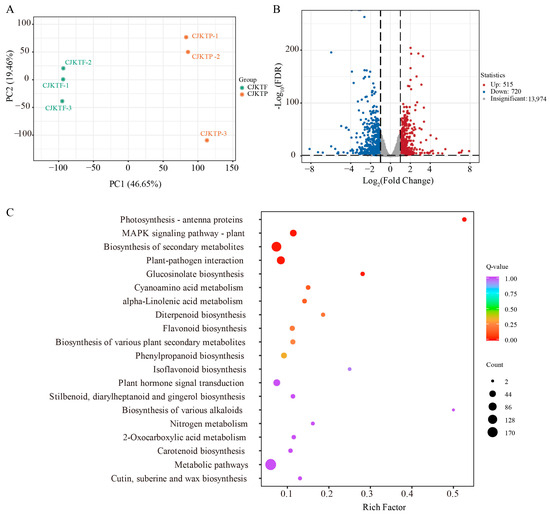
Figure 4.
Analysis of CJKTP and CJKTF transcript profiles. (A) PCA analysis based on gene transcript levels of CJKTP and CJKTF. (B) Volcano plot of differentially expressed genes between CJKTP and CJKTF. (C) KEGG analysis of differentially expressed genes between CJKTP and CJKTF.
3.5. Combined Analyses of Differential Metabolites and DEGs
Based on the differential metabolites from this study, as well as the KEGG annotation results of DEGs, the KEGG pathway found to be co-annotated by the two histologies includes both metabolic pathways and sesquiterpenoid and triterpenoid biosynthesis pathways. We will focus on the more specific sesquiterpenoid and triterpenoid biosynthesis pathway, which contains three genes Cs_ont_2g006430, Cs_ont_2g006440, and Cs_ont_2g006470, along with two associated VOCs, 1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl- (also known as nerolidol) and γ-cadinene (Figure 5A). Further correlation analysis showed that Cs_ont_2g006430 was significantly positively correlated with nerolidol and γ-cadinene, and Cs_ont_2g006440 and Cs_ont_2g006470 showed significant negative correlation with them, respectively (Pearson correlation coefficient > 0.9, p < 0.01; Figure 5A). Notably, nerolidol is the key differential VOC contributing to the aroma divergence between CJKTP and CJKTF mentioned above (Figure 3). It is a colourless sesquiterpene in citrus essential oils with a delicate green, floral aroma with citrus, woody, and waxy notes. γ-cadinene is characterized by a woody odor and is used in natural substances and extractives for its aromatic properties.
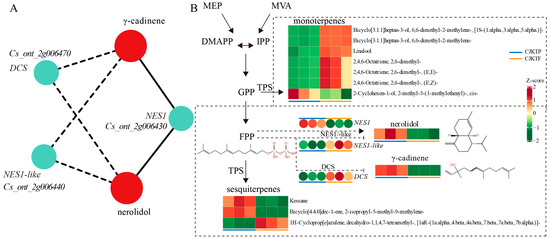
Figure 5.
Combined analysis of differentially expressed genes and differential VOCs. (A) Network map of VOC and gene correlations in the sesquiterpene biosynthesis pathway. The red circles represent metabolites and the cyan circles represent genes, with solid lines indicating positive correlations and dashed lines indicating negative correlations. (B) Heatmap of related gene expressions and VOC contents in terpene metabolic pathways between CJKTP and CJKTF.
In terms of content and type of aromatics, volatile terpenoids predominate in citrus fruits. The terpene biosynthesis pathway involves the production of terpenoids, which are natural compounds made up of isoprene units (C5). Terpenes are synthesized through two main pathways: the mevalonic acid (MVA) pathway and the methylerythritol phosphate (MEP) pathway. These pathways start with the basic building block of isoprene (C5), which can be extended to form compounds of varying lengths such as C10 (monoterpene), C15 (sesquiterpene), and other higher-order compounds (Figure 5B). Terpenoid biosynthesis in plants involves specialized enzymes known as terpene synthases that use these pathways to create a diverse array of terpenes [29]. The Cs_ont_2g006430, Cs_ont_2g006440, and Cs_ont_2g006470 genes encode three terpene synthases (TPS). Of these, Cs_ont_2g006430 encodes nerolidol synthase 1 (NES1), EC:4.2.3.48, which converts farnesyl diphosphate (FPP) and water into nerolidol (Figure 5B). Cs_ont_2g006440 is homologous to Cs_ont_2g006430 and encodes nerolidol synthase 1-like enzyme (NES1-like), which might also be involved in nerolidol synthesis (Figure 5B). Cs_ont_2g006470 encodes a cadinene synthase (DCS), EC:4.2.3.13, that catalyses the production of γ-cadinene from FPP, and Cs_ont_2g006470 may affect γ-cadinene synthesis.
In addition, different cultivation environments affect the metabolism of a wide range of yuzu terpene aroma substances, including monoterpenes and sesquiterpenes (Figure 5B). Interestingly, more differential monoterpene VOCs were higher in yuzu grown under field environments, whereas sesquiterpene VOCs were higher in yuzu grown under plastic greenhouses (Figure 5B). These differential changes may all be related to dynamic regulation by the relevant TPS enzymes. In conclusion, these results suggest that protective cultivation practices, such as plastic greenhouse cultivation, can be used to improve the aroma of yuzu in a good way.
4. Discussion
4.1. Effect of Plastic Greenhouse Cultivation on Yuzu Quality
There are many key indicators that make up an assessment of citrus fruit quality or flavour, including TSS, TA, aroma, and more. Protected cultivation systems have been shown to impact these quality traits across different crops. For instance, the use of net houses or poly-covered structures has been reported to alter TSS and TA levels in papaya, either positively or negatively, depending on the cultivation system employed [30]. A comparison of five early-ripening grape varieties under open field and plastic tunnel conditions, revealing significant differences in TSS/TA ratios between cultivation systems, although TSS and pH values themselves did not vary significantly [31]. In watermelon, protected cultivation with trellising yielded higher TSS levels compared to ground cultivation [32]. From previous studies, it was found that the effects of protected cultivation on fruit sugar and acid contents vary depending on species and growing conditions. In this study, yuzu fruits cultivated in plastic greenhouses exhibited a significant reduction in TA and a non-significant decline in TSS (Figure 1B). This aligns with observations in tomatoes, where fruits grown in non-heated greenhouses had lower TSS and TA compared to those grown in open fields [33]. The mechanisms driving these changes are complex and involve environmental factors such as temperature and light intensity. Plastic greenhouses provide enhanced thermal insulation and partial shading, often resulting in elevated internal temperatures and reduced light availability [34]. Elevated temperatures lead to reduced TSS and organic acid concentrations as plant respiration rates increase [35]. In addition, the shading effect of plastic covers may reduce light intensity for citrus. One study noted a significant reduction in light intensity of 21% and 50% under plastic cover under cloudy and sunny conditions, respectively [36]. This can lead to a reduction in photosynthetic activity, which affects the accumulation of fructose and glucose and reduces the accumulation of TSS. Since organic acids in citrus fruits are largely derived from carbohydrate metabolism, reduced photosynthetic efficiency also leads to a decline in acidity. In contrast, transcriptomic analysis in this study showed significant enrichment of DEGs associated with photosynthetic antenna proteins (Figure 4C). These proteins are crucial components of light-harvesting complexes, which facilitate the capture and transfer of solar energy during photosynthesis. Therefore, we propose that under protected cultivation conditions, the increase in temperature and the formation of shade increase the consumption of carbohydrates as well as reduce the accumulation of fructose and glucose, causing a decrease in the content of TSS and TA in yuzu.
For table fruits, sweetness and acidity are among the most important characteristics of interest to consumers, with TSS content mainly affecting the sweetness of the fruit and TA mainly affecting acidity. However, yuzu belongs to the sour orange group, which is primarily used for processing, where aroma becomes a more important quality attribute. Previous research has demonstrated that protected cultivation can substantially alter fruit aroma profiles. For example, ripe Dwarf Cavendish bananas showed significant differences in both quality and quantity of aroma compounds between open-field and protected cultivation systems [37]. Similarly, plastic-covered cultivation in feijoa altered the VOC profile by increasing the relative content of terpenes and reducing esters, leading to an herbaceous aroma in ‘Alcântara’ and ‘Nonante’ cultivars [38]. Additionally, the use of shade nets and greenhouses improved the aromatic quality of autumn-harvested tea leaves by increasing volatile content [39]. In our study, plastic greenhouse cultivation similarly modified the VOC profile in yuzu compared to field-grown fruit, resulting in improved aromatic quality. Environmental factors such as light intensity and temperature are known to regulate the biosynthesis of aroma-related metabolites [40]. For instance, volatile emissions in apples increase with rising temperature within the 0–30 °C range [41], while shading treatments alter aroma precursor levels and expression of aroma biosynthesis-related genes in cucumber [42]. In addition, some biotic factors also affect aroma compound production. On the one hand, insect damage changes in volatile emissions may be an indirect defence function that attracts natural enemies of herbivores [43]. On the other hand, some aromatic components, in addition to their attractive aroma, have insecticidal or antimicrobial properties of their own. Compared to the field environment, plastic greenhouses should have fewer biotic stresses, such as insect pests, due to the enclosed environment, in addition to temperature and light alterations. In this study, aromatic compounds such as 2,4,6-Octatriene, 2,6-dimethyl-, Benzene, isothiocyanato-, Linalool, and Cyclohexene, 2-ethenyl-1,3,3-trimethyl- were found, which are able to resist a wide range of pathogens or defend against a wide range of pests through toxicity, repellency, and inhibition of spawning [44,45,46,47]. It is noteworthy that the levels of these bioactive aromatics are much lower in plastic greenhouse-cultivated yuzu than in field-cultivated ones (Table 1), suggesting that yuzu in plastic greenhouses suffer from much fewer pests and pathogens. Moreover, transcriptomic data showed significant enrichment of DEGs involved in pathways associated with resistance to pests and pathogens, such as plant–pathogen interactions, glucosinolate biosynthesis, phenylpropanoid biosynthesis, and biosynthesis of various alkaloids (Figure 4C) [48,49,50]. These findings further support the hypothesis that the closed greenhouse environment leads to decreased pest-induced metabolic responses, indirectly affecting the aromatic composition of the fruit. Many volatile terpenes not only give off an aroma but also have the ability to repel or even kill pests. Consistent with the above hypothesis, all terpenes with reduced levels in CJKTP have biopesticide properties (Table 1). This results in more terpenes with higher levels in CJKTF, which seems to contradict the stronger aroma of CJKTP. However, the formation of odor is a complex system and is the result of a combination of VOC types and contents, and the odor components may interact with each other. There are four modes of interaction in the odor complex: integration, synergism, antagonism, and independence; this suggests that the odor outcome cannot be determined by the accumulation of VOC content alone [51]. The effect of changes in certain substances on the overall odor is not clear from this study. What is certain is that the overall odor quality of CJKTP is better than that of CJKTF.
In summary, plastic greenhouse cultivation significantly influences yuzu fruit quality, particularly by enhancing aromatic attributes while reducing TSS and TA levels. These changes are mediated by complex interactions among environmental (temperature, light), physiological (photosynthesis, respiration), and biotic (pathogen/pest pressure) factors. Given that yuzu is prized more for its aroma than for sweetness or acidity, the use of plastic greenhouses—an affordable and practical cultivation approach—offers a promising strategy for optimizing the aroma quality of fragrant yuzu.
4.2. Effect of Plastic Greenhouse Cultivation on Metabolic Pathways of Yuzu
Plastic greenhouse cultivation improves the quality of yuzu, particularly in terms of its aromatic profile. At the molecular level, this is driven by significant modulation of metabolic pathways, leading to altered concentrations of volatile organic compounds (VOCs) and subsequent changes in the fruit’s olfactory characteristics. Among the detected VOCs, terpenoids were the most abundant class, and they constituted the majority of differentially accumulated metabolites between CJKTP and CJKTF (Figure 2A,D). This highlights the central role of terpenoids as key volatile and aroma-active components in yuzu aroma formation [52]. Terpenoid biosynthesis begins with the formation of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), which are derived from the MVA or MEP pathways in plants. Terpene synthases catalyse the formation of terpenoids from these precursors [29]. In this study, nerolidol was the key differential aromatic compound between CJKTF and CJKTP (Figure 3). Nerolidol is synthesized from farnesyl diphosphate (FPP) via the action of nerolidol synthase 1 (NES1) [53]. Transcriptomic analysis revealed an upregulation of genes encoding nerolidol synthase in CJKTP, correlating with the increased accumulation of nerolidol, suggesting a positive regulatory relationship; conversely, expression of NES1-like genes was downregulated in CJKTP, indicating a potential antagonistic regulatory mechanism. These findings suggest that nerolidol levels may be jointly regulated by NES1 and NES1-like genes (Figure 5B). Levels of γ-cadinene and expression levels of genes encoding cadinene synthase showed a negative relationship in yuzu, suggesting that DCS may act as a hindrance to γ-cadinene biosynthesis. However, the existence and nature of these putative regulatory mechanisms require further experimental validation in future study. Many terpene synthases are bifunctional, allowing assembly-line catalysis, where multiple biosynthetic steps are carried out sequentially to catalyse the formation of a large number of di-/ester/triterpene skeletons. For example, transgenic Arabidopsis-expressing NES1 in strawberry produces (S)-linalool and its glycoside derivatives in addition to nerolidol [54]. Therefore, it cannot be ruled out whether the biosynthesis of nerolidol and γ-cadinene mentioned above is also influenced by other terpene synthases. Indeed, there are many DEGs enriched in the terpene biosynthetic pathway (Figure 4C). For terpenoids, the monoterpenes and sesquiterpenes generally volatilise if they are not conjugated to polar or large molecules. Higher-order terpenes are generally not volatile [55]. This raises the possibility that plastic greenhouse cultivation not only altered the levels of volatile low-molecular-weight terpenes but also impacted the biosynthesis of non-volatile terpenoids. Supporting this, DEGs involved in diterpene biosynthesis were also enriched under protected cultivation (Figure 4C, Table S2).
In addition to modifying aroma-related pathways, protected cultivation significantly influenced the biosynthesis of health-beneficial phytochemicals. Flavonoids, a class of polyphenolic compounds widely present in citrus, are known for their antioxidant properties, which help mitigate oxidative stress and inflammation. These bioactivities contribute to cardiovascular health and may lower the risk of chronic diseases [56]. Transcriptomic profiling revealed substantial enrichment of DEGs in both flavonoid and isoflavonoid biosynthesis pathways, along with the phenylpropanoid pathway, which serves as a precursor route to flavonoid biosynthesis (Figure 4C, Table S2). This suggests that flavonoid production is more extensively regulated under greenhouse conditions. Furthermore, DEGs related to the biosynthesis of α-linolenic acid, an essential omega-3 fatty acid vital for cardiovascular health [57], and carotenoids, which are key antioxidants and precursors of vitamin A [58,59], were also significantly enriched (Figure 4C). These variations in transcripts of genes related to flavonoid, Alpha-linolenic acid, and carotenoid biosynthesis indirectly suggest that the content of the corresponding nutrients is regulated by cultivation practices. Although this study focused primarily on the volatile metabolome, the observed transcriptional shifts imply that plastic greenhouse cultivation may influence the accumulation of a wide array of non-volatile nutrients as well. Given the limitations of the current metabolomics approach, which prioritized volatile compounds, direct quantification of these nutritional metabolites was not performed. However, future work will focus on targeted metabolite profiling to validate these transcriptomic findings.
Taken together, the results suggest that plastic greenhouse cultivation induces broad and complex reprogramming of yuzu metabolism. We hypothesize that the altered environmental conditions—such as modified temperature regimes, light intensity, and reduced biotic stress—under protected cultivation serve as upstream stimuli that regulate gene expression via pathways such as MAPK signaling and plant hormone signal transduction, ultimately reshaping the plant’s metabolic landscape (Figure 4C). This metabolic reconfiguration contributes to the enhanced aroma quality and potential nutritional benefits observed in greenhouse-grown yuzu.
5. Conclusions
This study revealed that protected cultivation can improve the quality of yuzu (Citrus junos cv. ‘Kitou’), especially in terms of aroma and its underlying molecular regulatory mechanisms. Both electronic nose and VOC metabolomic analyses revealed significant differences between plastic greenhouse-grown (CJKTP) and field-grown (CJKTF) samples. At the molecular level, 41 differentially accumulated volatile organic compounds (VOCs) and 1235 differentially expressed genes (DEGs) were identified. Notably, sesquiterpene-related compounds such as nerolidol and γ-cadinene, along with biosynthetic genes including NES1, NES1-like, and DCS, were co-annotated within the sesquiterpene biosynthesis pathway and showed strong correlations. These findings offer insights into the biochemical and molecular mechanisms of aroma enhancement in yuzu under protected cultivation conditions, offering a valuable reference for the targeted production of high-quality aromatic yuzu. Furthermore, beyond aroma improvement, the potential of protected cultivation to enhance other critical quality attributes of citrus fruit warrants further investigation in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11080945/s1, Table S1: All VOCs and rOAV values detected in CJKTP and CJKTF; Table S2: DEGs enriched in terpenes and flavonoid biosynthesis pathways.
Author Contributions
Conceptualization, F.K.; Data curation, C.C.; Formal analysis, L.S. and F.K.; Funding acquisition, C.C., L.S. and F.K.; Investigation, X.H., Z.N. and Y.Y.; Methodology, C.C., X.H., L.S., Z.N. and Y.Y.; Project administration, C.C.; Resources, X.H., L.S., Z.N. and F.K.; Software, C.C., L.W. and Y.Y.; Supervision, L.S. and F.K.; Validation, C.C., L.S. and L.W.; Visualization, C.C.; Writing—original draft, C.C.; Writing—review and editing, F.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a sub-project of the “Breeding New Fruit Cultivars Major Project of Zhejiang Province” (2021C02066-1); Taizhou Science and Technology Planning Project (24nya20); Zhejiang Provincial Natural Science Foundation of China under Grant No. LQN25C150007; Major Agricultural Technology Collaborative Promotion Plan Project of Zhejiang Province (2024ZDXT04-1).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The RNA-seq data on which this article is based are available on the National Center for Biotechnology Information website at https://www.ncbi.nlm.nih.gov/, (accessed on 16 July 2025) and can be accessed via PRJNA1292151.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VOCs | Volatile organic compounds |
| TSS | Soluble solids content |
| TA | Titratable acid |
| VIP | Variable importance in projection |
| rOAV | Relative odor activity value |
References
- Park, M.K.; Cha, J.Y.; Kang, M.C.; Jang, H.W.; Choi, Y.S. The effects of different extraction methods on essential oils from orange and tangor: From the peel to the essential oil. Food Sci. Nutr. 2024, 12, 804–814. [Google Scholar] [CrossRef]
- Lan Phi, N.T.; Sawamura, M. Characteristic aroma composition profile of mature stage Citrus junos (Yuzu) peel oil from different origins. Food Sci. Technol. Res. 2008, 14, 359–366. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, T.; Asakura, H.; Hayashi, T. Effects of olfactory stimulation from the fragrance of the Japanese citrus fruit Yuzu (Citrus junos Sieb. ex Tanaka) on mood states and salivary chromogranin a as an endocrinologic stress marker. J. Altern. Complem. Med. 2014, 20, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, H.; Hashimoto, S.; Hayashi, S. First identification of the odour-active unsaturated aliphatic acid (E)-4-methyl-3-hexenoic acid in yuzu (Citrus junos Sieb. ex Tanaka). Flavour Fragr. J. 2013, 28, 62–69. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kimura, T.; Hayashi, T. Aromatic effects of a Japanese citrus fruit—Yuzu (Citrus junos Sieb. ex Tanaka)—On psychoemotional states and autonomic nervous system activity during the menstrual cycle: A single-blind randomized controlled crossover study. BioPsychoSoc. Med. 2016, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Cho, H.S.; Jeong, H.; Lee, B.B.; Cho, Y.S.; Rameeza, F.; Eun, J.B. Physiochemical properties, dietary fibers, and functional characterization of three yuzu cultivars at five harvesting times. Food Sci. Biotechnol. 2021, 30, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, J.; Zhang, J.; Cheng, H.; Zhu, C.; Zheng, X. Changes and differential analysis of aroma composition of Citrus junos and Citrus ichangensis at different developmental periods. China Fruits 2023, 8, 58–65. (In Chinese) [Google Scholar]
- Liu, C.; Cheng, Y.; Zhang, H.; Deng, X.; Chen, F.; Xu, J. Volatile constituents of wild citrus Mangshanyegan (Citrus nobilis Lauriro) peel oil. J. Agric. Food Chem. 2012, 60, 2617–2628. [Google Scholar] [CrossRef]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef]
- Asikin, Y.; Taira, I.; Inafuku-Teramoto, S.; Sumi, H.; Ohta, H.; Takara, K.; Wada, K. The composition of volatile aroma components, flavanones, and polymethoxylated flavones in Shiikuwasha (Citrus depressa Hayata) peels of different cultivation lines. J. Agric. Food Chem. 2012, 60, 7973–7980. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhao, H.; Zhong, T.; Chen, D.; Wu, Y.; Xie, Z. Molecular regulatory mechanisms affecting fruit aroma. Foods 2024, 13, 1870. [Google Scholar] [CrossRef]
- Ke, F.; Nie, Z.; Huang, X.; Cui, C.; Yang, Y.; Xu, J.; Wang, L.; Sun, L. Investigating the Effect of Two Interstocks, Changshanhuyou and Ponkan, on the fruit quality and volatile flavor of cocktail grapefruit (Citrus paradisi Macf. cv. Cocktail). Horticulturae 2025, 11, 403. [Google Scholar] [CrossRef]
- Noda, T.; Daiou, K.; Mihara, T.; Murakami, H.; Nagano, Y. Efficient method for generating citrus hybrids with polyembryonic Satsuma mandarin as the female parent. Mol. Breed. 2022, 42, 51. [Google Scholar] [CrossRef]
- Pachiyappan, P.; Kumar, P.; Reddy, K.V.; Kumar, K.N.R.; Konduru, S.; Paramesh, V.; Rajanna, G.A.; Shankarappa, S.K.; Jaganathan, D.; Immanuel, S.; et al. Protected cultivation of horticultural crops as a livelihood opportunity in western India: An economic assessment. Sustainability 2022, 14, 7430. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Rahimi Devin, S.; Salazar, J.A.; López-Alcolea, J.; Rubio, M.; Martínez-García, P.J. Principles and prospects of prunus cultivation in greenhouse. Agronomy 2021, 11, 474. [Google Scholar] [CrossRef]
- Stanghellini, C.; Montero, J.I. Resource use efficiency in protected cultivation: Towards the greenhouse with zero emissions. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927, Lisbon, Portugal, 22–27 August 2010; pp. 91–100. [Google Scholar]
- Zhao, X.; Peng, J.; Zhang, L.; Yang, X.; Qiu, Y.; Cai, C.; Hu, J.; Huang, T.; Liang, Y.; Li, Z.; et al. Optimizing the quality of horticultural crop: Insights into pre-harvest practices in controlled environment agriculture. Front. Plant Sci. 2024, 15, 1427471. [Google Scholar] [CrossRef]
- Li, W.; Zheng, T.; Zhang, J.; Li, W.; Chen, K.; Zhang, K.; Fang, Y. Supplementary light with different wavelengths improved the monoterpenes aroma and quality traits of ‘Shine Muscat’ grape berries under facility cultivation. Food Chem. 2025, 474, 143255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Tian, X.; Yu, H.; Yu, Y. Optimization of sensor array and detection of stored duration of wheat by electronic nose. J. Food Eng. 2007, 82, 403–408. [Google Scholar] [CrossRef]
- Zhai, Y.; Guo, M.; Meng, Q.; Song, H. Characterization of key odor-active compounds in high quality high-salt liquid-state soy sauce. J. Food Compos. Anal. 2023, 117, 105148. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Xiao, J.; Liu, J.; Tang, N.; Zhou, A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, L.; Viau, C.; Lu, Y.; Salavati, R.; Basu, N.; Xia, J. MetaboAnalystR 4.0: A unified LC-MS workflow for global metabolomics. Nat. Commun. 2024, 15, 3675. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, P.; Yin, J.; Wang, W.; Zhang, J.; Wang, W.; Le, T.; Ni, D.; Jiang, H. Dynamic changes in volatile compounds of shaken black tea during its manufacture by GC × GC–TOFMS and multivariate data analysis. Foods 2022, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Quan, S.; Xiao, H. Towards efficient terpenoid biosynthesis: Manipulating IPP and DMAPP supply. Bioresour. Bioprocess. 2019, 6, 6. [Google Scholar] [CrossRef]
- Choudhury, S.; Islam, N.; Mustaki, S.; Uddain, J.; Azad, M.O.K.; Choi, K.Y.; Naznin, M.T. Evaluation of the different low-tech protective cultivation approaches to improve yield and phytochemical accumulation of papaya (Carica papaya L.) in bangladesh. Horticulturae 2022, 8, 210. [Google Scholar] [CrossRef]
- DurgaÇ, C.; Polat, A.A.; KamİLoĞLu, Ö. Comparison of open field and protected cultivation of five early table grape cultivars under Mediterranean conditions. Turk. J. Agric. For. 2011, 35, 491–499. [Google Scholar] [CrossRef]
- Adeeko, A.; Yudelevich, F.; Raphael, G.; Avraham, L.; Alon, H.; Zaaroor Presman, M.; Alkalai-Tuvia, S.; Fallik, E.; Paris, H.S.; Ziv, C. Trellising is advantageous over ground culture for out-of-season, protected production and storage of sweet acorn squash. Front. Hortic. 2024, 3, 1365147. [Google Scholar] [CrossRef]
- Brunele Caliman, F.R.; Henriques da Silva, D.J.; Stringheta, P.C.; Rezende Fontes, P.C.; Rodrigues Moreira, G.; Chartuni Mantovani, E. Quality of tomatoes grown under a protected environment and field conditions. Idesia 2010, 28, 75–82. [Google Scholar] [CrossRef]
- Ahemd, H.A.; Al-Faraj, A.A.; Abdel-Ghany, A.M. Shading greenhouses to improve the microclimate, energy and water saving in hot regions: A review. Sci. Hortic. 2016, 201, 36–45. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Jens, R.; Rolf, N.; Johannes, A.F.; Tor Henning, I. Influence of rain cover cultivation on taste and aroma quality of strawberries (Fragaria ananassa Duch.). J. Food Agric. Environ. 2004, 2, 74–82. [Google Scholar]
- Selli, S.; Gubbuk, H.; Kafkas, E.; Gunes, E. Comparison of aroma compounds in Dwarf Cavendish banana (Musa spp. AAA) grown from open-field and protected cultivation area. Sci. Hortic. 2012, 141, 76–82. [Google Scholar] [CrossRef]
- Hendges, M.V.; Moreira, M.A.; Steffens, C.A.; Talamini do Amarante, C.V. Aromatic profile of Feijoa (Feijoa sellowiana) fruit in protected cultivation, at harvest and after cold storage. Sci. Hortic. 2022, 293, 110691. [Google Scholar] [CrossRef]
- Liu, M.; Xie, F.; Cao, R.; Qi, X.; Chen, X. Effect of different cover cultivations in later summer on aroma constituents of autumn tea (Camellia sinensis L.). J. Agric. Chem. Environ. 2014, 03, 1–6. [Google Scholar] [CrossRef]
- Feng, J.; Ye, S.; Wang, J.; Wu, J.; Zhao, J.; Tian, W.; Pan, G.; Yu, B.; Qiu, D.; Lin, H.; et al. From water migration to aroma development: Revealing the influence of environmental airflow on the aroma of white tea during withering. Food Chem. 2025, 479, 143797. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A Review. New Zeal. J.Crop Hort Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Xue, W.; Liu, N.; Lu, P.; Yang, Y.; Chen, S. Photosynthesis and fatty acid metabolism reveals the effects of shading treatment on the fruit aroma quality of cucumber (Cucumis sativus L.). Sci. Hortic. 2024, 337, 113508. [Google Scholar] [CrossRef]
- Gouinguené, S.P.; Turlings, T.C.J. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef]
- Kang, Z.; Liu, F.; Zhang, Z.; Tian, H.; Liu, T. Volatile β-ocimene can regulate developmental performance of peach aphid myzus persicae through activation of defense responses in Chinese cabbage Brassica pekinensis. Front. Plant Sci. 2018, 9, 708. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Q.; Wang, L.; Lv, M.; Hou, Y.; Li, S. Linalool: A ubiquitous floral volatile mediating the communication between plants and insects. J. Syst. Evol. 2023, 61, 538–549. [Google Scholar] [CrossRef]
- Wiraswati, H.L.; Fauziah, N.; Pradini, G.W.; Kurnia, D.; Kodir, R.A.; Berbudi, A.; Arimdayu, A.R.; Laelalugina, A.; Supandi; Ma’ruf, I.F. Breynia cernua: Chemical profiling of volatile compounds in the stem extract and its antioxidant, antibacterial, antiplasmodial and anticancer activity in vitro and in silico. Metabolites 2023, 13, 281. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xu, Y.; Chen, T.; Li, B.; Zhang, Z.; Tian, S. Application and mechanism of benzyl-isothiocyanate, a natural antimicrobial agent from cruciferous vegetables, in controlling postharvest decay of strawberry. Postharvest Biol. Technol. 2021, 180, 111604. [Google Scholar] [CrossRef]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Dong, N.; Lin, H. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef]
- Wang, W.; Shen, X.; Zhang, S.; Lv, R.; Liu, M.; Xu, W.; Chen, Y.; Wang, H. Research on very volatile organic compounds and odors from veneered medium density fiberboard coated with water-based lacquers. Molecules 2022, 27, 3626. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yan, W.; Fan, Z.; Yang, Y.; Yu, A. Free volatile terpenoids and their interconversions in the pulp of Newhall navel orange during shelf life. J. Food Compos. Anal. 2025, 145, 107772. [Google Scholar] [CrossRef]
- Green, S.A.; Chen, X.; Nieuwenhuizen, N.J.; Matich, A.J.; Wang, M.Y.; Bunn, B.J.; Yauk, Y.K.; Atkinson, R.G. Identification, functional characterization, and regulation of the enzyme responsible for floral (E)-nerolidol biosynthesis in kiwifruit (Actinidia chinensis). J. Exp. Bot. 2012, 63, 1951–1967. [Google Scholar] [CrossRef]
- Aharoni, A.; Giri, A.P.; Deuerlein, S.; Griepink, F.; de Kogel, W.-J.; Verstappen, F.W.A.; Verhoeven, H.A.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Terpenoid metabolism in wild-type and transgenic arabidopsis plants. Plant Cell 2003, 15, 2866–2884. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, C.; Abodi, M.; D’Oria, V.; Milani, G.P.; Agostoni, C.; Mazzocchi, A. Alpha-linolenic acid and cardiovascular events: A narrative review. Int. J. Mol. Sci. 2023, 24, 14319. [Google Scholar] [CrossRef]
- Gürbüz, M.; Aktaç, Ş. Understanding the role of vitamin A and its precursors in the immune system. Nutr. Clin. Et Métab. 2022, 36, 89–98. [Google Scholar] [CrossRef]
- Bufka, J.; Vaňková, L.; Sýkora, J.; Křížková, V. Exploring carotenoids: Metabolism, antioxidants, and impacts on human health. J. Func. Foods 2024, 118, 106284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).