MdCDPK24 Encoding Calcium-Dependent Protein Kinase Enhances Apple Resistance to Colletotrichum gloeosporioides
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Microbial Strains
2.2. Identification of CDPK Genes in Apple
2.3. Chromosomal Locations of CDPKs in Apple
2.4. Phylogenetic Analysis
2.5. Conserved Motif and Domain Analysis
2.6. Gene Expression Analysis
2.7. Promoter Cis-Acting Element Analysis
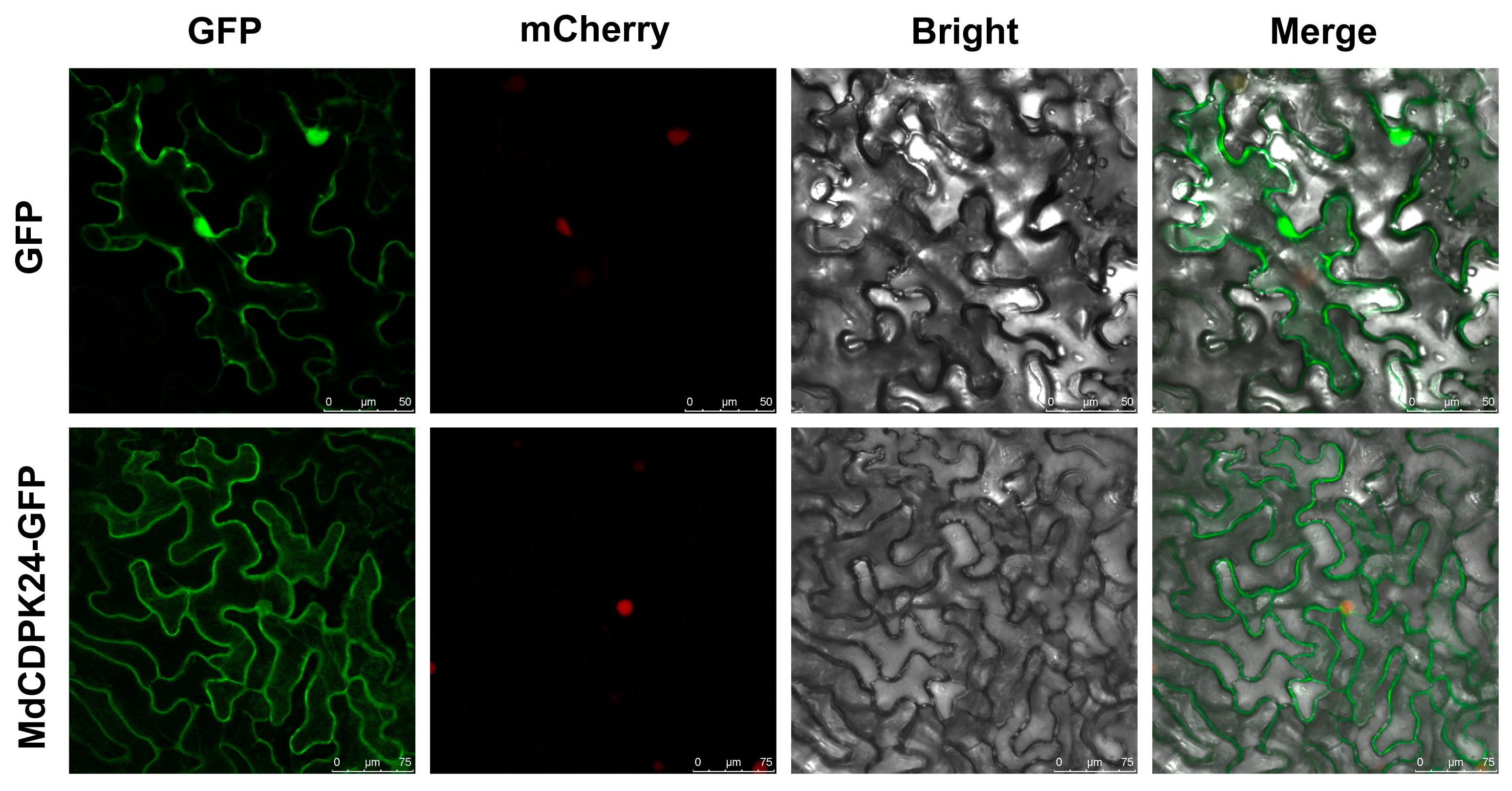
2.8. Subcellular Localization Analysis
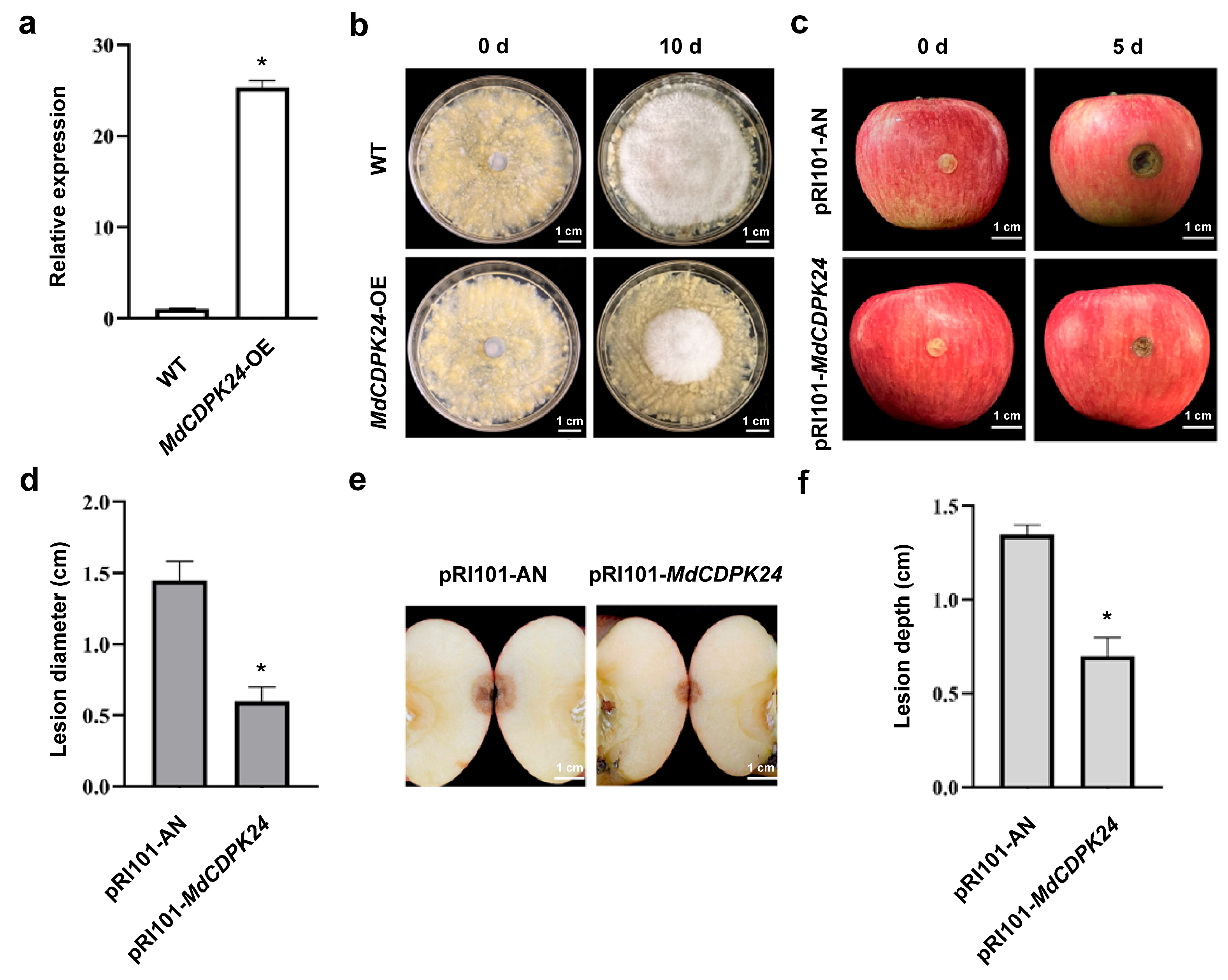
2.9. Generation of Transgenic Apple Calli and Fungal Treatment
2.10. Transient Overexpression of Genes in Fruit
2.11. Statistical Analysis
3. Results
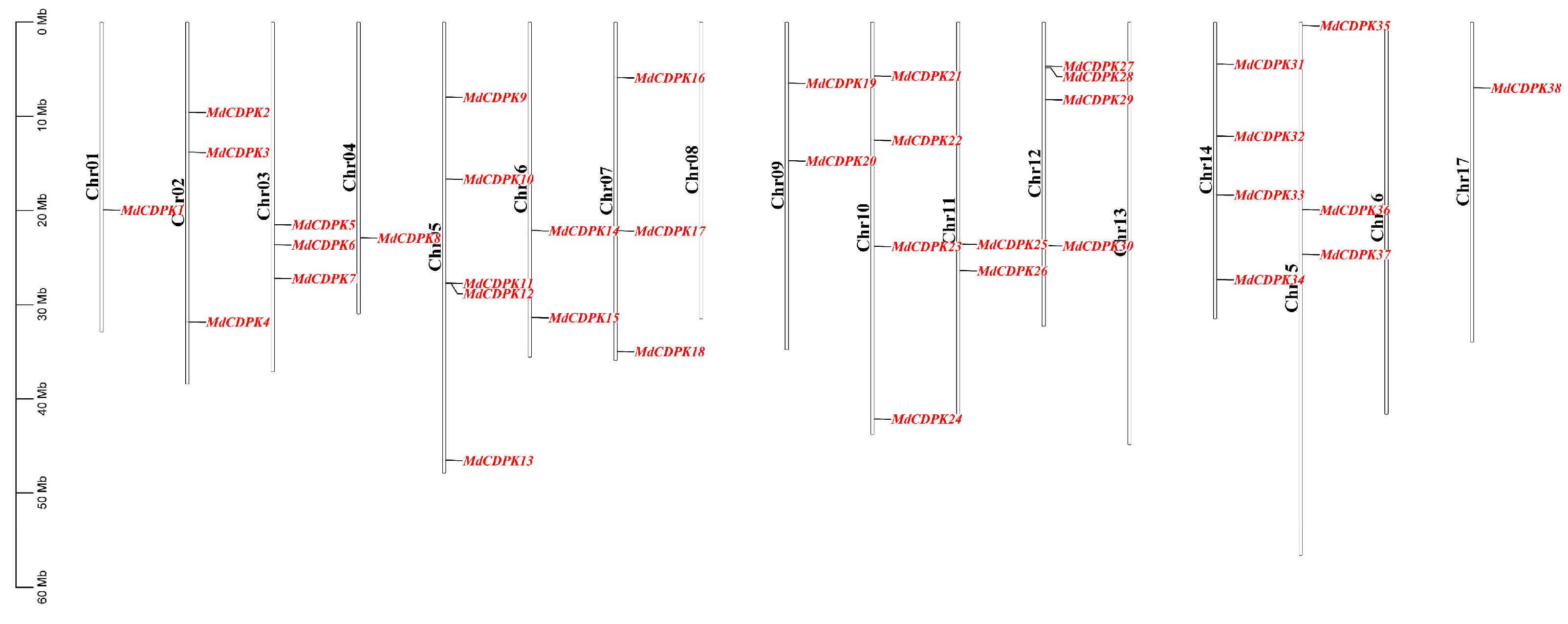
3.1. Identification and Chromosomal Distribution of CDPK Gene Family in Apple
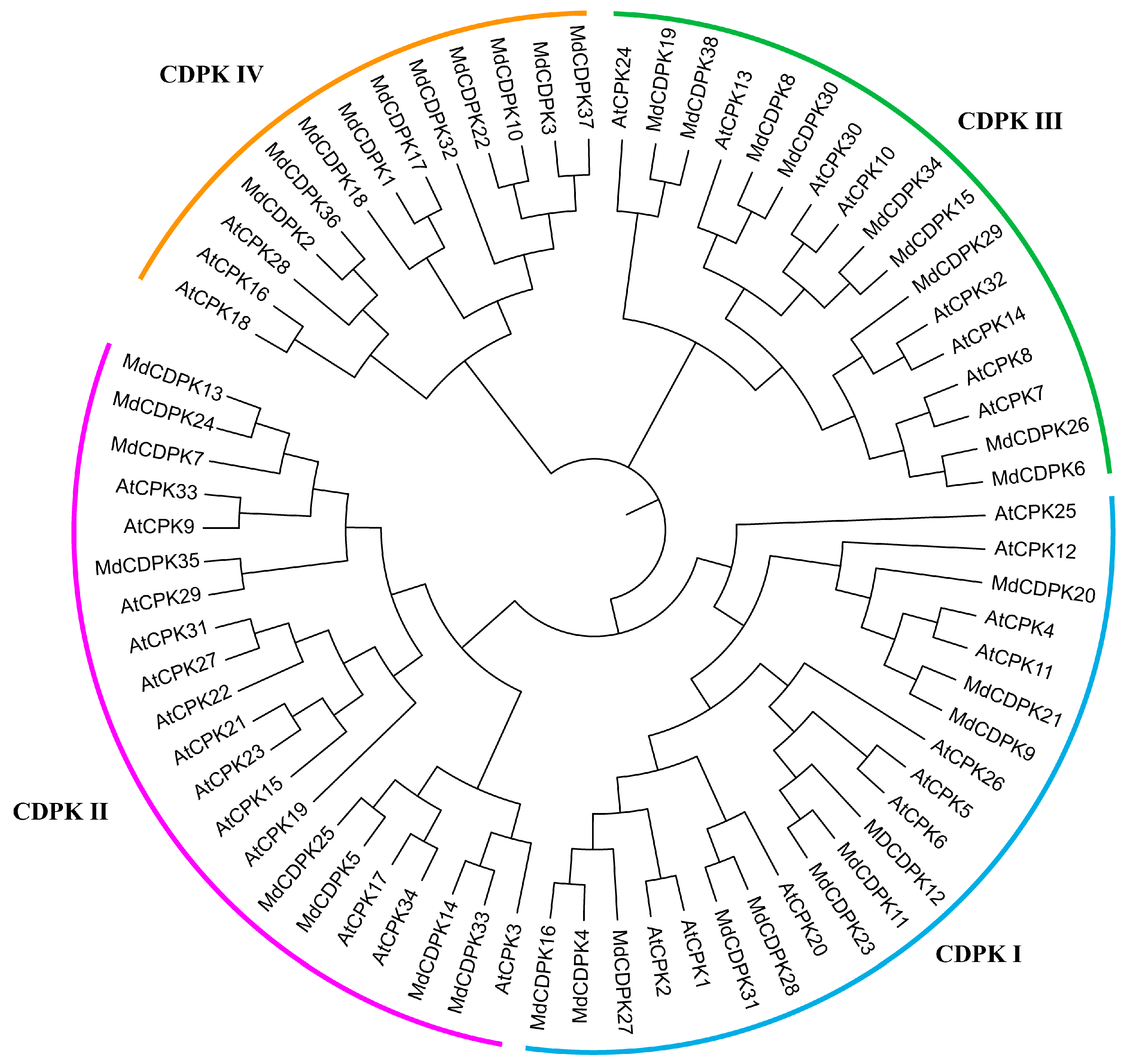
3.2. Phylogenetic Analysis of MdCDPKs
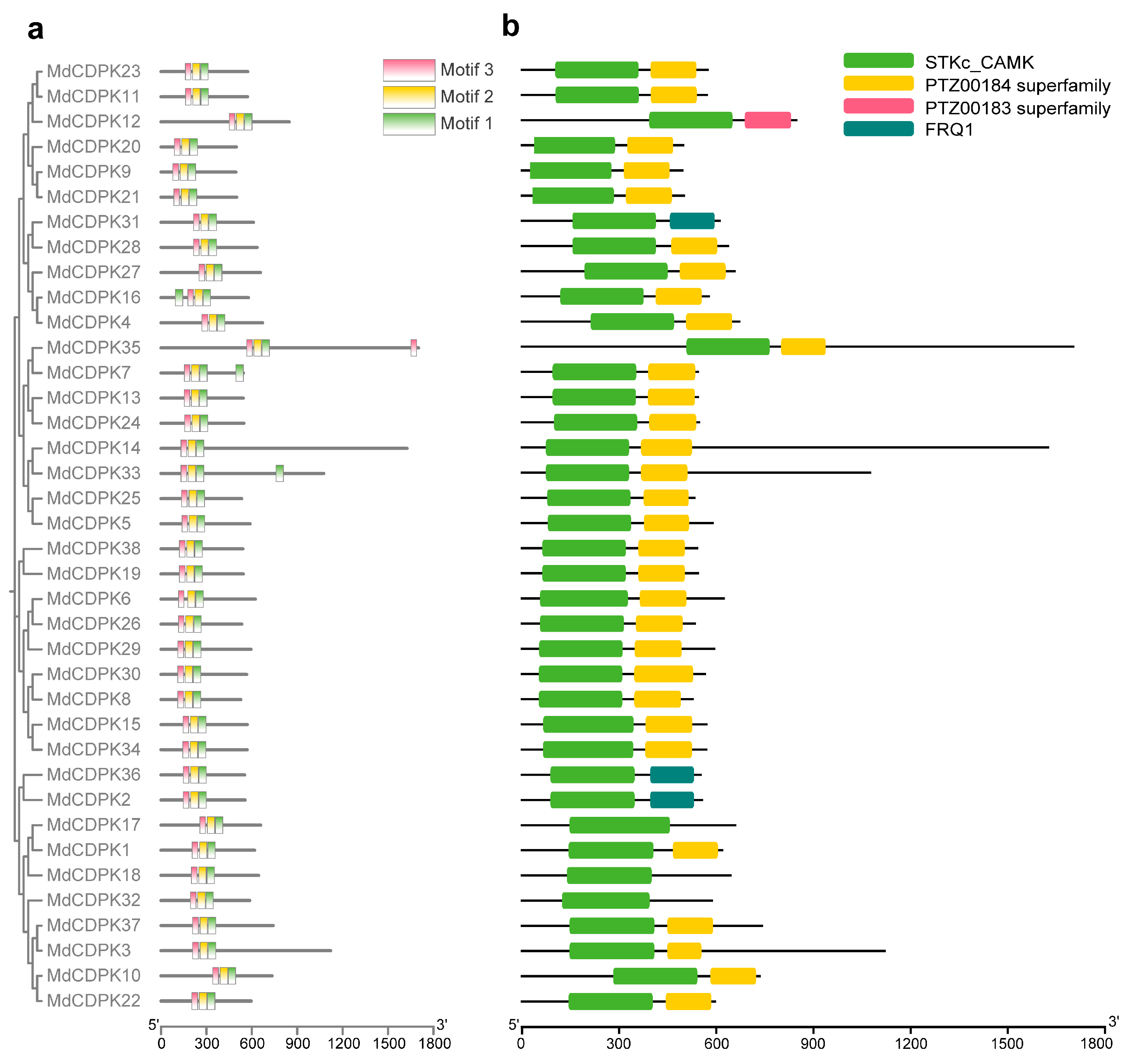
3.3. Conserved Motif and Domain Analysis of MdCDPKs
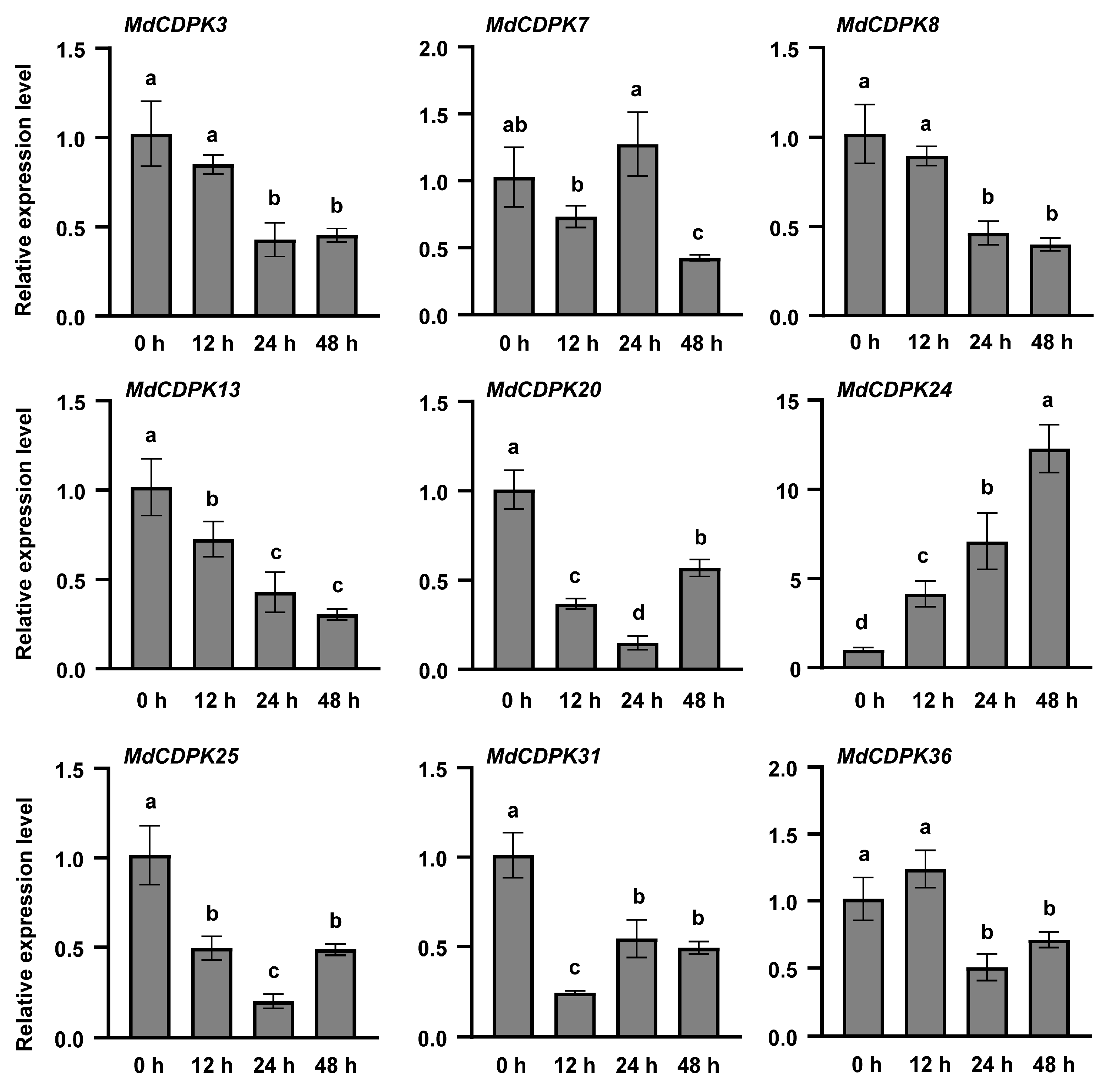
3.4. Expression Analysis of MdCDPKs During C. gloeosporioides Infection
3.5. MdCDPK24 Positively Regulates Apple Bitter Rot Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Wolf, J.; Zhang, F. Towards Sustainable Intensification of Apple Production in China—Yield Gaps and Nutrient Use Efficiency in Apple Farming Systems. J. Integr. Agric. 2016, 15, 716–725. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, R.; Gleason, M.L.; Sun, G. Sustainable Apple Disease Management in China: Challenges and Future Directions for a Transforming Industry. Plant Dis. 2022, 106, 786–799. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in Controlled Release Pesticide Formulations: Prospects to Safer Integrated Pest Management and Sustainable Agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Romeis, T.; Ludwig, A.A.; Martin, R.; Jones, J.D.G. Calcium-dependent Protein Kinases Play an Essential Role in a Plant Defence Response. EMBO J. 2001, 20, 5556–5567. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Han, C.; Qiao, Q.; Li, C.; Dong, H.; Wang, X.; Qi, K.; Xie, Z.; Huang, X.; Zhang, S. Genome-Wide Analysis of the Family 10 Plant Pathogenesis-Related Proteins in Pyrus Bretschneideri and Functional Analysis of PbrMLP for Colletotrichum Fructicola Resistance. Hortic. Adv. 2024, 2, 21. [Google Scholar] [CrossRef]
- Margaritopoulou, T.; Baira, E.; Anagnostopoulos, C.; Vichou, K.-E.; Markellou, E. Phospholipid Production and Signaling by a Plant Defense Inducer against Podosphaera Xanthii Is Genotype-Dependent. Hortic. Res. 2024, 11, uhae190. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and Current Challenges in Calcium Signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Romeis, T.; Herde, M. From Local to Global: CDPKs in Systemic Defense Signaling upon Microbial and Herbivore Attack. Curr. Opin. Plant Biol. 2014, 20, 1–10. [Google Scholar] [CrossRef]
- Bhar, A.; Chakraborty, A.; Roy, A. The Captivating Role of Calcium in Plant-Microbe Interaction. Front. Plant Sci. 2023, 14, 1138252. [Google Scholar] [CrossRef]
- Lee, J.; Rudd, J.J. Calcium-Dependent Protein Kinases: Versatile Plant Signalling Components Necessary for Pathogen Defence. Trends Plant Sci. 2002, 7, 97–98. [Google Scholar] [CrossRef]
- Bredow, M.; Monaghan, J. Cross-Kingdom Regulation of Calcium- and/or Calmodulin-Dependent Protein Kinases by Phospho-Switches That Relieve Autoinhibition. Curr. Opin. Plant Biol. 2022, 68, 102251. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Willmann, M.R.; Chen, H.-C.; Sheen, J. Calcium Signaling through Protein Kinases. The Arabidopsis Calcium-Dependent Protein Kinase Gene Family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef]
- Asano, T.; Tanaka, N.; Yang, G.; Hayashi, N.; Komatsu, S. Genome-Wide Identification of the Rice Calcium-Dependent Protein Kinase and Its Closely Related Kinase Gene Families: Comprehensive Analysis of the CDPKs Gene Family in Rice. Plant Cell Physiol. 2005, 46, 356–366. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Li, N.; Zhou, P.; Li, L. Genome-Wide Identification of Calcium-Dependent Protein Kinases (CDPKs) in Pear (Pyrus Bretschneideri Rehd) and Characterization of Their Responses to Venturia Nashicola Infection. Hortic. Environ. Biotechnol. 2022, 63, 903–915. [Google Scholar] [CrossRef]
- Dekomah, S.D.; Wang, Y.; Qin, T.; Xu, D.; Sun, C.; Yao, P.; Liu, Y.; Bi, Z.; Bai, J. Identification and Expression Analysis of Calcium-Dependent Protein Kinases Gene Family in Potato Under Drought Stress. Front. Genet. 2022, 13, 874397. [Google Scholar] [CrossRef]
- Zuo, R.; Hu, R.; Chai, G.; Xu, M.; Qi, G.; Kong, Y.; Zhou, G. Genome-Wide Identification, Classification, and Expression Analysis of CDPK and Its Closely Related Gene Families in Poplar (Populus Trichocarpa). Mol. Biol. Rep. 2013, 40, 2645–2662. [Google Scholar] [CrossRef] [PubMed]
- Yip Delormel, T.; Boudsocq, M. Properties and Functions of Calcium-Dependent Protein Kinases and Their Relatives in Arabidopsis Thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nakata, M.; Fukazawa, J.; Ishida, S.; Takahashi, Y. Alteration of Substrate Specificity: The Variable N-Terminal Domain of Tobacco Ca2+-Dependent Protein Kinase Is Important for Substrate Recognition. Plant Cell 2010, 22, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-Dependent Protein Kinases: Hubs in Plant Stress Signaling and Development. Plant Physiol. 2013, 163, 523–530. [Google Scholar] [CrossRef]
- Chandran, V.; Stollar, E.J.; Lindorff-Larsen, K.; Harper, J.F.; Chazin, W.J.; Dobson, C.M.; Luisi, B.F.; Christodoulou, J. Structure of the Regulatory Apparatus of a Calcium-Dependent Protein Kinase (CDPK): A Novel Mode of Calmodulin-Target Recognition. J. Mol. Biol. 2006, 357, 400–410. [Google Scholar] [CrossRef]
- Wernimont, A.K.; Artz, J.D.; Finerty, P.; Lin, Y.-H.; Amani, M.; Allali-Hassani, A.; Senisterra, G.; Vedadi, M.; Tempel, W.; Mackenzie, F.; et al. Structures of Apicomplexan Calcium-Dependent Protein Kinases Reveal Mechanism of Activation by Calcium. Nat. Struct. Mol. Biol. 2010, 17, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, Q.; Yang, J.; Xie, G.; Liu, J.-H. PtrCDPK10 of Poncirus Trifoliata Functions in Dehydration and Drought Tolerance by Reducing ROS Accumulation via Phosphorylating PtrAPX. Plant Sci. 2020, 291, 110320. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, H.; Wang, L.; Zhang, X.; He, W.; Xiang, Y. Comparative Genomic Analysis of the CPK Gene Family in Moso Bamboo (Phyllostachys Edulis) and the Functions of PheCPK1 in Drought Stress. Protoplasma 2023, 260, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Deng, C.; Deng, S.; Li, N.; Zhao, C.; Zhao, R.; Liang, S.; Chen, S. The Arabidopsis Ca2+-Dependent Protein Kinase CPK12 Is Involved in Plant Response to Salt Stress. Int. J. Mol. Sci. 2018, 19, 4062. [Google Scholar] [CrossRef]
- Borkiewicz, L.; Polkowska-Kowalczyk, L.; Cieśla, J.; Sowiński, P.; Jończyk, M.; Rymaszewski, W.; Szymańska, K.P.; Jaźwiec, R.; Muszyńska, G.; Szczegielniak, J. Expression of Maize Calcium-Dependent Protein Kinase (ZmCPK11) Improves Salt Tolerance in Transgenic Arabidopsis Plants by Regulating Sodium and Potassium Homeostasis and Stabilizing Photosystem II. Physiol. Plant. 2020, 168, 38–57. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, X.; Ren, S.; Zhao, J.; Zhu, Z.; Wang, Y. Overexpression of AcCDPK1 and AcCDPK5 from Allium Cepa. L Enhances Phytophthora nicotianae Resistance in Nicotiana benthamiana. Sci. Hortic. 2024, 326, 112743. [Google Scholar] [CrossRef]
- Mou, B.; Zhao, G.; Wang, J.; Wang, S.; He, F.; Ning, Y.; Li, D.; Zheng, X.; Cui, F.; Xue, F.; et al. The OsCPK17-OsPUB12-OsRLCK176 Module Regulates Immune Homeostasis in Rice. Plant Cell 2024, 36, 987–1006. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Shen, L.; Cao, J.; Liu, C.; Hu, J.; Guan, D.; He, S. A CaCDPK29–CaWRKY27b Module Promotes CaWRKY40-Mediated Thermotolerance and Immunity to Ralstonia Solanacearum in Pepper. New Phytol. 2022, 233, 1843–1863. [Google Scholar] [CrossRef]
- Jung, S.; Lee, T.; Cheng, C.-H.; Buble, K.; Zheng, P.; Yu, J.; Humann, J.; Ficklin, S.P.; Gasic, K.; Scott, K.; et al. 15 Years of GDR: New Data and Functionality in the Genome Database for Rosaceae. Nucleic Acids Res 2019, 47, D1137–D1145. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The Conserved Domain Database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The Proteomics Server for in-Depth Protein Knowledge and Analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, F.; Yang, S.; Zhang, Y.; Xue, H.; Wang, Y.; Yan, S.; Wang, Y.; Zhang, Z.; Ma, Y. MdWRKY100 Encodes a Group I WRKY Transcription Factor in Malus Domestica That Positively Regulates Resistance to Colletotrichum Gloeosporioides Infection. Plant Sci. 2019, 286, 68–77. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL Module Regulates Apple Salt Stress Tolerance by Activating MdWRKY100 Expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef]
- Liu, X.; Dai, H.; Zhang, F.; Wang, J.; Shi, J.; Chen, J.; He, P.; Wang, F.; Ma, Y. The miR7125-MdARF1 Module Enhances the Resistance of Apple to Colletotrichum Gloeosporioides by Promoting Lignin Synthesis in Response to Salicylic Acid Signalling. Plant Biotechnol. J. 2024, 22, 2741–2755. [Google Scholar] [CrossRef]
- Liese, A.; Romeis, T. Biochemical Regulation of in Vivo Function of Plant Calcium-Dependent Protein Kinases (CDPK). Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 1582–1589. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, H.-L.; Mei, C.; Wang, X.-J.; Yan, L.; Liu, R.; Zhang, X.-F.; Wang, X.-F.; Zhang, D.-P. The Arabidopsis Ca2+-Dependent Protein Kinase CPK12 Negatively Regulates Abscisic Acid Signaling in Seed Germination and Post-Germination Growth. New Phytol. 2011, 192, 61–73. [Google Scholar] [CrossRef]
- Almadanim, M.C.; Alexandre, B.M.; Rosa, M.T.G.; Sapeta, H.; Leitão, A.E.; Ramalho, J.C.; Lam, T.T.; Negrão, S.; Abreu, I.A.; Oliveira, M.M. Rice Calcium-Dependent Protein Kinase OsCPK17 Targets Plasma Membrane Intrinsic Protein and Sucrose-Phosphate Synthase and Is Required for a Proper Cold Stress Response. Plant Cell Environ. 2017, 40, 1197–1213. [Google Scholar] [CrossRef]
- Fantino, E.; Segretin, M.E.; Santin, F.; Mirkin, F.G.; Ulloa, R.M. Analysis of the Potato Calcium-Dependent Protein Kinase Family and Characterization of StCDPK7, a Member Induced upon Infection with Phytophthora Infestans. Plant Cell Rep. 2017, 36, 1137–1157. [Google Scholar] [CrossRef]
- Demir, F.; Horntrich, C.; Blachutzik, J.O.; Scherzer, S.; Reinders, Y.; Kierszniowska, S.; Schulze, W.X.; Harms, G.S.; Hedrich, R.; Geiger, D.; et al. Arabidopsis Nanodomain-Delimited ABA Signaling Pathway Regulates the Anion Channel SLAH3. Proc. Natl. Acad. Sci. USA 2013, 110, 8296–8301. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Yu, X.-C.; Wang, X.-J.; Zhao, R.; Li, Y.; Fan, R.-C.; Shang, Y.; Du, S.-Y.; Wang, X.-F.; Wu, F.-Q.; et al. Two Calcium-Dependent Protein Kinases, CPK4 and CPK11, Regulate Abscisic Acid Signal Transduction in Arabidopsis. Plant Cell 2007, 19, 3019–3036. [Google Scholar] [CrossRef]
- Yang, Z.; Rannala, B. Molecular Phylogenetics: Principles and Practice. Nat. Rev. Genet. 2012, 13, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-J.; Li, P.; Shimono, M.; Corrion, A.; Higaki, T.; He, S.Y.; Day, B. Arabidopsis Calcium-Dependent Protein Kinase 3 Regulates Actin Cytoskeleton Organization and Immunity. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An Integrative Web Server to Predict Subcellular Localization of Proteins. Nucleic Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef] [PubMed]
- Dammann, C.; Ichida, A.; Hong, B.; Romanowsky, S.M.; Hrabak, E.M.; Harmon, A.C.; Pickard, B.G.; Harper, J.F. Subcellular Targeting of Nine Calcium-Dependent Protein Kinase Isoforms from Arabidopsis. Plant Physiol. 2003, 132, 1840–1848. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liang, S.; Chen, X.; Liu, J.; Zhang, Y.; Wang, A. Identification of CDPK Gene Family in Solanum Habrochaites and Its Function Analysis under Stress. Int. J. Mol. Sci. 2022, 23, 4227. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, C.; Cheng, Y.; Ruan, M.; Ye, Q.; Wang, R.; Yao, Z.; Wan, H. Molecular Evolution and Functional Divergence of Stress-Responsive Cu/Zn Superoxide Dismutases in Plants. Int. J. Mol. Sci. 2022, 23, 7082. [Google Scholar] [CrossRef]
- Chmelová, Ľ.; Kraeva, N.; Saura, A.; Krayzel, A.; Vieira, C.S.; Ferreira, T.N.; Soares, R.P.; Bučková, B.; Galan, A.; Horáková, E.; et al. Intricate Balance of Dually-Localized Catalase Modulates Infectivity of Leptomonas Seymouri (Kinetoplastea: Trypanosomatidae). Int. J. Parasitol. 2024, 54, 391–400. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, P.; Lu, Y.; Bai, Y.; Wei, Y.; Liu, G.; Shi, H. MeRAV5 Promotes Drought Stress Resistance in Cassava by Modulating Hydrogen Peroxide and Lignin Accumulation. Plant J. 2021, 107, 847–860. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.-P.; Schulze, W.X.; Romeis, T. Calcium-Dependent Protein Kinase/NADPH Oxidase Activation Circuit Is Required for Rapid Defense Signal Propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Du, M.; Liang, X.; Fan, F.; Huang, G.; Zou, Y.; Bai, J.; Lu, D. The Calcium-Dependent Protein Kinase CPK28 Is Targeted by the Ubiquitin Ligases ATL31 and ATL6 for Proteasome-Mediated Degradation to Fine-Tune Immune Signaling in Arabidopsis. Plant Cell 2022, 34, 679–697. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Chen, K.; Xiao, Z.; Liang, X.; Lu, D. Phosphorylation Status of CPK28 Affects Its Ubiquitination and Protein Stability. New Phytol. 2023, 237, 1270–1284. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Y.; Yu, X.; Liu, J.; Yang, L.; Gao, Y.; Ke, G.; Zhou, M.; Mu, B.; Xiao, S.; et al. Overexpression of Two CDPKs from Wild Chinese Grapevine Enhances Powdery Mildew Resistance in Vitis Vinifera and Arabidopsis. New Phytol. 2021, 230, 2029–2046. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.; Dong, H.; Cai, B.; Tao, J. Characterization and Comparison of the CPK Gene Family in the Apple (Malus × Domestica) and Other Rosaceae Species and Its Response to Alternaria Alternata Infection. PLoS ONE 2016, 11, e0155590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Ma, Y.; Wang, D.; Wang, F. MdCDPK24 Encoding Calcium-Dependent Protein Kinase Enhances Apple Resistance to Colletotrichum gloeosporioides. Horticulturae 2025, 11, 942. https://doi.org/10.3390/horticulturae11080942
Shi J, Ma Y, Wang D, Wang F. MdCDPK24 Encoding Calcium-Dependent Protein Kinase Enhances Apple Resistance to Colletotrichum gloeosporioides. Horticulturae. 2025; 11(8):942. https://doi.org/10.3390/horticulturae11080942
Chicago/Turabian StyleShi, Jiajun, Yuxin Ma, Dajiang Wang, and Feng Wang. 2025. "MdCDPK24 Encoding Calcium-Dependent Protein Kinase Enhances Apple Resistance to Colletotrichum gloeosporioides" Horticulturae 11, no. 8: 942. https://doi.org/10.3390/horticulturae11080942
APA StyleShi, J., Ma, Y., Wang, D., & Wang, F. (2025). MdCDPK24 Encoding Calcium-Dependent Protein Kinase Enhances Apple Resistance to Colletotrichum gloeosporioides. Horticulturae, 11(8), 942. https://doi.org/10.3390/horticulturae11080942





