Abstract
The transition to ripening in non-climacteric species is governed by several signals, including hormones that enhance or counteract the abscisic acid (ABA)-promoting effect. The SQUAMOSA Promoter-binding protein-Like (SPL) transcription factors are involved in ripening through the modulation of anthocyanin biosynthesis. In sweet cherry fruits, several miR156-targeted PavSPLs are expressed before and during ripening. Recently, some PavSPLs were found in the transition from development to ripening in cultivars contrasting in maturity time. Additionally, several forms of miR156 were expressed in sweet cherry seeds of an early-season cultivar. In this work, we addressed the relevance of endocarp lignification and PavSPLs expression for the transition to ripening. First, we characterized early- and late-season sweet cherry cultivars, ‘Celeste’ and ‘Regina’, focusing on fruit and seed development, endocarp lignification, and PavSPL expression profile. Fruit growth dynamics revealed an earlier onset of color development and lignification in ‘Celeste’, while ‘Regina’ exhibited a prolonged lag phase and delayed embryo development. Transcript profiling at the light green stage showed a higher expression of PavSPL genes in fruits and identified cultivar-specific expressions, especially between ‘Regina’ and ‘Celeste’ seeds. Co-expression networks linked PavSPLs to genes involved in lignin and anthocyanin biosynthesis. We focused on PavSPL2 and PavSPL9, which were targeted by mtr-miR156a and gma-miR156f. Both PavSPLs and miRNAs were expressed in fruits and seeds at the yellow stage, an advanced point in the transition to ripening in sweet cherry. Exogenous application of auxin-related compounds in the mid-season cultivar ‘Lapins’ modulated endocarp lignification and pigmentation. Notably, p-IBA treatment, which enzymatically targets the lignin pathway, transiently increased anthocyanin accumulation and reduced lignin deposition, effects that correlated with the downregulation of PavSPL gene expression. These findings highlight the interplay between lignification, color evolution, and pigment biosynthesis during the transition from development to ripening in sweet cherry fruits, and suggest a role for PavSPL genes in this transition.
1. Introduction
Sweet cherry (Prunus avium L.) is a profitable crop whose production worldwide increased from 1.9 to 2.96 million metric tons between 2000 and 2023 [1]. This species is classified as a non-climacteric fruit, as it does not present significant ethylene production or a respiration burst at the onset of ripening [2]. Since the sweet cherry genome has been sequenced and annotated, this species is a good model for unveiling this type of ripening [3]. Sweet cherry depends on abscisic acid (ABA) for the triggering of ripening [4,5,6,7], which is similar to other non-climacteric species like strawberry and grapevine [8,9]. Other hormones modulate ripening in non-climacteric species. For instance, auxin exerts a negative ripening control in grapevine, whose content is higher in fruits with a high seed content [10]. However, Clayton-Cuch et al. reported that auxin can promote fruit ripening in sweet cherry [11]. This is supported by the finding that indole-3-acetic acid (IAA) treatment increased ABA concentration in sweet cherry fruits [7]. Hence, auxin in conjunction with ABA can positively modulate the ripening process in this species. On the other hand, cytokinins (CKs) increase fruit size and delay the fruit coloring process [12], and some CK pathway genes are regulated at the epigenomic level by ABA during ripening in sweet cherry [13]. Finally, gibberellin (GA) is a negative ripening regulator in sweet cherry, since the application of gibberellic acid (GA3) delayed ripening [14,15,16], suggesting a role in the control of maturity time. It is worth noting that GA3 treatment is a common agronomical practice, usually applied during the light green or straw yellow stages. This suggests that this moment is key for determining ripening initiation. In addition, cultivars with differences in maturity time have different lengths for these stages, as well as differences in hormone content and secondary metabolic profile [17]. Therefore, the evidence suggests that some signals occurring before the pink-stage-related ABA increase could modulate ripening initiation.
The growth of sweet cherry fruit is characterized by a double sigmoid curve, including three key phases. During stage I, intense cell division takes place; then a growth arrest occurs at stage II (also known as lag phase), where embryo development and pit hardening due to endocarp lignification occurs [18]; finally, during stage III a second exponential growth takes place [19]. At stage III, fruit ripening initiates, including the coloring process associated with changes in the expression of ABA and anthocyanin biosynthesis genes in the fruit skin [20]. However, other processes such as de-greening due to chlorophyll degradation occur some weeks earlier [21]. The lignification–ripening connection comes from the idea that a fully lignified endocarp indicates that the fruit is ripe and ready to disperse [22]. Thus, different signals are expected to coordinate the endocarp and mesocarp processes. In this regard, plant hormones control lignification, as shown in model organisms. For example, in Arabidopsis, auxin has been described as regulating lignin biosynthesis [23]. Stone cell development in pear (Pyrus pyrifolia) fruits is also affected by auxin [24].
Early-, mid-, and late-season sweet cherry cultivars differ in stage II duration, where lignification occurs [15,17,25]. As stage II is shorter in early-season cultivars, the embryo cannot reach its full size, since its development coincides with fruit ripening and senescence [25]. Thus, variations in the harvest time between cultivars could be associated with differences in the degree of embryo development. This is relevant considering that seeds have at least a one order of magnitude higher hormone content than the mesocarp, including auxin and GA3 [26]; hence, differences in seed development between cultivars may involve differences in hormone production, including auxin and GA, which in turn are key for controlling ripening initiation, as mentioned above. On the other hand, the endocarp lignification occurring at stage II could be a critical period, since profuse remodeling at the transcriptomic level occurs in fruits and seeds pre- versus post-lignification, including genes encoding transcription factors with hormone-specific responsiveness, as shown in Prunus persica [27].
Transcription factors are key in the regulation of plant processes. The SQUAMOSA Promoter-binding protein-Like (SPL) genes are part of a family of transcription factors acting as direct upstream regulators [28]. SPLs are regulated at the post-transcriptional level by miR156s [29], which repress their expression, resulting in the regulation of processes such as floral induction, shoot branching, and pigment biosynthesis [30,31,32]. SPL transcripts are part of a regulatory module, SPL/miR156, where their expression in a given process/tissue is controlled by specific miRNA156. Regulatory small RNA miR156 is part of a very conserved miRNA family, consisting of 20 nucleotides [33]. Overexpressing miR156 resulted in a prolonged juvenile stage and delayed flowering [34,35], showing a key role in regulating plant developmental transitions.
In fruit trees, SPLs are key in phase transitions such as the vegetative-to-adult reproductive phase, as Song et al. reported in pear (Pyrus spp.) [36]. There are 16 PavSPL encoding sequences in sweet cherry [29], with 12 of them predicted as targets of miR156 [37]. These PavSPLs are involved in flowering time and abiotic stress response. In the case of miR156 sequences, they were present during bud dormancy in sweet cherry [38].
The role of the SPL/miR156 module in the transition from development to ripening has recently gained attention. Functional analysis in Pyrus pyrifolia showed that this module is involved in anthocyanin accumulation during light-induced red peel coloration [39]. In that work, the abundance of several miR156s increased upon light-induced anthocyanin pathway activation, whereas PpySPL expression decreased, suggesting that SPLs are negative regulators of the coloring process and miRNA156 are positive regulators. This in line with findings showing that the expression of miR156 increases during fruit ripening in Solanum lycopersicum [40]. In blueberry (Vaccinium corymbosum), lines overexpressing the gene VcMIR156a increased anthocyanin accumulation, supporting their pro-ripening role, whereas VcSPL12 positively regulated chlorophyll accumulation, since it physically binds to the promoter of the chlorophyll biosynthetic gene VcDVR [41].
PavSPL genes and several miR156 sequences are present at color initiation in sweet cherry [42]. Their expression decreases in fruits during ripening, with differences starting from the de-greening to yellow stages [43], suggesting a role in the transition from development to ripening. PavSPL expression is affected by hormonal treatments. For instance, ABA decreased the transcript abundance of several PavSPLs, whereas GA—a ripening-antagonizing hormone—increased PavSPL gene expression [43], supporting the idea that they are negative ripening regulators. In a recent report, PavSPL genes were found to be expressed in fruits and seeds at the light green stage [44], where the seeds of the early-season cultivar ‘Celeste’ were highly abundant in several miR156 variants, consistent with the pro-ripening role of these miRNAs. Maldonado et al. showed several fruit–seed associations, suggesting that seeds may modulate the transition from development to ripening in sweet cherry [44].
As several PavSPLs have been found to have high expression levels in fruits, whereas miR156s have high abundance in seeds [44], here we hypothesize that the sweet cherry PSPL/miR156 module could have a role in inter-tissue post-transcriptional regulation. Long-distance movement of miRNAs in plant tissues has previously been discussed [45]. We argue that this could be addressed, at least from an exploratory perspective, by disturbing the lignification of the endocarp, which is located between the seed and fruit mesocarp tissues. This is supported by the fact that endocarp lignification is related to the ripening process, as mentioned above. Therefore, the aim of this work was to characterize the expression profile of sweet cherry miR156s and PavSPLs in seeds and fruits, and then to analyze the expression profile of selected PavSPL genes in response to treatments affecting lignification during the transition from development to ripening in sweet cherry.
To address this, we first analyzed the gene expression of sweet cherry PavSPLs in the fruits and seeds of early- and late-season cultivars. Then, we explored the effect of lignin modulators on fruit endocarp lignification and on ripening markers (chlorophyll, carotenoid, and anthocyanin contents) over two seasons in field conditions. The modulators included auxin-related compounds and a compound targeting the activity of a key lignification enzyme. Finally, the effect of these modulators on PavSPL gene expression was evaluated.
To our knowledge, the SPL response to lignin modulators at the level of gene expression has not been assessed in stone fruits, nor the physiological effect of disturbing lignification, specifically in relation to ripening markers in non-climacteric species at the transition from development to ripening.
2. Methods
2.1. Plant Material
2.1.1. Plant Material for Early- and Late-Season Cultivar Characterization
Sweet cherry (Prunus avium L.) fruits selected from trees growing in the Experimental Station INIA Los Tilos, located in Buin, Región Metropolitana, Chile (33°42′ S, 70°42′ W) were used during the 2022–2023 season. Two cultivars contrasting in maturity time were selected, the late-season ‘Regina’, and the early-season ‘Celeste’ (denomination Sumpaca®, 13S.24.28; licensed to McGrath Nurseries Ltd. in Hamilton, New Zealand). The ten-year-old trees were grafted on ‘Cap-6P’ rootstock. Plants were arranged in a 4 × 2 frame, with regular phytosanitary and fertirrigation management, and with hydrogen cyanamide treatment to break dormancy. Gibberellic acid was not applied in the orchard during the 2022–2023 season.
2.1.2. Plant Material for Mid-Season Cultivar Characterization
Sweet cherry fruits from trees established in 2006 and 2011 in the Experimental Station La Palma, Valparaíso, Chile (32°52′ S, 71°12′ W) were used in the 2023–2024 and 2024–2025 seasons, respectively. ‘Lapins’, a mid-season cultivar grafted on ‘Colt‘ rootstock was selected. The plant material in Section 2.1.1 and Section 2.1.2 corresponds to registered varieties and it was commercially obtained. The trees were in a 4 × 2.5 array, with similar productive management as mentioned in Section 2.1.1, except that the trees were covered with plastic bags when gibberellic acid (GA3) was applied, to avoid interactions with the treatments we applied to the plant material.
2.2. Experimental Design
2.2.1. Experimental Design for Early- and Late-Season Cultivar Characterization in the 2022–2023 Season
Three plants were randomly selected from ‘Celeste’ and ‘Regina’ cultivars, which were part of the arboretum of INIA Los Tilos. We considered each tree as a biological replicate; thus, three replicates (n = 3) were utilized. Sampling for qPCR analysis was at the straw yellow (SY) stage, which was 40 and 41 days after full bloom (DAFB) in ‘Celeste’ and ‘Regina’, respectively, with 0 DAFB when 50% flowers were open. For the qPCR analyses, eight representative fruits without apparent defects were sampled from each tree, separated into fruit (mesocarp) and seed tissues, and immediately frozen in liquid nitrogen until processing. Once in the laboratory, fruits and seeds were ground separately using liquid nitrogen. The endocarp tissue was carefully separated with tweezers and discarded. Ground samples were stored at −80 °C until the RNA extractions. In this work, ‘fruits’ corresponds to mesocarp-enriched tissues, whereas ‘seeds’ includes embryo and its surrounding tissues (endosperm and seed coat). Both, ‘seed’ and ‘fruit’ samples, were used for qPCR analysis. The fruit equatorial diameter of the same trees as used for sampling was measured during the season using 20 fruits per tree; lignification was evaluated at 21, 28, 35, and 42 DAFB in eight fruits per tree; and seed and embryo lengths were evaluated at 21, 28, 35, 42, and 49 DAFB by sampling three fruits per tree. The ripening parameter soluble solids content (SSC) was evaluated at the red (R) stage in five fruits per tree at 59 and 60 DAFB in ‘Celeste’ and ‘Regina’, respectively.
2.2.2. Experimental Design for the Treatments with Lignification Modulators in the 2023–2024 Season in the Mid-Season Cultivar ‘Lapins’
Four treatments with lignification modulators were performed in a randomized complete block design. For this, four trees of the ‘Lapins’ cultivar were randomly selected in a row, with the four blocks located in different rows, where the tree was the experimental unit. As four trees were selected (one of each row), four replicates per treatment (n = 4) were utilized. The treatments were NPA (naphtylphtalamic acid; Sigma-Aldrich, St. Louis, MO, USA), IAA (indol-3-acetic acid; Sigma-Aldrich, St. Louis, MO, USA), and p-IBA (p-iodobenzoic acid, Thermo Fisher Scientific, Waltham, MA, USA). All the treatments were at 40 µM and the control treatment corresponded to untreated plants. The treatments were applied at 25 DAFB, (T0), corresponding to 20% light green (LG)/80% green (G). Eight representative fruits without visible defects were collected from each replicate and frozen in liquid nitrogen. Grinding at the laboratory was carried out with a constant supply of liquid nitrogen, where every sample consisted of entire fruits (including endocarps and seeds). The samples for pigment content analyses correspond to 28 (T3), 46 (T21), 56 (T31), and 79 (T54) DAFB, with T3 corresponding to 50% LG/50% G; T21 to 90% LG/10% SY; T31 to 90% SY/10% pink (P); and T54 to 70% R/30% P. For qPCR analyses, only samples of the control and p-IBA treatments at T21 were selected. The samples were kept at −80 °C until they were used for qPCR and pigment content analyses. Sampling for the effect of the treatments on endocarp lignification was at 29 DAFB (T4). Eight fruits were sampled and fixed in FAA solution (10/50/5, formaldehyde (37%)/absolute ethanol/anhydrous acetic acid). The equatorial diameter of the fruit was recorded with 15 fruits per tree during the entire season. Ripening parameters, firmness, and SSC were evaluated at 71 DAFB in ten fruits per tree.
2.2.3. Experimental Design for the Treatments with Lignification Modulators in the 2024–2025 Season in the Mid-Season Cultivar ‘Lapins’
Five treatments with lignification modulators were performed in a randomized complete block design with three blocks. For this, five trees of the ‘Lapins’ cultivar were randomly selected per row, where each tree was the experimental unit. As three trees were selected (one for each row), three replicates per treatment (n = 3) were utilized. The treatments were p-IBA (Sigma-Aldrich, St. Louis, MO, USA) at 40 µM and 60 µM, and NAA (1-naphthaleneacetic acid; Sigma-Aldrich, St. Louis, MO, USA) at 40 µM and 100 µM. The water solutions were 1% Tween 20 (Thermo Fisher Scientific, Waltham, MA, USA). The control treatment corresponded to untreated plants. The treatments were applied at 25 DAFB (T0, where the tree phenology was 100% LG) through spraying until run-off. Eight representative fruits without visual defects were sampled for pigment analysis and immediately frozen in liquid nitrogen, another eight representative and healthy fruits were sampled and fixed in FAA solution (10/50/5, formaldehyde (37%)/absolute ethanol/anhydrous acetic acid). For endocarp lignification assessment and pigment analysis, the sample dates were 25 DAFB (before the treatments), 35 DAFB (T10, 90% LG/10% SY), and 50 DAFB (T25, 70% SY/30% P). At 68 DAFB, the fruit equatorial diameter of five fruits per tree was recorded.
2.3. Growth and Ripening Parameters Assessment
Non-destructive equatorial diameter measurement was performed in fruits of ‘Celeste’, ‘Regina’, and ‘Lapins’ with a caliper, using millimeters (mm) as the unit. SSC and firmness were recorded using a Pocket Brix-Acidity Meter (PAL-BX|ACID3, ATAGO Co., Ltd., Tokyo, Japan) and a durometer device (Durofel T.R. Turoni, Forlì, Italy), respectively. The durometer was placed on two opposite cheeks, according to San Martino et al. [46]. From these two quantifications, the average was calculated. Seed and embryo length were measured by longitudinally cutting the fruits and using mm as units. Then, the Q index was calculated by dividing the length of embryo by the length of seed [47]. Finally, to determine the color distribution of the fruits at harvest in the 2023–2024 season, a color chart (CTIFL, Paris, France) was used, where one is light red, two is red, three is red, and four is light mahogany.
2.4. RNA-Seq Expression Levels of PavSPL Genes and Gene Co-Expression Network Analysis
RNA-seq data from sweet cherry cultivars ‘Regina’ and ‘Celeste’ were used to determine PavSPL expression levels in fruits and seeds [44]. Samples were taken at the LG stage (34 DAFB) from the same trees as those indicated in Section 2.1.1. For the sequence processing, mapping, and RNAseq procedure visit the mentioned publication. For heatmap and clustering visualization of PavSPL gene expression, Morpheus software of the Broad Institute (https://software.broadinstitute.org/morpheus (accessed on 5 March 2025)) was utilized. Samples and genes were clustered by the ‘One minus Pearson correlation’ and average linkage method. Using the gene ontology categories of Prunus avium predicted genes (‘Tieton’ cv.; [48]), subgroups of genes related to flavonoid, anthocyanin, and lignin metabolism were selected. Then, their gene expression was extracted from [44] datasets (fruits and seeds of ‘Celeste’ and ‘Regina’). The gene expression of the selected gene datasets, as well as PavSPL gene expression, were used as input to the GENIE3 algorithm v.1.31.0 [49] using the R environment v.4.4.2 [50], for the inference of putative gene co-expression/interactions. The GENIE3 tool was run with standard parameters using 1000 decision trees, Random Forest as the tree-based method, and seeds of 123 for reproducibility. The output scores were used to create subnetworks based on the top 10% scores of PavSPL–gene pairs. Cytoscape v.3.10.21 was utilized to visualize the co-expression network maps [51].
2.5. RNA Extraction
For relative expression analyses using qPCR, total RNA was isolated from 0.5 g of mesocarp and seed tissue of ‘Celeste’ and ‘Regina’, using a Zymo extraction kit (Zymo Research, Irvine, CA, USA). In the case of ‘Lapins’ samples RNA was isolated from 0.5 g of whole fruit tissue using the CTAB method [52]. The integrity was evaluated by denaturant electrophoresis with MOPS buffer, using an agarose gel (1.5%) in order to detect two ribosomal bands (28S and 14S). The genomic DNA traces were eliminated using TURBOTM DNase (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. For all extractions, the purity values of the ratios A260/A230 and A260/A280 were close to 2.0.
2.6. cDNA Synthesis
For cDNA synthesis, reverse transcription was performed with 0.8 µg of RNA using a BIO-RAD iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Irvine, CA, USA), according to the manufacturer’s instructions. For microRNA (miRNA), reverse transcription was performed with 1 μg of RNA using a Mir-X miRNA First-Strand Synthesis Kit (Takara Bio, Kusatsu, Japan).
2.7. Selection of Candidate Genes for qPCR Expression Analyses
To select candidate genes, small RNA-seq and mRNA-seq datasets of fruits and seeds of ‘Celeste’ and ‘Regina’ were utilized [44]. From small RNA-seq data, [44] obtained a list of 25 miRNAs functionally annotated using miRbase [53]. Then, these 25 miRNAs were used to search in the online tool TarDB (http://www.biosequencing.cn/TarDB/ (accessed on 25 June 2024)). This database delivered the ID of 17 CDSs. Using a BLASTp tool on NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi, (accessed on 25 June 2024)), 15 sweet cherry ortholog genes were obtained. The Prunus avium cv. ‘Tieton’ Genome v2.0 assembly and annotation was used to identify the putative function of these orthologs [48]. We selected three sweet cherry SQUAMOSA Promoter-binding protein-Like (PavSPL) genes from the list of orthologs delivered by TarDB search [44], FUN_003506-T1 (PavSPL16), FUN_003521-T1 (PavSPL2), and FUN_037655-T1 (PavSPL9). These genes are included in the list of sweet cherry SPLs (Table S4). The three genes were orthologs (lowest E value) of the output sequences retrieved by TarDB (lowest p value) using as query miRNAs sequences zma-miR156j, mtr-miR156a, and gma-miR156f, respectively (here designated as miR156j, miR156a, and miR156f). Then, we selected only the PavSPLs with one allele in the cultivar ‘Tieton’ Genome v2.0 assembly; thus, PavSPL16 was excluded from the qPCR analyses. The psRNATarget tool (https://www.zhaolab.org/psRNATarget/ (accessed on 25 June 2024)) was used to predict the miR156-targeted PavSPL genes [54], showing that PavSPL2 and PavSPL9 were predicted targets of miR156a and miR156f, whereas miR156j was not targeting these genes; thus, miR156j was excluded from the expression analyses.
2.8. Phylogenetic Tree and Motif Discovery
BLASTx on NCBI was performed with PavSPL2 and PavSPL9 CDSs against Prunus spp. The best six hits sorted by E value and annotated as SPL were used for alignment. The nucleotide sequences were aligned with the online software ClustalW v.2.0.12, included on the website https://www.phylogeny.fr/ (accessed on 15 January 2025). We added the best hit against the Vitis vinifera genome in this alignment. MEGA software v.12 was utilized to construct the phylogenetic tree [55]. A neighbor-joining tree was generated using the bootstrap method for testing phylogeny, with 1000 replications, of which 977 were valid and 23 failed. For motif discovery, amino acid sequences were used as input on the online tool Motif Search (https://www.genome.jp/tools-bin/search_motif_lib (accessed on 15 January 2025)), selecting the Pfam database.
2.9. Primer Design for Gene Expression Analyses
To quantify mRNA abundance, specific qPCR primers were designed using the NCBI primer BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 25 July 2024)), as shown in Table S1. The site of primer alignment is shown in Figure S1. For miRNA abundance, primers were designed from the miRbase sequence database (https://www.mirbase.org/ (accessed on 25 July 2024)) [53], using the entire sequence of the mature miRNA (Table S2). Sweet cherry Actin V1 (XM_021976055.1) gene [7] was selected for the normalization of gene expression (Table S3), following the recommendations for the use of reference genes in Prunus spp. species [56]. Primers were evaluated using the online OligoAnalyzer tool from Integrated DNA Technologies (https://www.idtdna.com (accessed on 25 July 2024)). Every primer was tested for specificity using a dissociation curve analysis. Primer efficiency was determined using the LinRegPCR software v11.0 [57]. Primer efficiency was considered for relative transcript abundance calculations, as indicated by Pfaffl [58].
2.10. Quantification of Relative Gene Expression by qPCR
The qPCR analyses were performed on a QIAGEN Rotor-Gene Q system (QIAGEN, Hilden, Germany), using the conditions recommended in “Minimum information established for qRT-PCR experiments” (MIQE) [59] and “Golden Rules of Quantitative PCR” [60]. Takyon SYBR Green qPCR Master Mix (Eurogentec, Seraing, Belgium) was used, according to the manufacturer’s indications. For miRNA, TB Green Advantage® qPCR Premix (Takara, Japan) was used, according to the manufacturer’s instructions. For the mRNA and miRNA abundance reactions, 10 and 20 ng of cDNA were used, respectively. Three technical replicates were performed for each biological replicate.
2.11. Endocarp Lignification Assessment
Samples fixed in FAA solution and stored at 4 °C were washed three times with phosphate-buffered saline (PBS) solution (pH 7.0). Then, the fruits were longitudinally cut in half and immersed in a solution with one volume of concentrated HCl (37%) and two volumes of 3% phloroglucinol with absolute ethanol (phloroglucinol-HCl or Wiesner staining). After 90 s, the samples were washed with 70% ethanol [61]. For visualization, a 7×–45× stereo Binocular Microscope (AmScope, Irvine, CA, USA) was utilized.
2.12. Pigment Concentration Estimation
For pigment extraction, 30 mg of ground frozen tissue was mixed with 1.0 mL of 95% ethanol. The samples were incubated on an orbital shaker for 1 h, followed by overnight incubation at 4 °C. Subsequently, the samples were centrifuged at 16,000× g for 10 min to recover the supernatant. Chlorophyll (a and b) and carotenoid concentrations were quantified using a microplate spectrophotometer (Thermo Fisher Scientific, USA) by measuring the absorbance at 470, 648, 664, and 750 nm wavelengths. Total chlorophyll and carotenoid contents were calculated according to Lichtenthaler [62] and expressed as µg/g dry weight. Anthocyanin content was determined using the pH differential method described by Giusti and Wrolstad [63]. This method involved absorbance measurements of samples diluted in buffer solution at pH 1.0 and 4.5. Absorbance was measured at 520, 700, 900, and 975 nm. The content of anthocyanins was calculated following the equation described by Dyankova and Doneva [64], and contents were expressed as µg/g dry weight of cyanidin 3-O-glucoside.
2.13. Statistical Analysis
Data were subjected to a Shapiro–Wilk test to check the normality. The homogeneity of variances was evaluated with Levene’s test. For relative expression comparisons, Student’s t-test was used, with a significance level set at p < 0.05. SSC data comparisons between ‘Celeste’ and ‘Regina’ were analyzed using Student’s t-test (p < 0.05). One-way ANOVA followed by Tukey’s post hoc analysis (p < 0.05) was used to compare treatments performed on the ‘Lapins’ cultivar in both seasons. When indicated, Tukey’s post hoc test was used with a significance level of p < 0.05. INFOSTAT software, 2020 version obtained from https://www.infostat.com.ar/ (accessed on 25 July 2024), was used for statistical analyses (InfoStat, Córdoba, Argentina) and GraphPad Prism (GraphPad Software, Boston, MA, USA) software v.8.0.2 was used for visualizing the results.
3. Results
The early- and late-season cultivars ‘Celeste’ and ‘Regina’ were characterized during the 2022–2023 season. The fruit growth curves from 21 to 63 DAFB revealed that ‘Regina’ had a more pronounced lag phase, especially from 34 to 42 DAFB, while the pink coloration started earlier in the early-season cultivar ‘Celeste’ (Figure 1A). To determine differences between both cultivars regarding stone formation, lignin staining of the endocarp—the fruit tissue between mesocarp and seed—was performed. Differences were observed at 21 DAFB, with the lignification process of ‘Celeste’ starting earlier; additionally, the lignin staining was more marked in the early-season cultivar (Figure 1B). The ripening marker SSC was measured at the R stage, where ‘Regina’ had significantly less Brix° than ‘Celeste’ (Figure 1C).
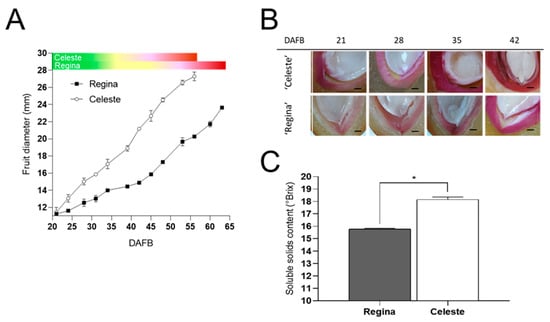
Figure 1.
Fruit growth, endocarp lignification, and soluble solids content (SSC) in the early-season cv. ‘Celeste’ and the late-season cv. ‘Regina’. (A) Equatorial diameter on different days after full bloom (DAFB). Phenology is presented as colored bars. (B) Endocarp lignin deposition as revealed by phloroglucinol-HCl staining. The black bars represent 1 mm. (C) SSC at the red (R) stage in ‘Celeste’ and ‘Regina’. In A and C, data are presented as the mean ± standard deviation (SD). In (C), the asterisk indicates statistical differences, as revealed by Student’s T-test with p < 0.05.
Regarding the seeds, at 28 DAFB, the embryo was observed, but only in the late-season cultivar ‘Regina’ (Figure 2A). At 42 DAFB, the embryo of ‘Regina’ was larger; finally, at 49 DAFB, ‘Regina’ had full embryo development, as the space inside the seed was completely occupied. The seed length was measured between 21 and 49 DAFB, with ‘Regina’ having bigger seeds throughout the season (Figure 2B). Finally, the Q index was assessed (Figure 2C), evaluating the relation between embryo length and seed length, where ‘Regina’ had higher values at the beginning of the season (Figure 2A).
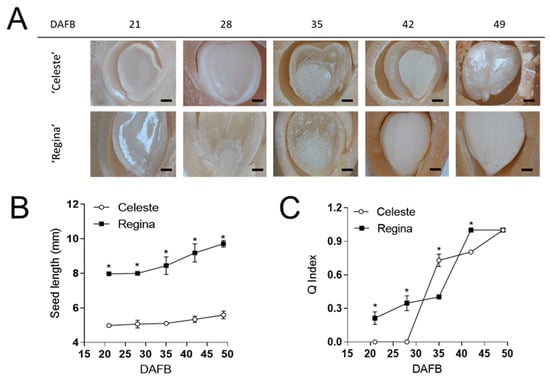
Figure 2.
Seed structure evolution in the early-season cv. ‘Celeste’ and the late-season cv. ‘Regina’. (A) Pictures showing seeds at different DAFB. The black bars represent 1 mm. (B) Quantification of seed length during development. (C) Q index estimation at different DAFB, corresponding to the ratio between embryo and seed lengths. The results in (B,C) are presented as mean ± SD. Zero values represent non-visible embryo; the asterisk indicates statistical differences at each date, as revealed by Student’s T-test with p < 0.05.
To determine differences between the cultivars at the molecular level, we used the expression levels of PavSPLs in the sweet cherry fruits and seeds at the transition from development to ripening of the early cultivar ‘Celeste’ and the late cultivar ‘Regina’, obtained in a previous transcriptome analysis performed at the LG stage [44]. First, we identified the PavSPLs already characterized in earlier works (Table S4) and searched for their expression level in the datasets. We observed clear differences between seeds and fruits, with PavSPLs being in general more abundant in fruits (Figure 3). Nevertheless, some PavSPLs showed differences between cultivars, including both PavSPL16 (FUN_003506-T1 and T2), PavSPL17 (FUN_021909-T1), and PavSPL9 (FUN_021909-T2; an allelic variant of PavSPL17 in the cv. ‘Tieton’ Genome v2.0 annotation), especially in seed tissues (CS versus RS).
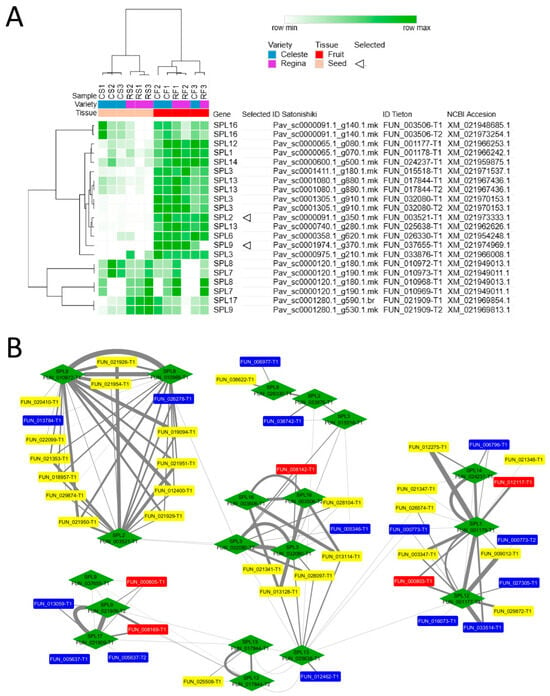
Figure 3.
Expression levels of PavSPL genes in seeds and fruits at the light green (LG) stage and co-expression of PavSPLs with secondary metabolism genes. (A) Heatmap for PavSPL expression in seeds and fruits of the cvs. ‘Celeste’ and ‘Regina’. For normalization, the trimmed mean of M-values were used. Hierarchical clustering was performed using one minus Pearson correlation with average, and clustering genes and samples. Names of the genes are related to NCBI accessions (Table S4). (B) Inferred co-expression/interaction networks of certain PavSPL genes (green) with flavonoid (blue), anthocyanin (red), and lignin (yellow) metabolism genes. Triangles indicate genes utilized in subsequent analysis.
The co-expression network shows connections with several transcripts found in the seed and fruit datasets of both cultivars. The transcripts included flavonoid, anthocyanin, and lignin metabolism genes that connected with PavSPLs (Figure 3B). PavSPL8 and PavSPL2 were co-expressed with several lignin related genes, whereas PavSPL9, PavSPL17 (and its related allelic variant), PavSPL12, PavSPL3, and PavSPL1 were connected with anthocyanin genes.
Based on the TarDB search using sequences of miR156 identified in fruit and seed samples [44], we obtained two candidates for expression analysis, PavSPL2 (FUN_003521-T1) and PavSPL9 (FUN_037655-T1). We analyzed in silico the predicted site for miR156 binding, and found that both PavSPLs were targeted for cleavage by miR156a and miR156f (Table 1). PavSPL2 and PavSPL9 were used for expression analyses. The sequence FUN_021909-T2 (also annotated as PavSPL9; Table S4) was not analyzed, as it corresponded to PavSPL17 allelic variant.

Table 1.
PavSPL sequences complementary to miR156a and miR156f.
To characterize PavSPL9 and PavSPL2, we assessed the presence of the SBP motif, which was found in both genes (Figure 4A). Then, we performed a phylogeny analysis and obtained that PavSPL9 and PavSPL2 grouped in different clades, having phylogenetic proximity with other Prunus spp. and Vitis vinifera SPL genes (Figure 4B).
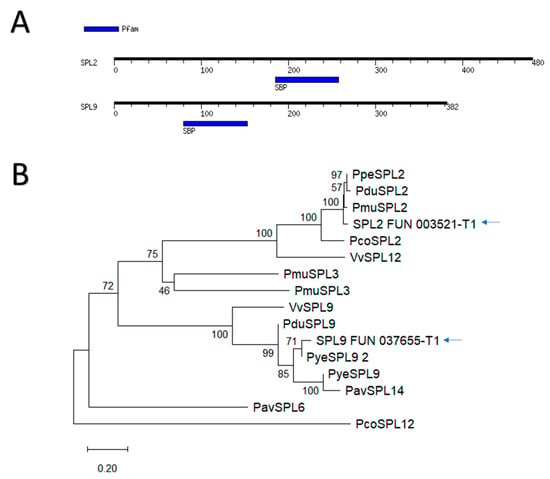
Figure 4.
PavSPL2 and PavSPL9 phylogeny and identification of the SBP motif. (A) SBP (for SQUAMOSA-PROMOTER BINDING PROTEIN) domain identified in the motif search performed using amino acidic sequences (blue colored boxes). (B) A bootstrap consensus neighbor-joining tree with 977 bootstrap replicates was generated. Blue arrows indicate genes utilized in subsequent gene expression analysis. Abbreviations of species are according to the Standard Nomenclature for Gene Designation in Rosaceae species [65]; Vv for Vitis vinifera.
We analyzed the PavSPL2 and PavSPL9 gene expression and miR156a and miR156f abundance in fruits and seeds at the SY stage of each cultivar. SY was chosen since we aimed to determine if they were expressed at a more advanced developmental point in the transition from development to ripening. Both PavSPLs were detected in all fruit samples (Figure 5A,B). In contrast, PavSPL2 was not detected in the seed tissues of the late-season cultivar ‘Regina’. Then, we analyzed the profile of miR156a and miR156f in the same samples (Figure 5C,D) and found that miR156a and miR156f were expressed in all the fruit and seed samples.
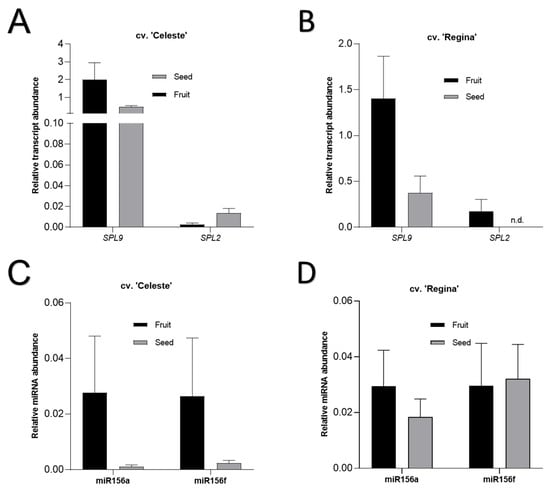
Figure 5.
Expression profile of sweet cherry SQUAMOSA Promoter-binding protein-Like (PavSPL) genes and miR156a and miR156f in seeds and fruits at the straw yellow (SY) stage. PavSPL9 and PavSPL2 transcript abundance of cvs. ‘Celeste’ and ‘Regina’ are presented in (A,B), respectively. The miRNA abundances of miR156a and miR156f are presented in (C,D), respectively. The Actin V1 (XM_021976055.1) gene was used as normalize for relative expression. PavSPL relative expression values were amplified by 103. The axis of ‘Celeste’ PavSPL was split in order to visualize the PavSPL2 expression in fruits. Data are presented as mean ± standard error of the mean (SEM). Non-detected genes are represented as n.d.
We evaluated the effect of lignification modulators in a mid-season cultivar during the 2023–2024 season. The IAA, NPA, and p-IBA treatments were performed at the initiation of the LG stage (25 DAFB). We measured color, pigments, and lignin staining (Figure 6).
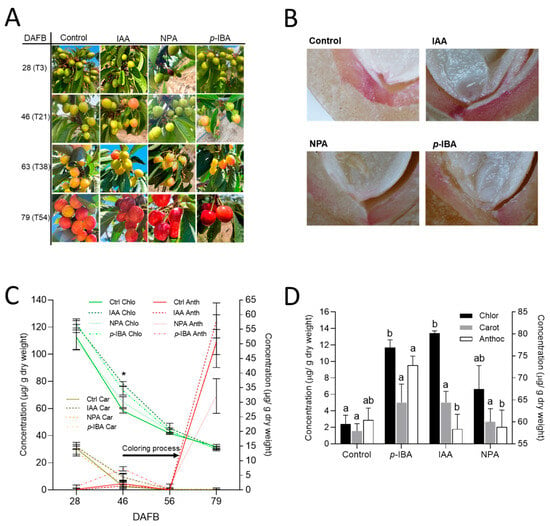
Figure 6.
Effect of treatments that modulated lignification on lignin deposition and coloring in the mid-season cv. ‘Lapins’ during the 2023–2024 season. Control corresponded to untreated trees, and 40 µM NPA, p-IBA and IAA treatments were applied at 25 DAFB (T0). (A) Sweet cherry fruit color at 46 DAFB (T21), corresponding to light green (LG) stage, i.e., 90% LG/10% straw yellow (SY). (B) Endocarp lignin deposition at 29 DAFB (T4), as revealed by phloroglucinol-HCl staining. (C) Quantification of total chlorophylls, carotenoids, and anthocyanins at different DAFB; * indicates the date with significant differences according to one-way ANOVA (comparisons at each date separately). (D) Quantification of total chlorophylls, carotenoids and total anthocyanins at 46 DAFB (T21). Data are presented as the mean ± SD, and different letters indicate statistical differences, as revealed by ANOVA Tukey’s post hoc test with p < 0.05, where each pigment was calculated independently.
We found that at 46 DAFB, three weeks after the treatments, an increase in the fruit coloration occurred in the IAA and p-IBA treatments (Figure 6A). Regarding lignin deposition, the p-IBA and NPA treatments presented less staining in the endocarp (Figure 6B). Chlorophyll and carotenoids reduced steadily during the season, whereas anthocyanins abruptly increased from 56 to 79 DAFB (Figure 6C). At 46 DAFB, there were significant differences when the pigments of all treatments were compared (Figure 6C). When we further analyzed these differences at this time point, we found that chlorophyll significantly increased in the p-IBA and IAA treatments (Figure 6D). Anthocyanins showed a trend of higher content in the p-IBA treatment compared to the control, though this was not significant. Fruit size significantly increased in the NPA treatment (Figure S2), whereas firmness and SSC did not change (Figure S3). The effect of the treatments at 46 DAFB was transient, as there were no differences between treatments at 56 DAFB (Figure 6C).
The effect of p-IBA and auxin treatments on lignification was assessed in the following season (2024–2025) in the same cultivar (Figure 7). The NAA analog was used instead of IAA, and both compounds, p-IBA and NAA, were tested at two doses. Thus, the five treatments further explored the effect of treatments that produced significant differences in the 2023–2024 season. Differences between treatments were observed at 48 DAFB (T23), where p-IBA increased red coloration in the transition to P stage (Figure 7A).
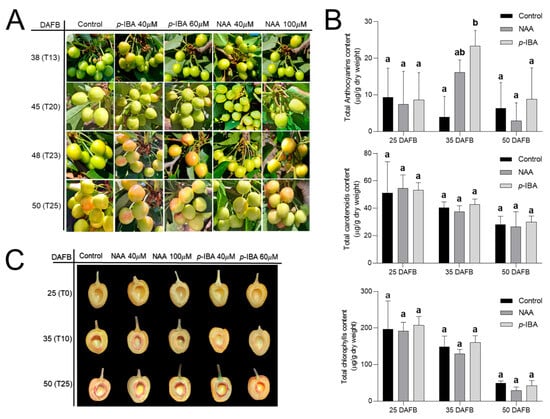
Figure 7.
Effect of treatments that modulate lignification on lignin deposition and coloring in the mid-season cv. ‘Lapins’ during the 2024–2025 season. Control corresponded to untreated trees, and 40 µM p-IBA, 60 µM p-IBA, 40 µM NAA, and 100 µM NAA were applied at 25 DAFB (T0). (A) Sweet cherry fruit color at 38, 45, 48, and 50 DAFB. (B) Total anthocyanins, carotenoids, and chlorophylls in the fruits at 25 DAFB (T0, 100% LG), 35 DAFB (T10, 90% LG/10% SY), and 50 DAFB (T25, 70% SY/30% P); p-IBA and NAA µM 40 were included. (C) Endocarp lignin deposition revealed by phloroglucinol-HCl staining at 25, 35, and 50 DAFB. In B, data are presented as the mean ± SD, and different letters indicate statistical differences, as revealed by one-way ANOVA and Tukey’s post-hoc test with p < 0.05.
These differences were not maintained over time, as the other treatments started to develop red color; thus, all the treatments were similar at 50 DAFB (T25). Regarding pigment content, significant differences were observed in anthocyanins versus control at 35 DAFB (T10) in the p-IBA treatment, whereas the chlorophyll and carotenoid content was not affected by any treatment (Figure 7B). Endocarp lignification analyses showed that at 25 DAFB (T0) there was no lignin deposition, whereas at 35 DAFB it was possible to observe lignification (Figure 7C). At this time point, the treatments presented differences, where both p-IBA treatments, and the NAA 100 µM treatment, had less lignin deposition (Figure 7C). However, these differences were transient, since at 50 DAFB all treatments had similar lignin staining. No differences were found in fruit diameter at 68 DAFB between the treatments (Figure S4).
Overall, lignification disturbance led to differences in pigment accumulation and color. In particular, anthocyanin content increased with p-IBA, which was significant in the 2024–2025 season. Thus, we aimed to associate these physiological effects with PavSPL expression, since they were co-expressed with lignin and anthocyanin genes (Figure 3B). We found that p-IBA treatment reduced the transcript abundance of both PavSPL genes, and this was significant in the case of SPL9 (Figure 8).
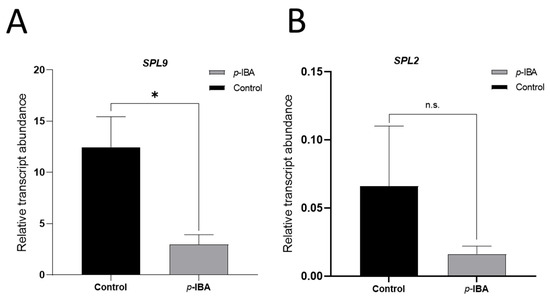
Figure 8.
Effect of p-IBA on PavSPL gene expression in the mid-season cv. ‘Lapins’ during the 2023–2024 season. Control corresponds to untreated trees, and p-IBA 40µM treatment was applied at 25 DAFB (T0). The expression was evaluated by qPCR at 46 DAFB (T21), corresponding to the light green stage (LG). For quantification of PavSPL9 (A) and PavSPL2 (B) relative transcript abundance, the Actin V1 (XM_021976055.1) gene was used as a normalizer. Data are presented as means ± SEM. Asterisk indicates statistical differences, as revealed by the Student’s T-test with p < 0.05. n.s., not significant.
4. Discussion
We characterized cultivars contrasting in harvest time, regarding fruit and seed development, and endocarp lignification (Figure 1). Differences were found in the stage II progression (Figure 1A). This is consistent with previous reports [15,17], where stage II had different lengths depending on the cultivar. Stage II can affect the timing of the following stage, by delaying the initiation of stage III, where SSC and anthocyanin accumulation occur. The shorter stage II in ‘Celeste’ in fact resulted in earlier sugar accumulation, as this cultivar presented more SSC (Figure 1C).
The endocarp lignification process also differed between cultivars (Figure 1B). According to previous reports, lignification may be a turning point in the onset of ripening at the molecular level, as profuse remodeling occurs at the transcriptomic level in fruits and seeds of Prunus persica before and after lignification [27]. The modulation of lignification is not so well understood, but it is possible that fruit or seed signals influence lignin deposition. Kondo et al. reported that the seed had higher levels of GA3 and auxin compared to the mesocarp [26]. On the other hand, the IAA flux is basipetal [66], suggesting that the IAA produced in the seeds moves to the fruit and pedicels. Auxin modulates lignification in Arabidopsis thaliana [23] and pear [24]. Thus, cultivars with differences in hormone content in fruits or seeds, especially auxin, might present differences in lignification. In this regard, Ponce et al. showed that fruits of an early-season cultivar had a higher mesocarp IAA content than a mid-season cultivar [17]. Whether this IAA comes from the fruit should be explored in future works. On the other hand, the increased lignin in the endocarp observed in the early-season phenotype could hinder the movement of GAs, which delay ripening [15].
Braak reported that the embryo of early-season cultivars does not reach full development since the ripening processes are advanced [25]. In this regard, we observed differences between ‘Celeste’ and ‘Regina’ in embryo and seed length (Figure 2). ‘Regina’ embryo was larger and reached full development. Possibly there are differences in hormone content associated with this differential degree of seed development between cultivars, though this must be addressed in future works. This could also influence lignification processes.
Then, we evaluated whether contrasting cultivars differed at the molecular level by focusing on PavSPL genes. SPL/miR156 module has been shown to be relevant for ripening initiation in other fruit species. In Pyrus spp., this module was implicated in the anthocyanin synthesis in response to light during fruit development [39]. In line with this, the authors showed that PpySPL10 and PpySPL13 proteins interacted with PpyMYB10, key in the regulation of anthocyanin structural genes. In blueberry, several SPLs expressed during ripening, and overexpression of miR156a led to more anthocyanin accumulation [41]. Regarding non-climacteric species, in fruits of litchi (Litchi chinensis Sonn.), SPLs co-express with anthocyanin genes [67].
We found that several PavSPLs were expressed at the transition from development to ripening, specifically at the LG (Figure 3), which was consistent with Wang et al. [42], though in this work an advanced stage was analyzed, corresponding to the initial R stage. However, Sun et al. showed a decrease in the expression of PavSPLs from LG onwards [43]. The decrease could be associated with hormonal regulation, since GA and ABA increased and decreased PavSPL expression, respectively [43]. GA is abundant at LG in sweet cherry [17], whereas ABA increases at the beginning of the P stage [5]. Thus, the reduction in PavSPL expression during ripening could be controlled by ABA, which in turn is key for anthocyanin accumulation [21]. Regarding GA, Liu et al. found that PavSPL14 protein (here PavSPL17 according to NCBI nomenclature; Table S4) interacted with PavDWARF8, a DELLA protein, part of the GA pathway [29].
PavSPLs are expressed in fruits of sweet cherry [29,43,44]; however, their expression in other tissues may also be important for fruit processes including ripening. Maldonado et al. showed that some PavSPLs also were expressed in the developing seed at the LG stage, consistent with our findings (Figure 3), though we profiled the complete gene family [44]. This allowed us to determine that most PavSPLs had higher expression in fruits than in seeds. This is consistent with findings in litchi fruits, where PavSPLs were more abundant in the pericarp than in the seed [67].
Interestingly, some PavSPLs showed differences between both cultivars, especially in seeds (Figure 3). For instance, PavSPL8 (FUN_010968-T1) and PavSPL17 (FUN_021909-T1) had more expression in ‘Regina’ seeds, whereas both alleles of PavSPL16 (FUN_003506-T1 and T2) were more abundant in ‘Celeste’ seeds. Whether this is related to differences in the hormone content of seeds should be explored in future works, but an influence of hormones is expected, according to the effect of ABA, GA, and methyl jasmonate (MeJA) treatments reported by Sun et al. [43]. In line with this, Maldonado et al. found differences in the content of several hormones (IAA, GAs, CKs, and JA) between ‘Celeste’ and ‘Regina’ seeds [44].
PavSPLs are targeted by several miR156 forms in sweet cherry [29,42]. Here, we identified two PavSPL genes, possibly targeted by miR156, using different in silico approaches (TarDB search using miR156 sequences; cleavage site predictions). We obtained complete complementarity between PavSPLs and miR156a and miR156f (Table 1). It is worth noting that PavSPL9 (FUN_037655-T1) corresponded to PavSPL16, while PavSPL2 (FUN_003521-T1) corresponded to PavSPL4, according to Liu et al. [29], but here we followed NCBI nomenclature, as in Wang et al. [42], as shown in Table S4. In the case of PavSPL9, we had additional indications that this gene was targeted by miR156, since it presented negative correlations (opposing expression patterns across the fruit and seed samples) with the hairpin bdi-MIR156a [44].
In the phylogenetic tree (Figure 4), PavSPL9 grouped with PavSPL14 (here PavSPL17). It is worth noting that, in NCBI, there is another PavSPL annotated as PavSPL9 (FUN_021909-T2); but this sequence corresponds to PavSPL15, which arose from PavSPL17 in a gene duplication event [29]; this explains the two allelic variants in the cv. ‘Tieton’ Genome v2.0 annotation.
PavSPL2 and PavSPL9 were also present at the SY stage (Figure 5). PavSPL2 was not detected in seed tissues of ‘Regina’, which coincided with a higher abundance of both miR156 variants in the seeds of this cultivar compared to ‘Celeste’ seeds, though these differences were not significant. Maldonado et al. [44] found that miR156 forms were highly abundant in seeds of the early cultivar ‘Celeste’, which could be related to the earlier stage analyzed (LG).
In line with the idea that lignified endocarp could act as a barrier for signal communication between the seed and the mesocarp, or it being a site of signal production itself, we aimed to disturb the lignification process and determine if PavSPL gene expression was altered, as well as the ripening process. In line with this, PavSPL2 and PavSPL9 were co-expressed with lignin and anthocyanin genes, respectively (Figure 3B). For this, we applied modulators of lignification in the 2023–2024 season (Figure 6). We used an enzymatic inhibitor of the CINNAMATE-4-HYDROXILASE [68], p-IBA, at 40 µM. We used this concentration as it affected lignification without producing a stress response [68]. We also utilized IAA, since it modulated ripening in sweet cherry [11]; in addition, Qu et al. [23] reported that IAA regulates lignin biosynthesis. We used a low dose (40 µM) in relation to a previous report in sweet cherry that utilized 1 mM [42], with fruit endogenous levels in the order of 2–4 µg per g of dry tissue [44] and 0.2 µg per g of fresh tissue [7]. Finally, we tested NPA, an inhibitor of polar auxin transport, in order to indirectly alter the IAA levels in the fruits [69]. NPA was also reported to modulate ripening in grapevine at a 40 µM dose [70].
As shown in Figure 6, the phloroglucinol-HCl staining was reduced in NPA and p-IBA treatments; on the other hand, it was more intense in the IAA treatment. In pear, the low dose (200 µM IAA) reduced stone cell content, while higher concentrations (>500 µM IAA) increased the stone cell content [24]. As such, our low dose treatment resembled the high dose effect found in pear, though it is worth noting that the lignification processes are likely different between both species and cellular types, especially considering that pear is climacteric. NPA caused the opposite effect to IAA in terms of lignification, and this is possibly related to a disturbance in auxin homeostasis, as polar auxin transport should be affected at this dose [66,70]. At 46 DAFB, IAA and p-IBA had a transient positive effect on the fruit coloration (Figure 6). It seems that this was due to different mechanisms, since p-IBA targets enzymatic activity, whereas IAA regulates several pathways involved in ripening, including ABA. Hence, though lignification increased with IAA, more color might be attributed to IAA regulation of ABA levels, as reported by Wang et al. [7]. On the other hand, the p-IBA-related color increase could be associated with lower lignin production, which opens the possibility of inter-tissue communication, under the idea that lignified endocarp represents a barrier between seeds and fruits, though this should be explored in future works. It is worth noting that p-IBA 40 µM was not associated with stress, since toxic responses usually occur within hours or a few days, and here we did not observe color differences at T3. Therefore, we propose that the effect on color of p-IBA could be related to lignification disturbance. Regarding pigments, the increase in anthocyanin content followed the chlorophyll decrease, as previously reported [21]. IAA did not produce the significant anthocyanin increase that was expected for more colored fruits. Regarding p-IBA, a trend of higher anthocyanin content compared to the control was observed, but it was not significant. Possibly this was due to sampling, since high heterogenicity was observed between fruits. Additionally, the low anthocyanin concentration at the P stage compared to R stage may have led to higher deviations between replicates. Finally, field and not controlled conditions could have influenced the treatment effect. Therefore, we repeated the assay the following season, 2024–2025, by focusing on treatments that improved color and with two concentrations.
We found a transient color increase with both concentrations of p-IBA versus control at 48 DAFB, accompanied with a significantly higher concentration of anthocyanins in the fruits in the 40 µM p-IBA treatment at 35 DAFB (Figure 7). NAA also improved color but only at 100 µM. Hence, similarly to the 2023–2024 season, less lignification was accompanied by more color in p-IBA, whereas more lignin deposition was related to color increase in NAA, suggesting different mechanisms of modulating color development. Interestingly, 40 µM NAA reduced lignin staining, which reinforced that the effect of auxin on the lignification process is dose-dependent, as shown in pear stone cells [24]. Similarly, 40 µM NAA reduced fruit color at 50 DAFB. Thus, only the 100 µM NAA dose resembled the 40 µM IAA treatment of the 2023–2024 season, regarding the positive effect on color and lignin deposition. The effect of IAA and p-IBA on the chlorophyll content, as observed in the 2023–2024 season, was not replicated; possibly, this could have been influenced by other environmental conditions. As in 2023–2024, the effect on lignification, color, and anthocyanins was transient for all the treatments, including p-IBA. Regarding NAA, this was different from the treatments using 100 µM that produced significantly increased anthocyanin content in sweet cherry [11]. This was possibly related to the application time, as the spraying was at the SY stage in Clayton-Cuch et al. [11], whereas we performed the treatments at LG in both seasons, where the ripening-related genes, including ABA signaling genes, are less expressed compared to SY [4]. Additionally, GA pathways exert a negative regulation on the ABA pathway when applied at LG [15,16]. Future works should also explore the effect of p-IBA at the SY.
It is worth noting that we detected an effect of p-IBA and IAA on the intensity of the phloroglucinol staining at T4 in the 2023–2024 season, and at T10 in the p-IBA and NAA treatments of the 2024–2025 season; thereafter, the color effect occurred around two weeks later. This is consistent with the work of Clayton-Cuch et al. [11], showing an effect of NAA on anthocyanin content three weeks after the treatment. Under the hypothesis that both processes—lignification and color development—were connected, a temporality should exist, where a treatment disturbs the lignification process and this, in turn, affects other processes that may lead to changes in the evolution of coloring.
We found that the treatments did not modulate ripening parameters differently than the color and pigment content, at least under our experimental conditions, since firmness and SSC were not affected, nor fruit diameter (Figures S2–S4). This suggests that color based on anthocyanins is partially independent of other ripening parameters. In line with this, desynchronization of color and firmness has been shown in other fruit crops. In ‘Hass’ avocado (Persea americana), when skin color develops coupled with firmness, certain markers are present, including ABA [50,71]. It would be interesting to apply these treatments to cultivars contrasting in maturity time, as they have a ten times difference in ABA fruit levels [17], in order to detect effects other than color.
The PavSPL gene expression was evaluated in response to p-IBA treatment (Figure 8). The downregulation of both genes—significant in the case of PavSPL9—was expected, as the p-IBA treated fruits presented more color and PavSPLs have been proposed to antagonize ripening, whereas miR156 is pro-ripening [39,41]. Several PavSPLs decrease their expression during sweet cherry fruit ripening and in response to ABA [43]; hence, it seems that the expression of these transcription factors has to be downregulated to allow the transition to ripening. In litchi and sweet cherry, SPLs are upregulated by GA applied to fruits [43,67]. Therefore, one possibility is that less lignin in the endocarp in the p-IBA treatment makes the endocarp more permeable to the flux of signals from the seed, including hormones such as IAA and GA, which modulate color development [7,16]. IAA from the seed could promote coloring through the ABA pathway, and ABA, in turn, could downregulate fruit PavSPLs; miR156 from the seed might contribute to PavSPL downregulation through post-transcriptional regulation (Figure 9). Thereafter, GA would increase the PavSPL levels, explaining the transient effect of p-IBA on color. This hypothesis is tempting but requires functional demonstration, as well as tracing of seed signals, including miRNAs and hormones.
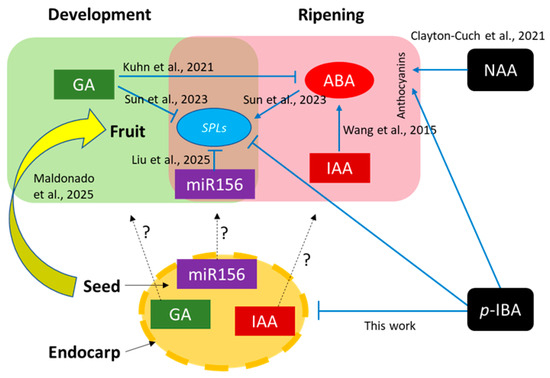
Figure 9.
Working model on the regulation of the sweet cherry transition to ripening, integrating hormone signals, the SPL/miR156 module, and the role of seed and endocarp. Works cited were performed in sweet cherry, and positive or negative effects are indicated in blue. Black dotted arrows refer to inter-tissue movement and interrogation signs denote that this should be demonstrated in future works. Large yellow arrow represents fruit–seed associations through multiomics analyses. Finally, the effect of exogenous treatments is indicated, where NAA and p-IBA promoted fruit color formation [7,11,16,29,43,44].
5. Conclusions
In this work, we contributed to the molecular understanding of the non-climacteric transition to ripening. Here, we characterized the expression of several sweet cherry SPL family members in the transition from development to ripening. This work also showed that PavSPLs, abundant in fruits, but also present in seeds, are co-expressed with anthocyanin and lignin genes. They were also found to be targeted by miR156 expressed in seeds and fruits, and downregulated in response to a treatment that impaired lignification and triggered color formation, though transiently. These treatments revealed that the endocarp lignification process is relevant to the transition to ripening. Future studies should further explore the effects of compounds that disrupt lignification, with the aim of developing growth regulators useful for orchard management. Finally, the SPL/miR156 module is a promising candidate for identifying genetic factors that could be used in marker-assisted breeding programs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11060706/s1, Figure S1: Position of the PavSPL Forward (Fw) and Reverse (Rv) primers on the sweet cherry cultivar ‘Tieton’ Genome v. 2.0; Figure S2: Effect of treatments that modulate lignification on fruit growth in the mid-season cultivar ‘Lapins’ at different DAFB. Figure S3: Effect on SSC and firmness of the lignification modulators treatments on the mid-season cultivar ‘Lapins’ at 71 DAFB (2023-2024 season); Figure 4: Effect on fruit size of the lignin modulators treatments on the mid-season cultivar ‘Lapins’ at 68 DAFB (2024-2025 season); Table S1: qPCR primer sequences with melting temperature (TM); Table S2: qPCR miRNA primer sequences with melting temperature (TM); Table S3: qPCR normalizer gene primer sequences with melting temperature (TM); Table S4. Accessions, IDs and PavSPL names.
Author Contributions
Conceptualization, N.K., J.E.M. and M.Z.; methodology, C.A., M.Z., O.A., M.M. (Mirna Melo) and M.M. (Marcela Menares); software, J.E.M., C.N.; validation, M.Z., C.N. and J.E.M.; formal analysis, R.P., M.Z. and M.M. (Marcela Menares); investigation, N.K., J.E.M., M.Z.; resources, L.A.M., N.K., J.E.M., R.P. and J.M.D.; data curation, J.E.M.; writing—original draft preparation, N.K., M.M. (Marcela Menares) and M.Z.; writing—review and editing, L.A.M., N.K., J.E.M., R.P. and J.M.D.; visualization, N.K., M.Z., J.E.M., C.N.; supervision, N.K., J.E.M.; project administration, N.K.; funding acquisition, L.A.M., N.K., J.E.M., R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondecyt Iniciación ANID 11221186, Fondecyt Regular ANID 1220223; Fondecyt Regular ANID 1231491; Fondecyt Regular ANID 1220097; SIA sa77210103. In addition, this research was partially supported by the supercomputing infrastructure of the NLHPC (ECM-02) (Powered@NLHPC). Romina Pedreschi was supported by ANID Millennium Science Initiative Program ICN2021_044. The APC was funded by Departamento de Investigación, Pontificia Universidad Católica de Valparaíso, and Proyecto InES de Género INGE220007, Pontificia Universidad Católica de Valparaíso.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Abscisic acid (ABA); indole-3-acetic acid (IAA); gibberellin (GA); cytokinin (CK); gibberellic acid (GA3); SQUAMOSA Promoter-binding protein-Like (SPL); microRNA (miRNA); straw yellow (SY); days after full bloom (DAFB); soluble solids content (SSC); p-IBA (p-iodobenzoic acid); green (G); light green (LG); pink (P); red (R); NAA (1-naphthaleneacetic acid); NPA (naphtylphtalamic acid); standard deviation (SD); standard error of the mean (SEM).
References
- Statista. Available online: https://www.statista.com/statistics/577489/world-cherry-production/ (accessed on 19 March 2025).
- Vignati, E.; Lipska, M.; Dunwell, J.M.; Caccamo, M.; Simkin, A.J. Fruit development in sweet cherry. Plants 2022, 11, 1531. [Google Scholar] [CrossRef] [PubMed]
- Dirlewanger, E.; Quero-Garcia, J.; Le Dantec, L.; Lambert, P.; Ruiz, D.; Dondini, L.; Arús, P. Comparison of the genetic determinism of two key phenological traits, flowering and maturity dates, in three Prunus species: Peach, apricot and sweet cherry. Heredity 2012, 109, 280–292. [Google Scholar] [CrossRef]
- Kuhn, N.; Ponce, C.; Arellano, M.; Time, A.; Multari, S.; Martens, S.; Meisel, L.A. ABA influences color initiation timing in P. avium L. fruits by sequentially modulating the transcript levels of ABA and anthocyanin-related genes. Tree Genet. Genomes 2021, 17, 20. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.; Ren, J.; Zhang, C.; Ding, Y.; Li, Z.; Leng, P. The role of ABA in the maturation and postharvest life of a nonclimacteric sweet cherry fruit. J. Plant Growth Regul. 2014, 33, 373–383. [Google Scholar] [CrossRef]
- Ren, J.; Chen, P.; Dai, S.J.; Li, P.; Li, Q.; Ji, K.; Leng, P. Role of abscisic acid and ethylene in sweet cherry fruit maturation: Molecular aspects. N. Z. J. Crop Hortic. Sci. 2011, 39, 161–174. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Sun, L.; Li, Q.; Dai, S.; Sun, Y.; Ji, K.; Li, Q.; Leng, P. Transcriptional regulation of PaPYLs, PaPP2Cs and PaSnRK2s during sweet cherry fruit development and in response to abscisic acid and auxin at onset of fruit ripening. Plant Growth Regul. 2015, 75, 455–464. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Blanco-Portales, R.; Molina-Hidalgo, F.J.; Cumplido-Laso, G.; García-Caparrós, N.; Moyano-Cañete, E.; Rodríguez-Franco, A. Extensive transcriptomic studies on the roles played by abscisic acid and auxins in the development and ripening of strawberry fruits. Funct. Integr. Genom. 2016, 16, 671–692. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.; Loveys, B.; Ford, C.; Davies, C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust. J. Grape Wine Res. 2009, 15, 195–204. [Google Scholar] [CrossRef]
- Gouthu, S.; Deluc, L.G. Timing of ripening initiation in grape berries and its relationship to seed content and pericarp auxin levels. BMC Plant Biol. 2015, 15, 46. [Google Scholar] [CrossRef]
- Clayton-Cuch, D.; Yu, L.; Shirley, N.; Bradley, D.; Bulone, V.; Böttcher, C. Auxin treatment enhances anthocyanin production in the non-climacteric sweet cherry (Prunus avium L.). Int. J. Mol. Sci. 2021, 22, 10760. [Google Scholar] [CrossRef]
- Zhang, C.; Whiting, M.D. Improving ‘Bing’ sweet cherry fruit quality with plant growth regulators. Sci. Hortic. 2011, 127, 341–346. [Google Scholar] [CrossRef]
- Kuhn, N.; Arellano, M.; Ponce, C.; Hodar, C.; Correa, F.; Multari, S.; Meisel, L.A. RNA-seq and WGBS analyses during fruit ripening and in response to ABA in sweet cherry (Prunus avium) reveal genetic and epigenetic modulation of auxin and cytokinin genes. J. Plant Growth Regul. 2025, 44, 1165–1187. [Google Scholar] [CrossRef]
- Kappel, F.; MacDonald, R.A. Gibberellic acid increases fruit firmness, fruit size, and delays maturity of ‘Sweetheart’ sweet cherry. J. Am. Pomol. Soc. 2002, 56, 219–222. [Google Scholar]
- Kuhn, N.; Ponce, C.; Arellano, M.; Time, A.; Sagredo, B.; Donoso, J.M.; Meisel, L.A. Gibberellic acid modifies the transcript abundance of ABA pathway orthologs and modulates sweet cherry (Prunus avium) fruit ripening in early- and mid-season varieties. Plants 2020, 9, 1796. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.; Maldonado, J.; Ponce, C.; Arellano, M.; Time, A.; Multari, S.; Meisel, L.A. RNAseq reveals different transcriptomic responses to GA3 in early and midseason varieties before ripening initiation in sweet cherry fruits. Sci. Rep. 2021, 11, 13075. [Google Scholar] [CrossRef]
- Ponce, C.; Kuhn, N.; Arellano, M.; Time, A.; Multari, S.; Martens, S.; Meisel, L.A. Differential phenolic compounds and hormone accumulation patterns between early-and mid-maturing sweet cherry (Prunus avium L.) cultivars during fruit development and ripening. J. Agric. Food Chem. 2021, 69, 8850–8860. [Google Scholar] [CrossRef] [PubMed]
- Gibeaut, D.M.; Whiting, M.D.; Einhorn, T. Time indices of multiphasic development in genotypes of sweet cherry are similar from dormancy to cessation of pit growth. Ann. Bot. 2017, 119, 465–475. [Google Scholar] [CrossRef]
- Zhang, C.; Whiting, M.D. Plant growth regulators improve sweet cherry fruit quality without reducing endocarp growth. Sci. Hortic. 2013, 150, 73–79. [Google Scholar] [CrossRef]
- Alkio, M.; Jonas, U.; Declercq, M.; Van Nocker, S.; Knoche, M. Transcriptional dynamics of the developing sweet cherry (Prunus avium L.) fruit: Sequencing, annotation and expression profiling of exocarp-associated genes. Hortic. Res. 2014, 1, 11. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, K.; Liu, L.; Zhang, K.; Yuan, H.; Liao, X.; Li, T. A role for PacMYBA in ABA-regulated anthocyanin biosynthesis in red-colored sweet cherry cv. Hong Deng (Prunus avium L.). Plant Cell Physiol. 2014, 55, 862–880. [Google Scholar] [CrossRef]
- Dardick, C.; Callahan, A.M. Evolution of the fruit endocarp: Molecular mechanisms underlying adaptations in seed protection and dispersal strategies. Front. Plant Sci. 2014, 5, 284. [Google Scholar] [CrossRef]
- Qu, G.; Peng, D.; Yu, Z.; Chen, X.; Cheng, X.; Yang, Y.; Zhou, B. Advances in the role of auxin for transcriptional regulation of lignin biosynthesis. Funct. Plant Biol. 2021, 48, 743–754. [Google Scholar] [PubMed]
- Wang, Y.; Wang, Q.; Zhang, F.; Han, C.; Li, W.; Ren, M.; Hou, C.; Tao, S. PbARF19-mediated auxin signaling regulates lignification in pear fruit stone cells. Plant Sci. 2024, 344, 112103. [Google Scholar] [CrossRef] [PubMed]
- Braak, J.P. The effect of flowering date and temperature on embryo development in sweet cherry (Prunus avium L.). Neth. J. Agric. Sci. 1978, 26, 13–30. [Google Scholar]
- Kondo, S.; Hayata, Y.; Iwasaki, N. Effects of indole-3-acetic acid and gibberellins on fruit development and maturation of sweet cherry. Acta Hortic. 2000, 514, 75–82. [Google Scholar] [CrossRef]
- Bonghi, C.; Trainotti, L.; Botton, A.; Tadiello, A.; Rasori, A.; Ziliotto, F.; Ramina, A. A microarray approach to identify genes involved in seed-pericarp cross-talk and development in peach. BMC Plant Biol. 2011, 11, 107. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Wu, M.F.; Yang, L.; Wu, G.; Poethig, R.S.; Wagner, D. The microRNA-regulated SBP-box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 2009, 17, 268–278. [Google Scholar] [CrossRef]
- Liu, X.; Sun, W.; Liu, H.; Wang, L.; Manzoor, M.A.; Wang, J.; Zhang, C. PavSPLs are key regulators of growth, development, and stress response in sweet cherry. Plant Sci. 2025, 350, 112279. [Google Scholar]
- Gou, J.Y.; Felippes, F.F.; Liu, C.J.; Weigel, D.; Wang, J.W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Wang, J.W.; Czech, B.; Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef]
- Wu, G.; Poethig, R.S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 2006, 133, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Z.; Zhao, X.; Cao, H.; Wang, L.; Liu, S.; Liu, Z. Superstar microRNA, miR156, involved in plant biological processes and stress response: A review. Sci. Hortic. 2023, 316, 112010. [Google Scholar] [CrossRef]
- Duan, Z.; Qin, Y.; Xia, X.; Yin, W. Overexpression of Populus euphratica peu-MIR156j gene enhancing salt tolerance in Arabidopsis thaliana. J. Beijing For. Univ. 2011, 33, 1–7. [Google Scholar]
- Sun, C.; Zhao, Q.; Liu, D.D.; You, C.X.; Hao, Y.J. Ectopic expression of the apple Md-miRNA156h gene regulates flower and fruit development in Arabidopsis. Plant Cell Tissue Organ Cult. 2013, 112, 343–351. [Google Scholar] [CrossRef]
- Song, M.; Wang, R.; Zhou, F.; Wang, R.; Zhang, S.; Li, D.; Yang, Y. SPLs-mediated flowering regulation and hormone biosynthesis and signaling accompany juvenile-adult phase transition in Pyrus. Sci. Hortic. 2020, 272, 109584. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Tang, G.; Huang, T. Small but powerful: Function of microRNAs in plant development. Plant Cell Rep. 2018, 37, 515–528. [Google Scholar] [CrossRef]
- Soto, E.; Sanchez, E.; Nuñez, C.; Montes, C.; Rothkegel, K.; Andrade, P.; Almeida, A.M. Small RNA differential expression analysis reveals miRNAs involved in dormancy progression in sweet cherry floral buds. Plants 2022, 11, 2396. [Google Scholar] [CrossRef]
- Qian, M.; Ni, J.; Niu, Q.; Bai, S.; Bao, L.; Li, J.; Teng, Y. Response of miR156-SPL module during the red peel coloration of bagging-treated Chinese sand pear (Pyrus pyrifolia Nakai). Front. Physiol. 2017, 8, 550. [Google Scholar] [CrossRef]
- Mohorianu, I.; Schwach, F.; Jing, R.; Lopez-Gomollon, S.; Moxon, S.; Szittya, G.; Dalmay, T. Profiling of short RNAs during fleshy fruit development reveals stage-specific sRNAome expression patterns. Plant J. 2011, 67, 232–246. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Zhai, L.; Cui, Y.; Tang, G.; Huo, J.; Bian, S. The miR156/SPL12 module orchestrates fruit colour change through directly regulating ethylene production pathway in blueberry. Plant Biotechnol. J. 2024, 22, 386–400. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Chang, H.; Zhou, J.; Luo, Y.; Zhang, K.; Wang, B. Sweet cherry fruit miRNAs and effect of high CO₂ on the profile associated with ripening. Planta 2019, 249, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Xiao, Y.; Zhang, X.; Du, B.; Turupu, M.; Li, T. Genome-wide identification of the SQUAMOSA promoter-binding protein-like (SPL) transcription factor family in sweet cherry fruit. Int. J. Mol. Sci. 2023, 24, 2880. [Google Scholar] [CrossRef]
- Maldonado, J.E.; Acevedo, O.; Melo, M.; Núñez, C.; Zavala, M.; Menares, M.; Kuhn, N. Seed-fruit multiomics integration of sweet cherry cultivars with different maturity time shows alternative molecular landscapes at the transition from development to ripening, unveiling a role of small RNAs, SPLs, lignin and inositol pathways. Sci. Hortic. 2025, 343, 114099. [Google Scholar]
- Marín-González, E.; Suárez-López, P. “And yet it moves”: Cell-to-cell and long-distance signaling by plant microRNAs. Plant Sci. 2012, 196, 18–30. [Google Scholar]
- San Martino, L.; Manavella, F.A.; García, D.A.; Salato, G. Phenology and fruit quality of nine sweet cherry cultivars in South Patagonia. In V International Cherry Symposium; ISHS: Leuven, Belgium, 2008; Volume 795, pp. 841–848. [Google Scholar]
- Hesse, C.O.; Kester, D.E. Germination of embryos of Prunus related to degree of embryo development and method of handling. Proc. Am. Soc. Hortic. Sci. 1955, 65, 251–264. [Google Scholar]
- Wang, J.; Liu, W.; Zhu, D.; Hong, P.; Zhang, S.; Xiao, S.; Wang, L.; Liu, Q. Chromosome-scale genome assembly of sweet cherry (Prunus avium L.) cv. Tieton obtained using long-read and Hi-C sequencing. Hortic. Res. 2020, 7, 36. [Google Scholar] [CrossRef]
- Huynh-Thu, V.A.; Irrthum, A.; Wehenkel, L.; Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 2010, 5, e12776. [Google Scholar] [CrossRef]
- Núñez-Lillo, G.; Ponce, E.; Arancibia-Guerra, C.; Carpentier, S.; Carrasco-Pancorbo, A.; Olmo-García, L.; Pedreschi, R. A multiomics integrative analysis of color de-synchronization with softening of ‘Hass’ avocado fruit: A first insight into a complex physiological disorder. Food Chem. 2023, 408, 135215. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Meisel, L.; Fonseca, B.; González, S.; Baeza-Yates, R.; Cambiazo, V.; Campos, R.; Silva, H. A rapid and efficient method for purifying high quality total RNA from peaches (Prunus persica) for functional genomics analyses. Biol. Res. 2005, 38, 83–88. [Google Scholar]
- Griffiths-Jones, S. miRBase: The microRNA sequence database. In MicroRNA Protocols; Humana Press: Totowa, NJ, USA, 2006; pp. 129–138. [Google Scholar]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetics Analysis Version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Gao, Z.; Wang, F.; Zhou, J.; Zhang, Z. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol. Biol. 2009, 10, 71. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; Van den Hoff, M.J.B.; Moorman, A. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Udvardi, M.K.; Czechowski, T.; Scheible, W.R. Eleven golden rules of quantitative RT-PCR. Plant Cell 2008, 20, 1736–1737. [Google Scholar] [CrossRef]
- Mitra, P.P.; Loqué, D. Histochemical staining of Arabidopsis thaliana secondary cell wall elements. J. Vis. Exp. 2014, 87, 51381. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Dyankova, S.; Doneva, M. Extraction and characterization of anthocyanin colorants from plant sources. Agric. Sci. Technol. 2016, 8, 85–89. [Google Scholar] [CrossRef]
- Jung, S.; Bassett, C.; Bielenberg, D.G.; Cheng, C.H.; Dardick, C.; Main, D.; Schaffer, R.J. A standard nomenclature for gene designation in the Rosaceae. Tree Genet. Genomes 2015, 11, 108. [Google Scholar] [CrossRef]
- Else, M.A.; Stankiewicz-Davies, A.P.; Crisp, C.M.; Atkinson, C.J. The role of polar auxin transport through pedicels of Prunus avium L. in relation to fruit development and retention. J. Exp. Bot. 2004, 55, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, J.; Jing, X.; Khan, F.S.; Chen, Y.; Chen, Z.; Zhao, X.; Wei, Y. Genome-wide identification of litchi SPL gene family and expression analysis in pericarp anthocyanin biosynthesis. Horticulturae 2024, 10, 762. [Google Scholar] [CrossRef]
- Van de Wouwer, D.; Vanholme, R.; Decou, R.; Goeminne, G.; Audenaert, D.; Nguyen, L.; Morsa, S.; Lammertyn, F.; Van Doorsselaere, J.; Boerjan, W. Chemical genetics uncovers novel inhibitors of lignification, including p-iodobenzoic acid targeting CINNAMATE-4-HYDROXYLASE. Plant Physiol. 2016, 172, 198–220. [Google Scholar] [CrossRef]
- Serrani, J.C.; Carrera, E.; Ruiz-Rivero, O.; Gallego-Giraldo, L.; Peres, L.E.P.; García-Martínez, J.L. Inhibition of auxin transport from the ovary or from the apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins. Plant Physiol. 2010, 153, 851–862. [Google Scholar] [CrossRef]
- Serrano, A.; Kuhn, N.; Restovic, F.; Meyer-Regueiro, C.; Madariaga, M.; Arce-Johnson, P. The glucose-related decrease in polar auxin transport during ripening and its possible role in grapevine berry coloring. J. Plant Growth Regul. 2023, 42, 365–375. [Google Scholar] [CrossRef]
- Arancibia-Guerra, C.; Núñez-Lillo, G.; Cáceres-Mella, A.; Carrera, E.; Meneses, C.; Kuhn, N.; Pedreschi, R. Color desynchronization with softening of ‘Hass’ avocado: Targeted pigment, hormone and gene expression analysis. Postharvest Biol. Technol. 2022, 194, 112067. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).