Abstract
Hericium coralloides is a highly valued gourmet and medicinal species with growing market demand across East Asia, though industrial production remains limited by cultivation challenges. This study investigated the molecular characteristics, biological traits, domestication potential, and cultivation protocols of Hericium coralloides strains collected from the Changbaishan Nature Reserve (Jiling, China). Optimal conditions for mycelial growth included mannose as the preferred carbon source, peptone as the nitrogen source, 30 °C incubation temperature, pH 5.5, and magnesium sulfate as the essential inorganic salt. The fruiting bodies had a protein content of 2.43% g/100 g (fresh sample meter). Total amino acids comprised 53.3% of the total amino acid profile, while essential amino acids accounted for 114.11% relative to non-essential amino acids, indicating high nutritional value. Under optimized domestication conditions—70% hardwood chips, 20% cottonseed hulls, 8% bran, 1% malic acid, and 1% gypsum—bags reached full colonization in 28 days, with a 15-day maturation phase and initial fruiting occurring after 12–14 days. The interval between flushes was 10–12 days. The average yield reached 318.65 ± 31.74 g per bag, with a biological conversion rate of 63.73%. These findings demonstrate that Hericium coralloides possesses significant potential for edible and commercial applications. This study provides a robust theoretical foundation and resource reference for its artificial cultivation, supporting its broader industrial and economic utilization.
1. Introduction
Hericium coralloides (Scop.) Pers. is a rare edible and medicinal fungus, primarily found in coniferous and mixed forests across Northeast, North, and Southwest China. It typically grows on semi-dead or decaying wood, with a fruiting season from early summer to early autumn each year [1]. Due to its strong dependence on specific ecological conditions, its natural distribution is limited and its wild resources are scarce. Consequently, H. coralloides has been designated a third-class protected species in China, and the scarcity of resources has constrained their development and utilization [2].
In addition to its nutritional value and culinary appeal, H. coralloides exhibits notable medicinal properties. Contemporary pharmacological and clinical studies have identified a range of bioactive compounds within this species, including polysaccharides, triterpenoids, proteins, amino acids, and sterols [3]. These constituents are associated with diverse pharmacological effects, such as immune enhancement, hypoglycemic activity, hepatogastric protection, anti-tumor effects, and antioxidation [4,5,6,7,8]. Among them, polysaccharides are particularly recognized for their strong antioxidant activity, capable of scavenging free radicals and delaying cellular aging [9]. Furthermore, H. coralloides has demonstrated inhibitory effects against various cancer cell lines, indicating promising anti-tumor potential [10,11]. As such, its development holds substantial economic value and broad application prospects in both the pharmaceutical and food industries. Despite its high value, the industrial utilization of H. coralloides remains limited due to its ecological specificity and resource scarcity. Research on its development, domestication, and utilization is relatively sparse [2]. In addition, current research on H. coralloides has primarily focused on chemical composition analysis [12] or optimizing single cultivation conditions [13,14]. In contrast, systematic domestication and cultivation, as well as the selection of high-yield strains, remain relatively scarce [15]. Therefore, breeding superior strains with strong resistance, high yield, and good quality is crucial for the industrialization of the H. coralloides industry. This study aims to achieve the efficient domestication of wild H. coralloides from Changbai Mountain for the first time by integrating germplasm innovation, breakthroughs in cultivation technology, and nutritional evaluation, thereby laying the foundation for their resource conservation and industrial application.
2. Materials and Methods
2.1. Test Strains
The test strain was wild Hericium coralloides, collected from a dead wood of Juglans mandshurica in the Changbaishan Nature Reserve (42°50′ N, 128°36′ E, Temperate continental monsoon climate, dark brown forest soil, 736 m above sea level). A total of one wild sample was collected, No. SH. The strain is now conserved in the Edible Mushroom Germplasm Resource Bank of Hangzhou Academy of Agricultural Sciences.
2.2. The Composition of the Medium
This experiment was conducted based on the formulation of Chen Shuang et al. [16] with parameter optimization. The composition of the media used was as follows: PDA solid medium—potato 200 g, glucose 20 g, agar 13 g, water 1.0 L, natural pH; carbon source medium—agar 13 g, peptone 4 g, variable carbon source 20 g, water 1.0 L; nitrogen source medium—agar 13 g, glucose 20 g, variable nitrogen source 4 g, water 1.0 L. The medium was subjected to autoclave sterilization (SX-300, Kagoshima seisakusyo Inc. (Kirishima-shi, Japan)) (0.105 MPa, 121 °C, 20 min).
2.3. Separation and Culture
This study used the tissue separation method [17]. After surface disinfection with 75% alcohol-soaked cotton, tissue samples were excised from the central part of the stipe near the flame of an alcohol lamp and inoculated onto a PDA medium. Tip mycelia were subsequently subcultured twice to purify the strain.
2.4. Morphological Identification Method
The macroscopic features of the collected fruiting bodies were systematically examined, with detailed documentation of their size, color, texture, and morphology. The number of primary and secondary branches, as well as the morphology and dimensions of the fungal spines, were recorded and categorized. Simultaneously, the morphology of the isolated mycelium was observed and documented [2].
2.5. Molecular Identification
Genomic DNA was extracted using a plant genome extraction kit (Kang Weishi, Taizhou City, China). Sequencing and analysis were conducted following the protocol described by Li Manling [18]. The resulting sequences were submitted to the NCBI database and analyzed using BLAST for similarity comparison with reference sequences, enabling taxonomic identification of the edible fungus and related species.
2.6. Biological Characteristics Test
To evaluate factors influencing the mycelial growth of H. coralloides, the effects of different carbon sources, nitrogen sources, temperatures, pH levels, and inorganic salts were assessed by measuring mycelial growth rate and vigor using the plate culture method. Three independent biological replicates were performed for each experimental condition. Within each biological replicate, at least three technical replicates were conducted for all measurements. Activated strains were inoculated onto media with varied formulations, and mycelial growth rate was determined by the cross-scratch method. Average growth rate and growth potential of mycelia were subsequently calculated [18].
Mycelial growth rate (cm/d) = colony diameter (cm)/mycelial growth days (d).
2.6.1. Carbon Source Test
Eight carbon sources—glucose, lactose, maltose, starch, sucrose, mannose, dextrin, and fructose—were each incorporated into separate media formulations. A carbon-free medium served as the control (CK).
2.6.2. Nitrogen Source Test
For nitrogen source evaluation, the following compounds were tested: yeast extract, ammonium nitrate (NH4NO3), potassium nitrate (KNO3), sodium nitrate (NaNO3), ammonium chloride (NH4Cl), peptone, ammonium sulfate ((NH4)2SO4), and urea. Each was added to the respective medium formulation, with a nitrogen-free medium used as the blank control (CK).
2.6.3. pH Experiment
The pH of the PDA medium was adjusted to 11 discrete levels—4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, and 9.0—using 1 mol/L NaOH or HCl. The adjusted media were sterilized (0.105 MPa, 121 °C, 20 min) and reserved for subsequent use.
2.6.4. Temperature Experiment
Temperature effects on growth were assessed by incubating H. coralloides cultures on PDA medium in the dark at seven temperatures: 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and 35 °C.
2.6.5. Inorganic Salt Experiment
To study the effect of inorganic salts, 4 g of peptone and 20 g of glucose were added to the basal medium as standard nitrogen and carbon sources. Six inorganic salts—ferrous sulfate (FeSO4), magnesium sulfate (MgSO4), zinc sulfate (ZnSO4), manganese sulfate (MnSO4), dipotassium hydrogen phosphate (K2HPO4), and potassium dihydrogen phosphate (KH2PO4)—were tested individually, with a salt-free medium serving as the blank control (CK).
2.6.6. Orthogonal Test
Based on the results of the single-factor experiments, three optimal levels were selected for each of the four factors—carbon source, nitrogen source, temperature, and pH—for use in an orthogonal design. An L9 (34) orthogonal test was conducted to evaluate the effects of these factors on mycelial growth, as detailed in Table 1.

Table 1.
Orthogonal test results.
2.7. Cultivation Experiment
The cultivation substrate consisted of 70% hardwood sawdust, 20% cottonseed hulls, 8% bran, 1% malic acid, and 1% gypsum, with the moisture content adjusted to 60–65%. The mixed substrate was packed into polypropylene bags (17 cm × 33 cm × 0.05 cm), with each bag containing 0.5 kg of dry material. Following autoclave sterilization, 20 mL of liquid inoculum was introduced under sterile conditions. Bags were incubated at 25 °C in darkness for 28 days. Once fully colonized, the cultures underwent a 15-day post-ripening phase, after which fruiting management was initiated. To induce primordium formation, the cultures were exposed to white light (500–800 Lux) for 8–10 h daily. After primordium emergence, an additional 3–5 h of blue light was applied each day at the same intensity. The first flush of H. coralloides fruiting bodies was harvested after 12–14 days. A second flush was produced after a further 10–12 days under identical conditions. Yields from both flushes were recorded, and the biological conversion rate was calculated using the following formula:
Biological conversion rate = (fresh weight of fruiting body/dry weight of culture medium) × 100%.
2.8. Composition Analysis of the Fruiting Body
The composition analysis methods for the fruiting body of H.coralloides are as follows: Coarse fiber content is determined using the national standard method GB/T 5009.10-2003 [19]. Protein content is determined using the standard GB 5009.5-2016 [20], and fat content is determined using the standard GB 5009.6-2016 [21]. Water content is determined according to GB 5009.3-2016 [22]. Amino acid content is determined using GB 5009.124-2016 [23].
2.9. Data Processing
Data analysis was performed using SPSS Statistics software (version 22.0, IBM Corp.). Differences between group means were assessed using Duncan’s multiple range test at a significance level of p < 0.05. The orthogonal experimental design was constructed and analyzed using the online platform SPSSAU. Figures were generated using Origin software (version 2021, OriginLab Corp.). Values in results represent means of technical replicates ± SD per biological replicate.
3. Results
3.1. Morphological Identification
Morphologically, the fruiting body of H. coralloides is coral-like, composed of up to five branching levels with four main branches. The diameter ranges from 12 to 25 cm, and the overall structure is pure white with a soft texture. The surface is densely covered with needle-like, fleshy spines measuring 1–3 cm in length and 1–2 mm in diameter, which are upright at the base and droop at the tips, resembling coral (Figure 1a). The mycelium is white, rope-like, and tightly interwoven, attaching firmly to the substrate with smooth, uniform colony margins (Figure 1b).
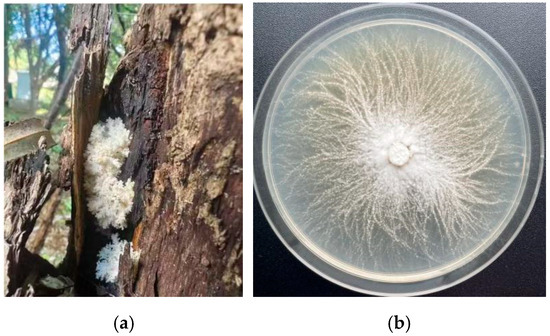
Figure 1.
Morphology of wild fruiting body and mycelium of H. coralloides: (a) Wild fruiting body of H. coralloides; (b) Mycelial growth of H. coralloides.
3.2. Molecular Biological Identification
The rDNA-ITS sequence analysis revealed a fragment size of 501 bp. This sequence has been submitted to GenBank under accession number PQ803194.1. BLAST homology analysis via the NCBI database indicated 100% sequence similarity with MG735348.1 and OR251284.1, all corresponding to H. coralloides. Based on both molecular data and morphological characteristics, the isolate was conclusively identified as H. coralloides.
3.3. Biological Characteristics
3.3.1. Effects of Different Carbon Sources on the Mycelial Growth of H. coralloides
As shown in Figure 2a,b, the mycelium of H. coralloides was able to grow on all tested carbon sources except lactose, with significantly higher growth rates than the control (p < 0.05). Among the carbon sources, maltose (0.734 ± 0.030 cm/day), mannose (0.731 ± 0.036 cm/day), and glucose (0.711 ± 0.041 cm/day) supported the fastest growth, with no statistically significant differences among them (p > 0.05). On maltose and glucose media, the mycelium appeared white, dense, and vigorously growing, with flocculent colony margins. On mannose, it also appeared white and dense, but exhibited filamentous margins. These results suggest that maltose, mannose, and glucose are the most favorable carbon sources for H. coralloides mycelial growth.
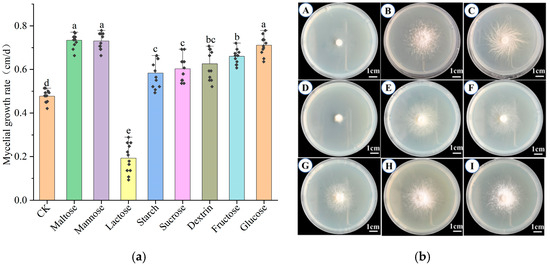
Figure 2.
Effects of different carbon sources on the mycelial growth of H. coralloides: (a) Growth rate of H. coralloides mycelium under different carbon source conditions; (b) Mycelial growth of H. coralloides under different carbon sources, (A): CK (PDA); (B): Maltose; (C): Mannose; (D): Lactose; (E): Starch; (F): Sucrose; (G): Dextrin; (H): Fructose; (I): Glucose. Different letters indicate significant differences (p < 0.05, Duncan’s multiple range test).
3.3.2. Effects of Different Nitrogen Sources on the Mycelial Growth of H. coralloides
Figure 3a,b demonstrated that, excluding urea, H. coralloides mycelium could grow on all tested nitrogen sources. Yeast extract, peptone, ammonium sulfate, and ammonium chloride significantly enhanced mycelial growth compared to the control (p < 0.05), while other nitrogen sources resulted in reduced growth (p > 0.05). The highest mycelial growth rate was observed with yeast extract (0.795 ± 0.021 cm/day), followed by peptone (0.744 ± 0.035 cm/day) and ammonium sulfate (0.365 ± 0.051 cm/day). Mycelium grown on yeast extract and peptone media was dense, white, and exhibited strong growth potential. In contrast, although ammonium sulfate supported relatively fast growth, the resulting mycelium was sparse and exhibited weaker vitality. Media containing ammonium nitrate, potassium nitrate, or ammonium sulfate resulted in both slower growth rates and sparse, weakly growing mycelium. Overall, yeast extract was identified as the optimal nitrogen source, followed by peptone.
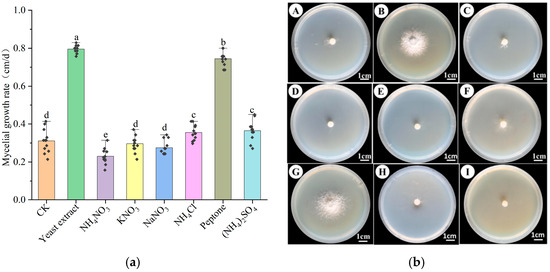
Figure 3.
Effect of different nitrogen sources on the mycelial growth of H. coralloides: (a) Growth rate of H. coralloides mycelium under different nitrogen source conditions; (b) Mycelial growth of H. coralloides under different nitrogen sources, (A): CK (PDA); (B): Yeast extract; (C): NH4NO3; (D): KNO3; (E): NaNO3; (F): NH4Cl; (G): Peptone; (H): (NH4)2SO4; (I): Urea. Different letters indicate significant differences (p < 0.05, Duncan’s multiple range test).
3.3.3. Effects of Different Temperatures on the Mycelial Growth of H. coralloides
Temperature is a key factor influencing the growth and development of H. coralloides mycelium. As shown in Figure 4a,b, no growth occurred at 5–10 °C, whereas sustained growth was observed between 15 °C and 35 °C. The mycelial growth rate exhibited a clear temperature dependence, initially increasing with temperature before declining. The optimal growth rate was recorded at 30 °C, reaching 1.088 ± 0.015 cm/day, which was significantly higher than that at other temperatures (p < 0.05). At 25 °C, the growth rate was 0.901 ± 0.047 cm/day, followed by 0.575 ± 0.051 cm/day at 20 °C. At 35 °C, growth was markedly inhibited, with a rate of only 0.271 ± 0.1 cm/day. Morphologically, mycelia appeared dense and white at 20 °C, 25 °C, and 30 °C, indicating strong growth potential at these temperatures. Collectively, these results suggest that 30 °C is the optimal temperature for H. coralloides mycelial growth, followed by 25 °C.
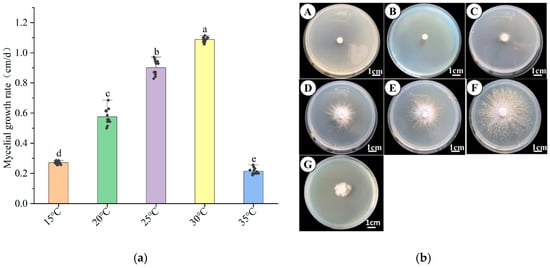
Figure 4.
Effect of different temperatures on the mycelial growth of H. coralloides: (a) Growth rate of H. coralloides mycelium under different temperature conditions; (b) Mycelial growth of H. coralloides under different temperature conditions, (A): 5 °C; (B): 10 °C; (C): 15 °C; (D): 20 °C; (E): 25 °C; (F): 30 °C; (G): 35 °C. Different letters indicate significant differences (p < 0.05, Duncan’s multiple range test).
3.3.4. Effects of Different pH on the Mycelial Growth of H. coralloides
Figure 5a,b illustrates that H. coralloides mycelia can grow across a range of pH conditions. The growth rate increased with rising pH up to a peak at pH 5.0 (1.011 ± 0.021 cm/day), which was significantly higher (p < 0.05) than at other pH levels, and then declined as pH increased further. Below pH 5.0, the growth rate exhibited a positive trend, whereas values above pH 5.0 led to gradual inhibition. The mycelium remained white, dense, and vigorous between pH 4.0 and 7.0. However, above pH 7.0, mycelial density decreased, and growth potential was noticeably weakened. These findings indicate that the optimal pH for H. coralloides mycelial growth is 5.0, with 5.5 as the second most favorable condition.
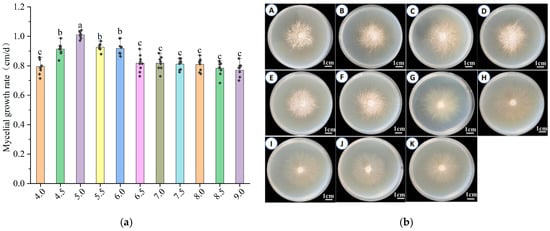
Figure 5.
Effect of different pH on the growth of mycelium of H. coralloides: (a) Growth rate of H. coralloides mycelium under different pH conditions; (b) Mycelial growth of H. coralloides under different pH conditions, (A): pH 4.0; (B): pH 4.5; (C): pH 5.0; (D): pH 5.5; (E): pH 6.0; (F): pH 6.5; (G): pH 7.0; (H): pH 7.5; (I): pH 8.0; (J): pH 8.5; (K): pH 9.0. Different letters indicate significant differences (p < 0.05, Duncan’s multiple range test).
3.3.5. Effects of Different Inorganic Salts on the Mycelial Growth of H. coralloides
As shown in Figure 6a,b, mycelial growth varied significantly across six different inorganic salt media. The highest growth rate was observed with magnesium sulfate, reaching 1.033 ± 0.044 cm/day—significantly higher than the control (0.893 ± 0.058 cm/day) (p < 0.05). Under this condition, the mycelium appeared white, dense, and robust, indicating enhanced growth performance. In contrast, dipotassium hydrogen phosphate and potassium dihydrogen phosphate yielded lower growth rates of 0.641 ± 0.026 cm/day and 0.838 ± 0.022 cm/day, respectively. Although the mycelia remained white and vigorous, these rates were significantly lower than the control (p < 0.05). Notably, no mycelial growth occurred when zinc sulfate, manganese sulfate, or ferrous sulfate was used, indicating that these salts inhibited development. Overall, magnesium sulfate proved to be the most suitable inorganic salt for promoting H. coralloides mycelial growth.
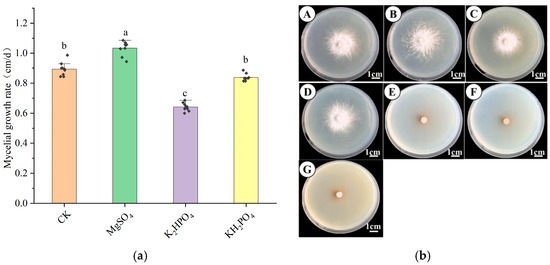
Figure 6.
Effect of different inorganic salts on the mycelial growth of H. coralloides: (a) Growth rate of H. coralloides mycelium under different inorganic salt conditions; (b) Mycelial growth of H. coralloides under different inorganic salt conditions, (A): CK (PDA); (B): MgSO4; (C): K2HPO4; (D): KH2PO4; (E): ZnSO4; (F): MnSO4; (G): FeSO4. Different letters indicate significant differences (p < 0.05, Duncan’s multiple range test).
3.4. Orthogonal Test Results
Orthogonal test results (Table 1 and Table 2; Figure 7) revealed significant effects of temperature, carbon source, pH, and nitrogen source on mycelial growth rate, ranked in influence as follows: temperature > carbon source > pH > nitrogen source. Temperature had the greatest impact, confirming it as the primary factor affecting growth, while nitrogen sources had the least influence—consistent with single-factor test results. Based on mean growth values, the optimal levels were as follows: carbon source (K2 > K3 > K1), nitrogen source (K2 > K3 > K1), temperature (K3 > K2 > K1), and pH (K2 > K3 > K1). The best factor combination was A2B2C3D2, corresponding to mannose as the carbon source, peptone as the nitrogen source, a temperature of 30 °C, and pH 5.5, which yielded optimal mycelial growth. Variance analysis (Table 3) further confirmed temperature as the most significant factor (highest F value is 800), followed by carbon source, nitrogen source, and pH. Temperature and carbon source also had the lowest p values, reinforcing their statistical significance.

Table 2.
Analysis of the results of orthogonal test.
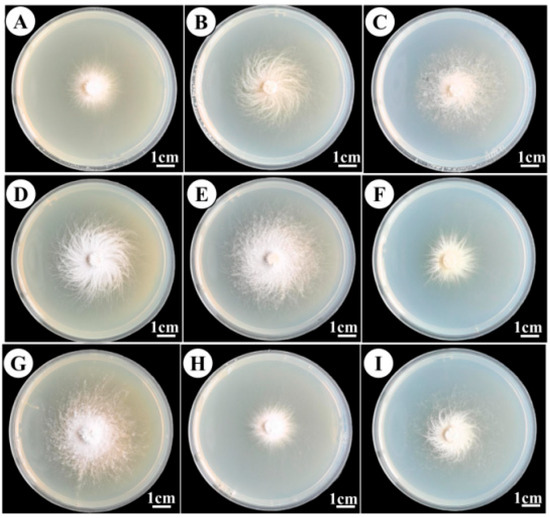
Figure 7.
Mycelial growth in the orthogonal test, (A–I) Culture medium formulations are shown in Table 2.

Table 3.
Variance analysis of the orthogonal experiment.
3.5. Domestication Cultivation
For cultivation, H. coralloides was grown on sawdust from broad-leaved trees. Following inoculation, culture bags were incubated in darkness at 25–26 °C. Mycelial colonization occurred within 28 days, after which bags were opened for fruiting. The first flush was harvested after 12–14 days, with a yield of 169.12 ± 23.91 g/bag and a biological conversion rate of 33.82%. The second flush followed after 10–12 additional days, yielding 149.53 ± 9.67 g/bag and a conversion rate of 29.91%. The total yield from both flushes reached 318.65 ± 31.74 g/bag, with an overall conversion rate of 63.73%. The fruiting bodies were white, coral-like, and of high commercial quality, measuring 169.74 ± 20.7 cm in length, 152.06 ± 5.85 cm in width, and 122.65 ± 9.06 cm in height (Figure 8).

Figure 8.
Domesticated and cultivated H. coralloides fruiting body.
3.6. Component Determination
Nutritional analysis (Table 4) revealed that H. coralloides fruiting bodies are rich in amino acids, polysaccharides, and proteins. Total amino acid (TAA) content was 1.76 g/100 g. The seven essential amino acids included methionine (0.02), leucine (0.15), valine (0.095), threonine (0.083), lysine (0.098), phenylalanine (0.08), and isoleucine (0.08) g/100 g, totaling 0.938 g/100 g of essential amino acids (EAA). Non-essential amino acids (NEAA) totaled 0.822 g/100 g. The contents of umami amino acids and sweet amino acids were 0.55 and 0.408 g/100 g, respectively. The EAA/TAA and EAA/NEAA ratios were 53.30 g/100 g and 114.11 g/100 g, both exceeding the ideal protein model recommended by FAO/WHO [24]. The total sugar content was 35.7%, indicating high nutritional value.

Table 4.
Nutrient content of the fruiting bodies of H. coralloides.
4. Discussion
Wild germplasm resources, shaped by long-term natural selection and evolution, possess rich genetic diversity and valuable traits. They represent a critical foundation for breeding and biotechnological innovation [25]. In recent years, biotechnological advances have facilitated increasing efforts to domesticate and cultivate wild fungal resources. Examples include the successful development of strains such as Panus conchatus [26], Pleurotus giganteus [18], and Ganoderma subflexipes [27]. These studies contribute not only to the conservation of wild fungal diversity but also to the enrichment of the edible fungi industry with new, high-quality varieties, thus playing a vital role in supporting industrial development.
This study investigated wild strains collected from the Changbai Mountain Nature Reserve and identified them as H. coralloides using a combination of morphological characteristics and molecular biology techniques. During the optimization of culture conditions, various nutritional and environmental factors—including carbon and nitrogen sources, pH, temperature, and inorganic salts—were found to significantly influence H. coralloides mycelial growth. Single-factor experiments revealed that the optimal carbon sources were maltose, mannose, and glucose; the preferred nitrogen sources were yeast extract and peptone; magnesium sulfate was the most effective inorganic salt; the optimal pH was 5.0; and the optimal temperature was 30 °C. Qu Na [14] reported glucose, starch, and mannitol as favorable carbon sources, while Zhang Weitong [13] found that corn flour and glucose supported optimal mycelial growth in liquid culture, aligning partially with the present findings and reflecting the broad carbon source adaptability of H. coralloides. Previous research on other wild fungi—including Agaricus bisporus [28], Trametes sanguinea (L.) Lloyd [29], Trametes elegans [30], and Sanghuangporus [31]—has also identified yeast extract and peptone as effective nitrogen sources, likely due to their rich content of amino acids, peptides, vitamins, and trace elements [32]. These results may also reflect the specific metabolic preferences of H. coralloides. This study further found that the optimal growth conditions differed slightly from Zhang Weitong’s [13] findings (the optimal temperature of 25 °C and pH 5.0), potentially due to strain-specific physiological differences [18]. Magnesium sulfate was confirmed as the optimal inorganic salt, consistent with Quna [14], this may be related to Mg2+ acting as an essential cofactor/activator for ATP-dependent kinases and phosphatases, which are crucial for driving cell wall synthesis and hyphal tip elongation [33], thereby explaining the promoting effect of MgSO4 on hyphal growth in this fungus. Based on both single-factor and orthogonal tests, the optimal culture conditions for strain SH were mannose as the carbon source, peptone as the nitrogen source, magnesium sulfate as the inorganic salt, a pH of 5.5, and a temperature of 30 °C. These results were similar to those reported by Lu Mengzhao [34] for Polyporus alveolaris.
Nutritional analysis indicates that the fruiting bodies of artificially cultivated H. coralloides possess high nutritional value (Table 4). The protein content of fresh samples (2.43%) is significantly higher than that of oyster mushrooms (1.66%), enoki mushrooms (2.18%), and button mushrooms (2.12%) [35,36]. The total essential amino acid (EAA) content (0.938 g/100 g) is also higher than that reported in the literature for bamboo fungus (0.54 g/100 g) and shiitake mushroom (0.528 g/100 g) [37]. More importantly, the EAA/TAA (53.3%) and EAA/NEAA (114.11%) ratios significantly exceed the ideal protein model recommended by the FAO/WHO (40% and 60%) [38], indicating that the amino acid composition of its protein is well-balanced and has high nutritional value. Additionally, it is rich in umami amino acids (glutamic acid + aspartic acid, 0.55 g/100 g) and sweet amino acids (glycine + alanine + serine + threonine, 0.408 g/100 g), accounting for 31.25% and 23.18% of total amino acids, respectively. These collectively contribute to its excellent flavor [39]. The umami amino acid content is comparable to or even higher than that of shiitake mushrooms (rich in glutamic acid) [40]. Overall, H. coralloides matches or even surpasses several significant domesticated edible fungi in key nutritional component indicators.
Nutritional composition is a key criterion for assessing the value of edible fungi. The protein content of the cultivated H. coralloides fruiting body reached 2.43%, which was significantly higher than that of several commonly consumed fungal species [35,36] such as Pleurotus eryngii (1.66%), Flammulina filiformis (2.18%), and Pleurotus ostreatus (2.12%). Amino acids, particularly those contributing to umami and sweetness, strongly influence the flavor. In this study, umami amino acids—glutamic acid and aspartic acid—accounted for 31.25% of the total amino acid content, while sweet amino acids—glycine, alanine, serine, and threonine—constituted 23.18%. The synergistic effects between these amino acid groups likely enhance the overall palatability of the mushroom [39]. The high concentration of characteristic amino acids imparts a rich, sweet, and tender flavor, indicating strong culinary value. Additionally, the content of seven essential amino acids reaches 0.938 g/100 g, surpassing that of Phallus indusiatus (0.54 g/100 g) and Lentinula edodes (0.528 g/100 g) [37]. According to the amino acid scoring model recommended by the World Health Organization and the Food and Agriculture Organization of the United Nations (FAO/WHO; with EAA/TAA ≥ 40% and EAA/NEAA ≥ 60%) [38], the amino acid profile of H. coralloides is superior to the reference model, highlighting its nutritional quality.
For domestication and cultivation, broadleaf sawdust, cottonseed hulls, and bran were used as the primary substrates. The average yield of two flushes reached 318.65 ± 31.74 g/bag, with a biological conversion rate of 63.73%. This is significantly higher than the typical levels found in many common edible fungi, such as straw mushroom Volvariella volvacea (21.73%) [41] and red-stemmed bamboo mushroom Dictyophora rubrovolvata (32%) [42], and also higher than that of the recently domesticated Pleurotus cystidiosus (27%) [43]. The cultivation cycle from inoculation to fruiting was 43 days, and each flush required 12–14 days, with subsequent flushes occurring within 10–12 days. Given its unique coral-like white fruiting body appearance and excellent commercial properties (Figure 8), strain SH of H. coralloides has demonstrated outstanding production performance and commercial application potential under existing domestication techniques.
5. Conclusions
This study systematically explored the biological characteristics and domestication potential of a wild H.coralloides strain isolated from the Changbaishan Nature Reserve. Morphological and molecular identification confirmed the strain as H. coralloides, providing a valuable germplasm resource for further utilization.
Biological trait analysis revealed that nutritional and environmental factors significantly affect mycelial growth. The optimal conditions for mycelial proliferation were determined as follows: mannose as the preferred carbon source, peptone as the optimal nitrogen source, magnesium sulfate as the essential inorganic salt, 30 °C cultivation temperature, and pH 5.5. Orthogonal tests confirmed temperature as the most critical factor, followed by carbon source, pH, and nitrogen source, with the combination of mannose, peptone, 30 °C, and pH 5.5 yielding the best growth.
Under optimized domestication conditions (70% hardwood chips, 20% cottonseed hulls, 8% bran, 1% malic acid, 1% gypsum), the strain exhibited excellent cultivation performance: complete colonization in 28 days, 15-day maturation, initial fruiting at 12–14 days, a 10–12 day interval between flushes, an average yield of 318.65 ± 31.74 g per bag, and a biological conversion rate of 63.73%. Nutritional analysis showed the fruiting bodies have high nutritional value, with 2.43% protein (fresh weight), essential amino acids accounting for 53.3% of total amino acids, and an essential/non-essential amino acid ratio of 114.11%, exceeding the FAO/WHO ideal protein standards.
These findings clarify the biological characteristics and optimal cultivation protocols for H. coralloides, verify its successful domestication, and confirm its significant potential for edible and commercial applications, providing a robust theoretical and practical basis for its artificial cultivation and industrial development.
Author Contributions
W.-D.Y. and B.Y. conceived of and designed this research. J.-L.S., Y.X., and Z.-F.Z. wrote this paper. X.-P.K. and Y.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Hangzhou Academy of Agricultural Sciences Agricultural Science and Technology Innovation and Demonstration Promotion Fund Peoject (2025HNCT-03); China Agriculture Research System (CARS-20); and Sannong Jiufang Project in Zhejiang Province (2023NJF025).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, F.; Wang, K.; Cai, L.; Zhao, M.; Kirk, P.M.; Fan, G.; Sun, Q.; Li, B.; Wang, S.; Yu, Z.; et al. Fungal names: A comprehensive nomenclatural repository and knowledge base for fungal taxonomy. Nucleic Acids Res. 2023, 51, D708–D716. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, Z.H.; Yu, S.T.; Guo, X.X.; Yang, N. New cultivar‘ZLsh-1’of Hericium coralloides. Mycosystema 2024, 43, 155–162. [Google Scholar] [CrossRef]
- Tu, X.Y.; Chu, L.L.; Wang, M.; Chen, B.Z.; Jiang, Y.J. Extraction of polysacchsride from hericium corallinum and analysis on its in vitro antioxidant activity. Biotechnol. Bull. 2023, 39, 276–286. [Google Scholar] [CrossRef]
- Yu, J.Q.; Wu, K.Y.; Chen, Z.R.; Wang, J.H.; Zhang, X.; Wang, C.Y. Study on the optimization of ultrasonicassisted extraction process of Hercium coralloides(Scop.) Pers. Polysaccharides and its anti-colorectal cancer activity. Biotechnol. Bull. 2024, 40, 78–87. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhao, L.; Shui, X.; Wang, L.A.; Wu, Y. Antioxidant and Anti-Aging Activities of Ethyl Acetate Extract of the Coral Tooth Mushroom, Hericium coralloides (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 561–570. [Google Scholar] [CrossRef]
- Vedenicheva, N.P.; Al-Maali, G.A.; Bisko, N.A.; Kosakivska, I.V.; Ostrovska, G.V.; Khranovska, N.M.; Gorbach, O.I.; Garmanchuk, L.V.; Ostapchenko, L.I. Effect of Cytokinin-Containing Extracts from Some Medicinal Mushroom Mycelia on HepG2 Cells In Vitro. Int. J. Med. Mushrooms 2021, 23, 15–28. [Google Scholar] [CrossRef]
- Williams, L.M.; Berthon, B.S.; Stoodley, I.L.; Williams, E.J.; Wood, L.G. Medicinal Mushroom Extracts from Hericium coralloides and Trametes versicolor Exert Differential Immunomodulatory Effects on Immune Cells from Older Adults In Vitro. Nutrients 2023, 15, 2227. [Google Scholar] [CrossRef]
- Meng, J.L.; Tian, M.; Feng, C.P.; Chang, M.C.; Cheng, H.Y. Components of Hericium coralloids and Effects of Polysaccharide on Immune and Antioxidant Function in Mice. J. Chin. Inst. Food Sci. Technol. 2016, 16, 50–55. [Google Scholar] [CrossRef]
- Tabibzadeh, F.; Alvandi, H.; Hatamian-Zarmi, A.; Kalitukha, L.; Aghajani, H.; Ebrahimi-Hosseinzadeh, B. Antioxidant activity and cytotoxicity of exopolysaccharide from mushroom Hericium coralloides in submerged fermentation. Biomass Convers. Biorefin 2022, 14, 26953–26963. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hou, X.X.; Li, Z.Y.; Shan, S.H.; Chang, M.C.; Feng, C.P.; Wei, Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. Int. J. Biol. Macromol. 2020, 157, 288–295. [Google Scholar] [CrossRef]
- Hou, X.X.; Liu, J.Y.; Li, Z.Y.; Chang, M.C.; Guo, M.; Feng, C.P.; Shi, J.Y. Fruiting body polysaccharides of Hericium erinaceus induce apoptosis in human colorectal cancer cells via ROS generation mediating caspase-9-dependent signaling pathways. Food Funct. 2020, 11, 6128–6138. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Han, A.L.; Yun, S.J.; Chang, M.C.; Meng, J.L.; Feng, C.R. Cholesterol-lowering effect of H.coralloides polysaccharides and ITS mechanism. Acta Nutr. Sin. 2018, 40, 172–176. [Google Scholar] [CrossRef]
- Zhang, W.T.; Yao, F.J.; Fang, M.; Zhang, Y.M.; Ma, X.X.; Huang, C.Y.; Zhang, Y. Study on liquid culture of Hericium Coralloides and its cultivation effects. J. Northeast. Agric. Sci. 2021, 46, 125–131. [Google Scholar] [CrossRef]
- Qu, N.; Liu, D.; Wang, H.; Wang, J.W.; Liu, Y.B.; Xu, T.J.; Hu, X. Study on the Optimal medium formula of wild coral Hericium coralloides(Scop.)Pers.in Changbai Motntain. Edible Fungi China 2020, 39, 17–19. [Google Scholar] [CrossRef]
- Fan, Y.G.; Bau, T. Cultivation techniques of wild edible mushroom Hericium coralloides (Scop.) Pers. collected from Changbaishan Nature reserve. Edible Fungi China 2010, 29, 10–11. [Google Scholar] [CrossRef]
- Chen, S.; Liu, S.J.; Gao, Y.; Song, Z.K.; Ma, H.X. Biological characteristics and domestic cultivation of wild Ganoderma gibbosum. Mycosystema 2023, 42, 2218–2230. [Google Scholar] [CrossRef]
- Ge, Y.H.; He, J.Q.; Han, Z.; Xu, D.; Liu, H.X. Biological characteristics and domestication cultivation of Sparassis subalpina. Mycosystema 2024, 43, 156–173. [Google Scholar] [CrossRef]
- Li, M.L.; Zhu, A.H.; Ma, G.Y.; Qu, Z.; Lu, X.H.; Ma, H.X.; Liu, Z.D. Biological characteristics and domestication of wild Pleurotus giganteus. Mycosystema 2025, 44, 65–78. [Google Scholar] [CrossRef]
- GB/T 5009.10-2003; Determination of Crude Fiber in Vegetable Foods. Standardization Administration of China: Beijing, China, 2003.
- GB 5009.5-2016; National Food Safety Standard—Determination of Protein in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard—Determination of Fat Content in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.3-2016; National Food Safety Standard—Determination of Moisture Content in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.124-2016; National Food Safety Standard—Determination of Amino Acids in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Chen, J.L.; Yang, Y.K.; Wu, G.F.; Liu, P.; Li, Y. Biological characteristics and domestic cultivation of a wild Bondarzewia dickinsii strain. Mycosystema 2025, 44, 33–43. [Google Scholar] [CrossRef]
- Zhai, L.N.; Tang, Q.J.; Zhou, B.J.; Zhou, S.Z.; Wang, H.J.; Yun, Y.; Han, Y.S.; Wang, Q.Y.; Yan, X.W.; Xing, F.N. Genetic diversity analysis and core germplasm construction of oryza rufipogon Griff.in Hainan. J. Plant Genet. Resour. 2024, 25, 1624–1636. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, J.L. Biological characterization and domestication of a wild Panus conchatus strain. Mycosystema 2024, 43, 148–155. [Google Scholar] [CrossRef]
- Tian, R.; Chrn, L.F.; Zhao, R.X.; Zeng, N.K. Biological characteristics and cultivation of Ganoderma subflexipes. Mycosystema 2024, 43, 48–65. [Google Scholar] [CrossRef]
- Liang, Q.Q.; Ding, L.Q.; Tian, L.W.; Wang, Y.Z.; Niu, X.; Dan, H.J. Domestication and cultivation of a wild brown strain of Agaricus bisporus collected from Qilian Mountains. Mycosystema 2025, 44, 119–131. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Han, H.L.; Feng, L.N.; Cheng, X.Y.; Zhang, J.Z.; Wang, X.H.; Sun, W.M. Domestic cultivation of Trametes sanguinea. Mycosystema 2024, 43, 174–188. [Google Scholar] [CrossRef]
- Li, M.M.; Lin, J.T.; Huang, Z.X.; Liang, Y.L.; Huang, F.C.; Liu, B. Biological characteristics and domestication cultivation of Trametes elegans. Mycosystema 2023, 42, 1506–1516. [Google Scholar] [CrossRef]
- Song, J.L.; Wang, W.K.; Cheng, J.W.; Lu, N.; Yan, J.; Kang, X.P.; Yuan, W.D. Isolation and cultivation of wild Sanghuangporue. J. Northwest A F Univ. 2020, 48, 129–136. [Google Scholar] [CrossRef]
- An, Q.; Wu, X.J.; Wu, B.; Dai, Y.C. Effects of carbon and nitrogen sources on lignocellulose decomposition enzyme activities in Flammulina velutipes. Mycosystema 2015, 34, 761–771. [Google Scholar] [CrossRef]
- Tatsuno, K.; Yamada-Okabe, H.; Takagi, M.; Arisawa, M.; Sudoh, M. Properties of yeast expressed Aspergillus nidulans chitin synthase B which is essential for hyphal growth. FEMS Microbiol. Lett. 1997, 149, 279–284. [Google Scholar] [CrossRef]
- Lu, M.Z.; Wang, S.W.; Xu, J.Z. Wild Polyporus alveolaris Separate Isolation and Identification of Biological Characteristics. J. Northeast. For. Univ. 2016, 44, 76–79. [Google Scholar] [CrossRef]
- Chu, X.Z.; Liu, X.; Gong, P.; Liu, G.; Wang, B.R.; Gao, X. Effects of different matrix on growth, decelopment, nutrient and mineral element content of Pleurotus ostreatus. China Cucurbits Veg. 2024, 37, 90–95. [Google Scholar] [CrossRef]
- Li, B.; Cen, G.H.; Qiu, Y.; Chen, B.Z.; Deng, Y.J.; Xie, B.G.; Liu, F. Breeding of Flammylina filiformis‘Nongwanjin 8’,F.filiformis‘Nongwanjin 9’and Hypsizygus marmireus‘Nongwanzhen 1’. Mycosystema 2024, 43, 259–265. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Chung, l.-M.; Lee, S.-J.; Ahn, J.-K.; Kim, E.-H.; Kim, M.-J.; Kim, S.-L.; Moon, H.-I.; Ro, H.-M.; Kang, E.-Y.; et al. Comparison of free amino acid, carbohydrates concentrations in Korean edible and medicinal mushrooms. Food Chem. 2009, 113, 386–393. [Google Scholar] [CrossRef]
- Yin, M.; Chen, M.; Yanagisawa, T.; Matsuoka, R.; Xi, Y.; Tao, N.; Wang, X. Physical properties, chemical composition, and nutritional evaluation of common salad dressings. Front. Nutr. 2022, 9, 978648. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.Q.; Yang, S.S.; Yu, C.X.; Li, H.H.; Lin, Q.Y.; Chen, M.J.; Li, C.H. Nutritional Evaluttion of wiil and corresponding cultivated Hericium erinaceus. Acta Edulis Fungi 2020, 27, 131–142. [Google Scholar] [CrossRef]
- Huang, W.H.; Chen, H.Y.; Bao, D.P.; Yang, R.H.; Tao, X.S.; Wu, G.F.; Huang, J.F. Agronomic traits of wild Lentinus edodes strains and amino acid nutritional profile of the fruit bodies. Shanghai Agric. Sci. 2023, 39, 32–39. [Google Scholar] [CrossRef]
- Lin, F.; Gao, Y.Y.; Zhao, Y.; Yan, S.Y.; Chen, M.J. Comparison of biological characteristics of different Volvariella volvacea strains. Guangdong Agric. Sci. 2014, 41, 29–32. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Liu, N.; Wang, Y.Z.; Pan, G.C.; Zou, F.L.; Yang, X.; Long, H.W. Study on Breeding Varieties of Dictyophora rubrovolvata. Edible Fungi China 2020, 39, 12–15. [Google Scholar] [CrossRef]
- Xiao, Z.T.; He, H.Q.; Peng, Y.Y.; Liu, M.; Xu, J. Identification and Domestication of a Wild Pleurotus cystidiosus. Spec. Wild Econ. Anim. Plant Res. 2023, 45, 108–115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).