Abstract
Wild edible fruit plants are integral to the cultural, nutritional, medicinal, and economic practices of Indigenous Isan communities in Roi Et Province, northeastern Thailand, a region characterized by plateau and lowland topography and a tropical monsoon climate. This study aimed to document the diversity, traditional uses, phenology, and conservation status of these species to inform sustainable management and conservation efforts. Field surveys and ethnobotanical interviews with 200 informants (100 men, 100 women; random ages) were conducted across 20 local communities to identify species diversity and usage patterns, while phenological observations and conservation assessments were performed to understand reproductive cycles and species vulnerability between January and December 2023. A total of 68 species from 32 families were recorded, with peak flowering in March–April and fruiting in May–June. Analyses of Species Use Value (0.19–0.48) and Relative Frequency of Citation (0.15–0.44) identified key species with significant roles in food security and traditional medicine. Uvaria rufa had the highest SUV (0.48) and RFC (0.44). Informant consensus on medicinal applications was strong for ailments such as gastrointestinal and lymphatic disorders. Economically important species were also identified, with some contributing notable income through local trade. Conservation proposed one species as Critically Endangered and several others as Vulnerable. The results highlight the need for integrated conservation strategies, including community-based initiatives and recognition of Other Effective area-based Conservation Measures (OECMs), to ensure the preservation of biodiversity, traditional knowledge, and local livelihoods.
1. Introduction
Wild edible fruit plants have long played a crucial role in the diets, traditional medicine, and cultural practices of Indigenous communities worldwide [1,2,3]. These plants serve as vital sources of essential nutrients, including vitamins, minerals, and bioactive compounds, contributing significantly to food security, health, and overall well-being, particularly in rural and forest-dependent populations [4,5]. In many Indigenous societies, knowledge of wild edible fruit plants has been passed down through generations, forming an integral part of local ecological and ethnobotanical traditions [6,7]. Beyond their nutritional value, many wild edible fruit plants possess medicinal properties and have been widely used in traditional medicine to treat ailments ranging from digestive disorders and infections to chronic conditions such as diarrhea and hypertension [8]. However, despite their importance, many of these plants remain underutilized and underexplored in modern scientific research, limiting their potential applications in sustainable agriculture, pharmaceuticals, and food industries [9,10]. This underrepresentation highlights the importance of localized studies to bridge the gap between traditional plant use and contemporary research.
Northeastern Thailand, commonly referred to as the Isan region, is a unique biogeographical zone characterized by diverse ecosystems, including dry dipterocarp forests, mixed deciduous forests, and seasonal wetlands [11]. This environmental diversity has contributed to the region’s rich botanical resources, including numerous wild fruit-bearing species that have been historically harvested and utilized by local communities [12,13]. Among the provinces in this region, Roi Et stands out due to its extensive agricultural landscapes and the deep-rooted reliance of its Indigenous Isan communities on wild plant resources [14]. Wild edible fruit plants have long served as supplementary food sources, particularly during seasonal food shortages, and are an essential part of the region’s subsistence strategies [15]. Additionally, many of these fruits are deeply embedded in traditional healing systems, where they are used to prepare herbal remedies, tonics, and therapeutic formulations [16]. This ethnomedicinal knowledge represents an invaluable asset, reflecting centuries of accumulated wisdom regarding plant properties, preparation methods, and applications in healthcare [17]. Despite the ethnobotanical richness of the Isan region, there remains a significant gap in documented research specifically focusing on wild edible fruit plants in Roi Et Province. Previous studies in Northeast Thailand and other countries have tended to focus on other regions or broader taxonomic groups, with limited attention given to localized traditions within Isan communities. This lack of targeted research underscores the novelty and necessity of the present study [8,12,13,14,15,16].
Despite the vital role of wild edible fruit plants in local food systems and medicinal traditions, their utilization is rapidly declining due to socio-economic and environmental changes [18]. The expansion of commercial monoculture farming, deforestation, urbanization, and shifting dietary habits toward processed foods have led to a reduced reliance on wild plant species [19]. Consequently, traditional knowledge associated with these plants is at risk of being lost, particularly among younger generations with limited exposure to foraging practices and traditional herbal medicine [20]. Furthermore, the lack of scientific documentation on wild edible fruit plants exacerbates challenges in preserving and promoting their sustainable use [21]. Many species remain poorly studied in terms of their phenology and conservation status, posing challenges for future sustainability [22,23]. This decline in use and transmission of knowledge makes documentation efforts both timely and urgent, especially in areas where traditional practices persist but are under threat. Global domestication efforts for tropical underutilized fruits increasingly use multi-trait selection to improve key agronomic and nutritional characteristics [22]. Additionally, metabolomic approaches offer powerful tools to prioritize candidate species for breeding and commercialization, linking ethnobotanical indices to selection pipelines [24].
Understanding the ethnomedicinal uses and horticultural potential of wild edible fruit plants is crucial for biodiversity conservation, food security, and sustainable development [24]. Many of these plants possess unique agronomic characteristics, such as resilience to drought, adaptability to poor soils, and resistance to pests and diseases, making them promising candidates for domestication and commercial cultivation [25]. Integrating wild edible fruit plants into agroforestry systems and small-scale farming could enhance rural livelihoods while reducing dependence on external food sources [26]. Additionally, scientific validation of their medicinal properties could pave the way for new pharmaceutical discoveries and functional food products that leverage traditional botanical knowledge [27]. Furthermore, involving local communities in the conservation and sustainable use of these resources can empower knowledge holders and contribute to biocultural resilience.
This study aims to document and analyze the ethnomedicinal knowledge associated with wild edible fruit plants among Indigenous Isan communities in Roi Et Province, Northeastern Thailand. It specifically seeks to identify and classify the wild edible fruit species traditionally used by Isan communities, examine their medicinal applications in local healing practices, and assess the horticultural potential of these species for sustainable cultivation and economic development. Additionally, this study will highlight conservation strategies to preserve traditional knowledge and promote the sustainable use of these wild edible fruit plants.
By integrating ethnobotanical research with horticultural science, this study contributes to ongoing efforts to bridge traditional plant wisdom with modern agricultural and medicinal advancements. The findings will provide valuable insights into the potential of wild edible fruit plants as functional foods, medicinal resources, and sustainable crops, ultimately supporting biodiversity conservation and the well-being of Indigenous communities. In doing so, this study not only fills a critical research gap but also lays the foundation for future efforts to safeguard and revitalize traditional knowledge systems in the face of rapid environmental and cultural change.
2. Materials and Methods
2.1. Study Area
Roi Et Province is located in the central part of Northeast Thailand (Figure 1), covering an area of approximately 8317 km2. It shares borders with the provinces of Kalasin, Mukdahan, Yasothon, Sisaket, and Surin. The province’s general topography is characterized by a plateau, ranging from 120 to 160 m above mean sea level. In the northern region, mountains connect to the Phu Phan Mountain range, while the central area consists of a rolling plain. The southern part of the province features a flat plain along the banks of the Mun River and its tributaries, including the Chi River, Plap Phla River, and Tao River. This extensive lowland area, known as Thung Kula Rong Hai, forms a basin-shaped plain. The region experiences a tropical monsoon climate, with heavy rainfall typically occurring from June to October, while the period from March to May is marked by hot and dry weather.
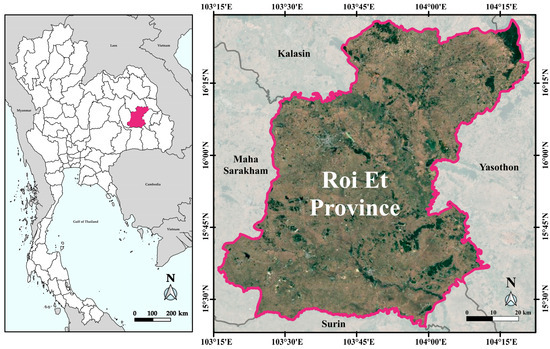
Figure 1.
Map showing the location of Roi Et Province in northeastern Thailand. The left panel highlights its position within the national context, while the right panel displays detailed provincial boundaries, major land cover, and neighboring provinces including Kalasin, Maha Sarakham, Yasothon, and Surin.
2.2. Ethnomedicinal Information
This study involved interviews with a diverse group of local informants, including community members, folk philosophers, herbalists, and local ethnobotanists. A total of 200 interviewees—equally divided between 100 males and 100 females from 20 Indigenous Isan communities in Roi Et Province—were interviewed to ensure balanced representation and inclusivity. Informants were selected based on a minimum of 5 years’ experience in wild plant collection, home gardening, or traditional medicinal practices. To ensure representativeness, participants were chosen to reflect gender balance and geographic coverage across the 20 districts of Roi Et Province. The goal was to gather in-depth knowledge about regional plant use. Structured questionnaires were used to collect detailed information on key aspects of each plant, such as local names, traditional uses, and perceived medicinal properties. This approach captured both practical knowledge and cultural perspectives, contributing to a comprehensive understanding of the plants’ ethnobotanical significance in the local context. However, since participants were randomly selected and the interviews focused solely on gathering information about plant names, parts used, and purposes of use, no personal or demographic data—including age—were recorded. On average, each informant mentioned approximately 7–10 plant taxa during the interviews. The interviews followed the ethical principles of the International Society of Ethnobiology Code of Ethics (ISE). Since no personal or sensitive information was collected, formal ethical approval was not required.
Field surveys were conducted from January 2023 to December 2023 to collect specimens and document wild edible fruit species throughout Roi Et Province. These surveys focused on collecting specimens in community forests. In protected areas and national parks, efforts were made to minimize ecological impact, with only photographic documentation and field notes being collected, and no specimens were taken. Specimens preserved in 70% ethanol were stored at the Vascular Plant Herbarium, Mahasarakham University (VMSU) in Thailand.
The morphological features of the collected wild edible fruit specimens were analyzed using a stereo microscope. These specimens were compared to protologue descriptions or other established species references, such as those found in the flora of Thailand, to confirm accurate species identification.
This process involved reviewing the primary taxonomic literature and major research databases, including Scopus, Web of Science, and Google Scholar. Additional information was gathered from the Kew Herbarium, the Plants of the World Online (accessed on 25 June 2025), and online collections, such as the Museum National d’Histoire Naturelle (https://www.mnhn.fr/en) (accessed on 25 June 2025). Furthermore, digital images and data from several renowned herbarium collections—including those at Aarhus University Herbarium (AU), The Forest Herbarium (BKF), Royal Botanic Garden Edinburgh Herbarium (E), Faculty of Forestry Herbarium (FOF), Royal Botanic Gardens Herbarium (K), National Museum of Natural History (P), Queen Sirikit Botanical Garden Herbarium (QBG), and Singapore Botanic Gardens Herbarium (SING)—were reviewed to ensure that the comparative analysis is based on reliable, accurate, and comprehensive data.
The purpose of this comparison was to highlight any changes that may have occurred between the existing literature and current medicinal data, as well as to identify the key species for future phytochemical, pharmaceutical, and nutritional analysis.
2.3. Phenology Study
This study on the phenology of wild edible fruit plants in Roi Et Province focused on documenting the flowering and fruiting patterns of these plants. Monthly field trips were conducted throughout the study year (January to December 2023), during which the phenological state (flowering and/or fruiting) of individual plants was recorded. For each species encountered, all individuals observed in flowering or fruiting stages were noted, and their phenological status was documented. Each month was assigned a numerical value from 1 to 12, corresponding to January through December, and phenological data were analyzed in relation to rainfall records and presented graphically to explore their connections. The number of individuals observed per species varied according to availability during field surveys.
2.4. Conservation Status Assessment
The conservation status of the wild edible fruit species in Roi Et Province was evaluated based on the criteria outlined by the International Union for Conservation of Nature (IUCN) Red List [28]. This assessment involved a thorough review of the species’ population sizes, distribution ranges, and potential threats to their survival, including habitat loss, over-exploitation, and environmental changes.
2.5. Data Analysis
This study employed multiple ethnobotanical indices to evaluate the importance of wild edible fruit plants from various dimensions. The Species Use Value (SUV) reflects the relative importance of each species based on the frequency of mention by informants. The Informant Consensus Factor (Fic) measures the level of agreement among informants regarding the use of plants for specific ailment categories. The Relative Frequency of Citation (RFC) indicates how commonly each species is mentioned across the sample population. Fidelity Level (FL) identifies the specificity of a plant’s use for a particular purpose. Together, these indices provide a multidimensional framework for prioritizing species with the greatest potential for further agronomic trials and sustainable utilization.
2.5.1. Species Use Value (SUV)
The Species Use Value (SUV) indicates the importance of a wild edible plant species as understood by the people of Roi Et Province. The SUV is calculated using the method outlined by Hoffman and Gallaher [29] and is expressed by the following formula:
In this equation, UVis represents the use values provided by individual informants for a specific wild edible plant species, while Ni represents the total number of informants. The SUV is calculated by adding the use values reported by all informants for a given species and dividing the sum by the total number of respondents.
2.5.2. Relative Frequency of Citation (RFC)
The Relative Frequency of Citation (RFC) was utilized to quantify the significance of various plant species based on the number of informants who recognized their use. The RFC index was calculated using the following formula [30]:
where FC represents the number of informants who mentioned a particular wild edible plant and N is the total number of participants surveyed. The RFC values range from 0 to 1, with higher values indicating greater recognition and importance of a species within the community. This index was instrumental in ranking the studied wild edible plants according to their relative significance, providing insights into the extent of their ethnobotanical use and local relevance.
RFC = FC/N
2.5.3. Informant Consensus Factor (Fic)
The Informant Consensus Factor (Fic) is calculated using the following formula [31]:
In this formula, nur refers to the total number of use reports for each disease category, and nt represents the number of species used within that category. Fic provides insight into the degree of agreement among informants regarding the use of edible wild fruits and assesses their consensus in selecting specific edible wild fruits for treating reported diseases. A high Fic value, approaching 1, suggests that widely recognized species are used by a significant portion of the local population due to their proven effectiveness in disease treatment. Conversely, a low Fic value, closer to 0, indicates a lack of agreement among informants, with species being chosen randomly to treat diseases.
2.5.4. Fidelity Level (%FL)
The Fidelity Level (FL) indicates the preference for a specific plant species in treating a particular disease or syndrome, as understood by the people of the region. The FL is expressed by the following formula [32]:
Here, Ip represents the number of informants who linked the plant to a specific health condition, while Iu denotes the total number of informants who acknowledged the plant’s medicinal use for any ailment.
2.5.5. The Economic Value of Wild Edible Fruits (EVWF)
The Economic Value of Wild Edible Fruits (EVWF) reflects the economic importance of wild edible plant species as perceived by traders and consumers in the study region. It is calculated using the following formula [33]:
where AP is the average price (THB/kg) of the species, SM is the total quantity sold by traders per month (kg), and SP is the number of months the species is traded annually. Multiplying these factors provides an estimate of the total annual economic value of each wild edible fruit species traded in the region.
3. Results
3.1. Diversity of Wild Edible Fruit Plants in Roi Et Province
This study identified a diverse range of wild edible fruit plants used in ethnomedicine by Indigenous Isan communities in Roi Et Province, comprising 68 species from 32 families (Table 1 and Figure 2). The Annonaceae family exhibited the highest diversity, with eight species, demonstrating its essential role in local diets. The Fabaceae family followed closely, with seven species, reflecting the importance of legumes in the community’s ethnobotanical practices. Other families with notable diversity included Ebenaceae, Phyllanthaceae, and Sapindaceae, each represented by four species. Additionally, the Moraceae and Burseraceae families contributed three and two species, respectively, indicating their significance in traditional uses. Other families, such as Anacardiaceae, Apocynaceae, Burseraceae, Malvaceae, Myrtaceae, Passifloraceae, Rhamnaceae, Rubiaceae, and Sapotaceae, were each represented by two species, while a few families contributed one species each.

Table 1.
Diversity of wild edible fruit plants used in ethnomedicine in Roi Et Province, Thailand, together with their vernacular name, distribution, flowering and fruiting periods, used parts, group of symptoms, SUV, RFC, conservation status, and voucher specimen numbers.
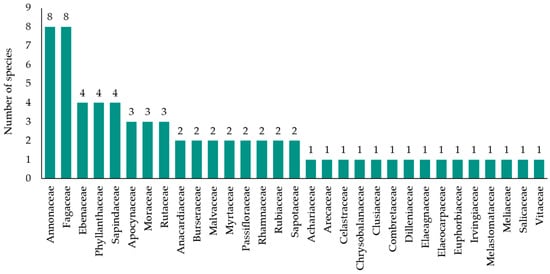
Figure 2.
Diversity of wild edible fruit plants in Roi Et Province.
Some representative examples of wild edible fruit plants documented in Roi Et Province are illustrated in Figure 3.

Figure 3.
Some representative examples of wild edible fruit plants documented in Roi Et Province: (a) Polyalthia evecta (Pierre) Finet & Gagnep.; (b) Uvaria rufa (Dunal) Blume; (c) Urceola polymorpha (Pierre ex Spire) D.J.Middleton & Livsh.; (d) Willughbeia edulis Roxb.; (e) Terminalia chebula Retz.; (f) Diospyros decandra Lour.; (g) Dialium cochinchinense Pierre; (h) Sindora siamensis Teijsm. ex Miq.; (i) Tamarindus indica L.; (j) Irvingia malayana Oliv. ex A.W.Benn.; (k) Sandoricum koetjape (Burm.f.) Merr.; (l) Syzygium antisepticum (Blume) Merr. & L.M.Perry; (m) Syzygium cumini (L.) Skeels; (n) Adenia viridiflora Craib; (o) Passiflora foetida L.; (p) Ziziphus mauritiana Lam.; (q) Ziziphus oenopolia (L.) Mill.; (r) Aegle marmelos (L.) Corrêa; (s) Nephelium hypoleucum Kurz; and (t) Ampelocissus martini Planch. Photographs by Tammanoon Jitpromma, except (d,f,h,k,o,r) by Thawatphong Boonma.
3.2. Phenology of Wild Edible Fruit Plants in Roi Et Province
The phenological patterns of wild edible fruit species in Roi Et Province exhibited distinct seasonal variations in both flowering and fruiting periods (Figure 4). The flowering period peaked in March and April, with the highest number of species (~45–50) recorded. Following this peak, the number of flowering species gradually declined, reaching the lowest point in October and November before showing a slight increase in December. The fruiting period showed a different trend, with a steady increase from January to May, reaching its highest point in May and June (~40 species). After this peak, fruiting remained relatively stable until August, followed by a gradual decline from September to December. The lowest fruiting activity was observed in October and November, corresponding with the lowest flowering activity.
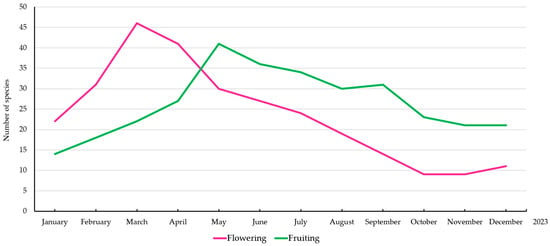
Figure 4.
Phenology of wild edible fruit plants in Roi Et Province.
3.3. Species Use Value (SUV) of Wild Edible Fruit Plants in Roi Et Province
A detailed list of medicinal plant species and their corresponding Species Use Values (SUVs) is provided in Table 1. The high Species Use Value (≥0.40) category is dominated by plants that play significant roles in traditional medicinal practices, with a high frequency of use for treating various health conditions. Among the most highly valued species is Aegle marmelos (SUV = 0.48), followed closely by Uvaria rufa (SUV = 0.48), both of which are crucial for their medicinal properties. Other plants with high SUV values (≥0.40) include Irvingia malayana (SUV = 0.41), Tamarindus indica (SUV = 0.40), and Mangifera caloneura (SUV = 0.38). These species are considered vital in local healthcare systems, being frequently used for a variety of ailments.
The medium Species Use Value (0.30–0.39) category includes plants that have a moderate but important role in traditional medicine. These species are commonly used in various regions but are not as universally essential as those in the high-use category. Notable species in this category include Phyllanthus emblica (SUV = 0.38), Ziziphus mauritiana (SUV = 0.38), and Xylopia vielana (SUV = 0.44), indicating their moderate yet notable presence in medicinal applications. Other species such as Diospyros decandra (SUV = 0.34), Cajanus cajan (SUV = 0.36), and Castanopsis piriformis (SUV = 0.34) are also valued for their therapeutic properties, though they may not be as frequently used as high SUV species.
The low Species Use Value category (<0.30) comprises medicinal plants that have relatively limited use in traditional healing systems. While these species may hold local significance and are sometimes used for specific treatments, their overall contribution to medicinal practices is minimal. These species include Streblus asper (SUV = 0.20), Adenanthera pavonina (SUV = 0.21), and Diospyros filipendula (SUV = 0.21). Although these plants may have specialized uses in certain cultures or regions, their overall medicinal utility is restricted when compared to species with higher SUV values.
3.4. Relative Frequency of Citation (RFC) of Wild Edible Fruit Plants in Roi Et Province
Based on the interview data (Table 2), the results indicate that certain wild edible fruit species hold significant cultural and practical importance in Roi Et Province. The Relative Frequency of Citation (RFC) values highlight the most frequently mentioned species, while the Species Use Value (SUV) reflects the extent of their applications within the community.

Table 2.
Comparison of the Top 12 RFCs with the SUV of wild edible fruit plants in Roi Et Province.
Among the species documented, Uvaria rufa had the highest SUV (0.48) and RFC (0.44), suggesting that it is widely recognized and frequently utilized. Xylopia vielana and Finlaysonia pierrei also exhibited high RFC values (0.41 and 0.40, respectively), indicating their strong presence in local knowledge systems. These species are commonly used for both dietary and medicinal purposes, reinforcing their importance in traditional practices.
Species such as Mangifera caloneura (SUV = 0.38, RFC = 0.33) and Calamus reinwardtii (SUV = 0.35, RFC = 0.33) were also frequently cited but had slightly lower SUV values, suggesting that while they are well known, their specific uses may be more limited compared to the highest-ranking species. Similarly, Terminalia chebula and Protium serratum had moderate and identical RFC (0.31) values, indicating their consistent presence in local practices, albeit with less versatility in usage.
Several other species, including Sandoricum koetjape, Passiflora foetida, Ziziphus mauritiana, and Nephelium hypoleucum, shared an RFC of 0.31 and similar SUV values, highlighting their steady but somewhat lower prominence within the community. The results suggest that species with high RFC values are more frequently mentioned, whereas those with higher SUV values are recognized for their multiple applications.
3.5. Informant Consensus Factor (Fic) of Wild Edible Fruit Plants in Roi Et Province
The Informant Consensus Factor (Fic) offers insight into the level of consensus among informants regarding the use of species for different health conditions (Table 3). The IAR values across various medical categories, along with the number of species used, indicate varying degrees of agreement.

Table 3.
Fic for medical categories of wild edible fruit plants in Roi Et Province.
The lymphatic system category showed the highest Fic of 0.91, based on just two species, suggesting a strong consensus among informants. The categories of poisoning and toxicology (7 species, Fic = 0.87) and gastrointestinal (35 species, Fic = 0.85) also demonstrated notable agreement, reflecting a general alignment in the use of species for these conditions. Infection, parasite, and immune system (28 species, Fic = 0.85) and musculoskeletal and joint diseases (13 species, Fic = 0.85) followed with similarly strong agreement, indicating consistency in informants’ knowledge.
Moderate agreement was seen in categories like obstetrics, gynecology, and urinary disorders (26 species, Fic = 0.83) and cancer (6 species, Fic = 0.84), where some variation in responses was present. Other categories, such as the respiratory system (8 species, Fic = 0.85) and skin system (15 species, Fic = 0.88), showed relatively strong agreement, though slightly more variability among informants’ reports.
The eyes category had the lowest Fic of 0.70, based on just four species, indicating less consensus compared to other conditions.
These findings reflect the varying levels of agreement across medical categories, showcasing the value of traditional ecological knowledge in the selection of species for health treatments. The results support the reliability of informant knowledge, although some categories show more variation than others.
3.6. Fidelity Level (FL) of Wild Edible Fruit Plants in Roi Et Province
A total of sixty-eight plant species were identified as medicinal in Roi Et Province. Among them, 14 species attained the highest FL value of 100, including Artabotrys spinosus, Dasymaschalon lomentaceum, Uvaria ferruginea var. cherrevensis, Xylopia vielana, Finlaysonia pierrei, Protium serratum, Diospyros decandra, Diospyros filipendula, Dialium cochinchinense, Castanopsis piriformis, Grewia hirsute, Passiflora foetida, and Canthium berberidifolium.
Tamarindus indica and Phyllanthus emblica are widely recognized for their medicinal properties, with different parts of these plants used for various health conditions. For T. indica, the bark exhibits the highest Fidelity Level (FL) at 18.92% and is primarily consumed for its antipyretic properties. The roots (16.22%) are used to treat infections, parasites, and immune system disorders, while the heartwood (13.51%) is consumed for obstetric, gynecological, and urinary issues. The inflorescences (13.51%) support cardiovascular health, and the seeds (13.51%) are used in cases of poisoning and toxicology. The leaves (10.81%) and fruits (5.41%) contribute to gastrointestinal health, while external applications (8.11%) are used for eye-related conditions. Similarly, Phyllanthus emblica is highly valued in traditional medicine. The bark (15.79%) is widely used for treating infections, parasites, and immune-related disorders, while the highest FL value is recorded for bathing applications (18.42%) to reduce fever. The inflorescences (15.79%) aid gastrointestinal health, and the fruits (13.16%) are beneficial for obstetric, gynecological, and urinary concerns. The leaves (13.16%) support cardiovascular health, while the roots (10.53%) are used in cancer treatment. External applications (5.26%) are used for eye-related conditions, and the seeds (7.89%) provide respiratory system benefits. Each part of these plants plays a significant role in traditional medicine, highlighting their importance in natural healing practices.
For the remaining plant species, fewer than eight categories of disease symptoms were documented, as shown in Table S1.
3.7. The Economic Value of Wild Edible Fruit Plants in Roi Et Province
This study identified 13 species of wild edible fruit plants harvested for trade (Table 4), with the fruit being the only part sold, representing 100% of the harvest. The wild edible fruit plants in Roi Et Province yield the highest value from Aegle marmelos, generating an average annual income of 100,800 THB per trader. This is attributed to its higher price range of 120 to 90 THB/kg and a trading period spanning around 3 months. Following this, Elaeocarpus hygrophilus, Phyllanthus emblica, and Sandoricum koetjape generate average annual earnings of 18,600 THB, 17,000 THB, and 12,000 THB per trader, respectively, with trading periods ranging from 4 to 8 months. These plants hold significant economic value primarily because they are cultivated as cash crops, enabling large-scale production and harvesting. Their abundance makes them readily available in the market, contributing to their widespread popularity and increasing demand for consumption. Other plants are also commonly consumed; however, the evaluation results indicate that they hold lower economic value. This is primarily due to the fact that they are not widely cultivated as large-scale cash crops, though they are grown on a smaller scale in some home gardens.

Table 4.
Scientific names of wild edible fruit plants and their prices and trading period in Roi Et Province, Thailand.
3.8. Conservation Status
Based on field surveys conducted in Roi Et Province, northeastern Thailand, Diospyros mollis is proposed to be assessed as Critically Endangered (CR C2a(i,ii), D1) under the IUCN Red List Categories and Criteria [28]. This assessment is based on the extremely small population size, estimated at fewer than 50 mature individuals, all confined to a single locality, with continuing decline observed in both the number of mature individuals and subpopulations. In addition, 24 species (Table 1) recorded during this study are proposed as Vulnerable (VU C2a(i), D1) due to their restricted population sizes (fewer than 1000 mature individuals), small subpopulations, and observed declines. Meanwhile, 37 species are proposed to be categorized as Least Concern (LC), as they are relatively widespread and exhibit no significant threats or declining trends at present.
4. Discussion
Diversity and Cultural Relevance: The diversity of wild edible fruit plants in Roi Et Province underscores their significant role in the ethnomedicine of Indigenous Isan communities. The identification of 68 species from 32 families highlights the rich botanical knowledge and reliance on these plants for both nutritional and medicinal purposes. This biodiversity emphasizes the cultural and ecological relevance of wild edible fruit plants within the Indigenous Isan communities [14,34]. Their ethnomedicinal applications, ranging from treating various ailments to supplementing daily nutritional intake, showcase the intricate relationship between the community and their surrounding flora [35].
Seasonal Phenology Patterns: The phenological patterns of wild edible fruit species in Roi Et Province exhibit distinct seasonal variations, with flowering activity peaking in March and April, and fruiting reaching a maximum in May and June. These trends align with studies in tropical and subtropical climates where environmental factors such as temperature, rainfall, and photoperiod regulate plant phenology [36,37,38,39,40,41,42,43]. The observed seasonality implies a reproductive strategy synchronized with favorable climatic conditions [44,45,46,47,48].
Cultural and Medicinal Use Value: The Species Use Value (SUV) analysis reveals varying degrees of medicinal importance among wild edible fruit plants. High SUV species are frequently used for diverse ailments, indicating their integral role in traditional medicine [49,50,51]. Medium and low SUV species contribute to traditional healthcare with specialized or occasional roles, highlighting the spectrum of ethnobotanical knowledge [15,52,53,54]. Understanding these different use levels can help prioritize species for conservation and further scientific validation [55,56,57].
Ethnobotanical Significance Based on Citation: The Relative Frequency of Citation (RFC) and SUV values jointly reveal species that are culturally prominent and functionally significant. Uvaria rufa, Xylopia vielana, and Finlaysonia pierrei scored high in both indices, emphasizing their role in food and medicine [58,59,60]. Species like Mangifera caloneura and Calamus reinwardtii, though slightly lower in SUV, are consistently referenced, suggesting cultural familiarity [61,62,63].
Informant Consensus and Medicinal Reliability: The Informant Consensus Factor (Fic) analysis shows high agreement in categories such as lymphatic and gastrointestinal disorders, indicating well-established medicinal applications [12,15,16,35,59,64,65,66,67,68,69,70,71]. A lower Fic in some categories, like eye-related conditions, may reflect limited knowledge or species availability. High Fic values suggest reliable traditional use and potential for future pharmacological studies [55,72]. The Informant Consensus Factor (Fic) values in this study show strong agreement among informants for most medicinal categories, with the highest consensus in the lymphatic system (0.91) and skin system (0.88). Lower Fic values for eyes (0.70) and cardiovascular system (0.82) suggest more diverse plant use or less uniform knowledge. Compared to the broader categories reported by Aswathi and Abdussalam [73], which had higher Fic values (0.92–0.97), our more specific medicinal categories naturally show slightly lower consensus due to greater diversity within each category. These high Fic values reflect reliable traditional knowledge and emphasize the value of detailed ethnobotanical studies to better understand plant use for specific ailments and guide future pharmacological research.
Fidelity Level and Species-Specific Applications: Fourteen species had a Fidelity Level (FL) of 100%, indicating consistent use for specific treatments. Tamarindus indica and Phyllanthus emblica stood out for their multiple medicinal applications using various plant parts, demonstrating a sophisticated understanding of plant pharmacology in the community [74,75,76,77].
Economic Importance and Horticultural Potential: Thirteen species are actively traded, contributing to local economies. Aegle marmelos leads in economic value, followed by Elaeocarpus hygrophilus and Phyllanthus emblica. These species’ cultivation as cash crops enhances their availability and market presence. Understanding these economic dynamics supports better decision making in sustainable horticultural practices and commercialization strategies [15,33,41,78]. Their dual role as both medicinal and edible plants highlights their significant horticultural potential, warranting further investigation for sustainable cultivation and broader utilization.
Conservation Priorities and Community-Led Strategies: Among the species recorded, Diospyros mollis is proposed as Critically Endangered, with 24 others classified as Vulnerable. This reflects habitat degradation and unsustainable resource use. While 37 species are Least Concern, they still warrant ongoing monitoring. Given that many threatened species are outside protected areas, incorporating Other Effective area-based Conservation Measures (OECMs) such as community forests and sacred groves can offer inclusive conservation strategies that integrate traditional land management practices [79,80,81].
Conclusion and Future Directions: This study underscores the ethnobotanical, ecological, and horticultural importance of wild edible fruit plants in Roi Et Province. Their diverse roles in healthcare, food systems, and local economies emphasize the need for conservation, sustainable management, and further research. Future studies should include phytochemical screenings, climate impact assessments, and exploration of underutilized species to enhance their application in both traditional and modern contexts.
5. Conclusions
This study highlights the profound ethnobotanical importance of wild edible fruit plants in Roi Et Province, northeastern Thailand. The documentation of 68 species from 32 families underscores the deep cultural, medicinal, ecological, and economic significance these plants hold for Indigenous Isan communities. Through their roles in traditional medicine, food security, and seasonal foraging, these species contribute to both the health and livelihoods of local people, while also supporting biodiversity and ecosystem resilience.
The phenological observations reveal distinct reproductive patterns, with flowering peaking in March–April and fruiting in May–June, suggesting strong alignment with seasonal climatic cues. These insights not only inform sustainable harvesting and agroforestry planning but also highlight the potential vulnerability of these species to climate change. The variation in Species Use Value (SUV) and Relative Frequency of Citation (RFC) further reflects the dynamic nature of traditional knowledge, where certain species serve as core elements in local healthcare and nutrition, while others fulfill more specialized roles.
Economic assessments indicate that some wild fruit species, particularly Aegle marmelos, contribute substantially to local income through small-scale trade, offering potential avenues for sustainable rural development. However, conservation assessments raise serious concerns, with species such as Diospyros mollis proposed as Critically Endangered and many others considered Vulnerable. These findings point to urgent threats from habitat loss, overharvesting, and land-use changes.
To safeguard this rich biocultural heritage, integrated conservation strategies are essential. The inclusion of Other Effective area-based Conservation Measures (OECMs), such as community forests and sacred groves, offers a practical and culturally respectful path to expand biodiversity protection beyond formal reserves. Furthermore, continued ethnobotanical research, phytochemical studies, and participatory conservation planning are needed to ensure the sustainable use and transmission of traditional knowledge.
In conclusion, wild edible fruit plants in Roi Et Province are more than biological resources—they are integral to cultural identity, health systems, local economies, and ecological sustainability. Protecting and valuing this botanical diversity is not only a conservation imperative but also a means of empowering local communities and reinforcing traditional practices in the face of rapid environmental and social change.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11080885/s1: Table S1: Fidelity Level (FL) of wild edible fruit plants used as medicinal plants in Roi Et Province.
Author Contributions
Conceptualization, P.S., S.S., T.B., A.J., M.K.N. and T.J.; methodology, P.S., S.S., T.B., A.J., M.K.N. and T.J.; software, T.B. and T.J.; validation, S.S., T.B. and T.J.; formal analysis, P.S., S.S., T.B., A.J., M.K.N. and T.J.; investigation, S.S., T.B., and T.J.; resources, S.S., T.B. and T.J.; data curation, S.S., T.B. and T.J.; writing—original draft preparation, T.B. and T.J.; writing—review and editing, P.S., S.S., T.B., A.J., M.K.N. and T.J.; visualization, T.B. and T.J.; supervision, P.S. and S.S.; project administration, P.S. and S.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was financially supported by Mahasarakham University.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors sincerely extend their gratitude to the Indigenous Isan communities in Roi Et Province for their invaluable knowledge, warm hospitality, and cooperation throughout this study. We deeply appreciate the local elders, herbalists, and community leaders for generously sharing their time and insights, which have been instrumental in documenting traditional practices. Our heartfelt thanks also go to Saisamorn Jitpromma and Sa-ngad Jitpromma for their invaluable assistance during the field trips conducted for this project. Additionally, we acknowledge the support from the Walai Rukhavej Botanical Research Institute, Mahasarakham University, for providing research facilities and funding assistance. We are also grateful for the guidance and constructive feedback from our colleagues and advisors, which have greatly contributed to the success of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Used parts | |
| Bark | (Ba) |
| Fruit | (Fr) |
| Heartwood | (Hw) |
| Inflorescence | (Fl) |
| Latex | (La) |
| Leave | (Le) |
| Root | (Ro) |
| Seed | (Se) |
| Stem | (St) |
| Whole plant | (Wh) |
| Group of symptoms | |
| Antipyretics | (Ant) |
| Cancer | (Can) |
| Cardiovascular system | (Car) |
| Central nervous system | (Cen) |
| Eyes | (Eye) |
| Gastrointestinal | (Gas) |
| Infection, parasite, and immune system | (Inf) |
| Lymphatic system | (Lym) |
| Musculoskeletal and joint diseases | (Mus) |
| Nutrition and blood | (Nut) |
| Obstetrics, gynecology, and urinary disorders | (Obs) |
| Poisoning and toxicology | (Poi) |
| Respiratory system | (Res) |
| Skin system | (Ski) |
| Conservation status | |
| Critically Endangered | (CR) |
| Data deficient | (DD) |
| Least Concern | (LC) |
| Near threatened | (NT) |
| Not evaluated | (NE) |
References
- Melaku, A.; Ebrahim, M.A. Critical review on wild-edible fruit species in Ethiopia. Int. J. For. Res. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Bhatt, I.D.; Rawat, S.; Badhani, A.; Rawal, R.S. Nutraceutical potential of selected wild edible fruits of the Indian Himalayan region. Foods 2017, 6, 84–91. [Google Scholar] [CrossRef]
- Suwardi, A.B.; Navia, Z.I.; Harmawan, T.; Syamsuardi, S.; Mukhtar, E. Wild edible fruits generate substantial income for local people of the Gunung Leuser National Park, Aceh Tamiang Region. Ethnobot. Res. Appl. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Heywood, V.H. Ethnopharmacology, food production, nutrition, and biodiversity conservation: Towards a sustainable future for indigenous peoples. J. Ethnopharmacol. 2011, 137, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C.F.R. Wild Edible plants: Nutritional and toxicological characteristics, retrieval strategies, and importance for Today’s Society. Food Chem. Toxicol. 2017, 110, 165–188. [Google Scholar] [CrossRef]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.K.; Kumar, K. Traditionally used wild edible plants of District Udhampur, J&K, India. J. Ethnobiol. Ethnomed. 2018, 14, 73. [Google Scholar] [CrossRef]
- Aryal, K.P.; Poudel, S.; Chaudhary, R.P.; Chettri, N.; Chaudhary, P.; Ning, W.; Kotru, R. Diversity and use of wild and non-cultivated edible plants in the Western Himalaya. J. Ethnobiol. Ethnomed. 2018, 14, 10. [Google Scholar] [CrossRef]
- Suwardi, A.B.; Harmawan, T.; Navia, Z.I.; Syamsuardi, S. The diversity of wild edible fruit plants and traditional knowledge in West Aceh Region, Indonesia. J. Med. Plants Stud. 2019, 7, 285–290. [Google Scholar]
- Talucder, M.S.A.; Ruba, U.B.; Robi, M.A.S. Potentiality of neglected and underutilized species (NUS) as a future resilient food: A systematic review. J. Agric. Food Res. 2024, 16, 101116. [Google Scholar] [CrossRef]
- Casanova-Pérez, L.; Cruz-Bautista, P.; San Juan-Martínez, A.; García-Alonso, F.; Barrios, F. Underutilized food plants and their potential contribution to food security: Lessons learned from the local context. Agroecol. Sustain. Food Syst. 2024, 48, 1265–1288. [Google Scholar] [CrossRef]
- Singh, M.; Griaud, C.; Collins, C.M. An evaluation of the effectiveness of protected areas in Thailand. Ecol. Indic. 2021, 125, 107536. [Google Scholar] [CrossRef]
- Saensouk, S.; Saensouk, P.; Ragsasilp, A.; Senakun, C.; Daovisan, H.; Setyawan, A.D.; Niamngon, T.; Niamngon, P.; Appamaraka, S. Medical ethnobotany and utilization of medicinal plants in the Don Pu Ta Forest Thai Yoi ethnic groups, Sakon Nakhon Province in the Northeastern Thailand. Biodiversitas 2024, 25, 3014–3031. [Google Scholar] [CrossRef]
- Cruz-Garcia, G.S.; Struik, P.C.; Johnson, D.E. Wild harvest: Distribution and diversity of wild food plants in rice ecosystems of Northeast Thailand. NJAS Wagening. J. Life Sci. 2016, 78, 1–11. [Google Scholar] [CrossRef]
- Niamngon, T.; Saensouk, S.; Saensou, P.; Junsongduang, A. Ethnobotanical study of the Lao Isan Ethnic Group in Pho Chai District, Roi Et Province, Northeastern Thailand. Trop. J. Nat. Prod. Res. 2024, 8, 6152–6181. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Hein, K.Z.; Appamaraka, S.; Maknoi, C.; Souladeth, P.; Koompoot, K.; Sonthongphithak, P.; Boonma, T.; Jitpromma, T. Diversity, ethnobotany, and horticultural potential of local vegetables in Chai Chumphol Temple Community Market, Maha Sarakham Province, Thailand. Horticulturae 2025, 11, 243. [Google Scholar] [CrossRef]
- Junsongduang, A.; Kasemwan, W.; Lumjoomjung, S.; Sabprachai, W.; Tanming, W.; Balslev, H. Ethnomedicinal knowledge of traditional healers in Roi Et, Thailand. Plants 2020, 9, 1177. [Google Scholar] [CrossRef]
- Zouraris, D.; Graikou, K.; Vasileiou, P.; Dimitrov, V.; Dajic Stevanovic, Z.; Bilia, A.R.; Zivkovic, J.; Dias, A.; Kasiotis, K.; Gardikis, K.; et al. EthnoHERBS: Harnessing traditional herbal knowledge for biodiversity conservation and innovative health solutions. Comput. Struct. Biotechnol. J. 2025, 29, 85–94. [Google Scholar] [CrossRef]
- González-Zamorano, L.; Cámara, R.M.; Morales, P.; Cámara, M. Harnessing edible wild fruits: Sustainability and health aspects. Nutrients 2025, 17, 412. [Google Scholar] [CrossRef]
- Gillani, S.W.; Ahmad, M.; Manzoor, M.; Waheed, M.; Iqbal, Z.; Ullah, R.; Pieroni, A.; Zhang, L.; Sulaiman, N.; Alrhmoun, M. The nexus between ecology of foraging and food security: Cross-cultural perceptions of wild food plants in Kashmir Himalaya. J. Ethnobiol. Ethnomed. 2024, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Oduor, F.; Kaindi, D.M.; Abong, G.; Thuita, F.; Termote, C. Community-based conservation strategies for wild edible plants in Turkana County, Kenya. Conservation 2025, 5, 1. [Google Scholar] [CrossRef]
- Sardeshpande, M.; Shackleton, C. Wild edible fruits: A systematic review of an under-researched multifunctional NTFP (non-timber forest product). Forests 2019, 10, 467. [Google Scholar] [CrossRef]
- Peduruhewa, P.; Jayathunge, L.; Liyanage, R. Potential of underutilized wild edible plants as the food for the future—A review. Sci. Rep. 2021, 9, 136–147. [Google Scholar] [CrossRef]
- Li, X.; Yadav, R.; Siddique, K.H.M. Neglected and underutilized crop species: The key to improving dietary diversity and fighting hunger and malnutrition in Asia and the Pacific. Front. Nutr. 2020, 7, 593711. [Google Scholar] [CrossRef]
- Cozzolino, A.; Motti, R.; Cartenì, F.; De Magistris, A.; Gherardelli, M.; Vitasović-Kosić, I. Horticultural food plants in traditional herbal medicine in the Mediterranean Basin: A review. Horticulturae 2024, 10, 684. [Google Scholar] [CrossRef]
- Hunter, D.; Borelli, T.; Beltrame, D.M.O.; Oliveira, C.N.S.; Coradin, L.; Wasike, V.W.; Wasilwa, L.; Mwai, J.; Manjella, A.; Samarasinghe, G.W.L.; et al. The potential of neglected and underutilized species for improving diets and nutrition. Planta 2019, 250, 709–729. [Google Scholar] [CrossRef] [PubMed]
- Yaregal, Y.; Sime, G. Traditional home garden agro-biodiversity dynamics, agro-ecosystem services, and management practices in smallholder farmers’ setting, South-Central Ethiopia. Food Energy Secur. 2024, 13, e569. [Google Scholar] [CrossRef]
- Khakurel, D.; Uprety, Y.; Ahn, G.; Cha, J.-Y.; Kim, W.-Y.; Lee, S.-H.; Rajbhandary, S. Diversity, distribution, and sustainability of traditional medicinal plants in Kaski District, western Nepal. Front. Pharmacol. 2022, 13, 1076351. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria Version 16. 2024. Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf (accessed on 1 January 2025).
- Hoffman, B.; Gallaher, T. Importance indices in ethnobotany. Ethnobotany Res. Appl. 2007, 5, 201–218. Available online: https://ethnobotanyjournal.org/index.php/era/article/view/130/115 (accessed on 1 July 2025). [CrossRef]
- Tardío, J.; Pardo-de-Santayana, M. Cultural importance indices: A comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain). Econ. Bot. 2008, 62, 24–39. [Google Scholar] [CrossRef]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. Medicinal plants in Mexico: Healers’ consensus and cultural importance. Soc. Sci. Med. 1998, 47, 1859–1871. [Google Scholar] [CrossRef]
- Friedman, J.; Yaniv, Z.; Dafni, A.; Palewitch, D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Phatlamphu, N.; Saensouk, S.; Saensouk, P.; Junsongduang, A.; Setyawan, A.D. Economic value assessment of edible plants in Muang District, Kalasin Province, Thailand. Biodiversitas 2023, 24, 3960–3967. [Google Scholar] [CrossRef]
- Edmondson, J.L. Sustainable urban horticulture—Providing more than just food. Cell Rep. Sustain. 2024, 1, 100011. [Google Scholar] [CrossRef]
- Qari, S.; Alqethami, A.; Qumsani, A. Ethnomedicinal evaluation of medicinal plants used for therapies by men and women in rural and urban communities in Makkah District. Saudi Pharm. J. 2023, 32, 101881. [Google Scholar] [CrossRef]
- Cho, L.-H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef]
- Gaudinier, A.; Blackman, B.K. Evolutionary processes from the perspective of flowering time diversity. New Phytol. 2020, 225, 1883–1898. [Google Scholar] [CrossRef] [PubMed]
- Saensouk, P.; Saensouk, S.; Rakarcha, S.; Boonma, T.; Jitpromma, T.; Sonthongphithak, P.; Ragsasilp, A.; Souladeth, P. Diversity and local uses of the Convolvulaceae family in Udon Thani Province, Thailand, with notes on its potential horticultural significance. Horticulturae 2025, 11, 312. [Google Scholar] [CrossRef]
- Satake, A.; Nagahama, A.; Sasaki, E. A cross-scale approach to unravel the molecular basis of plant phenology in temperate and tropical climates. New Phytol. 2022, 233, 2340–2353. [Google Scholar] [CrossRef]
- Rimpika; Jain, S.; Rathod, M.; Banjare, R.; Nidhi, N.; Sood, A.; Shilpa; Sharma, R. Physiological aspects of flowering, fruit setting, fruit development and fruit drop, regulation and their manipulation: A review. Int. J. Environ. Clim. Change 2023, 13, 205–224. [Google Scholar] [CrossRef]
- Jitpromma, T.; Saensouk, S.; Saensouk, P.; Boonma, T. Diversity, traditional uses, economic values, and conservation status of Zingiberaceae in Kalasin Province, Northeastern Thailand. Horticulturae 2025, 11, 247. [Google Scholar] [CrossRef]
- McLaren, K.P.; McDonald, M.A. Seasonal patterns of flowering and fruiting in a dry tropical forest in Jamaica. Biotropica 2005, 37, 584–590. [Google Scholar] [CrossRef]
- Bonada, M.; Edwards, E.J.; McCarthy, M.G.; Sepúlveda, G.C.; Petrie, P.R. Impact of low rainfall during dormancy on vine productivity and development. Aust. J. Grape Wine Res. 2020, 26, 325–342. [Google Scholar] [CrossRef]
- Arenas-Corraliza, M.G.; López-Díaz, M.L.; Rolo, V.; Cáceres, Y.; Moreno, G. Phenological, morphological and physiological drivers of cereal grain yield in Mediterranean Agroforestry Systems. Agric. Ecosyst. Environ. 2022, 340, 108158. [Google Scholar] [CrossRef]
- Ssali, F.; Sheil, D. Seasonality in the equatorial tropics: Flower, fruit, and leaf phenology of montane trees in the highlands of Southwest Uganda. Biotropica 2023, 55, 680–698. [Google Scholar] [CrossRef]
- Moegenburg, S.; Levey, D. Do frugivores respond to fruit harvest? An experimental study of short-term responses. Ecology 2003, 84, 2600–2612. [Google Scholar] [CrossRef]
- Austin, M.; Smith, A.; Olsen, K.; Hoch, P.; Krakos, K.; Schmocker, S.; Miller-Struttmann, N. Climate change increases flowering duration, driving phenological reassembly and elevated co-flowering richness. New Phytol. 2024, 243, 2486–2500. [Google Scholar] [CrossRef]
- Rashidi, P. An overview of different modeling approaches to prediction of the likely effects of climate change on range shifts of species. Int. J. Phys. Sci. 2012, 7, 1878–1883. [Google Scholar] [CrossRef]
- Savić, J.; Mačukanović-Jocić, M.; Jarić, S. Medical ethnobotany on the Javor Mountain (Bosnia and Herzegovina). Eur. J. Integr. Med. 2019, 27, 52–64. [Google Scholar] [CrossRef]
- Bibi, F.; Abbas, Z.; Harun, N.; Perveen, B.; Bussmann, R.W. Indigenous knowledge and quantitative ethnobotany of the Tanawal area, Lesser Western Himalayas, Pakistan. PLoS ONE 2022, 17, e0263604. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.J.; Xu, D.P.; Zhou, T.; Zhou, Y.; Li, S.; Li, H.B. Bioactivities and health benefits of wild fruits. Int. J. Mol. Sci. 2016, 17, 1258. [Google Scholar] [CrossRef]
- Yu, F.; Groen, T.A.; Wang, T.; Skidmore, A.K.; Huang, J.; Ma, K. Climatic niche breadth can explain variation in geographical range size of alpine and subalpine plants. Int. J. Geogr. Inf. Sci. 2016, 31, 190–212. [Google Scholar] [CrossRef]
- Constant, N.L.; Tshisikhawe, M.P. Hierarchies of knowledge: Ethnobotanical knowledge, practices and beliefs of the Vhavenda in South Africa for biodiversity conservation. J. Ethnobiol. Ethnomed. 2018, 14, 56. [Google Scholar] [CrossRef]
- Astutik, S.; Pretzsch, J.; Ndzifon Kimengsi, J. Asian medicinal plants’ production and utilization potentials: A review. Sustainability 2019, 11, 5483. [Google Scholar] [CrossRef]
- Derso, Y.D.; Kassaye, M.; Fassil, A.; Derebe, B.; Nigatu, A.; Ayene, F.; Tamer, M.; Van Damme, P. Composition, medicinal values, and threats of plants used in indigenous medicine in Jawi District, Ethiopia: Implications for conservation and sustainable use. Sci. Rep. 2024, 14, 23638. [Google Scholar] [CrossRef]
- Mudau, F.N.; Chimonyo, V.G.P.; Modi, A.T.; Mabhaudhi, T. Neglected and underutilised crops: A systematic review of their potential as food and herbal medicinal crops in South Africa. Front. Pharmacol. 2022, 12, 809866. [Google Scholar] [CrossRef]
- Dubale, S.; Kebebe, D.; Zeynudin, A.; Abdissa, N.; Suleman, S. Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J. Exp. Pharmacol. 2023, 15, 51–62. [Google Scholar] [CrossRef]
- Dapar, M.L.G.; Alejandro, G.J.D.; Meve, U.; Liede-Schumann, S. Quantitative ethnopharmacological documentation and molecular confirmation of medicinal plants used by the Manobo tribe of Agusan del Sur, Philippines. J. Ethnobiol. Ethnomed. 2020, 16, 14. [Google Scholar] [CrossRef]
- Bhagawan, W.S.; Suproborini, A.; Putri, D.L.P.; Nurfatma, A.; Putra, R.T. Ethnomedicinal study, phytochemical characterization, and pharmacological confirmation of selected medicinal plant on the northern slope of Mount Wilis, East Java, Indonesia. Biodiversitas 2022, 23, 4303–4313. [Google Scholar] [CrossRef]
- Junsongduang, A.; Saensouk, S.; Balslev, H. Amnat Charoen healers in Thailand and their medicinal plants. Plants 2025, 14, 602. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, X.; Guo, C.-A.; Zhang, X.; Feng, H.; Yang, H.; Wang, Y. An ethnobotanical study of wild edible plants used by the Tibetan in the Rongjia River Valley, Tibet, China. J. Ethnobiol. Ethnomed. 2023, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Ralte, L.; Sailo, H.; Singh, Y.T. Ethnobotanical study of medicinal plants used by the indigenous community of the Western Region of Mizoram, India. J. Ethnobiol. Ethnomed. 2024, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, J.; Gan, Q.; Ke, L.; Zhang, F.; Guo, H.; Zhao, F.; Wang, Y. An ethnobotanical study of forage plants in Zhuxi County in the Qinba Mountainous Area of Central China. Plant Divers. 2021, 43, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gunasekar, C.; Abu-Yousef, I.; Majdalawieh, A.; Srinivasan, N. Pharmacognostic evaluation of Terminalia chebula standard extracts and finished products. Med. J. Chem. 2019, 8, 441. [Google Scholar] [CrossRef][Green Version]
- Vanderplank, J. A Revision of Passiflora Section Dysosmia. Curtis’s Bot. Mag. 2013, 30, 318–387. [Google Scholar] [CrossRef]
- Luu, L.K.; Thangsiri, S.; Sahasakul, Y.; Aursalung, A.; Inthachat, W.; Temviriyanukul, P.; On-Nom, N.; Chupeerach, C.; Suttisansanee, U. Nutrients, phytochemicals and in vitro disease prevention of Nephelium hypoleucum Kurz fruit. Nutrients 2023, 15, 950. [Google Scholar] [CrossRef]
- Heineberg, M.; Hanazaki, N. Dynamics of the botanical knowledge of the Laklãnõ-Xokleng indigenous people in Southern Brazil. Acta Bot. Bras. 2019, 33, 254–268. [Google Scholar] [CrossRef]
- Bruschi, P.; Sugni, M.; Moretti, A.; Signorini, M.A.; Fico, G. Children’s versus adults’ knowledge of medicinal plants: An ethnobotanical study in Tremezzina (Como, Lombardy, Italy). Rev. Bras. Farmacogn. 2019, 29, 644–655. [Google Scholar] [CrossRef]
- Bhushan, S.; Dincă, I.; Shikha, S. Evaluating local livelihoods, sustainable forest management, and the potential for ecotourism development in Kaimur Wildlife Sanctuary, India. Front. For. Glob. Change 2024, 7, 1491917. [Google Scholar] [CrossRef]
- Gitima, G.; Gebre, A.; Berhanu, Y.; Wato, T. Exploring indigenous wisdom: Ethnobotanical documentation and conservation of medicinal plants in Goba District, Southwest Ethiopia. Sci. Afr. 2025, 27, e02571. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.; Kishor, A.; Singh, S.; Bhat, M.N.; Surmal, O.; Musarella, C.M. Exploring plant-based ethnomedicine and quantitative ethnopharmacology: Medicinal plants utilized by the population of Jasrota Hill in Western Himalaya. Sustainability 2020, 12, 7526. [Google Scholar] [CrossRef]
- Chashike, A.; Shibru, S.; Gebre, T.; Uncha, A. Ethnobotanical study of traditional medicinal plants and associated indigenous knowledge in Melokoza District, South Ethiopia. Trees For. People 2025, 20, 100849. [Google Scholar] [CrossRef]
- Aswathi, V.; Abdussalam, A.K. Determination of use value and informant consensus factor on ethnobotanic knowledge about wild legumes used by natives of Wayanad district, Kerala. Indian J. Tradit. Knowl. 2021, 20, 404–415. [Google Scholar] [CrossRef]
- Kidane, L.; Gebremedhin, G.; Beyene, T. Ethnobotanical study of medicinal plants in Ganta Afeshum District, Eastern Zone of Tigray, Northern Ethiopia. J. Ethnobiol. Ethnomed. 2018, 14, 64. [Google Scholar] [CrossRef]
- Ebifa-Othieno, E.; Mugisha, A.; Nyeko, P.; Kabasa, J.D. Knowledge, attitudes and practices in tamarind (Tamarindus indica L.) use and conservation in Eastern Uganda. J. Ethnobiol. Ethnomed. 2017, 13, 5. [Google Scholar] [CrossRef]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef]
- Haque, M.I.; Chowdhury, A.B.M.A.; Shahjahan, M.; Harun, M.G.D. Traditional healing practices in rural Bangladesh: A qualitative investigation. BMC Complement. Altern. Med. 2018, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Mbelebele, Z.; Mdoda, L.; Ntlanga, S.; Nontu, Y.; Gidi, L. Harmonizing traditional knowledge with environmental preservation: Sustainable strategies for the conservation of indigenous medicinal plants (IMPs) and their implications for economic well-being. Sustainability 2024, 16, 5841. [Google Scholar] [CrossRef]
- Shukla, S. Conservation of medicinal plant. J. Med. Bot. 2023, 7, 5–10. [Google Scholar] [CrossRef]
- IUCN WCPA. Report on the Regional Workshop on “Other Effective Area-Based Conservation Measures” (OECMs) in Southern and Eastern Mediterranean Region Identifying, Advancing and Reporting OECMs. Summary of Conclusions and Recommendations. Tunis, Tunisia, 10–11 February 2020; IUCN: Gland, Switzerland; Malaga, Spain, 2020; Available online: https://iucn.org/sites/default/files/content/documents/2020/oecms_regional_workshop_report-_0.pdf (accessed on 20 May 2025).
- Sharma, M.; Pasha, M.K.S.; Nightingale, M.; MacKinnon, K. Status of Other Effective Area-Based Conservation Measures (OECMs) in Asia; IUCN Asia Regional Office: Bangkok, Thailand, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).