Abstract
Pistachio nuts are among the 50 best foods with the highest antioxidant potential. They have a balanced content of mono- (~70%) and polyunsaturated (~20%) fatty acids, minerals, and bioactive compounds such as tocopherols, phytosterols, and phenolic compounds, which have shown rapid accessibility in the stomach. Pistachio consumption provides several health benefits, primarily due to its antioxidant properties and high content of essential nutrients. In this study, we analyzed the mineral composition, total phenolic content (TP), antioxidant activity (AA), and UHPLC/MS-MS polyphenolic profile of three Argentinian pistachio crops. Additionally, the physicochemical parameters and the elemental profiles of the growing soils were determined, as they influence mineral uptake and the synthesis of bioactive compounds in pistachio kernels. The TP was not significantly modified by the growing soils, with Crop3 presenting the highest TP content (276 ± 14 mg GA/100 g DW). Crop3 exhibited 18% higher TP content compared to Crop2. Similarly, FRAP values ranged from 28.0 to 36.5 mmol TE/100 g DW, with Crop1 showing a 30% increase compared to Crop2. DPPH values varied from 19.0 to 24.3 mmol TE/100 g DW, with Crop1 displaying 28% higher activity than Crop2. However, the polyphenolic profile was similar for all crops analyzed. Thirty compounds were identified; only Crop 1 contained the flavanone eriodyctiol and the isoflavone genistein, while the flavanone naringenin and the flavone luteolin were identified in Crop1 and Crop3. Regarding mineral content, the pistachio kernels mainly contained K, Ca, and Mg. Multivariate analyses revealed distinct elemental and antioxidant profiles among crops. LDA achieved classification accuracies of 77.7% for soils and 74.4% for kernels, with Pb, Zn, Cu, Rb, Sr, and Mn as key discriminants. CCA confirmed strong soil–kernel mineral correlations (r = 1), while GPA showed higher congruence between antioxidant traits and kernel composition than with soil geochemistry. These findings underscore the importance of soil composition in determining the nutritional quality of pistachio kernels, thereby supporting the beneficial health effects associated with pistachio consumption.
1. Introduction
The pistachio tree (Pistacia vera L.) is a member of the Anacardiaceae family and can grow in different soils, so that it can be cultivated in many countries, with Iran, the United States, Spain, and Turkey being the main producers [1]. Pistachios arrived in Argentina in 1980 thanks to two pioneering producers from the province of San Juan who brought plant material from Iran and California, United States. Since then, the number of planted hectares has increased significantly, with the area planted with pistachios growing by more than 500% in the past five years, particularly in the southern part of San Juan province and the northern part of Mendoza province. San Juan currently has 6500 hectares dedicated to pistachio production, representing 87% of the cultivated area in the country. It is estimated that the cultivated hectares will reach 10,000 in the coming years. Leading companies in the pistachio market produce in-shell and out-of-shell pistachios, as well as roasted and salted pistachios, pistachio flour and oil, and pistachio crunch. Production is destined for both domestic and international markets, and exported to Chile, Brazil, Uruguay, Paraguay, China, Germany, and Italy, among others. In a recent newspaper report, the representative of the country’s largest producer mentions that some 400,000 kilos are destined for the domestic market and 1.6 million kilos of pistachios are exported annually. The quality of pistachios produced in Argentina is highly valued in the local and international markets due to their healthy open kernels [2,3].
Over the past decade, in close collaboration between Pisté—a pioneer in pistachio production in Argentina—and Argentine universities, and with the goal of adding value to Argentine pistachio production, the first reports have been produced on the chemistry, biological activity, and nutritional value of natural, salted, and roasted pistachios, as well as their oils. In recent years, our research team, with the aim of contributing to the added value of Argentine pistachio production, has made contributions on the chemistry, biological activity, and nutritional value of some of the products [4,5,6,7,8].
However, factors such as soil type and texture, moisture levels, pH, and root distribution affect the nutritional requirements of pistachio trees. At the same time, they need 14 elements to grow, the macronutrients (N, P, K, Ca, Mg, and S) and the micronutrients (Fe, Mn, Cl, B, Cu, Zn, Ni, and Mo). These elements play an important role in plant physiology by providing disease resistance and improving fruit quality [9]. In relation to the mineral composition of pistachio, there are two main sources: a natural source, via the roots (related to parent rocks) and an anthropogenic source during handling and processing, as well as through agricultural practices (fertilizers and inorganic pesticides). Pistachios are an excellent source of proteins, fiber, monounsaturated fatty acids, minerals and vitamins, as well as carotene, phenolic acids, flavonoids, and anthocyanins. Several of these compounds have been associated with benefits for human health. Specifically, the polyphenols present in pistachios contribute significantly to their antioxidant and anti-inflammatory effect, as demonstrated in vitro, in vivo, and in clinical studies. Additionally, pistachio polyphenol extracts can affect the enzymes involved in glucose regulation and, consequently, type 2 diabetes. Regular pistachio consumption has been associated with a positive modulation of human intestinal microbiota and beneficial effects on skin health and cognitive function [10,11]. In addition, the awareness of healthy habits and the cult of healthy foods has contributed to enhancing the consumption of dried fruits in general, replacing crackers and bread as snacks. In this context, pistachios benefited, creating the need to introduce new cultivars. The province of San Juan, located in the center-west of Argentina, is a leading pistachio producer in Argentina [7]. The quality of pistachios produced is highly valued by local and international markets, due to their healthy open kernel.
Environmental conditions—such as climate, soil composition, irrigation source, and geographic location—play a key role in determining pistachio nut quality. Studies have shown that temperature, sunlight, and water availability can influence the phenolic profile, antioxidant activity, and lipid composition of pistachio kernels [12]. Similarly, soil nutrient levels, salinity, and pH affect mineral uptake and secondary metabolism in pistachio trees, which in turn modulate the accumulation of bioactive compounds such as tocopherols, phytosterols, and phenolics [13]. The combination of soil and climate factors was also studied to determine the geographic origin of pistachios and to distinguish between crops. Thus, it highlights the importance of local growing conditions in shaping the nutritional and functional characteristics of pistachio fruits [14].
Based on the above, there is a need to study new regions for cultivating pistachios and the fruits obtained from them. Previous studies have characterized pistachio cultivars based on their chemical composition [15,16,17,18] and physical traits [19], which help define nut quality and detect regional differences. Anderson and Smith [20] introduced a method that combines elemental analysis with classification techniques to trace the geographic origin of food products. Later, Anderson et al. [20] applied this strategy to pistachios, showing that mineral profiles can reliably indicate geographic origin. To date, no studies have demonstrated the relationship between the mineral composition of the soil and pistachio kernels. The purpose of this study was to evaluate how the soil-growing region affects the total phenolic content (TP), antioxidant activity (AA), polyphenol profile, and mineral concentration in Pistacia vera cv. Kerman from three Argentinean cultivars. Additionally, heavy metals were quantified in both soil and pistachio samples to evaluate safety for human consumption.
2. Materials and Methods
2.1. Samples
The soil and pistachio samples harvested in 2022 were provided by three pistachio-producing establishments from the province of San Juan, Argentina (Palos Blancos-Crop1, Pistachos de los Andes-Crop2, and Pisté SRL-Crop3).The three pistachio crops had similar environmental conditions: continental weather with hot summers (temperatures up to 42 °C), and cold winters (temperatures below 10 °C). Rainfall is scarce (<90 mm per year), requiring additional irrigation with either surface water (rivers formed by melting ice) or groundwater. The altitude of the three crops varied between 650 and 750 m.a.s.l.
Crop1 was in Punta de Agua (latitude: −32.0333°, longitude: −68.2000°), 25 de Mayo district. Crop2 was in Santa Rosa (latitude: −31.7333°, longitude: −68.3000°), 25 de Mayo district. Crop3 was located in Carpintería (latitude: −31.8167°, longitude: −68.5500°), Pocito district. The pistachios grew in large clusters and were harvested in late summer, when their covers were a pink color. After harvesting, pistachio covers were mechanically removed from the hard shells and dried in ovens until reaching a moisture content of 5–6%.
Soil samples were collected from Crop1 (n = 8), Crop2 (n = 8), and Crop3 (n = 8) using stainless steel shovels and were stored in individual black plastic bags in the dark. Soils were sampled within a radius of no more than 20 cm from the trunk, and in depths from 10 to 20 cm to avoid surface-soil pollution coming from the surrounding environment, reducing the effects of fertilizers, and variable organic matter content. Additionally, soil samples without implantation (control samples) were collected and analyzed at the same time to establish whether fertilizers used on crops might have modified the soil mineral profile: Crop1 (n = 4), Crop2 (n = 4), and Crop3 (n = 4). Each sample was studied in triplicate. The soil samples were dried at 30–40 °C, homogenized, and sieved through a <2 mm plastic sifter.
Control soil samples (non-implanted areas) were collected to assess whether fertilization and other agronomic practices might alter the mineral composition of the surrounding soil. However, no substantial differences were detected when compared to cultivated soils. Consequently, the control data were excluded from the final analysis to enhance clarity and maintain focus on the primary findings.
2.2. Pistachio
Pistachio (Pistacia vera L. cv. Kerman) kernels came from three industrial facilities in San Juan Province, Argentina: Palos Blancos (Crop1), Pistachos de los Andes (Crop2), and Pisté SRL (Crop3). The samples were taken during the final packaging stage, before their commercial distribution in domestic and international markets. At each facility, the nuts remained in light-protected, temperature-controlled warehouses under strict hygienic conditions to prevent microbial growth and preserve their organoleptic properties. From each crop, 5 kg of kernels were randomly selected, refrigerated at 4 °C during transport, and subsequently frozen at −40 °C in sealed black polyethylene bags for later analysis. Kernels were randomly taken from storage deposits and packed in black polyethylene bags at −40 °C in the dark. Three independent samples (×3 crops × 5 kg/sample) were used for the analysis. Kernels (100 g, randomly taken from each sample) were shelled (seed coat), weighed, and measured (long and wide) (Table 1). Ground pistachio (10 g; 40 mesh) was defatted using a Soxhlet apparatus with petroleum ether (3 × 200 mL) for 60 min each cycle, totaling three consecutive extractions. (PEE). Methanol acidified extract (MeOH-H+E) was obtained using acidified methanol with 0.1% HCl, v/v (3 × 200 mL, for 60 min each time). Solvents were evaporated under reduced pressure (40 °C), and dry extract yields (w/w) were calculated in terms of dry starting material. The acidified methanolic extracts were used for total phenolic (TP) and antioxidants assays.

Table 1.
Pistachio kernel characteristics, yield extracts, and TP content in pistachio crops.
A second set of pistachios was completely dehulled by hand, and representative samples (200 mg) of crushed skins were mixed with 2 mL acidified methanol (0.1% HCl, v/v), placed in tubes and sonicated (40 kHz, 30 min, 25 °C) (ultrasound bath model TB02TACA, TEST-LAB S.R.L., Buenos Aires, Argentina). Then, the homogenate was centrifuged at 10,000× g during 10 min using a Biofuge®28RS Heraeus Sepatech Centrifuge (Heraeus Instruments, Hanau, Hesse, Germany). The supernatant, acidified methanol skin extract (MeOH-H+SE), was separated, filtered (0.45 µm) and injected into a UPLC-PDA-HESI II-Orbitrap -MS/MS system for polyphenol analyses (see Section 2.6).
2.3. Physicochemical Parameters of Soils
The electrical conductivity (EC) and pH values were measured using a portable pH/ORP MeterHI 8424 (HANNA Instruments Argentina S.A., Buenos Aires, Argentina). One gram of soil was placed into a beaker, and then 10 mL of distilled water was added. The mixture was stirred and allowed to stand for 10 min, after which the pH and EC values were measured [21].
2.4. Elemental Analysis
The soil and pistachio samples (Crops 1–3) were prepared for elemental analysis. The bioavailable soil fraction was processed for elemental analysis as follows: 20 g soil sample was weighed into an Erlenmeyer flask, adding 50 mL of NH4NO3 (1 M). The resulting suspension was shaken for 2 h at room temperature. Subsequently, the suspension was allowed to settle for 1 h, filtered through 0.45 μm filters, and acidified with 0.5 mL of concentrated nitric acid (sub-boiling grade). This process was performed in duplicate for all samples. Three spiked samples were also prepared. Variable amounts of individual standard solutions (1000 mg/L in 1% nitric acid) were added to 40 g of dried sieved soil sample to double the starting concentration for each element. The rest of the procedure was the same as that used for non-spiked samples. All recoveries were between 85% and 115%.
Pistachios were dehulled, and kernels with their skin (seed coat) were milled using a grinder. Ground samples (particle size 0.5 mm) were accurately weighed and mineralized by acid digestion using a microwave oven (Anton Paar Multiwave 3000; Graz, Styria, Austria). Samples (0.2 g) were introduced into quartz vessels, followed by the addition of 8 mL concentrated nitric acid, keeping vessels open until no fumes were observed (2–3 h). Afterwards, vessels were cap-closed and heated using the following power sequence: starting with a 15 min ramp until they reached 600 W, held there for 45 min (maximum T = 169 °C; max pressure = 75 bar), and a final 15 min step disabled power to reach pressure equilibration. Mineralized samples were quantitatively transferred to 25 mL volumetric flasks, adjusting the volume with ultrapure water, followed by filtration using 0.45 µm filters. Spiked samples were also prepared by adding varying amounts of individual standard solutions (1000 mg/L in 1% nitric acid), doubling the starting concentration for each element. The rest of the procedure was the same as that used for non-spiked samples [19]. All recoveries were between 87% and 113%. A certified reference material (CRM: NIST 2548 a- typical diet) was analyzed for quality control using the same procedure. Recovery of elements measured in this work from CRM was between 85% and 110% of certified values.
Method validation parameters were evaluated to ensure analytical reliability. Calibration curves for all elements showed excellent linearity (R2 > 0.99). Repeatability was assessed by triplicate analysis, yielding relative standard deviations (RSD) below 8% for all elements. Limits of detection (LOD) and quantification (LOQ) were estimated as 3σ and 10σ of the blank signal, respectively. LOD values ranged from 0.02 to 0.25 µg/g, depending on the element. The analytical performance was consistent with expected standards for elemental analysis in food and soil matrices, as supported by the Horwitz ratio (HorRat < 2), indicating acceptable precision according to internationally recognized criteria [22,23].
Quantification of Elements by Q-ICP-MS
Eighteen elements were quantified in soil and pistachio samples: Ca, Cu, Fe, K, Mg, Mn, Na, Zn, B, Rb, Sr, Al, As, Pb, V, P, Se, and Cd. Elemental analyses were conducted using a Quadrupole Inductively Coupled Plasma Mass Spectrometer (Q-ICP-MS) Agilent 7500cx Series (Agilent Technologies, Santa Clara, CA, USA), equipped with an ASX-500 autosampler and a micro-flow concentric nebulizer. The sample introduction system consisted of a micro-flow concentric nebulizer, Peltier-cooled spray chamber, and 2.5 mm ID fixed injector torch. The RF power was 1500 W for all the experiments, and the interface was fitted with Ni sampling and skimmer cones designed for low polyatomic formation. Two operation modes were used: with and without collision cell technology (CCT). CCT mode measurements were performed for Mg, Al, K, Ca, V, Mn, Fe, Cu, Zn, As, Se, Rb, P, and Sr. For the CCT mode the collision cell was flushed with a collision gas (He). The elements B, Cd, and Pb were measured without operating the collision cell with gas, reaching full sensitivity. The oxide ratio and doubly charged species were maintained below 1% for both modes of operation. All measurements were performed using Sc, In, and Re as internal standards. Instrumental and procedural blanks were determined together with samples. Three replicates were obtained for each sample. Full quantitative analysis was performed from calibration plots, constructed using linear regression from standards for each element (R2 > 0.99). Sodium was quantified separately using Flame Atomic Absorption Spectrometry (FAAS), Perkin Elmer 3030 (Waltham, MA, USA) at a wavelength of 589 nm. All pistachio samples (digested) were diluted tenfold using HNO3 (2% sub-boiling grade in ultrapure water) before Q-ICPMS measurements. Standards and blanks were prepared using the same mixture (2% HNO3).
2.5. Total Phenolic Content (TP)
The total phenolic content (TP) was determined using the Folin–Ciocalteu method [24] by linear regression from a calibration plot constructed using gallic acid. The results were expressed as mg of gallic acid equivalents (GAE) per 100 g of pistachio on a dry weight (DW) basis (mg GAE/100 g DW).The values were obtained using a Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA) and are shown as the mean ± standard deviation (SD).
2.6. Identification of Phenolic Compounds in Pistachio Skin by UPLC-PDA-HESI II-Orbitrap-MS/MS
Equipment. A Thermo Scientific Dionex Ultimate 3000 UHPLC system and a rapid separations PDA detector controlled by Chromeleon 7.2 Software (Thermo Fisher Scientific, Waltham, MA, USA) hyphenated with a Thermo high-resolution Q Exactive focus mass spectrometer were used for analysis. The chromatographic system was coupled to the MS with a heated electrospray ionization source II (HESI II). Nitrogen (purity > 99.999%) obtained from a Zefiro nitrogen generator (Clantecnologica, Seville, Spain) was employed as both the collision and damping gas. Orbitrap parameters were calibrated as reported [25,26] and TraceFinder 3.2 (Thermo Fisher Scientific, San José, CA, USA) was used for UHPLC control and data processing, respectively. Q Exactive 2.0 SP 2 from Thermo Fisher Scientific was used to control the mass spectrometer.
LC parameters. Liquid chromatography using an UHPLC C18 column (Acclaim, 150 mm × 4.6 mm ID, 5 µm, Restek Corporation, Bellefonte PA, USA) operated at 25 °C with detection wavelengths at 254, 280, 320, and 440 nm, and PDA from 200 to 800 nm for peak characterization were employed. Mobile phases were water-formic acid solution 1% (A) and acetonitrile (B). The gradient (min, %B) was (0.00, 5); (5.00, 5); (10.00, 30); (15.00, 30); (20.00, 70); (25.00, 70); (35.00, 5), and 12 min for column equilibration. The flow was at 1.00 mL min−1 and injection volume: 10 µL. Extracts and standards dissolved in methanol were kept at 10 °C during storage.
MS parameters. The HESI and other mass spectrometry parameters were optimized as reported previously [25,26]. Detection and identification were based on retention time of target compounds and calculated exact mass, presented in section results 3.7. Table 4. The mass tolerance window was set to 5 mg/L for the two positive and negative modes used. A heated electrospray ionization source II (HESI II) was used as the ionization source, operating in both positive and negative ion modes. Full-scan MS and data-dependent MS/MS experiments were carried out over a mass range of m/z 100–1000, with a resolving power of 70,000 FWHM (at m/z 200). The spray voltage was set to 3.5 kV, capillary temperature to 320 °C, sheath gas at 40 arbitrary units, and auxiliary gas at 10 arbitrary units. The mass tolerance window for compound identification was set to 5 ppm. Detection was performed using the Q Exactive Focus hybrid quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), and data were processed with TraceFinder 3.2 and Chromeleon 7.2 software.
2.7. Antioxidant Activity
2.7.1. Free Radical Scavenging Activity on DPPH
Free radical scavenging effects of the MeOH-H+SE were assessed by the fade of a methanolic solution of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) according to the procedure described by Brand-Williams et al. [27]. Scavenging activities were evaluated at 517 nm in Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA). The loss of color (fade percentage) indicated the free radical scavenging efficiency of the substances. Trolox (0–20 mM) was used as a standard antioxidant. The activity of the extracts was obtained by interpolating the absorbance on a calibration plot and expressed as millimolar Trolox equivalent (mM TE) per 100 g of pistachio on a dry weight (DW) basis (mM TE/100 g DW).
2.7.2. Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay measures the reducing capability of the samples by evaluating the conversion of a Fe3+/ferricyanide complex to Fe2+. The iron-reducing power of the samples was tested using the assay reported by Benzie [28] with some modifications [29]. Briefly, 10 µL extract (10 mg/mL) was added to 190 µL of a mixed solution composed of acetate buffer (300 mM, pH 3.6, 83.3% v/v), potassium ferricyanide (10 mM, 8.3% v/v), and iron trichloride (20 mM, 8.3% v/v). The mixture was incubated at room temperature for 30 min and then the absorbance was read at 540 nm using Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA)). Trolox (1–20 mM) was used as a standard antioxidant and the activity of the extracts was expressed as millimolar trolox equivalent (mM TE) per 100 g of pistachio on a dry weight (DW) basis (mM TE/100 g DW).
2.8. Statistical Analysis
We performed all univariate and multivariate analyses using Python v3.11 to ensure consistency and reproducibility across datasets. We applied key libraries including pandas, scipy, scikit-learn, and matplotlib.
We calculated means and standard deviations for each parameter. For univariate comparisons, we applied one-way ANOVA followed by Fisher’s LSD test at a significance level of p < 0.05. We indicated sample sizes in each figure or table caption as n = 10 for morphological and TP analyses, n = 8 for soil mineral composition, and n = 3 for kernel elemental profiles.
We selected and applied the following multivariate methods.
- Linear Discriminant Analysis (LDA)
We used LDA to classify samples based on their mineral composition and to identify the most discriminant variables. We implemented repeated stratified 5-fold cross-validation (10 runs), calculated classification accuracy and confusion matrices, and visualized projections in LD1–LD2 space. We computed standardized loadings to determine the contribution of each feature to the discriminant functions.
- Canonical Correlation Analysis (CCA)
We applied CCA to examine correlations between soil and kernel mineral profiles. We standardized both datasets, matched variables, and generated canonical projections. We addressed the difference in sample sizes (soil: n = 8; kernels: n = 3) by running multiple three-point combinations from each soil group.
- Generalized Procrustes Analysis (GPA)
We conducted GPA to integrate mineral profiles from soils, kernels, and TP-AA matrices into a consensus configuration. We used standardization, Procrustes alignment, disparity metrics, and two-dimensional visualizations to compare patterns across data domains.
Together, these methods allowed us to detect meaningful relationships, groupings, and markers associated with crop origin. We selected them for their ability to uncover both correlations and classification trends within complex chemical datasets.
3. Results
3.1. Pistachio Kernel Morphological and Chemical Characterization
Table 1 shows the morphological and chemical characteristics of pistachio kernels from the three Argentine crops. Regarding kernel length, Crop1 exhibited significantly greater values compared to Crop3, while Crop2 showed intermediate values without statistical difference from the other two. In terms of kernel width, no significant differences were observed among crops (p > 0.05).
Kernel weight per unit was significantly higher in Crop1 compared to Crop2 and Crop3, which did not differ statistically.
Concerning extractive yield, the PEE extract was significantly lower in Crop1 compared to Crop3, whereas Crop2 showed an intermediate value without significant difference. The MeOH-H+E extract yield showed no significant differences among the three crops.
Notably, total phenolic content (TP) showed significant variation across crops, with Crop3 and Crop1 presenting the highest TP levels, whereas Crop2 exhibited a significantly lower content.
3.2. Soils Physicochemical Measurement
The pH values of soil samples were 8.0 ± 0.3, 8.0 ± 0.2, and 7.8 ± 0.2 for Crop1, 2, and 3, respectively, not showing significant differences between them.
Soil salinity is considered as an important limitation; it produces an adverse impact on plant physiology [30]. In this work, the EC varied between 127 and 4743 μS/cm in crops analyzed. The highest value was found in Crop1 (3720 ± 1023 μS/cm), followed by Crop2 (700 ± 245 μS/cm), and Crop3 (172 ± 45 μS/cm). These values are within the tolerance limit, and the cultivars were not affected by these parameters.
3.3. Multi-Elemental Composition
We analyzed the elemental composition of soil and pistachio kernel samples from the three crops to evaluate potential associations between them. The results appear in Table 2 and Table 3.

Table 2.
Concentration of elements in crop soil.

Table 3.
Concentration of elements in pistachio kernels (mg/100 g DW).
Soil mineral content. The elemental analysis of soil samples revealed that Fe, Ca, and Al were the most abundant elements across all three crops. Significant differences were also observed in the levels of potentially toxic heavy metals (As, Cd, Co, Ni, and Pb), with Pb being the most abundant on average, followed by As, Ni, Co, and Cd (Table 2). Crop3 exhibited the lowest overall concentrations, while Crop1 and Crop2 showed higher levels of Pb and As. Statistically significant differences were observed for As, Se, and Ni (p < 0.05), indicating a heterogeneous geochemical profile among the sampled sites.
Then, we performed linear discriminant analysis (LDA) using the mineral dataset from soil samples to classify them according to their geographic origin. The model achieved an average cross-validated accuracy of 77.70% (±5.78%), confirming the effectiveness of this approach. The two canonical discriminant functions, LD1 and LD2, explained 93.05% and 6.95% of the total variance, respectively. LD1 captured most of the variability and was primarily influenced by differences in Pb, Zn, and Cu concentrations, highlighting these elements as key drivers of soil differentiation. LD2 contributed additional separation, though to a lesser extent. Figure 1 reveals the projection of samples in the LD1–LD2 space, illustrating clear geographic separation among the three soil profiles and supporting the presence of distinct geochemical patterns linked to crop origin.
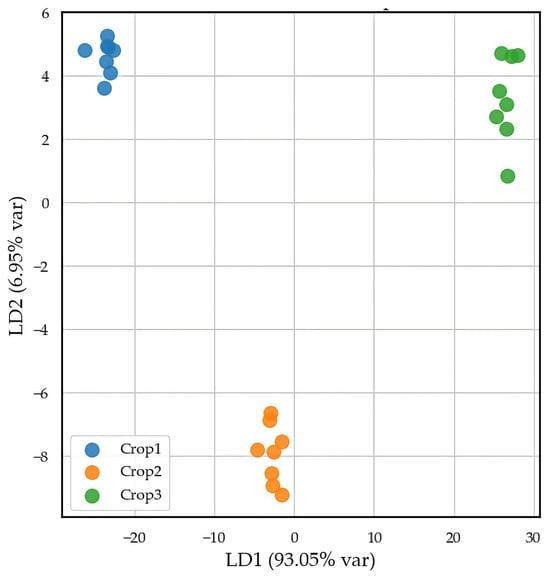
Figure 1.
LDA score plot of soil samples based on mineral composition from the three pistachio crop sites.
Pistachio mineral content.
Table 3 summarizes the elemental composition of pistachio kernels across the three cultivation sites. Potassium (K), calcium (Ca), and magnesium (Mg) represented the most concentrated minerals, with comparable levels in all samples. Among the minor and trace elements, sodium (Na), iron (Fe), zinc (Zn), copper (Cu), manganese (Mn), strontium (Sr), and rubidium (Rb) appeared in decreasing order of concentration. Na levels reached 13 ± 2 mg/100 g DW in Crop3, exceeding those measured in the other crops. Rb concentrations were higher in Crops1 and 3 than in Crop2, while Crop1 contained the highest Zn value. The K/Rb and Ca/Sr ratios differed markedly among crops. Crop2 recorded the highest K/Rb value but the lowest Ca/Sr ratio. These elemental ratios offer reliable markers for distinguishing between growing locations.
LDA applied to the elemental profiles of pistachio kernels yielded an average classification accuracy of 74.44% ± 10.00% across 10 cross-validation runs. Classification results remained consistent for Crops 1 and 2, whereas several samples from Crop 3 appeared grouped with other classes. The first and second canonical functions (LD1 and LD2) accounted for 84.64% and 15.36% of the total variance, respectively. The most influential variables in the model were rubidium (Rb), strontium (Sr), and manganese (Mn), followed by copper (Cu) and zinc (Zn). Figure 2 displays the distribution of kernel samples in the LD1–LD2 projection space.
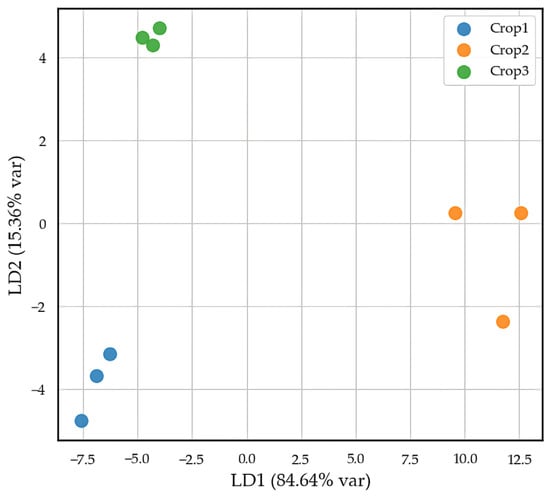
Figure 2.
Distribution of elements in pistachio nuts from Crop1, Crop2, and Crop3 in the plane defined by the first two canonical functions of LDA.
The kernel samples underwent screening for aluminum (Al), selenium (Se), arsenic (As), cadmium (Cd), cobalt (Co), nickel (Ni), lead (Pb), and vanadium (V). All measured concentrations fell below the method’s detection limits, indicating minimal transfer from soil and no associated toxicological concerns.
All detected values for potentially toxic elements were below the maximum levels established by the FAO/WHO Codex Alimentarius for food and feed safety. That confirms that the analyzed pistachio kernels posed no concern regarding heavy metal contamination, aligning with international food safety standards [31].
CCA of soil and kernel elemental composition explored the relationship between pistachio kernel composition and the mineral content of the soils in which they were grown. Due to differences in sample sizes (n = 3 for kernels, n = 8 for soils), the analysis included every possible set of three soil values per crop to ensure consistent comparison.
Several combinations yielded very high canonical correlation values (r1 = 1). These results show a clear correspondence between soil composition and the mineral profile of the kernel.
The findings highlight a consistent trend across cultivation sites, with soil composition closely reflecting the elemental characteristics of the corresponding pistachio kernels.
3.4. Yield Extracts and Total Phenolic Content (TP)
In Table 1, the results of the extracted yield and TP content are presented.
3.5. Antioxidant Capacity
In the DPPH assay, Crop1 exhibited the highest activity (24 ± 5 b mmol TE/100 g DW), followed by Crop2 (19 ± 2 ab) and Crop3 (22 ± 0.5 a), indicating statistically significant differences. In contrast, FRAP values showed a descending trend—Crop1 (37 ± 1), Crop2 (28 ± 9), and Crop3 (19 ± 9)—but no statistically significant differences were found among them (p > 0.05).These findings reflect crop-dependent variation in antioxidant activity, particularly under DPPH assay conditions.
3.6. Generalized Procrustes Analysis (GPA)
To explore the geometric relationship among the mineral composition of pistachio nuts, soil constituents, and antioxidant parameters, a generalized Procrustes analysis (GPA) was applied to standardized data matrices.
Based on the results of the previous elemental analyses, the variables selected for the GPA were Pb, Zn, and Cu for the soil, and Rb, Sr, and Mn for the pistachio kernels.
In the case of soil, Pb and Zn exhibited statistically significant differences among crop sites (Table 2). Cu did not show considerable variation, but it was one of the most discriminating variables in the LDA model, contributing to the geographic separation of the soil profiles (Figure 1).
Regarding the pistachio kernels, Rb, Sr, and Mn were selected due to their high discriminant power in the kernel LDA analysis (Figure 2) and their significant variation across crops (Table 3). These elements also showed spatial correspondence with total phenolic content and antioxidant activity, reinforcing their relevance as functional and geochemical markers.
This target selection of variables allowed the GPA to integrate soil and kernel data with phenolic-related traits in a coherent structural framework, facilitating the identification of cross-matrix correlations.
As illustrated in Figure 3, the GPA configuration revealed a close geometric alignment between antioxidant traits (total phenolic content, DPPH, and FRAP) and the mineral profile of the kernels, particularly in Crops 1 and 3. The Procrustes distance between these two datasets was minimal (0.070), suggesting a high level of structural congruence. In contrast, the distance between kernel and soil mineral compositions was larger (0.585), indicating a more limited spatial correspondence.
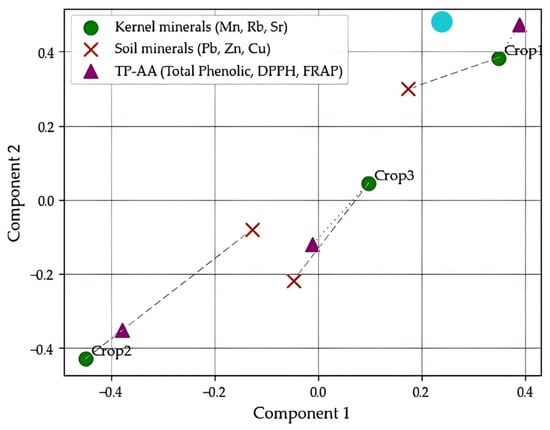
Figure 3.
GPA configuration showing the spatial alignment among mineral profiles of pistachio kernels and soils, and antioxidant parameters (TP, DPPH, FRAP) in Crops 1, 2, and 3.
These results clearly differentiate the three pistachio crops and emphasize a stronger association between antioxidant capacity and internal mineral composition than with the characteristics of the cultivation soil. The high Procrustes congruence, supported by the canonical correlation value (r = 1), suggested that the structural alignment between kernel composition and antioxidant traits was not only geometrically robust but also biologically meaningful.
3.7. UPLC-PDA-HESI II-Orbitrap-MS/MS: Compounds Identified in Pistachio Skin
A total of 30 compounds were detected and provisionally identified using high-resolution Orbitrap MS and PDA detection (Table 4), based on comparisons with HR-MS and PDA data from the literature. The UHPLC-MS fingerprints generated are depicted in Figure 4 while examples of the full MS spectra, and structures of representative compounds are depicted in Figure S1 (Supplementary Materials). The detailed identification is explained below.

Table 4.
Compounds identified in pistachio by UPLC-PDA-HESI II-Orbitrap-MS/MS.
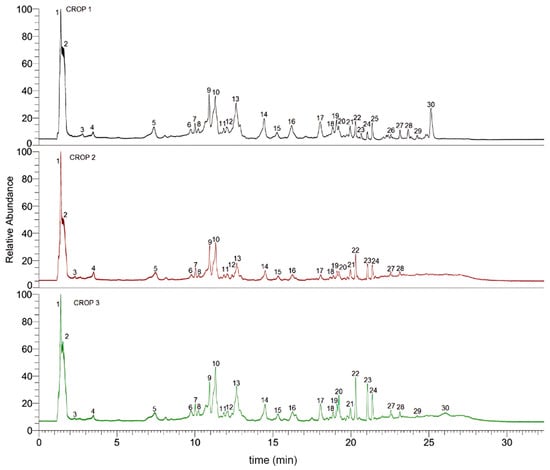
Figure 4.
UHPLC-Orbitrap MS (TIC, total ion current) chromatograms of Crop 1, Crop 2, and Crop 3.
Phenolic acids. Regarding phenolic acids, 3-O-caffeoylquinic acid, quinic acid, and its isomer are reported for the first time in these kernels. Gallic acid was previously reported in pistachios from Argentina [4]. Peak 1 with a [M-H]- ion at m/z 353.0894 and daughter characteristic ions at m/z 191.0361 (quinic acid) was identified as chlorogenic acid [25]. Peak 2 and peak 3 with [M-H]- ions at m/z 191.0191 and 191.0192 were identified as quinic acid and its positional isomer (C7H11O6−) [21], while peak 4 and peak 5 with [M-H]- ions at m/z 169.0135 were identified as gallic acid (Figure S1a) and its isomer [3]. Accordingly, peak 13 with a [M-H]− ion at m/z 449.1982 and daughter characteristic ions at m/z 287.06 [M-H]− (eriodictyol C15H11O6−) was identified as eriodictyol-O-hexoside, as reported in [4,31]. Peak 14 and 20 (Figure S1c) with a [M-H]− ion at m/z 447.0929 and daughter characteristic ions at m/z 285.0405 [M-H]− (3,7-dihydroxy-2-(4-hidroxyphenyl)-4-oxo-4h-chromen-5-olate C15H9O6−) were identified as kaempferol-3-glucoside and kaempferol-3-galactoside, respectively. Peaks 15 and 17 with a [M-H]− ion at m/z 449.1085 and 449.1082 were identified as cyanidin-O-galactoside (Figure S1e) isomers [32]. Peaks 19 and 21 with a [M-H]- ion at m/z 463.0877 were identified as quercetin-O-hexoside isomers showing characteristic daughter ions at m/z 300.0281 [M-2H]2− C15H8O72−) [30].
Flavonols and derivatives. Several flavonoids have previously been detected in Pistacia vera kernels in crops from San Juan province, Argentina [4]. In addition, new flavonoids compounds have been found in the present work. Isomers detected by peaks 6, 7, and 12 with [M-H]− ions at m/z 577.1340 producing daughter ions at m/z 289.07 (catechin monomer C15H13O6−), were identified as procyanidin dimers such as procyanidin B2 (Figure S1b, Supplementary Materials). Isomer compounds with peaks 8 and 11 with an [M−H]− ion at around m/z 1153.2505 were identified as procyanidin tetramers producing an [M−H]− ion at m/z 865.19 [M-H]− (catechin trimer C45H37O18−), 577.13 [M-H]− (catechin dimer C30H25O12-) 289.07 [M-H]− (catechin monomer C15H13O6-) [33]. Peak 9 was identified as procyanidin trimer with [M-H]− ions at m/z 865.1940 producing a MS2 ions at m/z 577.13 [M-H]− (catechin dimer C30H25O12-) and 289.07 [M-H]− (catechin monomer C15H13O6−) [32]. Catechin isomers were detected by peaks 10 and 16 with [M-H]− ions at m/z 289.0715 and 289.0713 [4]. Peak 23 was identified as rutin with [M-H]− ions at m/z 609.1449 and daughter quercetin characteristic ion at m/z 301.0339 (C15H9O7−). Peak 24 with [M-H]− ion at m/z 317.0301 (Figure S1h) was identified as myricetin, while peak 25 was identified as the flavanone eriodyctiol (Figure S1i) with [M-H]− ion at m/z 288.0590, and daughter characteristic ions at m/z 151.0391 (C7H3O42−) and 133.0443 (C8H5O22−). Peaks 18, 22, and 28 were identified as quercetin (Figure S1f) and its isomers with [M-H]− ions at m/z 301.0353, 301.0348, and 301.0352, respectively. Peak 26 was identified as the isoflavone genistin with [M+H]− ions at m/z 433.1138 and daughter ion at m/z 271.0965. Peak 27 was identified as daidzein with [M+H]− ions at m/z 253.0503. Peak 29 with an [M−H]− ion at m/z 271.0608 and daughter ion at m/z 243.0659 was identified as the flavanone naringenin [30,32]. Finally, peak 30 with [M+H]− ion at m/z 285.0401, and daughter ions at m/z 151.0029 (C7H3O42−); 133.0287 (C8H5O22−) was identified as luteolin.
In this study on nuts of Andean pistachio cultivars, 30 compounds were identified, of which 15 compounds update the chemical composition in relation to previous reports [4,8]. Most of them were flavonoids or glycosides with O-glycosylation, which is rapidly bioaccessible in the stomach, maximizes their transformation and absorption process in the upper small intestine and promotes health effects [34,35]. Moreover, the chemistry composition showed slight differences between crops: the flavanone eriodyctiol and the isoflavone genistin were identified in Crop1, while the flavanone naringenin and the flavone luteolin were detected in Crop1 and Crop3 (Figure 4).
4. Discussion
4.1. Total Phenolic Content
The total phenolic content did not show significant differences between pistachio cultivars. These values were similar to those reported by Aliakbarkhaniet al. [32] in 20 genotypes of pistachios from Persia and higher than those in Italian pistachio kernels (81.7 ± 1.9 mg GAE/100 g) [36]. However, a greater concentration of TP was reported by Tsantili et al. [18] (1620 to 790 mg GAE/100 g) and Kornsteiner et al. [36] (492–1442 mg GAE/100 g) in pistachios from Greece.
4.2. Kernel Mineral Composition and Its Relation to Antioxidant Properties
Potassium was the most abundant mineral in pistachio kernels (946 ± 78 to 1064 ± 33 mg/100 g DW), followed by calcium (121 ± 2 to 131 ± 5 mg/100 g DW), highlighting physiological importance in kernel development. Rb and Sr, while not essential, behave as analogs of K and Ca, respectively. Rb accumulation may reflect potassium uptake and transport. Supporting this, previous studies in Iranian pistachio cultivars have found positive correlations between potassium levels and total phenolic content, suggesting a link between potassium availability and enhanced antioxidant potential.
The generalized Procrustes analysis (GPA) revealed a strong structural alignment between the mineral composition of pistachio kernels (Mn, Rb, Sr) and their antioxidant properties (total phenolics, DPPH, FRAP), particularly in Crop1 and Crop3. In contrast, the spatial congruence between soil mineral content (Pb, Zn, Cu) and kernel composition was lower, suggesting that the internal nutrient profile of the fruit is more directly associated with its functional traits.
Mn plays a well-established physiological role as a cofactor in mitochondrial Mn-superoxide dismutase (Mn-SOD), which catalyzes the dismutation of superoxide radicals [37]. Although Mn itself does not react with DPPH or FRAP reagents, it contributes to the plant’s oxidative stress defense system and may indirectly enhance the biosynthesis of antioxidant compounds, such as polyphenols and flavonoids [38].
These observations suggest that kernel mineral composition—particularly Mn and K-associated elements—may contribute to phenolic accumulation and antioxidant capacity in pistachio kernels.
4.3. Soil Physicochemical Parameters
The physicochemical characterization of soils from the three cultivation sites revealed slightly alkaline pH values and moderate electrical conductivity (EC) levels. These results are consistent with the tolerance profile of Pistacia vera, which thrives in arid regions with high salinity and limited rainfall. The pH values observed in our study align with those reported by Havlin et al. [39], and the species’ known tolerance to salinity—up to 6000 μS/cm of electrical conductivity [40]—further supports the robustness of pistachios to edaphic stressors and their ability to maintain nutrient uptake efficiency under varied soil conditions.
4.4. Antioxidant Capacity and Phenolic Composition
Although FRAP values showed a general decline from Crop1 to Crop3, differences were not statistically significant, suggesting similar electron-donating capacity among extracts. In contrast, DPPH results showed significant variation, with Crop1 exhibiting the highest radical scavenging activity. This aligns with its elevated levels of total phenolics and the presence of specific antioxidant compounds.
UHPLC-MS analysis identified more than 30 phenolic compounds, including gallic acid, quinic acid, rutin, catechin, myricetin, and procyanidins. Many of these, particularly gallic acid, catechin, rutin, and quercetin derivatives, are well-known for their hydrogen-donating properties, which directly influence DPPH assay outcomes. Their presence supports the higher antioxidant potential observed in Crop1 and, to a lesser extent, in Crop3.
Furthermore, the detection of unique flavonoids like eriodyctiol and genistin in Crop1, and luteolin and naringenin in Crops 1 and 3, reveals subtle compositional differences that likely contribute to variation in antioxidant response. These molecules, despite not altering total phenolics significantly, may exhibit high specific activity in scavenging assays, highlighting the relevance of phenolic quality beyond mere quantity.
Therefore, the antioxidant behavior of pistachio extracts reflects both the diversity and concentration of their phenolic profiles—especially those flavonoids and acids confirmed by UHPLC-MS—which explains the stronger DPPH activity observed in Crop1. The characterization of the chemical compounds present in Argentine pistachios reveals the presence of polyphenols, recognized for their beneficial properties for human health, including anti-inflammatory, antioxidant, and probiotic effects. A recent in vivo investigation supported that quinic acid could improve the dysbiosis of gut microbiota, alleviate the pathological symptoms of dextran sodium sulfate-induced colitis, and inhibit intestinal inflammation, suggesting that quinic acid could be used as a potential supplement in the treatment of patients with inflammatory bowel disease, which includes Crohn’s disease and ulcerative colitis. It has also been reported that quinic acid could improve high-fat-diet-induced neuroinflammation, ameliorate ulcerative colitis in rats, arrest cell proliferation, and can inhibit vascular inflammation and atherosclerosis [41,42,43,44,45]. The antioxidant or free radical scavenging properties of catechins have been supported by numerous in vitro and in vivo studies, including clinical studies. Catechins and their diastereomers possess phenolic hydroxyl groups in their structure, which can react with ROS and RNS by donating a hydrogen atom and/or an electron, resulting in the formation of a relatively stable flavonoid radical [46]. Helicobacter pylori bacterium is associated with various gastric diseases, including gastric cancer, with a high prevalence in developing countries due to poor health conditions. Current treatment involves proton pump inhibitors combined with antibiotics. Some of the flavonoids detected in Argentine pistachios, such as kaempferol, rutin, quercetin, myricetin, catechin, and epicatechin, have been shown to regulate the expression of key H. pylori genes, alter cell membrane permeability, and affect proton efflux. Some flavonoids have demonstrated the potential to inhibit H. pylori even in resistant strains. Gene expression and molecular docking studies reveal how these flavonoids interact with the membrane, bacterial genes, and proteins, affecting host cell transcription, translation, and bacterial adhesion. Quercetin, rutin, and myricetin have shown antimicrobial and anti-inflammatory properties, with all three enhancing antibiotic efficacy. The potential synergistic effects observed when flavonoids are co-administered with antibiotics provide an avenue to enhance therapeutic efficacy and combat antibiotic resistance [47].
5. Conclusions
This study offers a comprehensive characterization of three Andean pistachio crops by integrating morphological traits, mineral composition, phenolic content, antioxidant capacity, and multivariate statistical analysis. Among all analyzed minerals, potassium was the most abundant, while Mn, Rb, and Sr were especially relevant due to their variation across crops and their strong association with antioxidant traits.
The high Procrustes congruence, together with a canonical correlation value of r = 1, demonstrates that internal physiological and biochemical mechanisms shape the kernel’s functional traits more directly than soil geochemistry does. Notably, Crop1 exhibited the highest antioxidant activity in both DPPH and FRAP assays, suggesting that the antioxidant capacity appears to be governed not only by the quantity of total phenolics but also by qualitative differences in their chemical profiles. This interpretation is supported by the presence of specific phenolic compounds (e.g., eriodictyol, luteolin, and genistin) in Crop1 and Crop3, as identified through UHPLC-MS.
Furthermore, generalized Procrustes analysis (GPA) revealed a robust structural alignment between antioxidant parameters (TP, DPPH, FRAP) and internal mineral composition—particularly Mn, Rb, and Sr—while the correspondence with soil mineral composition was notably weaker. The strong Procrustes congruence, together with the canonical correlation value of r = 1, shows that internal physiological and biochemical mechanisms shape the kernel’s functional traits more directly than soil geochemistry does.
Additionally, linear discriminant analysis (LDA) applied to both soil and kernel elemental profiles demonstrated high classification accuracy (77.7% and 74.4%, respectively), validating the presence of distinct geochemical and nutritional fingerprints among the crops. Rb, Sr, Mn, and Pb emerged as discriminant elements in the separation of growing sites. These chemometric results reinforce the relevance of both internal and external mineral markers in distinguishing pistachio origin and quality.
Altogether, these findings underscore the interplay between geochemical and biochemical markers in characterizing the nutraceutical value of pistachios. Such integrative insights are valuable for crop selection, nutritional labeling, and the development of functional food products aimed at health-conscious consumers.
In practical terms, this work supports the strategic use of elemental and phenolic profiles to optimize pistachio cultivation practices and to reinforce geographic traceability in the food industry. Future research should explore long-term soil–plant dynamics and genotype-specific responses to environmental factors, potentially expanding this approach to other nut crops in diverse agroecological regions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11080925/s1, Figure S1: Full Orbitrap HR-MS spectra and structures of (a) peak 4, (b) peak 6, (c) peak 14, (e) peak 15, (f) peak 18, (g) peak 19, (h) peak 24, (i) peak 25.
Author Contributions
Conceptualization, D.Z.-G., M.J.S., A.T., and M.P.F.; methodology, D.Z.-G., M.J.S., J.G., A.T., and M.P.F.; formal analysis, D.Z.-G., M.J.S., A.T., and M.P.F.; investigation, D.Z.-G., M.J.S., J.G., A.T., and M.P.F.; resources, D.Z.-G., M.J.S., and M.P.F.; data curation, D.Z.-G., M.J.S., J.G., A.T., and M.P.F.; writing—original draft preparation, D.Z.-G., M.J.S., A.T., and M.P.F.; writing—review and editing, D.Z.-G., M.J.S., A.T., and M.P.F.; visualization M.P.F. and D.Z.-G.; supervision, D.Z.-G., M.P.F., and M.J.S.; project administration, D.Z.-G., M.J.S., and M.P.F.; funding acquisition, D.Z.-G., M.J.S., J.G., and M.P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PROJOVI ((J.G.) Resolución Nº 1500-23-R and PDTS-CICITCA-UNSJ Resolución Nº 1499-23-R); Argentina. M.J.S. received financial support from Fondecyt (Grant 1220075) and Fondequip (EQM170172), Chile.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
Authors would like to express our gratitude to Pisté SRL, Pistachos de los Andes, and Palos Blancos for providing pistachio samples. J.G. thanks CONICET-Argentina for his postdoctoral fellowship. D.Z.G. and M.P.F. are research members of CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Argentina. The authors also thank Ana Laura Paroldi Sanchez for language editing and valuable suggestions regarding the writing and presentation of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Interempresas Media, S.L. Origen Y Producción del Pistacho. Available online: https://www.frutas-hortalizas.com/Frutas/Origen-produccion-Pistacho.html (accessed on 21 July 2025).
- (INTA) Instituto Nacional de Tecnología Agropecuaria. El INTA Realizó La Primera Zonificación Agroclimática Del Pistachero. Available online: https://www.argentina.gob.ar/noticias/el-inta-realizo-la-primera-zonificacion-agroclimatica-del-pistachero (accessed on 20 July 2025).
- Argentina Forbes Quiénes Lideran el Boom del Pistacho en. Argentina. Available online: https://www.forbesargentina.com/money/quienes-lideran-boom-pistacho-argentina-n66120 (accessed on 20 July 2025).
- Fabani, M.P.; Luna, L.; Baroni, M.V.; Monferran, M.V.; Ighani, M.; Tapia, A.; Wunderlin, D.A.; Feresin, G.E. Pistachio (Pistacia vera Var Kerman) from Argentinean Cultivars. A Natural Product with Potential to Improve Human Health. J. Funct. Foods 2013, 5, 1347–1356. [Google Scholar] [CrossRef]
- Penci, M.C.; Martinez, M.L.; Fabani, M.P.; Feresin, G.E.; Tapia, A.; Ighani, M.; Ribotta, P.D.; Wunderlin, D.A. Matching Changes in Sensory Evaluation with Physical and Chemical Parameters. Food Bioproc. Tech. 2013, 6, 3305–3316. [Google Scholar] [CrossRef]
- Martínez, M.L.; Fabani, M.P.; Baroni, M.V.; Huaman, R.N.M.; Ighani, M.; Maestri, D.M.; Wunderlin, D.; Tapia, A.; Feresin, G.E. Argentinian Pistachio Oil and Flour: A Potential Novel Approach of Pistachio Nut Utilization. J. Food Sci. Technol. 2016, 53, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Zalazar-García, D.; Feresin, G.E.; Rodriguez, R. Optimal Operational Variables of Phenolic Compound Extractions from Pistachio Industry Waste (Pistacia Vera Var. Kerman) Using the Response Surface Method. Biomass Convers. Biorefin. 2022, 12, 3761–3770. [Google Scholar] [CrossRef]
- Piñeiro, M.; Parera, V.; Ortiz, J.E.; Llalla-Cordova, O.; Manrique, S.; Castro, B.; Ighani, M.; Luna, L.C.; Feresin, G.E. Agro-Industrial Waste from Pistacia Vera: Chemical Profile and Bioactive Properties. Plants 2025, 14, 1420. [Google Scholar] [CrossRef]
- Beede, R.; Brown, P.; Kallsen, C.; Weinbaum, S. Diagnosing and Correcting Nutrient Deficiencies. In Pistachio Production Manual; Division of Agriculture and Natural Resources, University of California: Oakland, CA, USA, 2005; pp. 147–157. [Google Scholar]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants 2022, 11, 18. [Google Scholar] [CrossRef]
- United States Department of Agriculture. USDA Nutrient Database for Standard Reference; United States Department of Agriculture: Washington, DC, USA, 2005.
- Moreno-Rojas, J.M.; Velasco-Ruiz, I.; Lovera, M.; Ordoñez-Díaz, J.L.; Ortiz-Somovilla, V.; De Santiago, E.; Arquero, O.; Pereira-Caro, G. Evaluation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia. Antioxidants 2022, 11, 609. [Google Scholar] [CrossRef]
- Karimi, S.; Tavallali, V. Interactive Effects of Soil Salinity and Boron on Growth, Mineral Composition and CO2 Assimilation of Pistachio Seedlings. Acta Physiol. Plant. 2017, 39, 242. [Google Scholar] [CrossRef]
- Yarahmadi, J.; Amini, A. Determining Land Suitability for Pistachio Cultivation Development Based on Climate Variables to Adapt to Drought. Theor. Appl. Climatol. 2021, 143, 1631–1642. [Google Scholar] [CrossRef]
- Arena, E.; Campisi, S.; Fallico, B.; Maccarone, E. Distribution of Fatty Acids and Phytosterols as a Criterion to Discriminate Geographic Origin of Pistachio Seeds. Food Chem. 2007, 104, 403–408. [Google Scholar] [CrossRef]
- Catalán, L.; Alvarez-Ortí, M.; Pardo-Giménez, A.; Gómez, R.; Rabadán, A.; Pardo, J.E. Pistachio Oil: A Review on Its Chemical Composition, Extraction Systems, and Uses. Eur. J. Lipid Sci. Technol. 2017, 119, 1600126. [Google Scholar] [CrossRef]
- Seferoglu, S.; Seferoglu, H.G.; Tekintas, F.E.; Balta, F. Biochemical Composition Influenced by Different Locations in Uzun Pistachio Cv. (Pistacia vera L.) Grown in Turkey. J. Food Compos. Anal. 2006, 19, 461–465. [Google Scholar] [CrossRef]
- Tsantili, E.; Takidelli, C.; Christopoulos, M.; Lambrinea, E.; Rouskas, D.; Roussos, P. Physical, Compositional and Sensory Differences in Nuts among Pistachio (Pistachia vera L.) Varieties. Sci. Hortic. 2010, 125, 562–568. [Google Scholar] [CrossRef]
- Rabadán, A.; Pardo, J.E.; Gómez, R.; Alvarruiz, A.; Álvarez-Ortí, M. Usefulness of Physical Parameters for Pistachio Cultivar Differentiation. Sci. Hortic. 2017, 222, 7–11. [Google Scholar] [CrossRef]
- Anderson, K.A.; Smith, B.W. Chemical Profiling To Differentiate Geographic Growing Origins of Coffee. J. Agric. Food Chem. 2002, 50, 2068–2075. [Google Scholar] [CrossRef]
- Fernández Linares, L.; Rojas Avelizapa, N.; Roldan Carrillo, T.; Islas, M.; Zerraga Martinez, H.; Hernandez Uribe, R.; Reyes Avila, R.; Flores Hernandez, D.; Arce Ortega, J. Manual de Técnicas de Análisis de Suelos Aplicadas a la Remediación de Sitios Contaminados; Instituto Nacional de Ecología, Ed.; Instituto Mexicano De Petróleo: Mexico City, Mexico, 2006. [Google Scholar]
- ICH International Council for Harmonisation. Q2 (R1): Validation of Analytical Procedures: Text and Methodology—Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Re; ICH International Council for Harmonisation: Geneva, Switzerland, 2005; Volume 2. [Google Scholar]
- Horwitz, W.; Albert, R. The Horwitz Ratio (HorRat): A Useful Index of Method Performance with Respect to Precision. J. AOAC Int. 2006, 89, 1095–1109. [Google Scholar] [CrossRef]
- Heldrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepúlveda, B. Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC–Q/Orbitrap/MS/MS and Its Antioxidant Properties. Molecules 2016, 21, 245. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast Detection of Phenolic Compounds in Extracts of Easter Pears (Pyrus communis) from the Atacama Desert by Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC–Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Kruse, M.S.; Gamarra-Luques, C.; Lima, B.; Zaragosa, J.; Piñeiro, M.; Tapia, A.; Coirini, H.; Rey, M. Oxalis Erythrorhiza Gillies Ex Hooker et Arnott (Oxalidaceae): Chemical Analysis, Biological In Vitro and In Vivo Properties and Behavioral Effects. Antioxidants 2024, 13, 1494. [Google Scholar] [CrossRef]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant Activity and Phenolic Profile of Pistachio (Pistacia vera L., Variety Bronte) Seeds and Skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- CXS 1993-1995; Codex Alimentarius Commission General Standard for Contaminants and Toxins in Food and Feed. FAO/WHO: Rome, Italy, 1995.
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of Phenolic Composition and Antioxidant Activity in Fruits, Rhizomes and Leaves of the White Strawberry (Fragaria chiloensis Spp. Chiloensis Form Chiloensis) Using HPLC-DAD–ESI-MS and Free Radical Quenching Techniques. J. Food Compos. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Paini, M.; Casazza, A.A.; Perego, P. Effect of Encapsulating Agent on Physical-Chemical Characteristics of Olive Pomace Polyphenols-Rich Extracts. Chem. Eng. Trans. 2015, 43, 97–102. [Google Scholar] [CrossRef]
- Bulló, M.; Juanola-Falgarona, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Nutrition Attributes and Health Effects of Pistachio Nuts. Br. J. Nutr. 2015, 113, S79–S93. [Google Scholar] [CrossRef]
- Xiao, J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and Total Phenolics in 10 Different Nut Types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Messant, M.; Hennebelle, T.; Guérard, F.; Gakière, B.; Gall, A.; Thomine, S.; Krieger-Liszkay, A. Manganese Excess and Deficiency Affects Photosynthesis and Metabolism in Marchantia Polymorpha. Plant Biol. 2022. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A Comprehensive Review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Sanden, B.L.; Ferguson, L.; Reyes, H.C.; Grattan, S.R. Effect of Salinity on Evapotranspiration and Yield of San Joaquin Valley Pistachios. Acta Hortic. 2004, 664, 583–589. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Luo, L.; Zhang, Y.; Huang, K.; Guan, X. Millet Quinic Acid Relieves Colitis by Regulating Gut Microbiota and Inhibiting MyD88/NF-ΚB Signaling Pathway. Foods 2025, 14, 2267. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, Y.; Guan, T.; Zhang, Y.; Huang, K.; Zhang, Z.; Cao, W.; Guan, X. Quinic Acid Alleviates High-Fat Diet-Induced Neuroinflammation by Inhibiting DR3/IKK/NF-ΚB Signaling via Gut Microbial Tryptophan Metabolites. Gut Microbes 2024, 16, 2374608. [Google Scholar] [CrossRef]
- Jang, S.A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.H.; Koo, H.J.; Kang, S.C. Quinic Acid Inhibits Vascular Inflammation in TNF-α-Stimulated Vascular Smooth Muscle Cells. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Lorigooini, Z.; Amini-Khoei, H.; Sabzevary-Ghahfarokhi, M.; Rafieian-Kopaei, M. Quinic Acid Ameliorates Ulcerative Colitis in Rats, through the Inhibition of Two TLR4-NF-ΚB and NF-ΚB-INOS-NO Signaling Pathways. Immun. Inflamm. Dis. 2023, 11, e926. [Google Scholar] [CrossRef]
- Mortelé, O.; Jörissen, J.; Spacova, I.; Lebeer, S.; van Nuijs, A.L.N.; Hermans, N. Demonstrating the Involvement of an Active Efflux Mechanism in the Intestinal Absorption of Chlorogenic Acid and Quinic Acid Using a Caco-2 Bidirectional Permeability Assay. Food Funct. 2021, 12, 417–425. [Google Scholar] [CrossRef]
- Stoeva, S.; Hvarchanova, N.; Georgiev, K.D.; Radeva-Ilieva, M. Green Tea: Antioxidant vs. Pro-Oxidant Activity. Beverages 2025, 11, 64. [Google Scholar] [CrossRef]
- Tan, A.; Scortecci, K.C.; Boylan, F. A Review on Flavonoids as Anti-Helicobacter Pylori Agents. Appl. Sci. 2025, 15, 3936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).