Nursery Propagation Systems for High-Quality Strawberry (Fragaria × ananassa Duch.) Plug Plant Production from Micropropagated, Soilless-Grown Mother Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Strawberry Mother Plant: Cultivars and Selection
2.2. Production of Micropropagated and In Vivo Mother Plants
2.3. Soilless Cultivation of the Mother Plants
2.4. Nursery Tips Production of cv ‘Dina’
2.5. Data Collected to Identify the Stoloniferous Efficiency of the Different Mother Plants
2.6. Qualitative Response of ‘Dina’ Nursery Tip Production
2.7. Statistical Analysis
3. Results and Discussion
3.1. Comparing Stoloniferous Capacity of In Vivo and Micropropagated Mother Plants of the Everbearing ‘AN12,13,58’ Breeding Selection
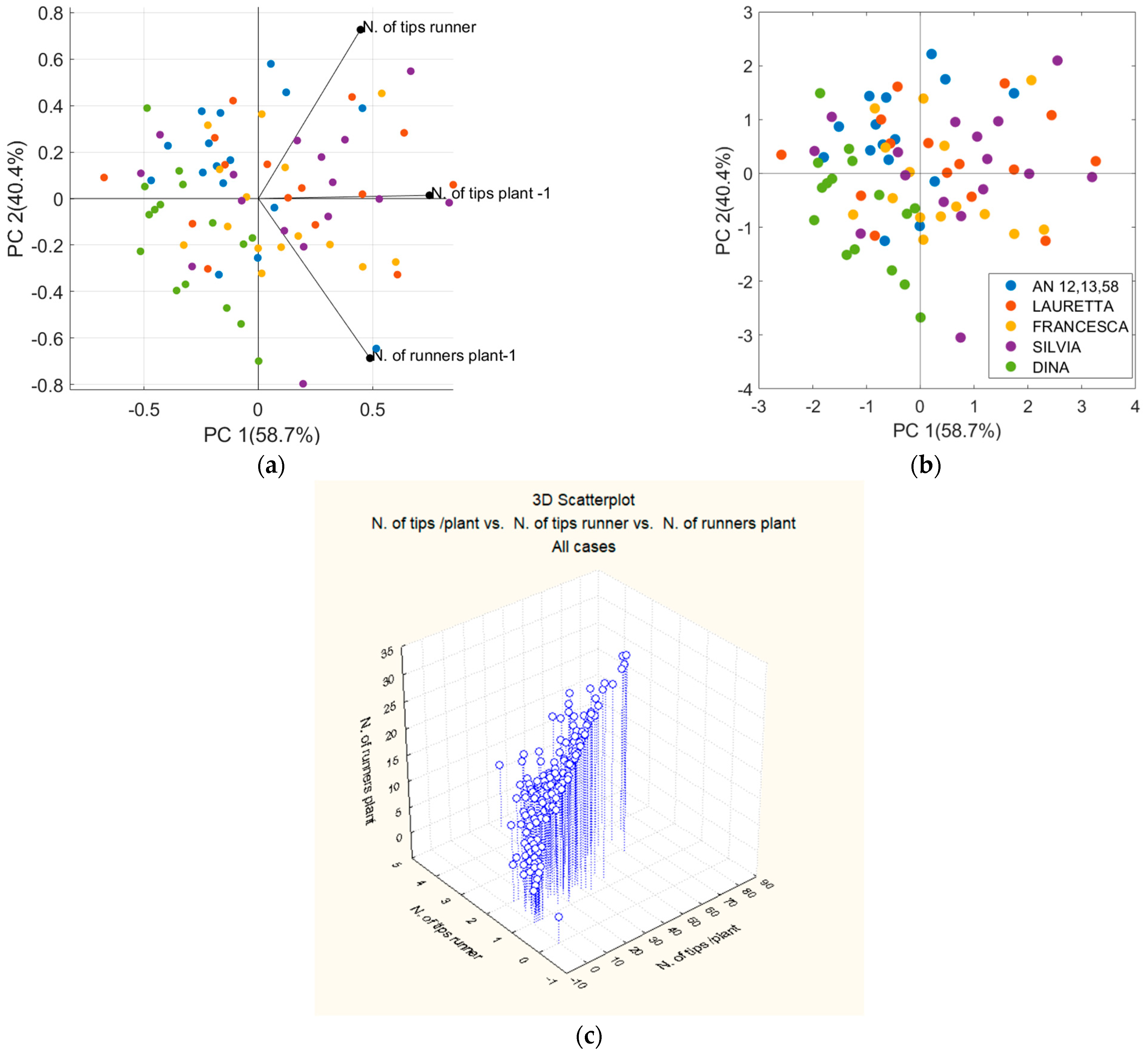
3.2. Stolonifer Capacity of Micropropagated Mother Plants of Different Genotypes
3.3. Stolonifer Capacity of Micropropagated Mother Plant of cv. ‘Dina’ Transplanted on Two Dates
3.4. Four Fertilization Strategy: Plant Architecture of ‘Dina’ Plug Plants’ Epigeal Apparatus
3.4.1. Leaf Fresh and Dry Weight of ‘Dina’ Plug Plants
3.4.2. L*C*h° Values of Three Leaves of ‘Dina’ Rooted Tips
3.4.3. Root System of ‘Dina’ Plug Plants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparacino, A.; Ollani, S.; Baima, L.; Oliviero, M.; Borra, D.; Rui, M.; Mastromonaco, G. Analyzing strawberry preferences: Best–worst scaling methodology and purchase styles. Foods 2024, 13, 1474. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, A.; Petit, A.; Lesemann, S.; Rey-Serra, P.; Mazzoni, L.; Masny, A.; Sánchez-Sevilla, J.F.; Potier, A.; Gaston, A.; Klamkowski, K.; et al. Strawberry phenotypic plasticity in flowering time is driven by the interaction between genetic loci and temperature. J. Exp. Bot. 2024, 75, 5923–5939. [Google Scholar] [CrossRef]
- Hytönen, T.; Kurokura, T. Control of flowering and runnering in strawberry. Hortic. J. 2020, 89, 96–107. [Google Scholar] [CrossRef]
- Judd, L.A.; Jackson, B.E.; Fonteno, W.C. Advancements in root growth measurement technologies and observation capabilities for container-grown plants. Plants 2015, 4, 369–392. [Google Scholar] [CrossRef]
- Senger, E.; Osorio, S.; Olbricht, K.; Shaw, P.; Denoyes, B.; Davik, J.; Predieri, S.; Karhu, S.; Raubach, S.; Lippi, N.; et al. Towards smart and sustainable development of modern berry cultivars in Europe. Plant J. 2022, 111, 1238–1251. [Google Scholar] [CrossRef]
- Capocasa, F.; Balducci, F.; Mazzoni, L.; Marcellini, M.; Pergolotti, V.; Mezzetti, B. Preliminary results of different strawberry cultivars in multi-cropping soilless cultivation. Acta Hortic. 2021, 1309, 579–584. [Google Scholar] [CrossRef]
- Rahman, M.; Samtani, J.; Baker, A. New Insights in the Detection and Management of Major Foliar and Root Diseases of Strawberries. Preprints 2023. [Google Scholar] [CrossRef]
- Dale, A.; Hughes, B.R.; Donnelly, D. The role of micropropagation in producing specific pathogen-tested plants. HortScience 2008, 43, 74–77. [Google Scholar] [CrossRef]
- Freeman, S. Detection and characterization of soilborne diseases of strawberry and their management. In ISHS—X International Strawberry Symposium (ISS2025) Book of Abstracts; Yancheng, China, 2025; (Book of Abstracts, publisher not formally listed.). [Google Scholar]
- Milinkovic, M.; Merriman, P.R.; Greenhalgh, F.C.; Mattner, S.W.; Horstra, C.B. Evaluation of soil-less systems for strawberry transplant production in Australia. Acta Hortic. 2017, 1176, 53–64. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kataoka, S.; Shirasawa, K.; Noguchi, Y.; Isobe, S. Genomic selection for F1 hybrid breeding in strawberry (Fragaria × ananassa). Front. Plant Sci. 2021, 12, 645111. [Google Scholar] [CrossRef]
- Neri, J.C.; Meléndez-Mori, J.B.; Tejada-Alvarado, J.J.; Vilca-Valqui, N.C.; Huaman-Huaman, E.; Oliva, M.; Goñas, M. An optimized protocol for micropropagation and acclimatization of strawberry (Fragaria × ananassa Duch.) variety ‘Aroma’. Agronomy 2022, 12, 968. [Google Scholar] [CrossRef]
- Quiroz, K.A.; Berríos, M.; Carrasco, B.; Retamales, J.B.; Caligari, P.D.; García-Gonzáles, R. Meristem culture and subsequent micropropagation of Chilean strawberry (Fragaria chiloensis (L.) Duch.). Biol. Res. 2017, 50, 20. [Google Scholar] [CrossRef]
- Boxus, P.; Damiano, C.; Brasseur, E.; Ammirato, D.A.; Evans, P.V.; Sharp, W.R.; Yamada, Y. Handbook of Plant Cell Culture; Macmillan Publishing Company: New York, NY, USA, 1984. [Google Scholar]
- Boxus, P. Review on strawberry mass propagation. Acta Hortic. 1988, 265, 309–320. [Google Scholar] [CrossRef]
- Mukherjee, E.; Gantait, S. Strawberry biotechnology: A review on progress over past 10 years. Scien-626 Tia Hortic. 2024, 338, 113618. [Google Scholar] [CrossRef]
- Capocasa, F.; Balducci, F.; Marcellini, M.; Bernardini, D.; Navacchi, O.; Mezzetti, B. Comparing nursery behavior, field plant yield and fruit quality of in vitro and in vivo propagated strawberry mother plants. Plant Cell Tissue Organ Cult. 2019, 136, 65–74. [Google Scholar] [CrossRef]
- Balducci, F.; Marcellini, M.; Schrey, S.; Linnemannstöns, L.; Capocasa, F.; Mezzetti, B.; Mazzoni, L. “Francesca”, “Lauretta”, “Silvia” and “Dina”: Four new strawberry cultivars for northern and southern European cultivation conditions from the Marche Polytechnic University breeding programme. Acta Hortic. 2021, 1309, 205–208. [Google Scholar] [CrossRef]
- Sabbadini, S.; Marcellini, M.; Mezzetti, B.; Capocasa, F. Establishing micropropagation protocols for new strawberry (Fragaria × ananassa) breeding lines. Acta Hortic. 2021, 1309, 573–578. [Google Scholar] [CrossRef]
- Rowley, D.; Black, B.; Drost, D. Strawberry Plug Plant Production. USU Extension Publication Horticulture/High Tunnels/2010-02pr. 2010. Available online: https://digitalcommons.usu.edu/psc_facpub/511/ (accessed on 23 July 2025).
- Durner, E.F.; Poling, E.B.; Maas, J.L. Recent advances in strawberry plug transplant technology. HortTechnology 2002, 12, 545–550. [Google Scholar] [CrossRef]
- Xu, X. Optimizing Environmental Parameters for Precision Indoor Propagation of Day-Neutral Strawberry. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2019. [Google Scholar] [CrossRef]
- Al-Madhagi, I. The habit of strawberry flowering is the key for runner propagation, where the photoperiod is the main environmental factor-A review. Adv. Hortic. Sci. 2023, 37, 433–449. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Wei, H.; Jeong, B.R. A long-day photoperiod and 6-benzyladenine promote runner formation through upregulation of soluble sugar content in strawberry. Int. J. Mol. Sci. 2020, 21, 4917. [Google Scholar] [CrossRef]
- Kadir, S.; Sidhu, G.; Al-Khatib, K. Strawberry (Fragaria × ananassa Duch.) growth and productivity as affected by temperature. HortScience 2006, 41, 1423–1430. [Google Scholar] [CrossRef]
- Keskin, M.; Sekerli, Y.E.; Gunduz, K. Influence of leaf water content on the prediction of nutrient stress in strawberry leaves using chromameter. Int. J. Agric. Biol. 2018, 20, 2103–2109. [Google Scholar] [CrossRef]
- León, A.P.; Viña, S.Z.; Frezza, D.; Chaves, A.; Chiesa, A. Estimation of Chlorophyll Contents by Correlations between SPAD-502 Meter and Chroma Mete. Commun. Soil Sci. Plant Anal. 2007, 38, 2877–2885. [Google Scholar] [CrossRef]
- Madeira, A.C.; Ferreira, A.; de Varennes, A.; Vieira, M.I. SPAD meter versus tristimulus colorimeter to estimate chlorophyll content and leaf color in sweet pepper. Commun. Soil Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Tomás-Barberán, F.A.; Gil, M.I. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Application of tristimulus colorimetry to estimate the carotenoids content in ultrafrozen orange juices. J. Agric. Food Chem. 2003, 51, 7266–7270. [Google Scholar] [CrossRef]
- Arias, R.; Lee, T.C.; Logendra, L.; Janes, H. Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Hyman, J.R.; Gaus, J.; Foolad, M.R. A rapid and accurate method for estimating tomato lycopene content by measuring chromaticity values of fruit puree. J. Am. Soc. Hortic. Sci. 2004, 129, 717–723. [Google Scholar] [CrossRef]
- Jeffery, R.P.; Simpson, R.J.; Lambers, H.; Orchard, S.; Kidd, D.R.; Haling, R.E.; Ryan, M.H. Contrasting communities of arbuscule-forming root symbionts change external critical phosphorus requirements of some annual pasture legumes. Appl. Soil Ecol. 2018, 126, 88–97. [Google Scholar] [CrossRef]
- Hochmuth, G.; Cantliffe, D.; Chandler, C.; Stanley, C.; Bish, E.; Waldo, E.; Legard, D.; Duval, J. Containerized strawberry transplants reduce establishment-period water use and enhance early growth and flowering compared with bare-root plants. HortTechnology 2006, 16, 46–54. [Google Scholar] [CrossRef]



| SAMPLE | Concentration (g/L) | EC (mS dm−1) | pH |
|---|---|---|---|
| C: CONTROL | 0 g/L | 0.65 | 7.50 |
| Sol_1: NPK 12-52-5 | 1 g/L | 1.26 | 5.72 |
| Sol_2: NPK 15-31-10 | 1 g/L | 1.39 | 6.27 |
| Sol_3: NPK 20-20-20 | 1 g/L | 0.99 | 6.14 |
| Parameters | In Vivo Mother Plant | In Vitro Mother Plant | ||||
|---|---|---|---|---|---|---|
| Days from Transplanting | T20 | T40 | T60 | T20 | T40 | T60 |
| No. of runners p−1 | 0.75 ± 0.77 b | 2.75 ± 1.06 b | 4.06 ± 1.24 b | 5.00 ± 1.13 a | 10.42 ± 3.49 a | 13.42 ± 6.19 a |
| No. of tips per r−1 | 0.69 ± 0.68 b | 1.67 ± 0.84 b | 2.56 ± 1.15 b | 1.35 ± 0.22 a | 3.21 ± 0.41 a | 4.26 ± 0.51 a |
| No. of tips p−1 | 1.00 ± 1.26 b | 4.75 ± 2.24 b | 10.24 ± 1.68 b | 6.75 ± 1.84 a | 30.58 ± 11.01 a | 34.75 ± 12.38 a |
| Length of runner | 6.19 ± 6.90 b | 48.00 ± 18.36 b | 98.88 ± 20.56 b | 17.08 ± 4.40 a | 64.5 ± 11.88 a | 107.5 ± 13.60 a |
| Prop. System | No. Tips at T_60 | Estimated RT m−1 | Estimated No. of Mother Plants | Estimated No. Bags and Linear Meter (8 Plants b−1 of 1 m) |
|---|---|---|---|---|
| In vivo | 10.24 ± 1.68 b | 82 | 9766 | 1220 |
| In vitro | 34.75 ± 12.38 a | 278 | 2878 | 359 |
| Genotypes | No. of Runners Plant−1 | No. of Tips Per Runner−1 | No. of Tips Plant−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T20 | T40 | T60 | T20 | T40 | T60 | T20 | T40 | T60 | |
| ‘AN12,13,58’ | 5.00 ± 0.22 a | 10.42 ± 0.41 a | 13.4 ± 1.69 ns | 1.35 ± 0.22 a | 3.21 ± 0.41 a | 4.03 ± 1.69 a | 6.75 ± 1.84 a | 30.58 ± 11.01 a | 34.75 ± 12.38 ab |
| ‘Dina’ | 1.34 ± 0.21 c | 6.72 ± 0.19 bc | 15.16 ± 2.35 ns | 0.91 ± 0.39 c | 1.70 ± 0.70 b | 3.50 ± 1.55 b | 1.41 ± 0.91 d | 12.97 ± 10.28 b | 26.84 ± 6.98 b |
| ‘Francesca’ | 0.75 ± 0.58 c | 2.63 ± 0.30 c | 17.56 ± 1.33 ns | 0.69 ± 0.48 d | 1.29 ± 0.30 c | 3.81 ± 1.33 a | 0.75 ± 0.58 d | 3.50 ± 1.79 b | 43.81 ± 13.40° |
| ‘Lauretta’ | 2.44 ± 0.39 b | 3.88 ± 0.70 bc | 15.56 ± 1.55 ns | 1.02 ± 0.08 bc | 1.43 ± 0.25 bc | 3.81 ± 1.94 a | 2.50 ± 0.97 c | 5.50 ± 1.75 b | 45.06 ± 20.46° |
| ‘Silvia’ | 3.19 ± 0.08 b | 4.94 ± 0.25 b | 17.25 ± 1.94 ns | 1.19 ± 0.21 ab | 1.60 ± 0.19 bc | 4.06 ± 2.35 a | 3.88 ± 1.59 b | 8.00 ± 2.22 b | 47.19 ± 18.74° |
| Genotypes | No. of Tips at T_60 | Estimated RT m−1 | Estimated No. of Mother Plant | Estimated No. Bags and Linear Meter (8 Plants b−1 of 1 m) |
|---|---|---|---|---|
| ‘AN12,13,58’ | 34.75 ± 12.38 ab | 278 | 2878 | 359 |
| ‘Dina’ | 26.84 ± 6.98 b | 215 | 3726 | 465 |
| ‘Francesca’ | 43.81 ± 13.40 a | 350 | 2286 | 285 |
| ‘Lauretta’ | 45.06 ± 20.46 a | 360 | 2219 | 277 |
| ‘Silvia’ | 47.19 ± 18.74 a | 378 | 2119 | 264 |
| Parameters | April 13th | May 5th | ||||
|---|---|---|---|---|---|---|
| Days from Transplanting | T20 | T40 | T60 | T20 | T40 | T60 |
| No. of runners p−1 | 1.13 ± 0.81 ns | 3.19 ± 0.91 b | 15.13 ± 6.13 ns | 1.56 ± 0.73 ns | 10.25 ± 3.59 a | 15.19 ± 4.49 ns |
| No. of tips per r−1 | 0.75 ± 0.45 b | 1.13 ± 0.14 b | 3.38 ± 1.89 ns | 1.06 ± 0.25 a | 1.99 ± 0.43 a | 4.25 ± 1.89 ns |
| No. of tips p−1 | 1.13 ± 0.81 ns | 3.69 ± 1.35 b | 33.25 ± 7.54 ns | 1.69 ± 0.95 ns | 22.25 ± 5.72 a | 29.06 ± 5.79 ns |
| Length of two runner | 5.88 ± 4.64 b | 29.91 ± 11.31 b | 75.03 ± 10.76 b | 9.75 ± 5.59 a | 58.38 ± 18.81 a | 101.13 ± 11.78 a |
| Transplanting Dates | No. of Tips at T_60 | Estimated RT m−1 | Estimated No. of Mother Plants | Estimated No. of Bags and Linear Meter (8 Plants b−1 of 1 m) |
|---|---|---|---|---|
| 13/04/2022 | 33.25 ± 7.54 ns | 266 | 3007 | 376 |
| 05/05/2022 | 29.06 ± 5.79 ns | 232 | 3441 | 430 |
| Parameters | Fertilization | T0 | T7 | T14 | T21 | T28 |
|---|---|---|---|---|---|---|
| Lightness (L*) | C | 38.06 ± 2.40 ns | 36.34 ± 1.44 ns | 36.36 ± 2.82 a | 36.73 ± 2.20° | 36.54 ± 2.24 a |
| Sol_1 | 38.61 ± 2.45 ns | 33.65 ± 1.82 ns | 35.63 ± 2.02 b | 32.30 ± 2.73 bc | 34.38 ± 2.02 b | |
| Sol_2 | 39.40 ± 3.01 ns | 34.97 ± 1.84 ns | 36.16 ± 2.80 b | 34.23 ± 3.09 b | 34.55 ± 2.12 b | |
| Sol_3 | 39.70 ± 3.46 ns | 34.47 ± 2.01 ns | 36.09 ± 2.45 b | 31.88 ± 2.13 c | 33.40 ± 2.07 b | |
| Chroma (C*) | C | 25.38 ± 4.43 ns | 22.20 ± 1.83 ns | 24.52 ± 4.67 ns | 25.67 ± 2.85° | 25.59 ± 3.41 a |
| Sol_1 | 24.45 ± 4.4 ns | 22.05 ± 3.20 ns | 24.47 ± 3.45 ns | 19.44 ± 3.20 c | 19.41 ± 2.82 bc | |
| Sol_2 | 27.65 ± 4.75 ns | 22.52 ± 1.92 ns | 23.48 ± 2.47 ns | 19.75 ± 2.44 bc | 20.87 ± 3.54 b | |
| Sol_3 | 26.69 ± 5.0 ns | 20.31 ± 2.72 ns | 23.83 ± 3.15 ns | 21.71 ± 2.35 b | 18.21 ± 3.09 c | |
| Hue angle (h°) | C | 126.38 ± 1.61 ns | 127.83 ± 1.11 ns | 127.01 ± 1.82 ns | 126.18 ± 1.81 b | 126.86 ± 1.52 b |
| Sol_1 | 126.97 ± 1.53 ns | 128.03 ± 1.32 ns | 126.31 ± 1.47 ns | 128.94 ± 1.17° | 130.15 ± 1.74 a | |
| Sol_2 | 125.70 ± 1.89 ns | 127.71 ± 1.30 ns | 127.46 ± 0.81 ns | 128.65 ± 1.59° | 128.71 ± 1.52 c | |
| Sol_3 | 126.06 ± 1.92 ns | 128.94 ± 1.38 ns | 127.31 ± 1.17 ns | 127.99 ± 0.95° | 129.97 ± 0.91 a | |
| leaf petiole fresh weight (g) | C | 1.95 ± 0.42 ns | 3.38 ± 0.37 ns | 4.45 ± 1.11 ab | 5.59 ± 1.22 ns | 5.71 ± 0.98 b |
| Sol_1 | 2.00 ± 0.37 ns | 2.94 ± 0.39 ns | 4.05 ± 0.51 b | 7.58 ± 1.01 ns | 8.90 ± 0.49 a | |
| Sol_2 | 2.43 ± 0.27 ns | 2.86 ± 0.37 ns | 3.13 ± 0.43 b | 6.49 ± 1.28 ns | 6.05 ± 1.40 b | |
| Sol_3 | 1.90 ± 0.18 ns | 4.02 ± 0.73 ns | 3.92 ± 0.81 a | 7.67 ± 0.73 ns | 8.27 ± 1.53 a | |
| leaf petiole dry weight (g) | C | 0.36 ± 0.10 b | 0.65 ± 0.09 ns | 0.87 ± 0.24 ab | 1.14 ± 0.30 ns | 1.30 ± 0.26 ab |
| Sol_1 | 0.41 ± 0.11 ab | 0.50 ± 0.07 ns | 0.68 ± 0.13 b | 1.41 ± 0.16 ns | 1.59 ± 0.04 a | |
| Sol_2 | 0.50 ± 0.06 a | 0.52 ± 0.06 ns | 0.58 ± 0.09 b | 1.23 ± 0.25 ns | 1.05 ± 0.31 b | |
| Sol_3 | 0.38 ± 0.04 ab | 0.69 ± 0.15 ns | 0.67 ± 0.15 a | 1.38 ± 0.21 ns | 1.54 ± 0.37 a |
| Parameters | Fertilization | T0 | T7 | T14 | T21 | T28 |
|---|---|---|---|---|---|---|
| Root length (cm) | C | 97.61 ± 16.25 ns | 208.53 ± 59.63 ns | 285.10 ± 71.94 ns | 626.89 ± 149.15 ns | 649.19 ± 198.47 ns |
| Sol_1 | 120.64 ± 38.29 ns | 162.07 ± 57.27 ns | 208.12 ± 44.15 ns | 548.40 ± 39.49 ns | 499.36 ± 150.95 ns | |
| Sol_2 | 105.32 ± 10.13 ns | 162.57 ± 47.18 ns | 236.58 ± 37.30 ns | 498.08 ± 34.08 ns | 462.30 ± 131.85 ns | |
| Sol_3 | 110.29 ± 14.97 ns | 258.89 ± 29.39 ns | 281.59 ± 31.07 ns | 728.65 ± 46.88 ns | 651.80 ± 130.86 ns | |
| root diameter (mm) | C | 0.42 ± 0.06 ns | 0.44 ± 0.01 ns | 0.44 ± 0.03 ns | 0.37 ± 0.02 ns | 0.43 ± 0.04 ns |
| Sol_1 | 0.43 ± 0.03 ns | 0.42 ± 0.01 ns | 0.47 ± 0.05 ns | 0.40 ± 0.02 ns | 0.50 ± 0.04 ns | |
| Sol_2 | 0.47 ± 0.06 ns | 0.42 ± 0.02 ns | 0.42 ± 0.02 ns | 0.43 ± 0.04 ns | 0.46 ± 0.05 ns | |
| Sol_3 | 0.42 ± 0.04 ns | 0.45 ± 0.05 ns | 0.41 ± 0.03 | 0.43 ± 0.08 ns | 0.47 ± 0.03 ns | |
| root volume (mm3) | C | 137.25 ± 33.95 ns | 313.00 ± 89.91 ns | 432.75 ± 76.53 ab | 686.75 ± 269.70 ns | 986.75 ± 453.22 ns |
| Sol_1 | 175.50 ± 63.38 ns | 216.75 ± 65.95 ns | 359.25 ± 122.45 b | 685.75 ± 69.48 ns | 993.00 ± 442.57 ns | |
| Sol_2 | 187.00 ± 36.23 ns | 222.75 ± 50.78 ns | 325.25 ± 81.72 b | 718.00 ± 162.08 ns | 759.50 ± 255.58 ns | |
| Sol_3 | 156.50 ± 48.35 ns | 409.75 ± 85.09 ns | 376.00 ± 87.05 a | 937.25 ± 180.65 ns | 1132.25 ± 354.25 ns | |
| root fresh weight (g) | C | 0.08 ± 0.05 b | 0.30 ± 0.16 ns | 0.68 ± 0.39 ns | 0.78 ± 0.30 ns | 1.21 ± 0.54 ns |
| Sol_1 | 0.24 ± 0.08 a | 0.27 ± 0.10 ns | 0.36 ± 0.13 ns | 0.91 ± 0.22 ns | 1.30 ± 0.42 ns | |
| Sol_2 | 0.24 ± 0.09 a | 0.23 ± 0.07 ns | 0.34 ± 0.17 ns | 1.01 ± 0.36 ns | 1.08 ± 0.57 ns | |
| Sol_3 | 0.16 ± 0.04 ab | 0.46 ± 0.17 ns | 0.49 ± 0.12 ns | 1.19 ± 0.26 ns | 1.37 ± 0.50 ns | |
| root dry weight (g) | C | 0.03 ± 0.02 ns | 0.05 ± 0.02 ns | 0.09 ± 0.06 ns | 0.14 ± 0.05 ns | 0.22 ± 0.10 ns |
| Sol_1 | 0.05 ± 0.02 ns | 0.03 ± 0.01 ns | 0.04 ± 0.02 ns | 0.11 ± 0.02 ns | 0.66 ± 1.02 ns | |
| Sol_2 | 0.04 ± 0.01 ns | 0.04 ± 0.01 ns | 0.04 ± 0.01 ns | 0.13 ± 0.02 ns | 0.12 ± 0.06 ns | |
| Sol_3 | 0.04 ± 0.01 ns | 0.05 ± 0.01 ns | 0.05 ± 0.02 ns | 0.14 ± 0.03 ns | 0.20 ± 0.07 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morresi, V.; Capocasa, F.; Balducci, F.; Diamanti, J.; Mezzetti, B. Nursery Propagation Systems for High-Quality Strawberry (Fragaria × ananassa Duch.) Plug Plant Production from Micropropagated, Soilless-Grown Mother Plants. Horticulturae 2025, 11, 888. https://doi.org/10.3390/horticulturae11080888
Morresi V, Capocasa F, Balducci F, Diamanti J, Mezzetti B. Nursery Propagation Systems for High-Quality Strawberry (Fragaria × ananassa Duch.) Plug Plant Production from Micropropagated, Soilless-Grown Mother Plants. Horticulturae. 2025; 11(8):888. https://doi.org/10.3390/horticulturae11080888
Chicago/Turabian StyleMorresi, Valentina, Franco Capocasa, Francesca Balducci, Jacopo Diamanti, and Bruno Mezzetti. 2025. "Nursery Propagation Systems for High-Quality Strawberry (Fragaria × ananassa Duch.) Plug Plant Production from Micropropagated, Soilless-Grown Mother Plants" Horticulturae 11, no. 8: 888. https://doi.org/10.3390/horticulturae11080888
APA StyleMorresi, V., Capocasa, F., Balducci, F., Diamanti, J., & Mezzetti, B. (2025). Nursery Propagation Systems for High-Quality Strawberry (Fragaria × ananassa Duch.) Plug Plant Production from Micropropagated, Soilless-Grown Mother Plants. Horticulturae, 11(8), 888. https://doi.org/10.3390/horticulturae11080888










