Characterization and Selection of Lycium barbarum Cultivars Based on Physicochemical, Bioactive, and Aromatic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Location, and Growing Conditions
2.2. Methods
2.2.1. Physicochemical Analysis
2.2.2. Content of Sugar and Organic Acids
2.2.3. Total Phenolic Content
2.2.4. Total Antioxidant Activity
2.2.5. Individual Phenolic Compounds
2.2.6. Total and Individual Carotenoid Compounds
2.2.7. Ascorbic Acid Content
2.2.8. Analysis of Volatile Aromatic Compounds (VOCs)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Quality Characteristics
3.2. Composition of Sugar and Organic Acids
3.3. Bioactive Quality Characteristics
3.3.1. Phenolic Compounds
3.3.2. Carotenoid Compounds
3.3.3. Antioxidant Activity
3.3.4. Correlation of Antioxidant Activity and Bioactive Compounds
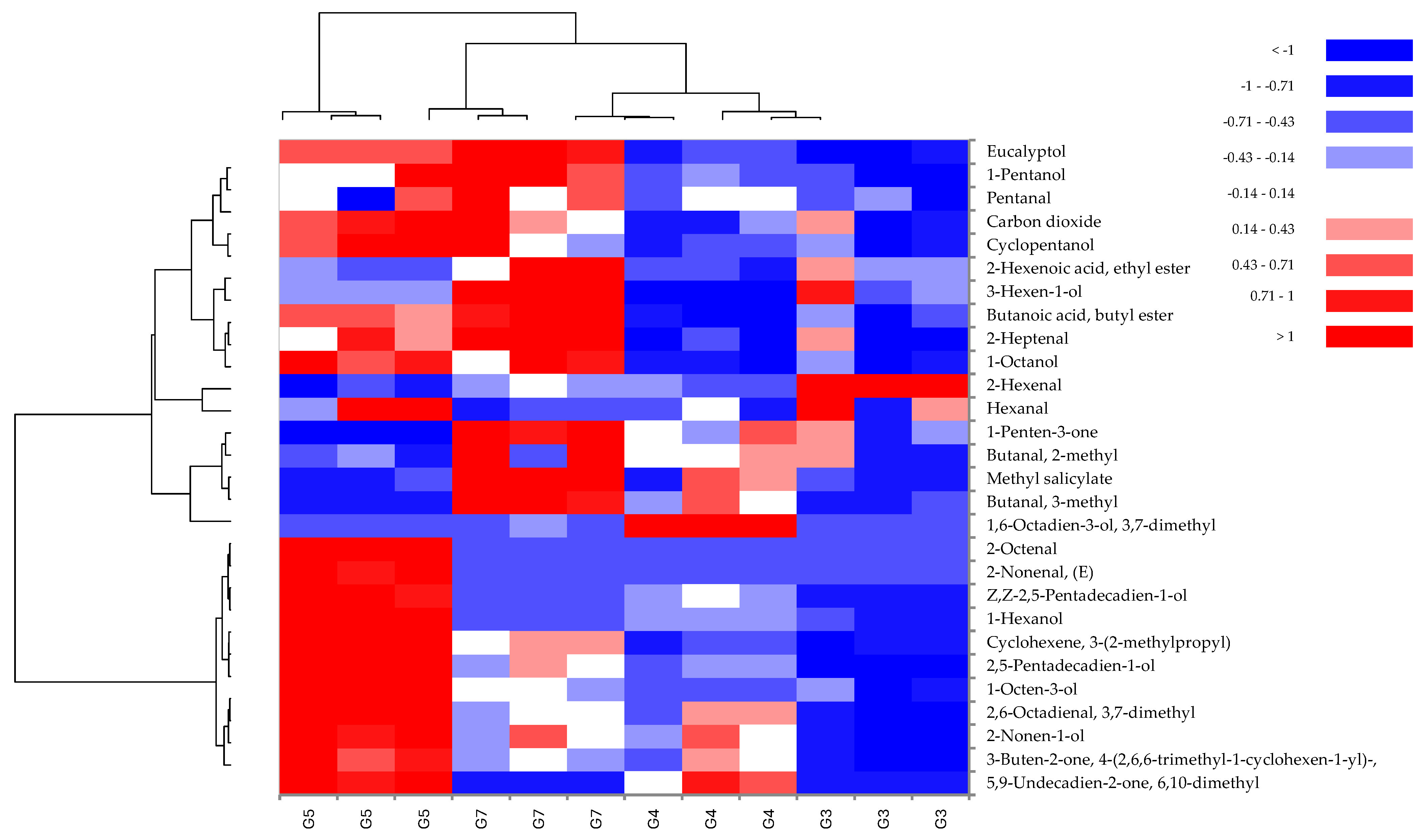
3.4. Volatile Organic Compounds (VOCs)
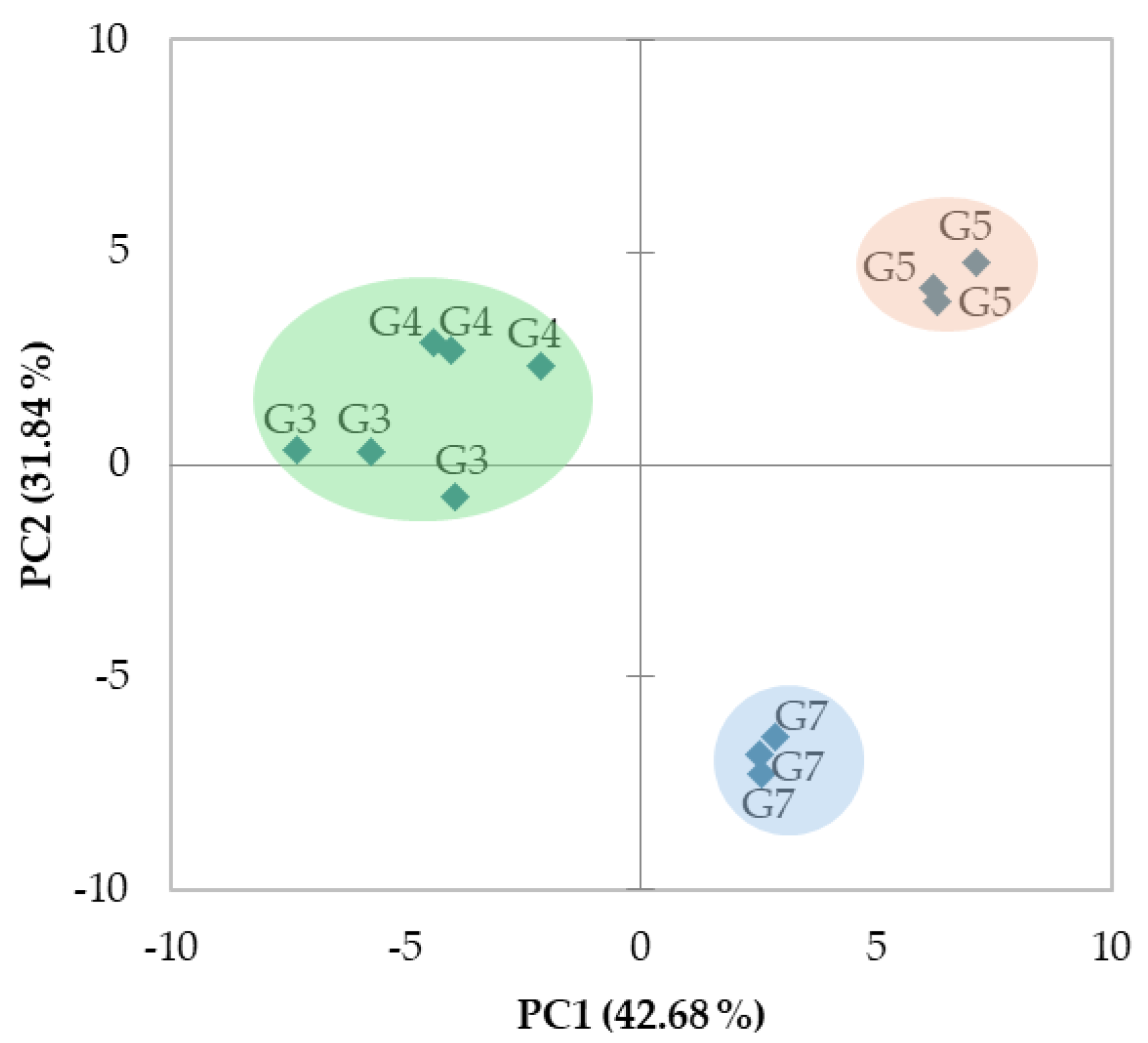
3.5. PCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-Car | α-Carotene |
| β-Car | β-carotene |
| β-Crp | β-Cryptoxanthin |
| Cap | Capsanthin |
| AClo | Chlorogenic Acid |
| AnClo | Neoclorogenic Acid |
| ApC | p-coumaroylquinic |
| ApCou | p-coumaric acid |
| GB | Goji Berry |
| LRI | Linear Retention Index |
| PCA | Principal Component Analysis |
| TCC | Total Carotenoid Content |
| TPC | Total Phenolic Compounds |
| Rut | Ruthin |
| t-Fer | t-ferulic acid |
| TSS | Total Soluble Solid |
| TA | Titratable acidity |
| VOC | Volatile Organic Compound |
| Zea | Zeaxanthin |
References
- Antonelli, M.; Donelli, D. Health-Promoting Effects of Goji Berries (Lycium barbarum): A Literature Overview. Biol. Life Sci. Forum 2024, 40, 1. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.; Pešić, M.B. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Zheng, Y.; Fu, L. Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.). Appl. Sci. 2025, 15, 262. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). What Are Healthy Diets? Joint Statement by the Food and Agriculture Organization of the United Nations and the World Health Organization; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024. [Google Scholar]
- Ceccarini, M.R.; Codini, M.; Cataldi, S.; Fioretti, B.; Brecchia, G.; Albi, E.; Beccari, T. Licium Barbarum Cultivated in Italy: Chemical Characterization and Nutritional Evaluation. Ital. J. Food Sci. 2022, 34, 59–65. [Google Scholar] [CrossRef]
- Rubio, R.M.; García, M.R.; Barroso, N.N.; Iñiguez, F.M.S.; Gómez, M.J.R.; Magro, P.C. Classification of Goji Berry (Lycium barbarum L.) Varieties According to Physicochemical and Bioactive Signature. Eur. Food Res. Technol. 2025, 251, 355–365. [Google Scholar] [CrossRef]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Yiu, G.; Hackman, R.M. Goji Berry Intake Increases Macular Pigment Optical Density in Healthy Adults: A Randomized Pilot Trial. Nutrients 2021, 13, 4409. [Google Scholar] [CrossRef]
- Sun, Q.; Du, M.; Kang, Y.; Zhu, M.J. Prebiotic Effects of Goji Berry in Protection against Inflammatory Bowel Disease. Crit. Rev. Food Sci. Nutr. 2023, 63, 5206–5230. [Google Scholar] [CrossRef]
- Yu, J.; Yan, Y.; Zhang, L.; Mi, J.; Yu, L.; Zhang, F.; Lu, L.; Luo, Q.; Li, X.; Zhou, X.; et al. A Comprehensive Review of Goji Berry Processing and Utilization. Food Sci. Nutr. 2023, 11, 7445–7457. [Google Scholar] [CrossRef]
- Fernández-Ríos, A.; Laso, J.; Aldaco, R.; Margallo, M. Superfoods: A Super Impact on Health and the Environment? Curr. Opin. Environ. Sci. Health 2023, 31, 100410. [Google Scholar] [CrossRef]
- Aguilera, J.M. Berries as Foods: Processing, Products, and Health Implications. Annu. Rev. Food Sci. Technol. 2024, 15, 1–26. [Google Scholar] [CrossRef]
- Peng, Q.; Huang, J.; Li, S.; Massou, B.B.; Chen, Z.; Zhu, Q.; Xie, G. Comprehensive Origin Authentication of Wolfberry Pulp (Lycium barbarum L.) Using Multimodal Sensory Analysis and Chemometrics. Ind. Crop. Prod. 2024, 219, 119023. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Detection and Analysis of Volatile Flavor Compounds in Different Varieties and Origins of Goji Berries Using HS-GC-IMS. LWT 2023, 187, 115322. [Google Scholar] [CrossRef]
- Oğuz, İ.; Oğuz, H.İ.; Ürün, İ.; Attar, Ş.H.; Atasever, S.; Kafkas, N.E. Determination of Aroma and Protein Contents in Organic Lycium barbarum L. and Lycium chinense Miller Fruits in Different Ripening Periods. Erwerbs-Obstbau 2023, 65, 1171–1183. [Google Scholar] [CrossRef]
- de Freitas Santos Júnior, A.; Mota, M.D.; de Aragão Tannus, C.; de Souza Dias, F.; de Andrade Santana, D.; Moura, H.F.S.; Magalhães, H.I.F. Phytochemical, Biological and Technological Aspects of Phenolic Bioactives in Goji Berries. In Phytochemicals in Goji Berries; CRC Press: Boca Raton, FL, USA, 2020; pp. 15–37. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Moisture in Malt Gravimetric Method. (935.29). In Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Lozano, M.; Vidal-Aragón, M.C.; Hernández, M.T.; Ayuso, M.C.; Bernalte, M.J.; García, J.; Velardo, B. Physicochemical and Nutritional Properties and Volatile Constituents of Six Japanese Plum (Prunus salicina Lindl.) Cultivars. Eur. Food Res. Technol. 2009, 228, 403–410. [Google Scholar] [CrossRef]
- Fatchurrahman, D.; Amodio, M.L.; Colelli, G. Quality of Goji Berry Fruit (Lycium barbarum L.) Stored at Different Temperatures. Foods 2022, 11, 3700. [Google Scholar] [CrossRef]
- Capotorto, I.; Amodio, M.L.; Diaz, M.T.B.; de Chiara, M.L.V.; Colelli, G. Effect of Anti-Browning Solutions on Quality of Fresh-Cut Fennel during Storage. Postharvest Biol. Technol. 2018, 137, 21–30. [Google Scholar] [CrossRef]
- Savran, A.; Zengin, G.; Aktumsek, A.; Mocan, A.; Glamoćlija, J.; Ćirić, A.; Soković, M. Phenolic Compounds and Biological Effects of Edible Rumex Scutatus and Pseudosempervivum Sempervivum: Potential Sources of Natural Agents with Health Benefits. Food Funct. 2016, 7, 3252–3262. [Google Scholar] [CrossRef]
- Manzano Durán, R.; Sánchez, J.E.F.; Velardo-Micharet, B.; Gómez, M.J.R. Multivariate Optimization of Ultrasound-Assisted Extraction for the Determination of Phenolic Compounds in Plums (Prunus salicina Lindl.) by High-Performance Liquid Chromatography (HPLC). Instrum. Sci. Technol. 2020, 48, 113–127. [Google Scholar] [CrossRef]
- Zacarías-Garcia, J.; Carlos, G.; Gil, J.V.; Navarro, J.L.; Zacarías, L.; Rodrigo, M.J. Juices and By-Products of Red-Fleshed Sweet Oranges: Assessment of Bioactive and Nutritional Compounds. Foods 2023, 12, 400. [Google Scholar] [CrossRef]
- Bohoyo-Gil, D.; Dominguez-Valhondo, D.; García-Parra, J.J.; González-Gómez, D. UHPLC as a Suitable Methodology for the Analysis of Carotenoids in Food Matrix. Eur. Food Res. Technol. 2012, 235, 1055–1061. [Google Scholar] [CrossRef]
- Campos, F.M.; Ribeiro, S.M.R.; Della Lucia, C.M.; Pinheiro-Sant’Ana, H.M.; Stringheta, P.C. Optimization of Methodology to Analyze Ascorbic and Dehydroascorbic Acid in Vegetables. Quim. Nova 2009, 32, 87–91. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Quan, J.; An, W.; Zhao, J.; Xi, W. Identification of Characteristic Aroma Volatiles of Ningxia Goji Berries (Lycium barbarum L.) and Their Developmental Changes. Int. J. Food Prop. 2017, 20, S214–S227. [Google Scholar] [CrossRef]
- Ma, R.H.; Zhang, X.X.; Ni, Z.J.; Thakur, K.; Wang, W.; Yan, Y.M.; Cao, Y.L.; Zhang, J.G.; Rengasamy, K.R.R.; Wei, Z.J. Lycium barbarum (Goji) as Functional Food: A Review of Its Nutrition, Phytochemical Structure, Biological Features, and Food Industry Prospects. Crit. Rev. Food Sci. Nutr. 2023, 63, 10621–10635. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, X.; Ali, M.M.; Rizwan, H.M.; Li, B.; Li, H.; Jia, K.; Yang, X.; Ma, S.; Li, S.; et al. Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora edulis f. flavicarpa) and Purple (Passiflora edulis f. edulis) Passion Fruits. Int. J. Mol. Sci. 2021, 22, 5765. [Google Scholar] [CrossRef]
- Polat, M.; Mertoglu, K.; Eskimez, I.; Okatan, V. Effects of the Fruiting Period and Growing Seasons on Market Quality in Goji Berry (Lycium barbarum L.). Folia Hortic. 2020, 32, 229–239. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Xi, W.; An, W.; Niu, L.; Cao, Y.; Wang, H.; Wang, Y.; Yin, Y. Changes in Sugars and Organic Acids in Wolfberry (Lycium barbarum L.) Fruit during Development and Maturation. Food Chem. 2015, 173, 718–724. [Google Scholar] [CrossRef]
- Mutyam, S.; Chilakala, S.; Tallapally, M.; Upadhyayula, V.V.R. Gas Chromatography–Mass Spectrometric Determination of Organic Acids in Fruit Juices by Multiwalled Carbon Nanotube–Based Ion-Pair Dispersive Solid-Phase Extraction and in Situ Butylation. Rapid Commun. Mass Spectrom. 2021, 35, e9165. [Google Scholar] [CrossRef]
- Kafkas, N.E.; Oğuz, H.İ.; Oğuz, İ. Evaluation of Fruit Characteristics of Various Organically-Grown Goji Berry (Lycium barbarum L., Lycium Chinense Miller) Species during Ripening Stages. J. Food Compos. Anal. 2021, 101, 103846. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Compounds Profile, Nutritional Compounds and Bioactive Properties of Lycium barbarum L.: A Comparative Study with Stems and Fruits. Ind. Crop. Prod. 2018, 122, 574–581. [Google Scholar] [CrossRef]
- Jeepipalli, S.P.K.; Xu, B. Phenolic Profiles and Antioxidant Properties of Goji Berries (Lycium barbarum). In Phytochemicals in Goji Berries; CRC Press: Boca Raton, FL, USA, 2020; pp. 225–232. [Google Scholar] [CrossRef]
- Poggioni, L.; Romi, M.; Guarnieri, M.; Cai, G.; Cantini, C. Nutraceutical Profile of Goji (Lycium barbarum L.) Berries in Relation to Environmental Conditions and Harvesting Period. Food Biosci. 2022, 49, 101954. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Breniere, T.; Fanciullino, A.L.; Dumont, D.; Le Bourvellec, C.; Riva, C.; Borel, P.; Landrier, J.F.; Bertin, N. Effect of Long-Term Deficit Irrigation on Tomato and Goji Berry Quality: From Fruit Composition to in Vitro Bioaccessibility of Carotenoids. Front. Plant Sci. 2024, 15, 1339536. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, M.; Zhang, D.; Xie, Z. Characterization of Goji Quality at Different Harvest Stages in Qaidam Basin Based on Transcriptome and Widely Targeted Metabolome. J. Food Biochem. 2024, 2024, 1139944. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional Constituents and Antioxidant Activities of Eight Chinese Native Goji Genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Su, Z.; Jia, H.; Sun, M.; Cai, Z.; Shen, Z.; Zhao, B.; Li, J.; Ma, R.; Yu, M.; Yan, J. Integrative Analysis of the Metabolome and Transcriptome Reveals the Molecular Mechanism of Chlorogenic Acid Synthesis in Peach Fruit. Front. Nutr. 2022, 9, 961626. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, Natural Sources, Dietary Intake and Pharmacokinetic Properties of Ferulic Acid: A Review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical Characterization, Antioxidant and Antimicrobial Properties of Goji Berries Cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative Studies on Phenolic Profiles, Antioxidant Capacities and Carotenoid Contents of Red Goji Berry (Lycium barbarum) and Black Goji Berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Tekin-Cakmak, Z.H.; Kayacan Çakmakoglu, S.; Karasu, S.; Kasapoglu, M.Z.; Avci, E. Effect of Different Drying Techniques on Total Bioactive Compounds and Individual Phenolic Composition in Goji Berries. Processes 2023, 11, 754. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Manríquez, D.; Luengwilai, K.; González-Agüero, M. Chapter 1 Aroma Volatiles: Biosynthesis and Mechanisms of Modulation During Fruit Ripening. Adv. Bot. Res. 2009, 50, 1–37. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Forney, C.F.; Song, J. Flavors and Aromas: Chemistry and Biological Functions. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; Volume 1, pp. 515–539. [Google Scholar] [CrossRef]
- Chen, F.; Su, Y.; Zhang, F.; Guo, Y. Low-Temperature Headspace-Trap Gas Chromatography with Mass Spectrometry for the Determination of Trace Volatile Compounds from the Fruit of Lycium barbarum L. J. Sep. Sci. 2015, 38, 670–676. [Google Scholar] [CrossRef]
- Endes, Z.; Uslu, N.; Musa Özcan, M.; Fatif, E. Physico-Chemical Properties, Fatty Acid Composition and Mineral Contents of Goji Berry (Lycium barbarum L.) Fruit. J. Agroaliment. Process. Technol. 2015, 21, 36–40. [Google Scholar]
- Zhu, H.; Wei, M.; Zhang, Y.; Tao, X. Analysis of Volatile Organic Compounds of Different Types of Peppers (Capsicum annuum L.) Using Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry. eFood 2024, 5, e70027. [Google Scholar] [CrossRef]
- Do, S.; Kim, Y.; Yim, J.; Lee, K.G. Analysis of Volatile Compounds, Betaine, and Antioxidant Effect in Goji Berry (Lycium barbarum L.) Powder Extracted by Various Drying Methods and Extraction Solvents. Curr. Res. Food Sci. 2024, 9, 100798. [Google Scholar] [CrossRef]
| G3 | G4 | G5 | G7 | p | |
|---|---|---|---|---|---|
| Moisture (g 100 g−1 fw) | 83.44 ± 0.18 a | 84.74 ±0.91 a | 83.05 ±0.79 a | 78.96 ± 0.89 b | *** |
| pH | 4.91 ± 0.15 b | 5.06 ± 0.12 b | 5.29 ± 0.25 a | 5.23 ± 0.15 a | *** |
| TSS (°Brix) | 20.27 ± 0.01 a | 20.17 ± 0.01 a | 18.77 ± 0.05 b | 17.73 ± 0.01 c | *** |
| TA (%) | 0.42 ± 0.01 a | 0.39 ± 0.00 a | 0.34 ± 0.00 b | 0.40 ± 0.00 a | *** |
| RI | 48.80 ± 1.05 c | 51.16 ± 0.20 b | 54.84 ± 0.65 a | 44.46 ± 0.26 d | *** |
| G3 | G4 | G5 | G7 | p | |
|---|---|---|---|---|---|
| Sugars (g 100 g−1 dw) | |||||
| Glucose | 44.10 ± 0.82 a | 44.04 ± 1.28 a | 26.89 ± 0.92 b | 26.36 ± 0.41 b | * |
| Fructose | 44.24 ± 0.89 ab | 48.77 ± 1.45 a | 44.06 ± 2.31 ab | 28.99 ± 0.46 b | * |
| Total | 88.34 ± 1.67 a | 92.81 ± 2.73 a | 70.95 ± 3.98 b | 55.36 ± 0.87 c | *** |
| Organic acids (g 100 g−1 dw) | |||||
| Oxalic | 0.02 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.02 ± 0.00 a | ** |
| Citric | 1.60 ± 0.12 b | 3.57 ± 0.17 a | 4.45 ± 0.57 a | 0.68 ± 0.02 c | *** |
| Tartaric | 1.53 ± 0.10 a | 1.52 ± 0.06 a | 1.31 ± 0.04 b | 1.00 ± 0.04 c | *** |
| Malic | 1.73 ± 0.17 a | 0.91 ± 0.06 b | 0.47 ± 0.18 c | 1.09 ± 0.06 b | *** |
| Ascorbic | 0.46 ± 0.03 a | 0.51 ± 0.03 a | 0.38 ± 0.03 b | 0.26 ± 0.02 c | *** |
| Succinic | 0.96 ± 0.09 | 0.69 ± 0.06 | 0.68 ± 0.04 | 0.65 ± 0.06 | NS |
| Total | 6.29 ± 0.36 ab | 7.19 ± 0.25 a | 7.31 ± 0.81 ab | 3.71 ± 0.18 b | * |
| G3 | G4 | G5 | G7 | p | |
|---|---|---|---|---|---|
| Total phenolic content (TPC) (mg GAE g−1 dw) | 17.86 ± 1.04 a | 19.11 ± 2.18 a | 14.83 ± 0.97 c | 16.79 ± 1.20 b | * |
| Individual phenolic compounds (µg phenol g−1 dw) | |||||
| Chlorogenic acid (AClo) | 199.9 ±19.85 a | 163.86 ± 17.46 a | 213.3 ± 27.4 a | 89.85 ± 6.77 b | * |
| Neoclorogenic acid (AnClo) | 621.5 ± 84.2 c | 890.3 ± 42.6 b | 1214.4 ± 47.5 a | 287.5 ± 14.0 d | *** |
| p-coumaroylquinic (ApC) | 89.74 ± 4.26 b | 90.26 ± 9.79 b | 209.9 ± 9.8 a | 41.28 ± 3.12 c | ** |
| p-coumaric acid (ApCou) | n.d. b | n.d. b | 7.03 ± 1.10 a | n.d. b | ** |
| t-ferulic acid (t-Fer) | 30.50 ± 3.29 b | 40.58 ± 1.89 a | 42.92 ± 1.60 a | 45.29 ± 5.06 a | *** |
| Ruthin (Rut) | n.d. b | n.d. b | 12.67 ± 3.74 a | n.d. b | ** |
| Total carotenoid content (TCC) (mg βCE g−1 dw) | 1.97 ± 0.01 ab | 2.45 ± 0.23 a | 1.38 ± 0.32 b | 1.13 ± 0.09 b | ** |
| Individual carotenoid compounds (µg g−1 dw) | |||||
| Capsanthin (Cap) | 147.8 ± 58.3 a | 49.02 ± 23.07 b | 32.06 ± 4.46 b | 95.76 ± 11.48 ab | * |
| Zeaxanthin (Zea) | 1398 ± 514.1 | 1722.6 ± 768.7 | 1212.0 ± 268.9 | 1026.7 ± 98.3 | NS |
| β-Cryptoxanthin (β-Crp) | 36.83 ± 11.87 | 31.28 ± 13.24 | 15.01 ± 2.67 | 26.69 ± 2.57 | NS |
| α-carotene (α-Car) | 163.8 ± 49.6 a | 149.2 ± 74.2 ab | 44.48 ± 9.50 b | 74.67 ± 11.01 ab | ** |
| β-carotene (β-Car) | 1.47 ± 0.65 ab | 2.40 ± 0.57 a | 1.17 ± 0.18 b | 1.47 ± 0.17 ab | * |
| Antioxidant activity (mg TE g−1 dw) | |||||
| DPPH | 12.42 ± 1.11 a | 10.04 ± 0.71 b | 12.15 ± 0.55 a | 12.34 ± 0.71 a | * |
| CUPRAC | 102.9 ± 3.0 | 126.0 ± 19.2 | 106.9 ± 10.08 | 104.7 ± 5.2 | NS |
| DPPH | CUPRAC | TPC | Aclo | Anclo | ApC | ApCou | t-Fer | Rut | TCC | Cap | Zea | β-Crp | α-Car | β-Car | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | 1 * | ||||||||||||||
| CUPRAC | −0.437 | 1 * | |||||||||||||
| TPC | −0.317 | 0.553 | 1 * | ||||||||||||
| Aclo | 0.035 | 0.014 | −0.010 | 1 * | |||||||||||
| Anclo | −0.206 | 0.245 | −0.246 | 0.760 * | 1 * | ||||||||||
| ApC | 0.091 | −0.050 | −0.501 | 0.759 * | 0.912 * | 1 * | |||||||||
| ApCou | 0.200 | −0.160 | −0.690 * | 0.513 | 0.766 * | 0.935 * | 1 * | ||||||||
| t-Fer | −0.102 | 0.250 | −0.168 | −0.409 | 0.011 | 0.071 | 0.272 | 1 * | |||||||
| Rut | 0.167 | −0.068 | −0.660 * | 0.512 | 0.738 * | 0.898 * | 0.919 * | 0.304 | 1 * | ||||||
| TCC | −0.521 | 0.551 | 0.736 * | 0.401 | 0.267 | −0.081 | −0.387 | −0.422 | −0.374 | 1 * | |||||
| Cap | 0.599 * | −0.181 | 0.227 | −0.134 | −0.562 | −0.501 | −0.544 | −0.593 * | −0.516 | 0.048 | 1 * | ||||
| Zea | −0.053 | 0.743 * | 0.542 | 0.236 | 0.256 | 0.021 | −0.185 | −0.171 | −0.088 | 0.616 * | 0.271 | 1 * | |||
| β-Crp | 0.200 | 0.330 | 0.654 * | −0.071 | −0.354 | −0.506 | −0.664 * | −0.473 | −0.612 * | 0.507 | 0.786 * | 0.735 * | 1 * | ||
| α-Car | −0.019 | 0.473 | 0.727 * | 0.129 | −0.139 | −0.367 | −0.591 * | −0.549 | −0.554 | 0.710 * | 0.634 * | 0.810 * | 0.946 * | 1 * | |
| β-car | −0.482 | 0.763 * | 0.679 * | −0.142 | 0.038 | −0.336 | −0.460 | −0.012 | −0.406 | 0.677 * | −0.039 | 0.560 | 0.412 | 0.523 | 1 * |
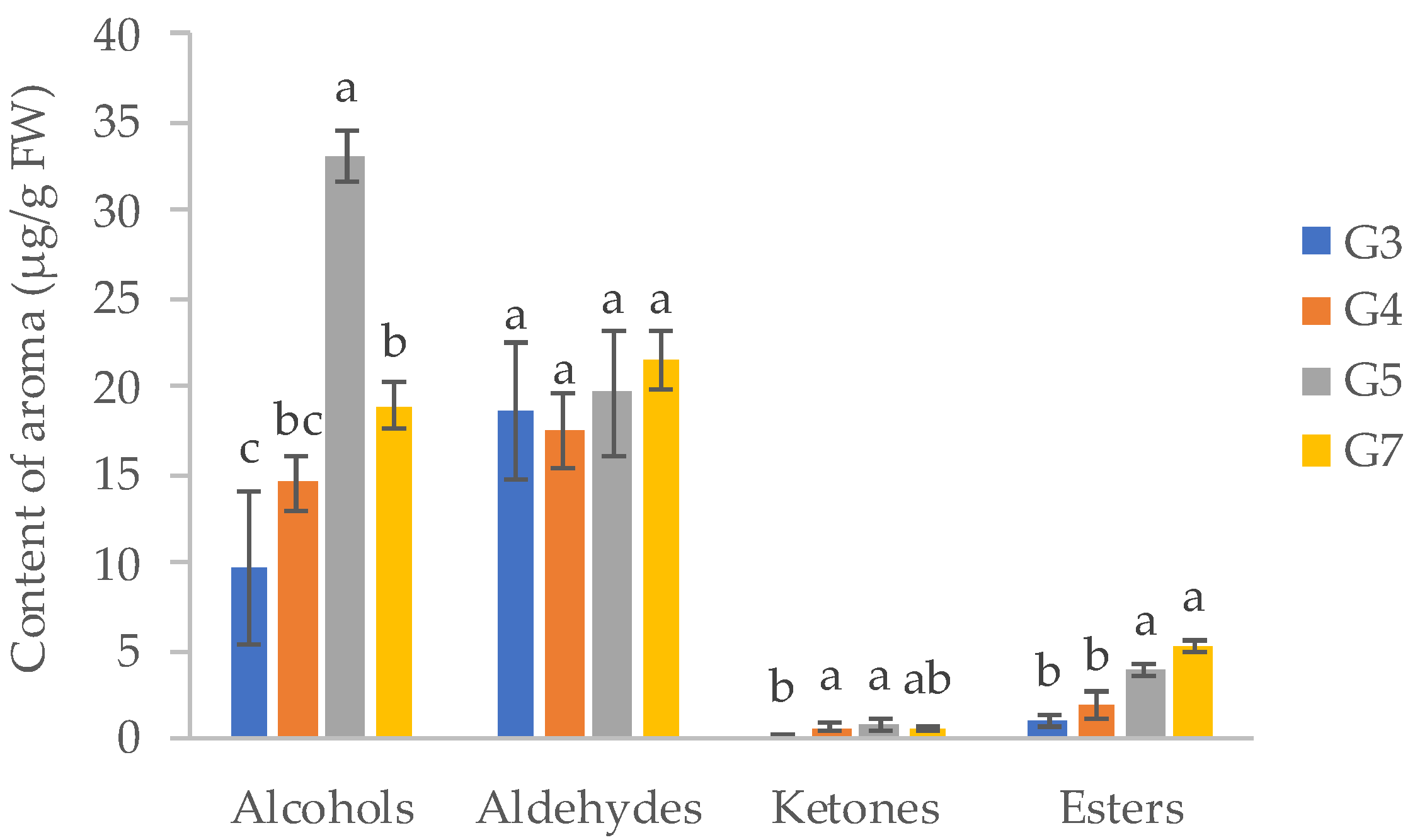
| LRI | Volatile Organic Compounds | Id. Method | G3 | G4 | G5 | G7 | Aroma Quality |
|---|---|---|---|---|---|---|---|
| Alcohols | |||||||
| 660.0 | Cyclopentanol | NIST | 0.14 ± 0.09 b | 0.17 ± 0.03 b | 0.63 ± 0.12 a | 0.40 ± 0.18 ab | Fruit, green |
| 746.5 | 1-Pentanol | Sigma–Aldrich | 0.27 ± 0.19 b | 0.53 ± 0.08 ab | 0.89 ± 0.35 a | 1.14 ± 0.25 a | Fruit, sweet |
| 853.9 | 3-Hexen-1-ol | Sigma–Aldrich | 2.08 ± 0.92 ab | 0.77 ± 0.06 b | 1.65 ± 0.01 b | 3.86 ± 0.39 a | Green, floral, earthy |
| 870.2 | 1-Hexanol | Sigma–Aldrich | 1.17 ± 0.65 d | 3.00 ± 0.32 b | 10.49 ± 1.18 a | 2.28 ± 0.01 c | Green, floral, oily |
| 980.5 | 1-Octen-3-ol | NIST | 5.58 ± 2.34 c | 7.00 ± 0.38 bc | 16.71 ± 0.84 a | 9.34 ± 0.69 b | Mushroom like |
| 1058.6 | 2,5-Pentadecadien-1-ol | NIST | 0.07 ± 0.04 c | 0.37 ± 0.09 b | 1.11 ± 0.08 a | 0.54 ± 0.15 b | |
| 1071.4 | 1-Octanol | NIST | 0.30 ± 0.12 b | 0.27 ± 0.03 b | 0.78 ± 0.13 a | 0.75 ± 0.17 a | Orange, rose-like, green |
| 1098.1 | 1,6-Octadien-3-ol, 3,7-dimethyl | NIST | 0.07 ± 0.04 b | 2.02 ± 0.49 a | 0.07 ± 0.01 b | 0.21 ± 0.04 a | |
| 1103.0 | 2-Nonen-1-ol | NIST | 0.09 ± 0.06 c | 0.33 ± 0.08 b | 0.54 ± 0.07 a | 0.34 ± 0.06 b | Sweet, fatty, melon-like |
| 1300.8 | Z,Z-2,5-Pentadecadien-1-ol | NIST | n.d. | 0.05 ± 0.01 b | 0.21 ± 0.06 a | 0.02 ± 0.00 c | |
| Aldehydes | |||||||
| 637.2 | Butanal, 3-methyl | Sigma–Aldrich | 0.63 ± 0.16 c | 2.64 ± 1.09 b | 0.59 ± 0.20 c | 5.48 ± 1.65 a | Apple and peach |
| 644.1 | Butanal, 2-methyl | Sigma–Aldrich | 0.93 ± 0.45 a | 1.36 ± 0.17 a | 0.83 ± 0.19 a | 2.17 ± 1.05 a | Musty, fermented baking |
| 672.9 | Pentanal | Sigma–Aldrich | 0.38 ± 0.09 b | 0.49 ± 0.05 ab | 0.49 ± 0.19 ab | 0.74 ± 0.26 a | Almond, malt, pungent |
| 786.1 | Hexanal | Sigma–Aldrich | 14.04 ± 3.20 a | 11.88 ± 1.18 a | 15.93 ± 3.29 a | 11.43 ± 0.71 a | Green, grassy |
| 849.1 | 2-Hexenal | NIST | 2.20 ± 0.18 a | 0.62 ± 0.12 c | 0.35 ± 0.14 c | 0.87 ± 0.15 b | Green, bitter |
| 958.8 | 2-Heptenal | NIST | 0.37 ± 0.12 b | 0.33 ± 0.04 b | 0.53 ± 0.08 ab | 0.66 ± 0.03 a | Pungent, green, fatty |
| 973.3 | 2-Nonenal, (E) | NIST | n.d. | n.d. | 0.18 ± 0.06 a | n.d. | Citrus, green |
| 974.8 | 2-Octenal | NIST | n.d. | n.d. | 0.41 ± 0.04 a | n.d. | Fresh, cucumber, green |
| 1098.6 | 2,6-Octadienal, 3,7-dimethyl | NIST | 0.06 ± 0.04 c | 0.20 ± 0.05 b | 0.33 ± 0.03 a | 0.19 ± 0.01 b | |
| Ketones | |||||||
| 663.1 | 1-Penten-3-one | NIST | 0.18 ± 0.09 bc | 0.24 ± 0.10 b | n.d. | 0.49 ± 0.10 a | Fresh, pungent |
| 1458.3 | 5,9-Undecadien-2-one, 6,10-dimethyl | NIST | n.d. | 0.31 ± 0.11 b | 0.52 ± 0.17 a | n.d. | |
| 1494.1 | 3-Buten-2-one | NIST | 0.02 ± 0.02 b | 0.12 ± 0.05 b | 0.28 ± 0.10 a | 0.11 ± 0.03 b | Pungent |
| Esters | |||||||
| 989.9 | Cyclohexene, 3-(2-methylpropyl) | NIST | 0.14 ± 0.05 b | 0.06 ± 0.03 c | 0.08 ± 0.02 c | 0.36 ± 0.16 a | |
| 995.3 | Butanoic acid, butyl ester | NIST | 0.15 ± 0.09 b | 0.08 ± 0.04 b | 0.37 ± 0.01 a | 0.52 ± 0.08 a | Butter, cheese, stinky |
| 1194.6 | Methyl salicylate | NIST | 0.24 ± 0.15 b | 0.53 ± 0.12 b | 2.73 ± 0.34 a | 1.38 ± 0.24 b | Sweet, aromatic, green |
| 1046.1 | 2-Hexenoic acid, ethyl ester | NIST | 0.14 ± 0.05 a | 0.06 ± 0.03 b | 0.08 ± 0.02 b | 0.36 ± 0.16 a | Sweat |
| Aromatic Compounds | |||||||
| 1027.0 | Eucalyptol | Sigma–Aldrich | 1.43 ± 0.51 d | 2.86 ± 0.26 c | 5.74 ± 0.14 b | 7.47 ± 0.89 a | Herbal, camphor-like |
| Others | |||||||
| <600.0 | Carbon dioxide | NIST | 0.34 ± 0.17 a | 0.37 ± 0.05 a | 0.64 ± 0.05 a | 0.59 ± 0.13 a | Pungent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomando González, J.C.; Rodríguez Gómez, M.J.; Ramos García, M.; Nicolás Barroso, N.; Calvo Magro, P. Characterization and Selection of Lycium barbarum Cultivars Based on Physicochemical, Bioactive, and Aromatic Properties. Horticulturae 2025, 11, 924. https://doi.org/10.3390/horticulturae11080924
Solomando González JC, Rodríguez Gómez MJ, Ramos García M, Nicolás Barroso N, Calvo Magro P. Characterization and Selection of Lycium barbarum Cultivars Based on Physicochemical, Bioactive, and Aromatic Properties. Horticulturae. 2025; 11(8):924. https://doi.org/10.3390/horticulturae11080924
Chicago/Turabian StyleSolomando González, Juan Carlos, María José Rodríguez Gómez, María Ramos García, Noelia Nicolás Barroso, and Patricia Calvo Magro. 2025. "Characterization and Selection of Lycium barbarum Cultivars Based on Physicochemical, Bioactive, and Aromatic Properties" Horticulturae 11, no. 8: 924. https://doi.org/10.3390/horticulturae11080924
APA StyleSolomando González, J. C., Rodríguez Gómez, M. J., Ramos García, M., Nicolás Barroso, N., & Calvo Magro, P. (2025). Characterization and Selection of Lycium barbarum Cultivars Based on Physicochemical, Bioactive, and Aromatic Properties. Horticulturae, 11(8), 924. https://doi.org/10.3390/horticulturae11080924






