Effect of Biostimulant Applications on Eco-Physiological Traits, Yield, and Fruit Quality of Two Raspberry Cultivars
Abstract
1. Introduction
2. Materials and Methods
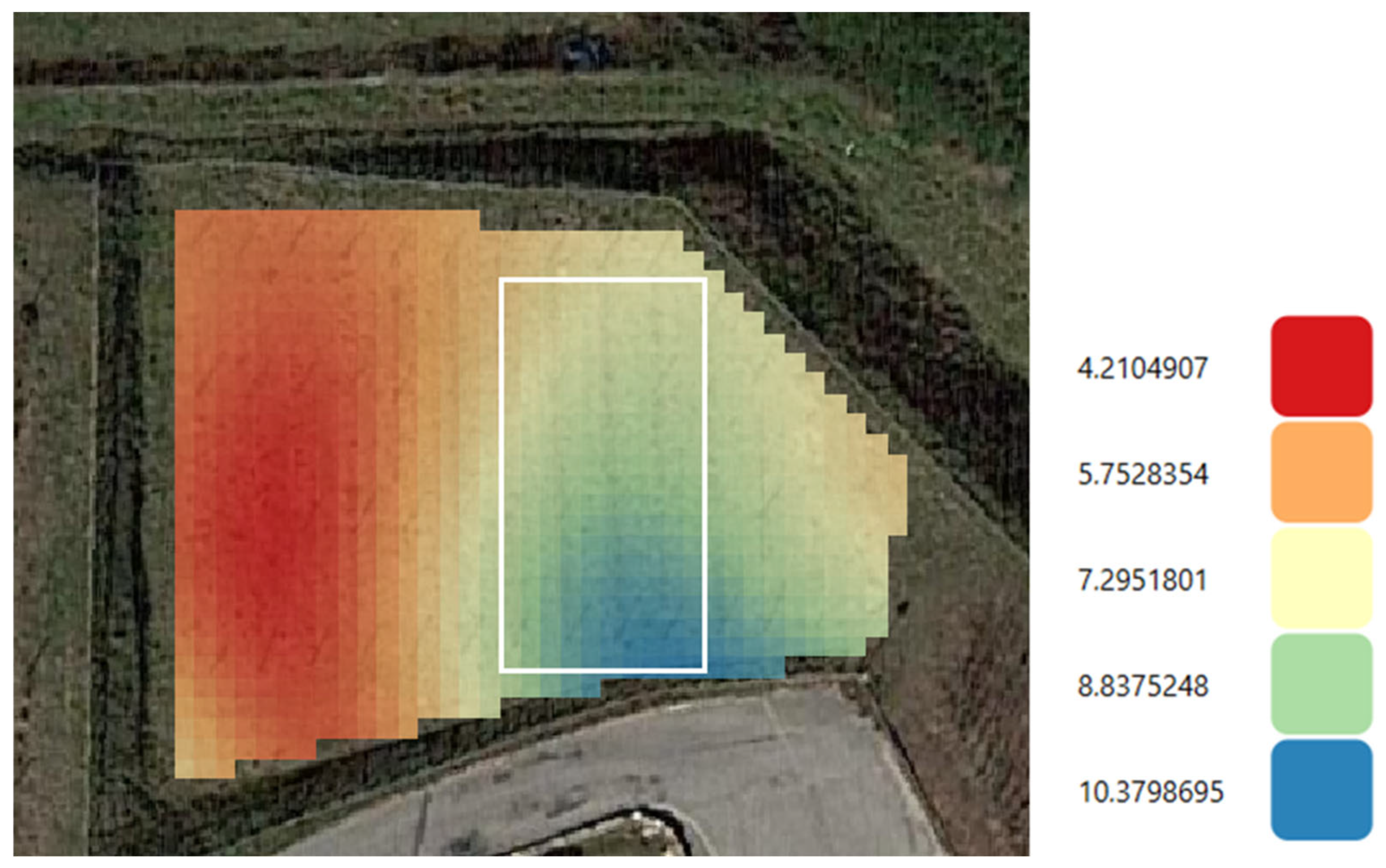
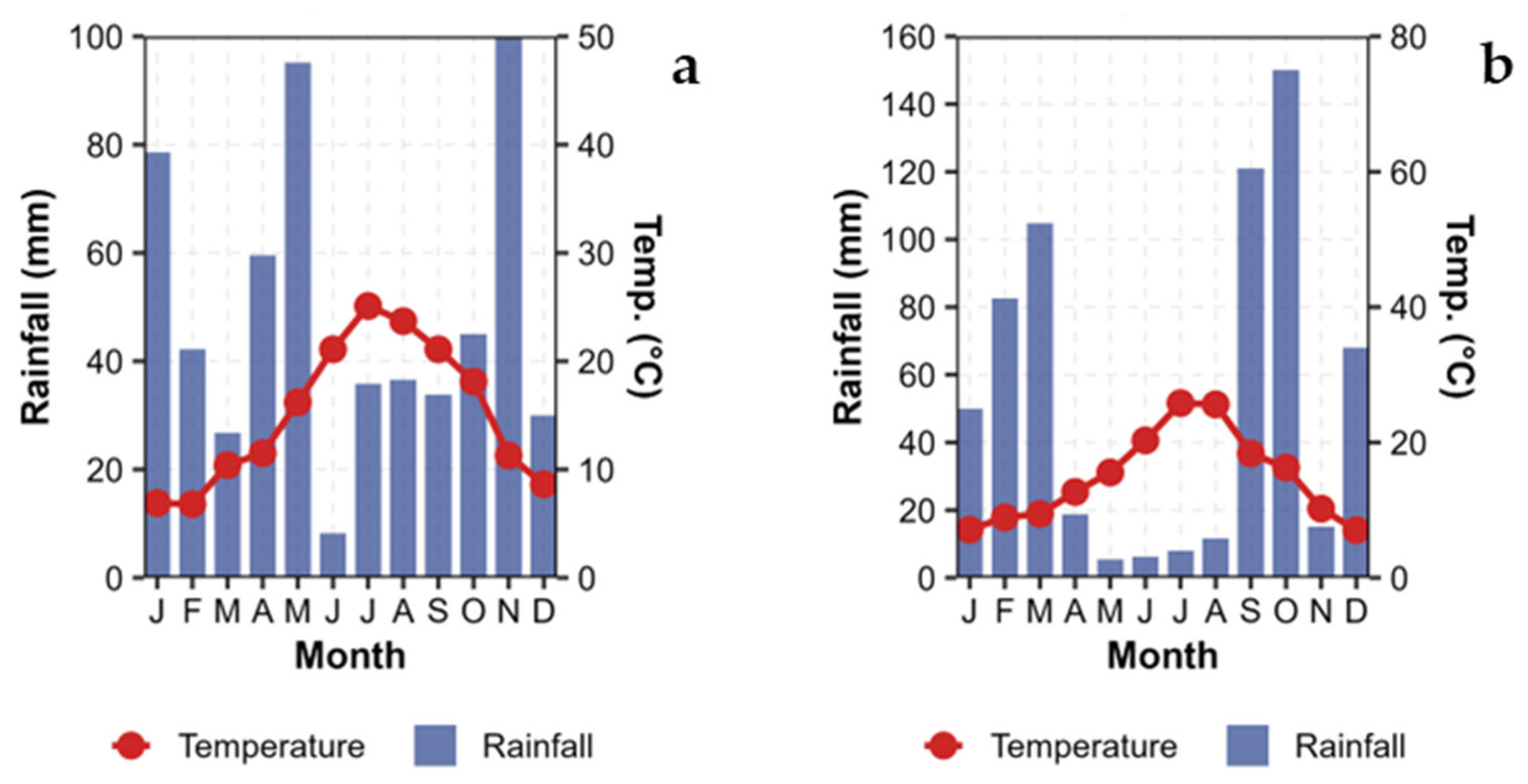
2.1. Site Description
2.2. Biostimulant Treatments
2.3. Eco-Physiological Leaf Measurements
2.4. Yield and Berry Qualitative Traits
2.5. Statistical Analyses
3. Results
3.1. Eco-Physiological Measurements
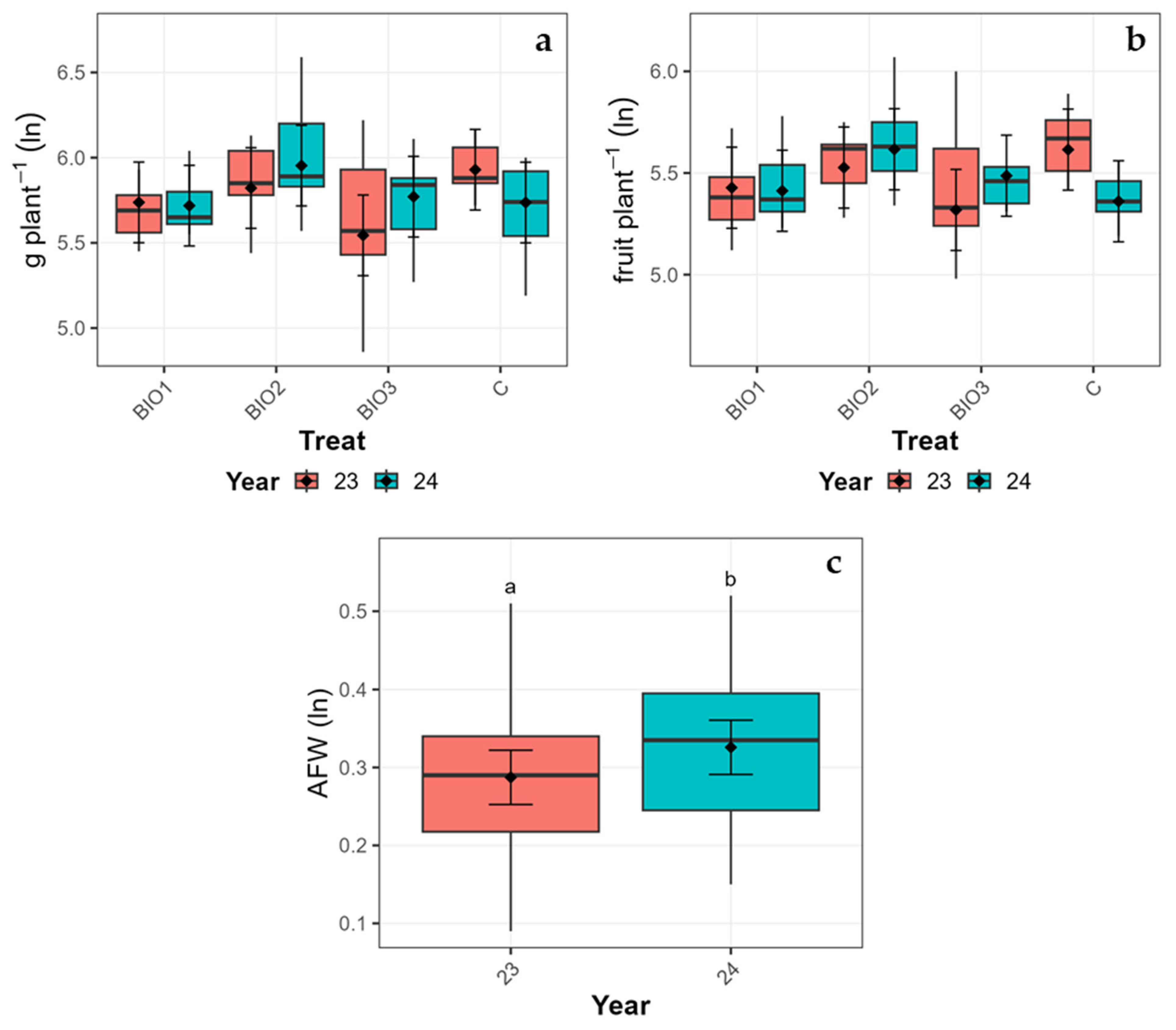
3.2. Yield Performance
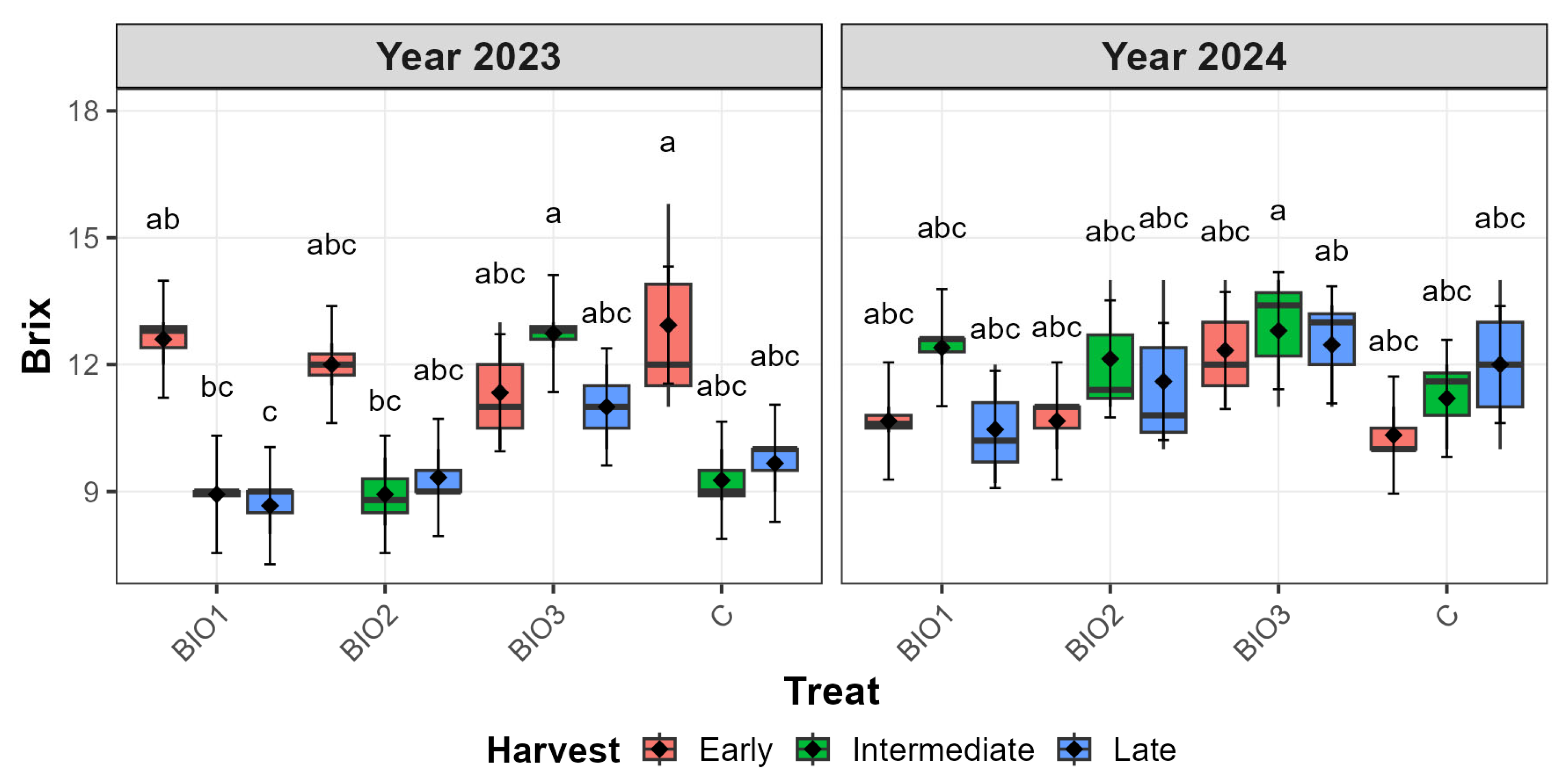
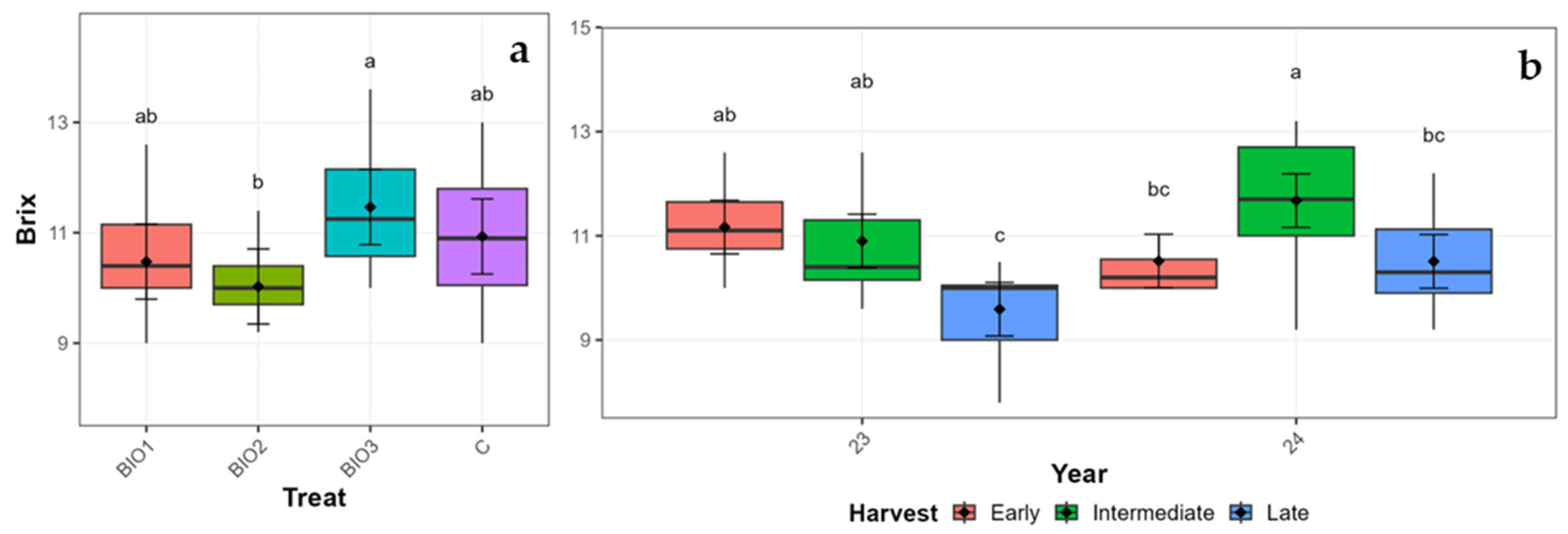
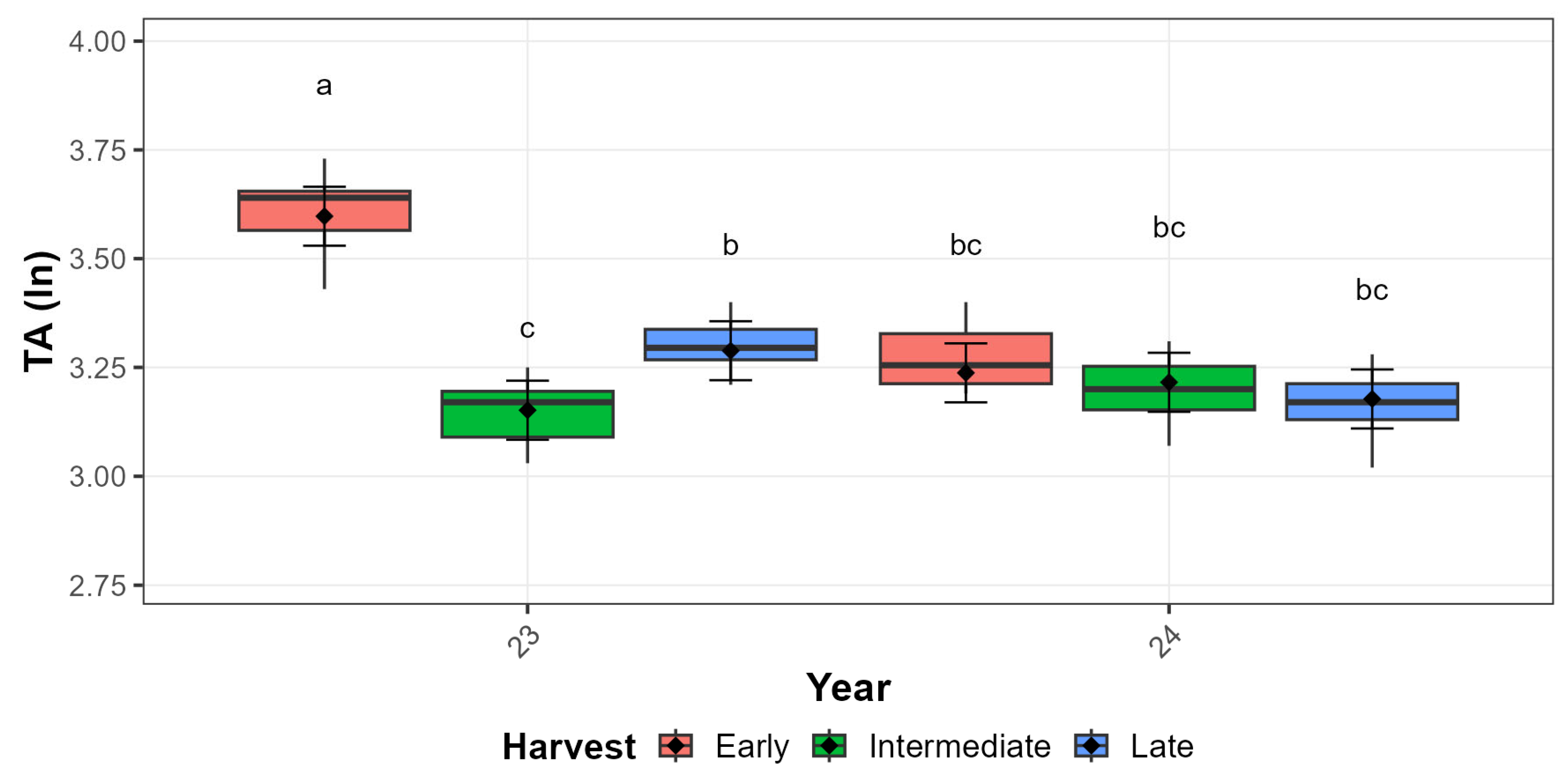
3.3. Berries Total Soluble Solid, pH, and Titratable Acidity
3.3.1. ‘Autumn Bliss’ Qualitative Traits
3.3.2. ‘Zeva’ Qualitative Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- UN DESA UN DESA Databases. Available online: https://www.un.org/en/desa/products/un-desa-databases (accessed on 14 November 2023).
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Ponder, A.; Hallmann, E. The Effects of Organic and Conventional Farm Management and Harvest Time on the Polyphenol Content in Different Raspberry Cultivars. Food Chem. 2019, 301, 125295. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 9 June 2025).
- Andreotti, C.; Rouphael, Y.; Colla, G.; Basile, B. Rate and Timing of Application of Biostimulant Substances to Enhance Fruit Tree Tolerance toward Environmental Stresses and Fruit Quality. Agronomy 2022, 12, 603. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- La Torre, A.; Battaglia, V.; Caradonia, F. An Overview of the Current Plant Biostimulant Legislations in Different European Member States. J. Sci. Food Agric. 2016, 96, 727–734. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Mandal, S.; Anand, U.; López-Bucio, J.; Radha; Kumar, M.; Lal, M.K.; Tiwari, R.K.; Dey, A. Biostimulants and Environmental Stress Mitigation in Crops: A Novel and Emerging Approach for Agricultural Sustainability under Climate Change. Environ. Res. 2023, 233, 116357. [Google Scholar] [CrossRef]
- Mounaimi, S.; Hamim, A.; El Boukhari, M.E.M.; Elarroussi, H.; Lyamlouli, K. A Comprehensive Review of Integrating Biostimulants and Biopesticides for Organic Berry Farming: Exploring Challenges and Opportunities for Africa. Front. Sustain. Food Syst. 2024, 8, 1452823. [Google Scholar] [CrossRef]
- Trzciński, P.; Frąc, M.; Lisek, A.; Przybył, M.; Frąc, M.; Sas-Paszt, L. Growth Promotion of Raspberry and Strawberry Plants by Bacterial Inoculants. Acta Sci. Pol. Hortorum Cultus 2021, 20, 71–82. [Google Scholar] [CrossRef]
- Balcı, G.; Keles, H.; Cakmakcı, R. The Effects of Biofertilizers on Some Physological Responses in Heritage Raspberries. KSU J. Agric. Nat. 2020, 23, 1422–1427. [Google Scholar] [CrossRef]
- Kazakov, P.; Alseekh, S.; Ivanova, V.; Gechev, T. Biostimulant-Based Molecular Priming Improves Crop Quality and Enhances Yield of Raspberry and Strawberry Fruits. Metabolites 2024, 14, 594. [Google Scholar] [CrossRef]
- Drobek, M.; Cybulska, J.; Zdunek, A.; Sas-Paszt, L.; Frąc, M. Effect of Microbial Biostimulants on the Antioxidant Profile, Antioxidant Capacity and Activity of Enzymes Influencing the Quality Level of Raspberries (Rubus idaeus L.). Food Chem. 2024, 454, 139746. [Google Scholar] [CrossRef] [PubMed]
- Makonya, G.M.; Bryla, D.R.; Hardigan, M.A.; Hoashi-Erhardt, W.; DeVetter, L.W. Biostimulants with Glycine Betaine or Kelp Extract Alleviate Heat Stress in Red Raspberry (Rubus Idaeus). Sci. Rep. 2025, 15, 2251. [Google Scholar] [CrossRef]
- Aguilar, N.A.S.; Fuentes, J.A.G.; Luis Alonso, V.A.; López Pérez, M.G.; MacIas, J.M.; Benavides-Mendoz, A.; Morales, S.G. Effect of Elicitors and Biostimulants on the Content of Bioactive Compounds in Raspberry Fruits. Hortic. Sci. 2023, 50, 101–111. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 9 May 2025).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B.; Jensen, S.P. lmerTest: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R.V.; Banfai, B.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; et al. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 9 May 2025).
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S. Multcomp: Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Wadas, W.; Dziugieł, T. Changes in Assimilation Area and Chlorophyll Content of Very Early Potato (Solanum tuberosum L.) Cultivars as Influenced by Biostimulants. Agronomy 2020, 10, 387. [Google Scholar] [CrossRef]
- Franzoni, G.; Ferrante, A. Plant Extract Improves Quality Traits of Green and Red Lettuce Cultivars. Heliyon 2024, 10, e39224. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Francesca, S.; Najai, S.; Zhou, R.; Decros, G.; Cassan, C.; Delmas, F.; Ottosen, C.-O.; Barone, A.; Rigano, M.M. Phenotyping to Dissect the Biostimulant Action of a Protein Hydrolysate in Tomato Plants under Combined Abiotic Stress. Plant Physiol. Biochem. 2022, 179, 32–43. [Google Scholar] [CrossRef]
- Kravic, N.; Babic, V.; Vukadinovic, J.; Ristic, D.; Dragicevic, V.; Mladenovic Drinic, S.; Andjelkovic, V. Alteration of Metabolites Accumulation in Maize Inbreds Leaf Tissue under Long-Term Water Deficit. Biology 2021, 10, 694. [Google Scholar] [CrossRef]
- Neill, S.O.; Gould, K.S. Anthocyanins in Leaves: Light Attenuators or Antioxidants? Funct. Plant Biol. 2003, 30, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Shu, Z.; Sun, S.-L.; Peng, C.-L.; Wang, X.-J.; Lin, Z.-F. Antioxidation of Anthocyanins in Photosynthesis Under High Temperature Stress. J. Integr. Plant Biol. 2007, 49, 1341–1351. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A New Optical Leaf-Clip Meter for Simultaneous Non-Destructive Assessment of Leaf Chlorophyll and Epidermal Flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, F.; Chen, X.; Li, Z.; Mulla, D.J. Nitrogen Balance Index Prediction of Winter Wheat by Canopy Hyperspectral Transformation and Machine Learning. Remote Sens. 2022, 14, 3504. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A Meta-Analysis and Review of Plant-Growth Response to Humic Substances: Practical Implications for Agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Ciriello, M.; Fusco, G.M.; Woodrow, P.; Carillo, P.; Rouphael, Y. Unravelling the Nexus of Plant Response to Non-Microbial Biostimulants under Stress Conditions. Plant Stress 2024, 11, 100421. [Google Scholar] [CrossRef]
- Aghaeifard, F.; Babalar, M.; Fallahi, E.; Ahmadi, A. Influence of Humic Acid and Salicylic Acid on Yield, Fruit Quality, and Leaf Mineral Elements of Strawberry (Fragaria × Ananassa Duch.) Cv. Camarosa. J. Plant Nutr. 2016, 39, 1821–1829. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P. The Effect of a Preparation Containing Humic Acids on the Growth, Yield, and Quality of Strawberry Fruits (Fragaria × Ananassa (Duchesne Ex Weston) Duchesne Ex Rozier). Agronomy 2023, 13, 1872. [Google Scholar] [CrossRef]
- Ladyzhenskaya, O.; Aniskina, T.; Simakhin, M.; Donskih, V.; Pashutin, V.; Tazin, I.; Kryuchkova, V. Productivity of Modern Raspberry Varieties and Prospects for Their Selection. Crops 2025, 5, 1. [Google Scholar] [CrossRef]
- Ortega-Farias, S.; Meza, S.E.; López-Olivari, R.; Araya-Alman, M.; Carrasco-Benavides, M. Effects of Four Irrigation Regimes on Yield, Fruit Quality, Plant Water Status, and Water Productivity in a Furrow-Irrigated Red Raspberry Orchard. Agric. Water Manag. 2022, 273, 107885. [Google Scholar] [CrossRef]
- Morales, C.G.; Pino, M.T.; Del Pozo, A. Phenological and Physiological Responses to Drought Stress and Subsequent Rehydration Cycles in Two Raspberry Cultivars. Sci. Hortic. 2013, 162, 234–241. [Google Scholar] [CrossRef]
- Sonnewald, U.; Fernie, A.R. Next-Generation Strategies for Understanding and Influencing Source–Sink Relations in Crop Plants. Curr. Opin. Plant Biol. 2018, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Spasojević, S.; Maksimović, V.; Milosavljević, D.; Djekić, I.; Radivojević, D.; Sredojević, A.; Milivojević, J. Variation in Chemical, Textural and Sensorial Traits Among Remontant Red Raspberry (Rubus idaeus L.) Cultivars Maintained in a Double-Cropping System. Plants 2024, 13, 3382. [Google Scholar] [CrossRef]
- Janick, J. Plant Breeding Reviews, Volume 32: Raspberry Breeding and Genetics; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-59381-3. [Google Scholar]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological Effects of Humic Substances on Higher Plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical Structure and Biological Activity of Humic Substances Define Their Role as Plant Growth Promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Milivojević, J.; Maksimović, V.; Nikolić, M.; Bogdanović, J.; Maletić, R.; Milatović, D. Chemical and Antioxidant Properties of Cultivated and Wild Fragaria and Rubus Berries. J. Food Qual. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sønsteby, A. Raspberry Fruit Quality Changes during Ripening and Storage as Assessed by Colour, Sensory Evaluation and Chemical Analyses. Sci. Hortic. 2015, 195, 216–225. [Google Scholar] [CrossRef]





| Treatment Code | Commercial Product | Manufacturer | Main Components | Commercial Claim | Dosage (10 L) |
|---|---|---|---|---|---|
| BIO1 | EVEO® | Thermoflora, Pordenone, Italy | Fulvic and humic acids | Improved abiotic stress response | 20 g |
| BIO2 | BLACKJAK BIO® | Sipcam Italia Spa, Italy | Leonardite (humic acids) | Vegetative growth, photosynthesis enhancement, stress mitigation | 20 mL |
| BIO3 | MEGAFOL® | Valagro Spa, Atessa, Chieti, Italy | Plant-based extracts (vinasse, corn steep liquor, potassium acetate, urea) | Improved abiotic stress response | 60 mL |
| Chl | Flav | Anth (Ln) | NBI (Ln) | g Plant−1 (Ln) | Fruits Plant−1 (Ln) | g Fruit−1 (Ln) | |
|---|---|---|---|---|---|---|---|
| Cultivar | 1.43 * | 1.16 | 0.02 | 0.07 | 0.02 | 0.05 | 0.06 |
| Treatment | 1.43 | 1.89 | 1.45 | 0.22 | 0.94 | 0.76 | 1.47 |
| Year | 101.97 *** | 1161.48 *** | 44.89 *** | 203.91 *** | 0.57 | 0.004 | 8.39 ** |
| C × T | 0.07 | 0.68 | 0.27 | 0.24 | 0.3 | 0.41 | 0.69 |
| C × Y | 1.73 | 0.27 | 1.31 | 0.59 | 0.71 | 0.34 | 1.38 |
| T × Y | 32.80 *** | 7 65 *** | 8.75 *** | 8.20 *** | 3.56 * | 4.16 * | 1.33 |
| C × T × Y | 15.08 *** | 4.48 *** | 3.23 *** | 3.85 ** | 0.02 | 1.23 | 1.41 |
| °Bx | pH | TA (Ln) | °Bx | pH | TA (Ln) | |
|---|---|---|---|---|---|---|
| Autumn Bliss | Zeva | |||||
| Treatment | 5.7 * | 2.77 | 8.48 *** | 4.37 * | 6.81 *** | 0.76 |
| Year | 12.05 ** | 0.4 | 7.42 ** | 3.51 | 0.32 | 31.49 *** |
| Harvest | 3.94 * | 2.15 | 26.62 *** | 15.24 *** | 0.94 | 34.7 *** |
| T × Y | 0.4 | 0.04 | 2.5 | 0.48 | 6.16 ** | 0.44 |
| T × H | 1.69 | 3.37 ** | 1.48 | 1.49 | 0.53 | 0.78 |
| Y × H | 15.32 *** | 10.92 *** | 23.33 *** | 7.29 ** | 59.47 *** | 25.95 *** |
| T × Y × H | 2.47 * | 5.89 *** | 1.31 | 0.52 | 0.33 | 1.21 |
| Factor | Level | Chlorophyll Content (µg cm−2) | Flavonols (A.U.) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||
| Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | ||
| Cultivar | AB | 22.21 | 0.20 | 21.80 | 22.62 | 1.93 | 0.02 | 1.89 | 1.97 |
| Z | 21.70 | 0.14 | 21.41 | 21.99 | 1.91 | 0.01 | 1.88 | 1.94 | |
| Year | 23 | 22.52 | 0.13 | 22.24 | 22.79 | 2.05 | 0.01 | 2.03 | 2.08 |
| 24 | 21.39 | 0.13 | 21.12 | 21.66 | 1.79 | 0.01 | 1.76 | 1.81 | |
| Treatment | BIO1 | 21.87 | 0.25 | 21.37 | 22.37 | 1.93 | 0.02 | 1.88 | 1.97 |
| BIO2 | 22.08 | 0.25 | 21.57 | 22.58 | 1.91 | 0.02 | 1.87 | 1.96 | |
| BIO3 | 21.59 | 0.25 | 21.09 | 22.09 | 1.88 | 0.02 | 1.84 | 1.93 | |
| C | 22.28 | 0.25 | 21.77 | 22.78 | 1.96 | 0.02 | 1.91 | 2.01 | |
| Factor | Level | Anthocyanins (A.U.) | NBI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||
| Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | ||
| Cultivar | AB | 0.18 | 0.00 | 0.17 | 0.18 | 11.11 | 0.15 | 10.80 | 11.42 |
| Z | 0.18 | 0.00 | 0.17 | 0.18 | 12.53 | 0.15 | 12.22 | 12.84 | |
| Year | 23 | 0.19 | 0.00 | 0.18 | 0.19 | 11.84 | 0.24 | 11.35 | 12.33 |
| 24 | 0.17 | 0.00 | 0.17 | 0.17 | 11.80 | 0.17 | 11.45 | 12.14 | |
| Treatment | BIO1 | 0.18 | 0.00 | 0.17 | 0.18 | 11.69 | 0.29 | 11.09 | 12.28 |
| BIO2 | 0.17 | 0.00 | 0.17 | 0.18 | 12.01 | 0.29 | 11.41 | 12.61 | |
| BIO3 | 0.18 | 0.00 | 0.18 | 0.19 | 11.91 | 0.29 | 11.31 | 12.50 | |
| C | 0.18 | 0.00 | 0.17 | 0.18 | 11.67 | 0.29 | 11.08 | 12.27 | |
| Factor | Level | Plant Yield (g Plant−1) | Fruit Set (Fruit Plant−1) | Avg. Fruit Weight (g Fruit−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | |||||||||||
| Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | ||
| Cultivar | AB | 332.48 | 28.75 | 273.60 | 391.37 | 241.96 | 17.33 | 206.45 | 277.47 | 1.37 | 0.04 | 1.30 | 1.44 |
| Z | 343.41 | 20.33 | 301.77 | 385.05 | 249.98 | 12.26 | 224.87 | 275.09 | 1.36 | 0.03 | 1.31 | 1.41 | |
| Year | 23 | 330.35 | 19.45 | 291.04 | 369.66 | 246.48 | 12.00 | 222.28 | 270.68 | 1.34 | 0.02 | 1.29 | 1.38 |
| 24 | 345.54 | 19.45 | 306.23 | 384.85 | 245.46 | 12.00 | 221.26 | 269.66 | 1.39 | 0.02 | 1.34 | 1.44 | |
| Treatment | BIO1 | 314.80 | 35.21 | 242.68 | 386.91 | 230.12 | 21.23 | 186.64 | 273.61 | 1.37 | 0.04 | 1.28 | 1.46 |
| BIO2 | 377.83 | 35.21 | 305.71 | 449.95 | 270.12 | 21.23 | 226.64 | 313.61 | 1.38 | 0.04 | 1.29 | 1.47 | |
| BIO3 | 310.28 | 35.21 | 238.16 | 382.39 | 236.96 | 21.23 | 193.47 | 280.45 | 1.29 | 0.04 | 1.20 | 1.38 | |
| C | 348.88 | 35.21 | 276.76 | 421.00 | 246.67 | 21.23 | 203.18 | 290.15 | 1.42 | 0.04 | 1.33 | 1.51 | |
| Cultivar | Factor | Level | SSC (°Brix) | pH | TA (g L−1 Citric Acid) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | ||||||||||||
| Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | |||
| AB | Year | 23 | 10.62 | 0.20 | 10.21 | 11.03 | 2.98 | 0.01 | 2.95 | 3.01 | 26.51 | 0.48 | 25.55 | 27.47 |
| 24 | 11.59 | 0.20 | 11.18 | 12.00 | 2.97 | 0.01 | 2.94 | 3.00 | 24.35 | 0.48 | 23.39 | 25.31 | ||
| Harvest | Early | 11.61 | 0.24 | 11.11 | 12.10 | 2.95 | 0.02 | 2.91 | 2.98 | 27.73 | 0.58 | 26.55 | 28.90 | |
| Inter. | 11.05 | 0.24 | 10.56 | 11.54 | 2.99 | 0.02 | 2.96 | 3.03 | 22.37 | 0.58 | 21.20 | 23.55 | ||
| Late | 10.65 | 0.24 | 10.16 | 11.14 | 2.98 | 0.02 | 2.94 | 3.01 | 26.19 | 0.58 | 25.01 | 27.36 | ||
| Treatment | BIO1 | 10.62 | 0.29 | 9.96 | 11.28 | 2.95 | 0.02 | 2.90 | 3.01 | 27.23 | 0.68 | 25.87 | 28.58 | |
| BIO2 | 10.78 | 0.29 | 10.12 | 11.44 | 3.00 | 0.02 | 2.94 | 3.05 | 22.97 | 0.68 | 21.61 | 24.33 | ||
| BIO3 | 12.11 | 0.29 | 11.45 | 12.77 | 3.01 | 0.02 | 2.96 | 3.07 | 25.29 | 0.68 | 23.93 | 26.65 | ||
| C | 10.90 | 0.29 | 10.24 | 11.56 | 2.93 | 0.02 | 2.87 | 2.98 | 26.23 | 0.68 | 24.87 | 27.59 | ||
| 95% CI | 95% CI | 95% CI | ||||||||||||
| Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | |||
| Z | Year | 23 | 10.55 | 0.17 | 10.18 | 10.92 | 2.96 | 0.01 | 2.94 | 2.97 | 28.99 | 0.62 | 27.66 | 30.32 |
| 24 | 10.90 | 0.17 | 10.53 | 11.27 | 2.96 | 0.01 | 2.95 | 2.98 | 24.99 | 0.62 | 23.65 | 26.32 | ||
| Harvest | Early | 10.84 | 0.20 | 10.43 | 11.25 | 2.95 | 0.01 | 2.93 | 2.97 | 31.25 | 0.70 | 29.80 | 32.70 | |
| Inter. | 11.29 | 0.20 | 10.88 | 11.70 | 2.96 | 0.01 | 2.95 | 2.98 | 24.23 | 0.70 | 22.77 | 25.68 | ||
| Late | 10.05 | 0.20 | 9.64 | 10.46 | 2.97 | 0.01 | 2.95 | 2.98 | 25.48 | 0.70 | 24.03 | 26.93 | ||
| Treatment | BIO1 | 10.48 | 0.29 | 9.80 | 11.16 | 2.96 | 0.01 | 2.94 | 2.98 | 27.36 | 1.07 | 24.90 | 29.82 | |
| BIO2 | 10.03 | 0.29 | 9.35 | 10.71 | 2.99 | 0.01 | 2.97 | 3.01 | 25.78 | 1.07 | 23.32 | 28.24 | ||
| BIO3 | 11.47 | 0.29 | 10.79 | 12.15 | 2.96 | 0.01 | 2.94 | 2.98 | 26.82 | 1.07 | 24.36 | 29.28 | ||
| C | 10.93 | 0.29 | 10.25 | 11.61 | 2.93 | 0.01 | 2.91 | 2.95 | 27.99 | 1.07 | 25.53 | 30.45 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanelli, F.; Silvestri, C.; Cristofori, V. Effect of Biostimulant Applications on Eco-Physiological Traits, Yield, and Fruit Quality of Two Raspberry Cultivars. Horticulturae 2025, 11, 906. https://doi.org/10.3390/horticulturae11080906
Giovanelli F, Silvestri C, Cristofori V. Effect of Biostimulant Applications on Eco-Physiological Traits, Yield, and Fruit Quality of Two Raspberry Cultivars. Horticulturae. 2025; 11(8):906. https://doi.org/10.3390/horticulturae11080906
Chicago/Turabian StyleGiovanelli, Francesco, Cristian Silvestri, and Valerio Cristofori. 2025. "Effect of Biostimulant Applications on Eco-Physiological Traits, Yield, and Fruit Quality of Two Raspberry Cultivars" Horticulturae 11, no. 8: 906. https://doi.org/10.3390/horticulturae11080906
APA StyleGiovanelli, F., Silvestri, C., & Cristofori, V. (2025). Effect of Biostimulant Applications on Eco-Physiological Traits, Yield, and Fruit Quality of Two Raspberry Cultivars. Horticulturae, 11(8), 906. https://doi.org/10.3390/horticulturae11080906








