Peltate Glandular Trichomes in Relation to Their Parameters, Essential Oil Amount, Chemotype, Plant Sex and Habitat Characteristics in Thymus pulegioides
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil Content, Chemotype, and Sex of Thymus pulegioides Plants
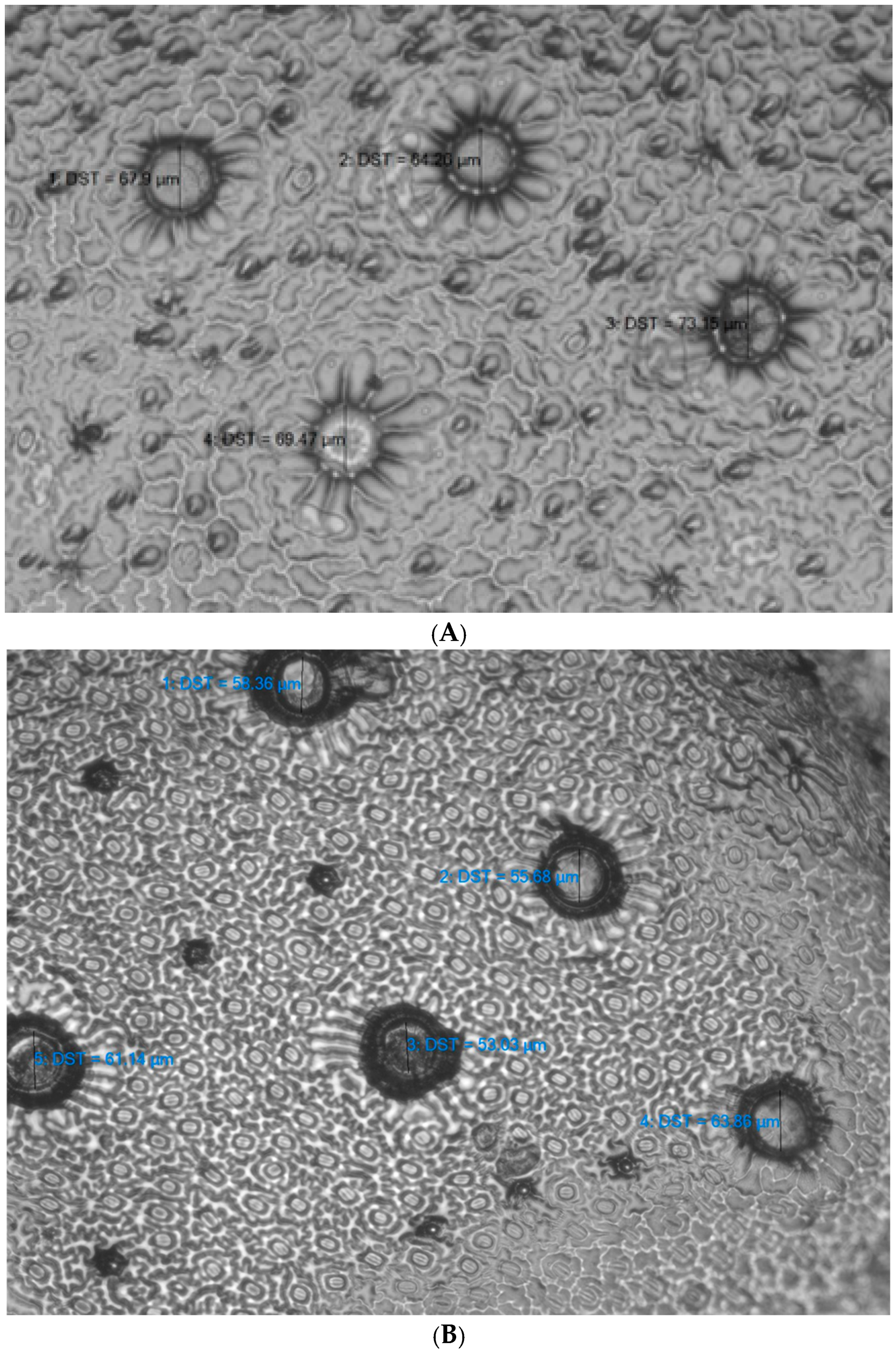
2.2. Analysis of Peltate Glandular Trichomes
2.3. Analysis of Habitats
2.4. Dynamics of Density and Diameter of Peltate Glandular Trichomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Thymus pulegioides Habitats
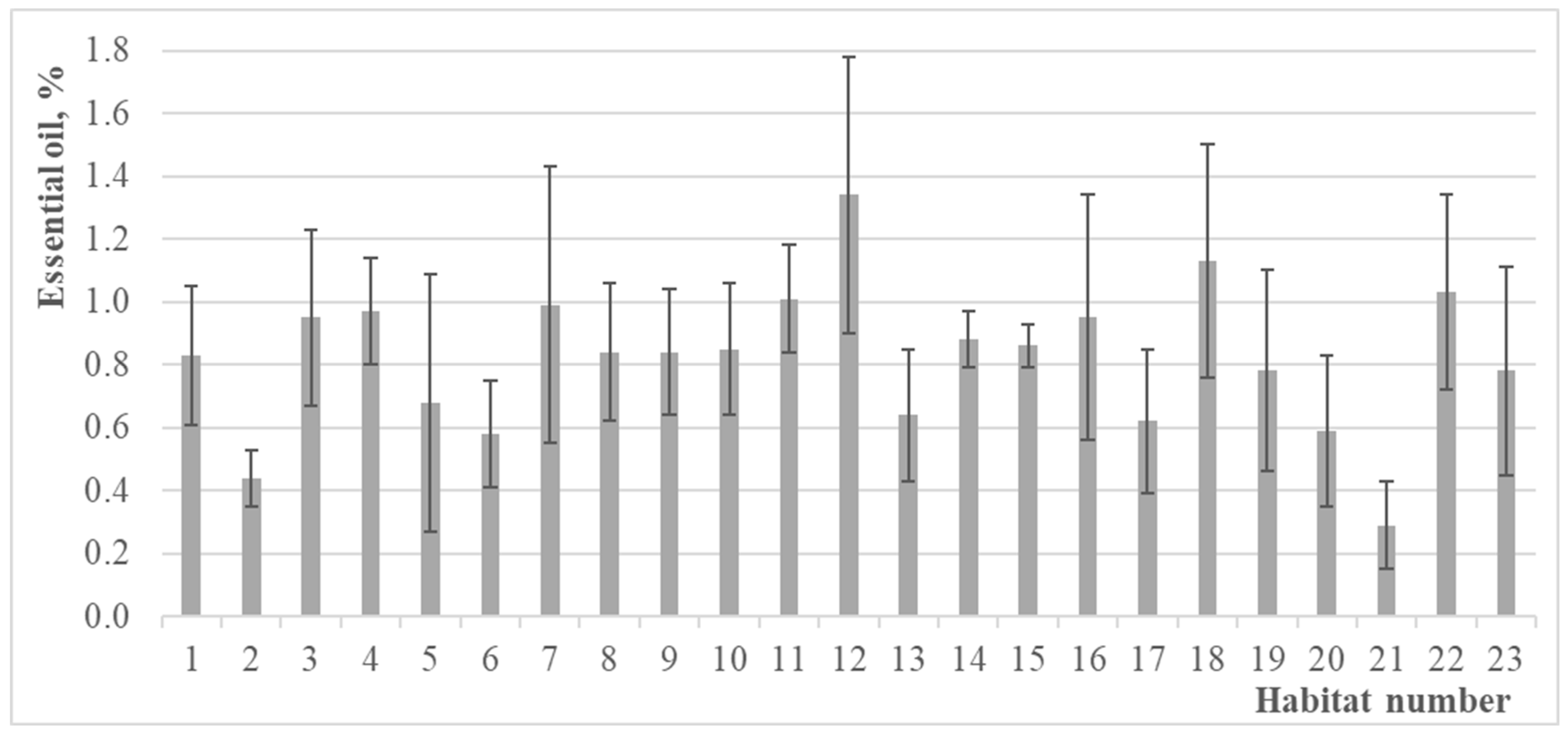
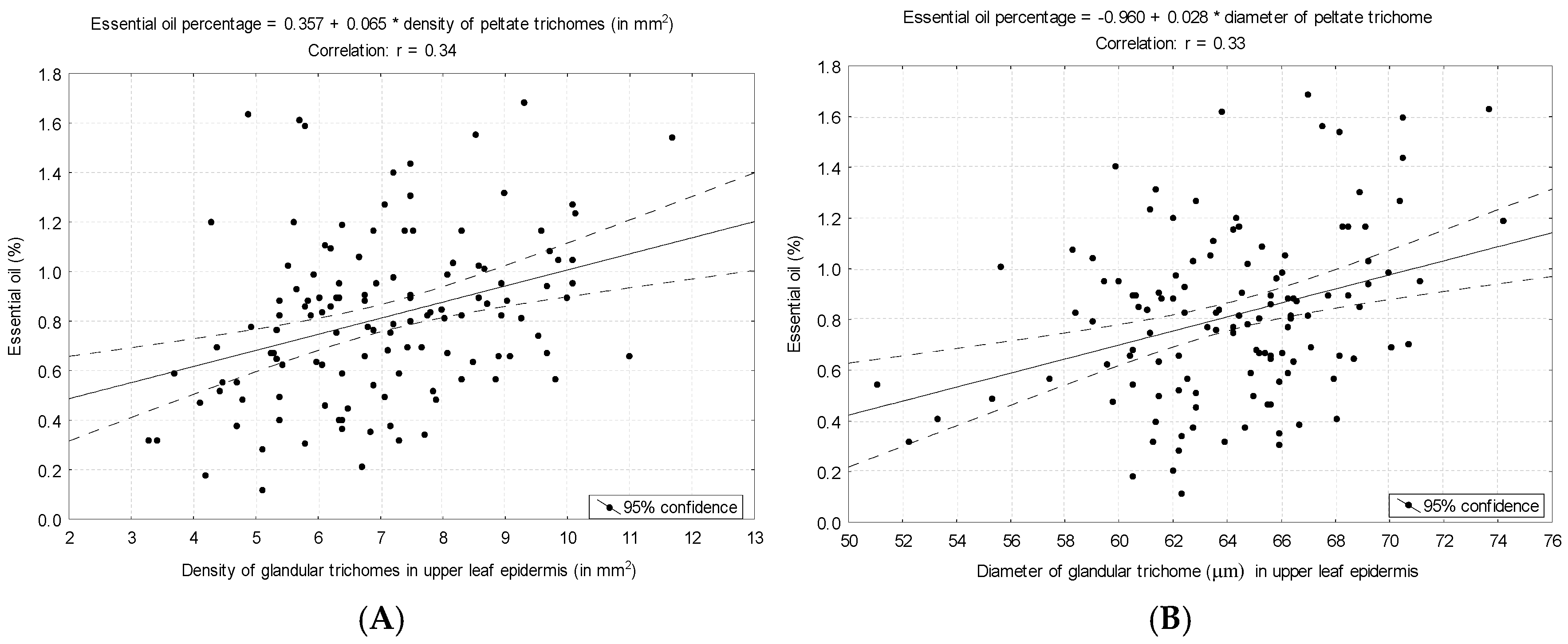
3.2. Essential Oil Amounts of Thymus pulegioides in Different Habitats, Chemotypes, and Sexes
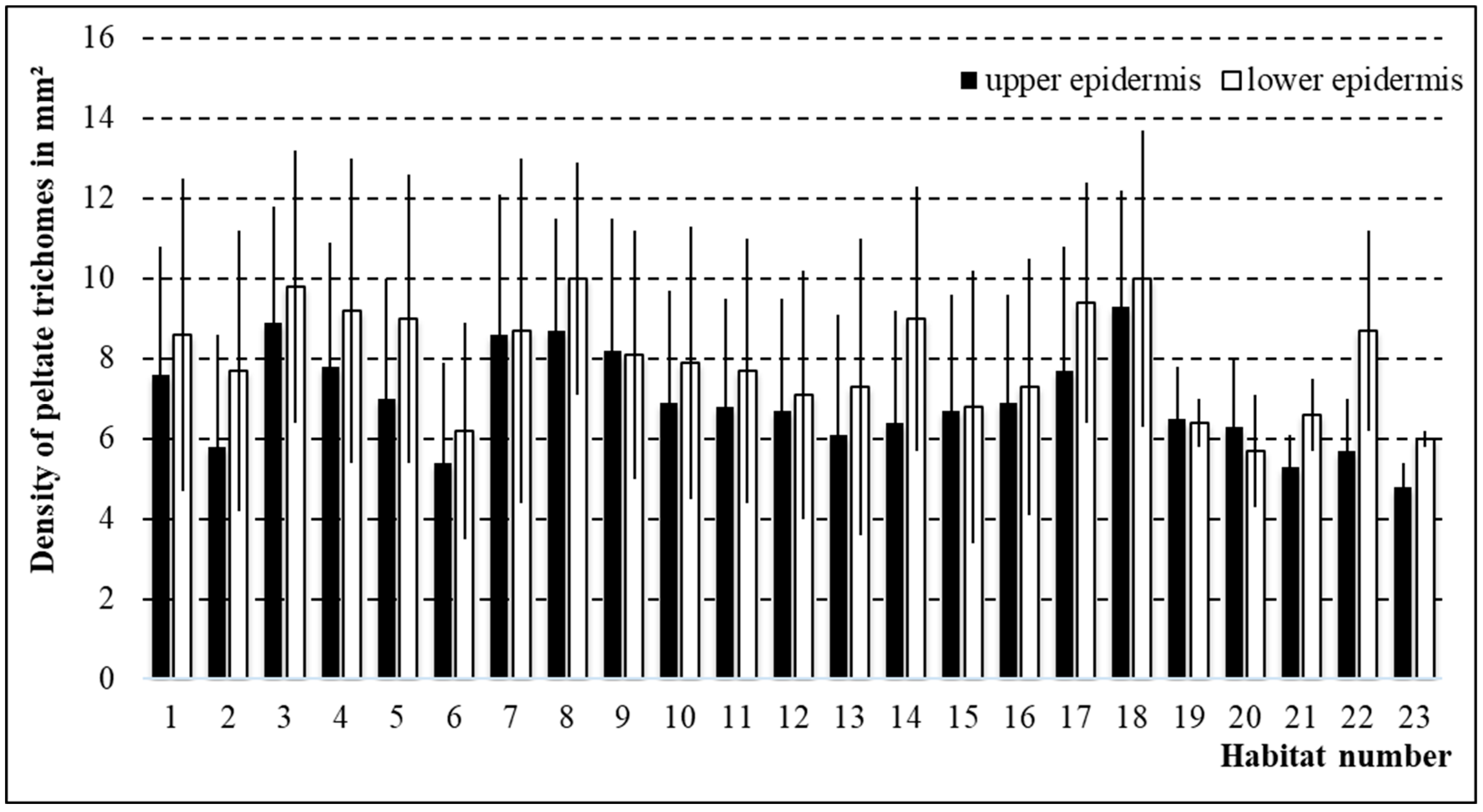
3.3. Density of Peltate Glandular Trichomes in Thymus pulegioides Growing in Different Habitats and Belonging to Different Chemotypes and Sexes
3.4. Diameter of Peltate Glandular Trichomes in Thymus pulegioides Growing in Different Habitats and Belonging to Different Chemotypes and Sexes
3.5. Dynamics of Density and Diameter of Peltate Glandular Trichomes During Leaf Vegetation
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamani, S.; Fathi, M.; Ebadi, M.-T.; Máthé, Á. Global trade of medicinal and aromatic plants. A review. J. Agric. Food Res. 2025, 21, 101910. [Google Scholar] [CrossRef]
- Franz, C.; Novak, J. Sources of Essential Oils. In Handbook of Essential Oils: Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 39–82. [Google Scholar]
- Lawrence, B.M.; Tucker, A. The genus Thymus as a source of commercial products. In Thyme: The Genus Thymus; Stahl-Biskup, E., Sáez, F., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 252–262. [Google Scholar]
- Nieto, G. A Review on applications and uses of Thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- György, Z.; Incze, N.; Pluhár, Z. Differentiating Thymus vulgaris chemotypes with ISSR molecular markers. Biochem. Syst. Ecol. 2020, 92, 104118. [Google Scholar] [CrossRef]
- Stakelienė, V.; Ložienė, K. Gynodioecy in Thymus pulegioides L., T. serpyllum L. and their hybrid T. × oblongifolius Opiz (Lamiaceae): Flower size dimorphism, female frequency and effect of environmental factors. Plant Biosyst. 2014, 148, 49–57. [Google Scholar] [CrossRef]
- Gang, D.R.; Wang, J.; Dudareva, N.; Nam, K.H.; Simon, J.R.; Lewinsohn, E.; Pichersky, E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 2001, 125, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Naidoo, Y.; Nicholas, A. The foliar trichomes of Plectranthus laxiflorus Benth [Lamiaceae]: An important medicinal plant. N. Z. J. Bot. 2010, 48, 55–61. [Google Scholar] [CrossRef]
- The Councile of Europe. European Pharmacopoeia, 6th ed.; European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2020; Volume 1. [Google Scholar]
- Marquesa, N.T.; Filipe, A.; Pinto, P.; Barroso, J.; Trindade, H.; Power, D.M.; Figueiredo, A.C. Trichome density in relation to volatiles emission and 1,8-cineole synthase gene expression in Thymus albicans vegetative and reproductive organs. Chem. Biodivers. 2020, 17, e1900669. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D. Population structure and the spatial dynamics of genetic polymorphism in thyme. In Thyme: The Genus Thymus; Stahl-Biskup, E., Sáez, F., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 44–74. [Google Scholar]
- Boz, I.; Gille, E.; Necula, R.; Dunca, S.; Zamfirache, M.M. Chemical composition and antibacterial activity of essential oils from five populations of Thymus pulegioides L. Cellulose Chem. Technol. 2015, 49, 169–174. [Google Scholar]
- Ložienė, K.; Venskutonis, P.R.; Vaičiūnienė, J. Chemical diversity of essential oil of Thymus pulegioides L. and Thymus serpyllum L. growing in Lithuania. Biologija 2002, 1, 62–64. [Google Scholar]
- Groendahl, E.; Ehlers, B.K.; Keefover-Ring, K. A New cis-Sabinene Hydrate Chemotype Detected in Large Thyme (Thymus pulegioides L.) Growing Wild in Denmark. J. Essent. Oil Res. 2008, 20, 40–41. [Google Scholar] [CrossRef]
- Wester, P.; Möseler, B.M.; Knöss, W. Intra-population terpene polymorphism of Thymus pulegioides L.: Evidence for seven chemotypes in a German limestone grassland. Biochem. Syst. Ecol. 2020, 93, 104173. [Google Scholar] [CrossRef]
- Ložienė, K. Selection of fecund and chemically valuable clones of thyme (Thymus) species growing wild in Lithuania. Ind. Crops Prod. 2009, 29, 502–508. [Google Scholar] [CrossRef]
- Radušienė, J.; Janulis, V. Improvement of diversity, trade and conservation of medicinal and aromatic plants. Medicina 2004, 40, 705–709. [Google Scholar] [PubMed]
- Mockutė, D.; Bernotienė, G. The α-terpinyl acetate chemotype of essential oil of Thymus pulegioides L. Biochem. Syst. Ecol. 2001, 29, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mockutė, D.; Bernotienė, G. Chemical composition of the essential oils and the odor of Thymus pulegioides L. growing wild in Vilnius. J. Essent. Oil Res. 2005, 17, 415–441. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzuge der Vegetationskunde, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964. [Google Scholar]
- Balevičienė, J.; Kizienė, B.; Lazdauskaitė, Ž.; Patalauskaitė, D.; Rašomavičius, V.; Sinkevičienė, Z.; Tučienė, A.; Venckus, Z. Vegetation of Lithuania: 1. Meadows; Šviesa: Vilnius, Lithuania, 1998. [Google Scholar]
- Pavel, M.; Ristic, M.; Stevic, T. Essential oils of Thymus pulegioides and Thymus glabrescens from Romania: Chemical composition and antimicrobial activity. J. Serb. Chem. Soc. 2010, 75, 27–34. [Google Scholar] [CrossRef]
- Martínez-Natarén, D.A.; Villalobos-Perera, P.A.; Munguía-Rosas, M.A. Morphology and density of glandular trichomes of Ocimum campechianum and Ruellia nudiflora in contrasting light environments: A scanning electron microscopy study. Flora 2018, 248, 28–33. [Google Scholar] [CrossRef]
- Radonic, A.; Mastelic, J. Essential oil and glycosidically bound volatiles of Thymus pulegioides L. growing wild in Croatia. Croat. Chem. Acta 2008, 81, 599–606. [Google Scholar]
- Pinto, E.; Pina–Vaz, C.; Salgueiro, L.; Concalves, M.J.; Costa-de-Oliveira, S.; Cavaleiro, C.; Palmaeira, A.; Rodrigues, A.; Martinez-de-Oliveira, J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermophyte species. J. Med. Microbiol. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, M.H.; Papajani, V.; Zelikovic, S.C.; Matevski, V. Essential oil analysis of Two Thymus spp. growing wild in Kosowo. J. Essent. Oil Bear. Plants 2014, 17, 832–837. [Google Scholar] [CrossRef]
- Farhat, M.B.; Jordan, M.J.; Chaouch-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Phenophase effects of sage (Salvia officinalis) yield and composition of essential oil. J. Appl. Res. Med. Aromat. Plants 2016, 3, 87–93. [Google Scholar] [CrossRef]
- Miguel, M.G.; Guerrero, C.; Rodrigues, C.; Broto, J.C.; Duarte, F.; Valencio, F.; Tavares, R. Main components of essential oils from wild Portuguese Thymus mastichina L. (L) spp. mastichina in different development stages or under culture conditions. J. Essent. Oil. Res. 2004, 16, 111–114. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Ayad, H.S.; Hendawy, S.F. Effect of potassium humate and nitrogen fertilizer on herb and essential oil of oregano under different irrigation intervals. Ozean J. Appl. Sci. 2009, 2, 319–323. [Google Scholar]
- Said-Al Ahl, H.A.H.; Wahby, M.S. Effect of water stress and potassium humate on the productivity of oregano plant using saline and fresh water irrigation. Ozean J. Appl. Sci. 2010, 3, 125–141. [Google Scholar]
- Bakhy, K.; Benlhabib, O.; Bighelli, A.; Casanova, J.; Tomi, F.; Faiz, C.A. Yield and chemical variability of essential oil isolated from aerial parts of wild Origanum compactum Benth. from Moroccan Western Rif. Am. J. Essent. Oil Nat. Prod. 2014, 4, 9–17. [Google Scholar]
- Mansoorkhani-Roghaye, A.; Shahriari, Z.; Mohaselli, V.; Osfoori, M.; Shahriari, A.G. Effect of graded levels of NPK on herb oil, yield and oil composition of basil (Ocimum basilicum L.). Glob. J. Res. Med. Plants Indig. Med. 2012, 1, 258–264. [Google Scholar]
- Vaičiulytė, V.; Ložienė, K.; Taraškevičius, R. Impact of Edaphic and Climatic Factors on Thymus pulegioides Essential Oil Composition and Potential Prevalence of Chemotypes. Plants 2022, 11, 2536. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Fridman, E.; Gang, D.R.; Vassao, D.G.; Jackson, B.L.; Kosh, C.M.; Orlova, I.; Spassova, S.M.; Lewis, N.G.; Noel, J.P.; et al. Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc. Natl. Acad. Sci. USA 2006, 103, 10128–10133. [Google Scholar] [CrossRef] [PubMed]
- Malenci, D.R.; Kevesan, Z.S.; Popovic, M.T. Mineral composition of selected Salvia species growing wild in the Vojvodina province. Zb. Matice Srp. Prir. Nauk. 2003, 105, 25–33. [Google Scholar] [CrossRef]
- Gul, H.; Said, A.; Saeed, B.; Mohammad, F.; Ahmad, I. Effects of foliar application of nitrogen, potassium and zinc on wheat growth. J. Agric. Biol. Sci. 2011, 6, 56–58. [Google Scholar]
- Tabata, M. Genetics of monoterpene biosynthesis in perilla plants. Plant Biotechnol. J. 2000, 17, 237–280. [Google Scholar] [CrossRef]
- Singh, P.; Misra, A.; Srivastava, N.K. Influence of Mn deficiency on growth, chlorophyll content, physiology and essential monoterpene oils in genotypes of spearmint (Mentha spicata). Photosynthetica 2001, 39, 473–476. [Google Scholar] [CrossRef]
- Maffei, M.; Chialva, F.; Sacco, T. Glandular trichomes and essential oils in developing peppermint leaves. New Phytol. 1989, 111, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Kamašina, V.; Ložienė, K. The evaluation of phenotypic diversity of Thymus × oblongifolius according to some anatomical characters and comparison with parent species. Acta Bot. Hung. 2009, 51, 85–97. [Google Scholar] [CrossRef]
- Dhawan, S.S.; Shukla, P.; Gupta, P.; Lal, R.K. A cold-tolerant evergreen interspecific hybrid of Ocimum kilimandscharicum and Ocimum basilicum: Analyzing trichomes and molecular variations. Protoplasma 2016, 253, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.M.; Mahdiyeh, M.; Nohooji, M.G.; Akhani, M. Analysis of trichome morphology and density in Salvia nemorosa L. (Lamiaceae) of Iran. Botanica 2018, 24, 49–58. [Google Scholar] [CrossRef]
- Kowalski, R.; Kowalska, G.; Jankowska, M.; Nawrocka, A.; Kałwa, K.; Pankiewicz, U.; Włodarczyk-Stasiak, M. Secretory structures and essential oil composition of selected industrial species of Lamiaceae. Acta Sci. Pol. Hortorum Cultus 2019, 18, 53–69. [Google Scholar] [CrossRef]
- Singh, R.; Singh, G. Aphids. In Polyphagous Pests of Crops; Omkar, Ed.; Springer: Singapore, 2021; pp. 105–182. [Google Scholar]
- Lee, Y.L.; Ding, P. Production of essential oil in plants: Ontogeny, secretory structures and seasonal variations. Pertanika J. Sch. Res. Rev. 2016, 2, 1–10. [Google Scholar]
- Kimura, M.; Ishii, M.; Yoshimi, M.; Ichimura, M.; Tomitaka, Y. Essential oils and glandular trichomes of perilla seedlings as affected by light intensity. Acta Hortic. 2000, 515, 219–226. [Google Scholar] [CrossRef]
- Boz, I.; Lobiuc, A.; Tănase, C. Chemical composition of essential oils and secretory hairs of Thymus dacicus Borbás related to harvesting time. Cellul. Chem. Technol. 2017, 51, 813–819. [Google Scholar]
- Rodrigues, L.; Monteiro, P.; Póvoa, O.; Teixeira, G.; Moldão, M.; Figueiredo, A.C.; Monteiro, A. Morphology of secretory structures and essential oil composition in Mentha cervina L. from Portugal. Flavour Fragr. J. 2008, 23, 340–347. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Sabzalian, M.R. Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem. 2020, 307, 125521. [Google Scholar] [CrossRef] [PubMed]

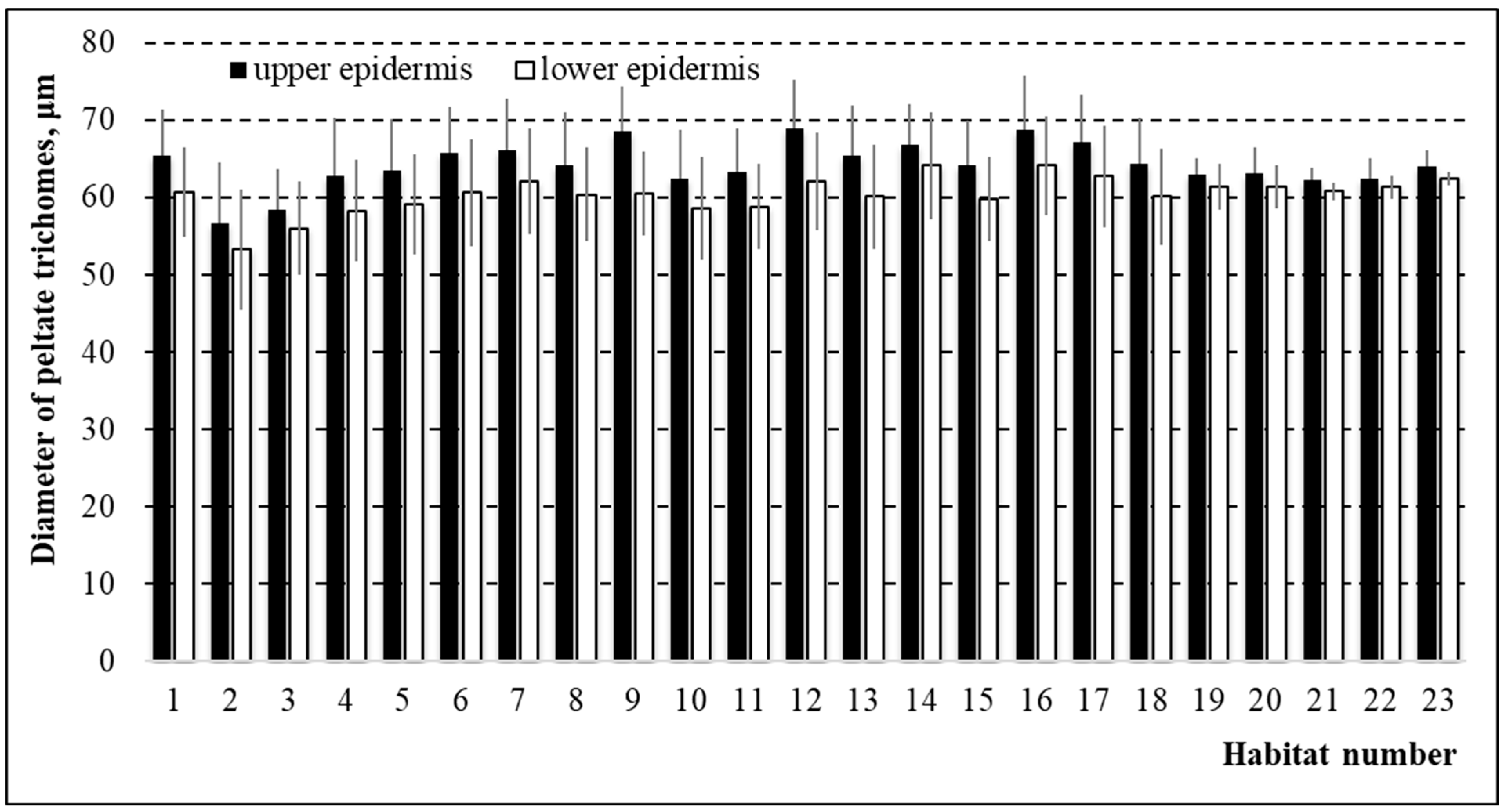
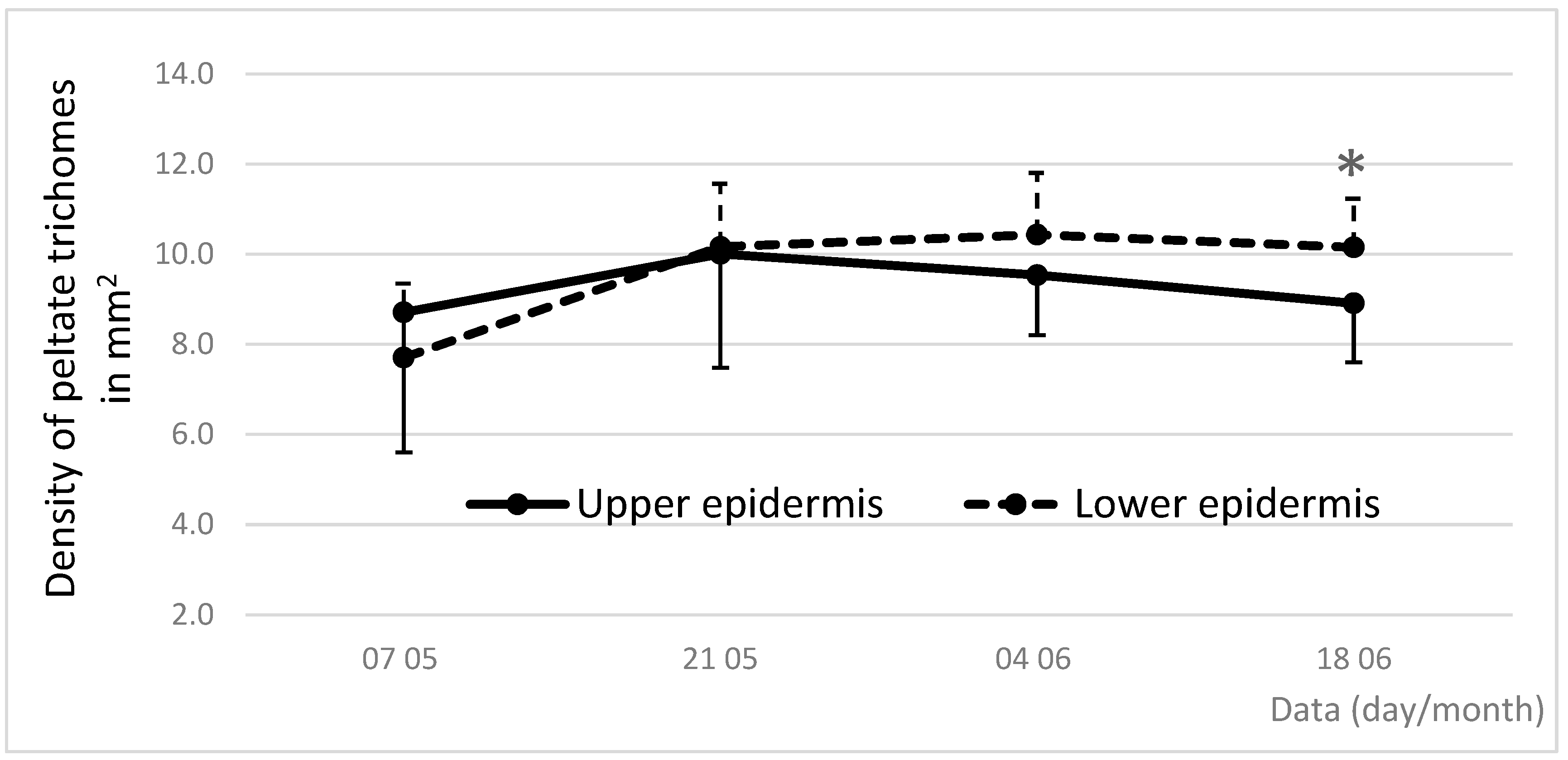
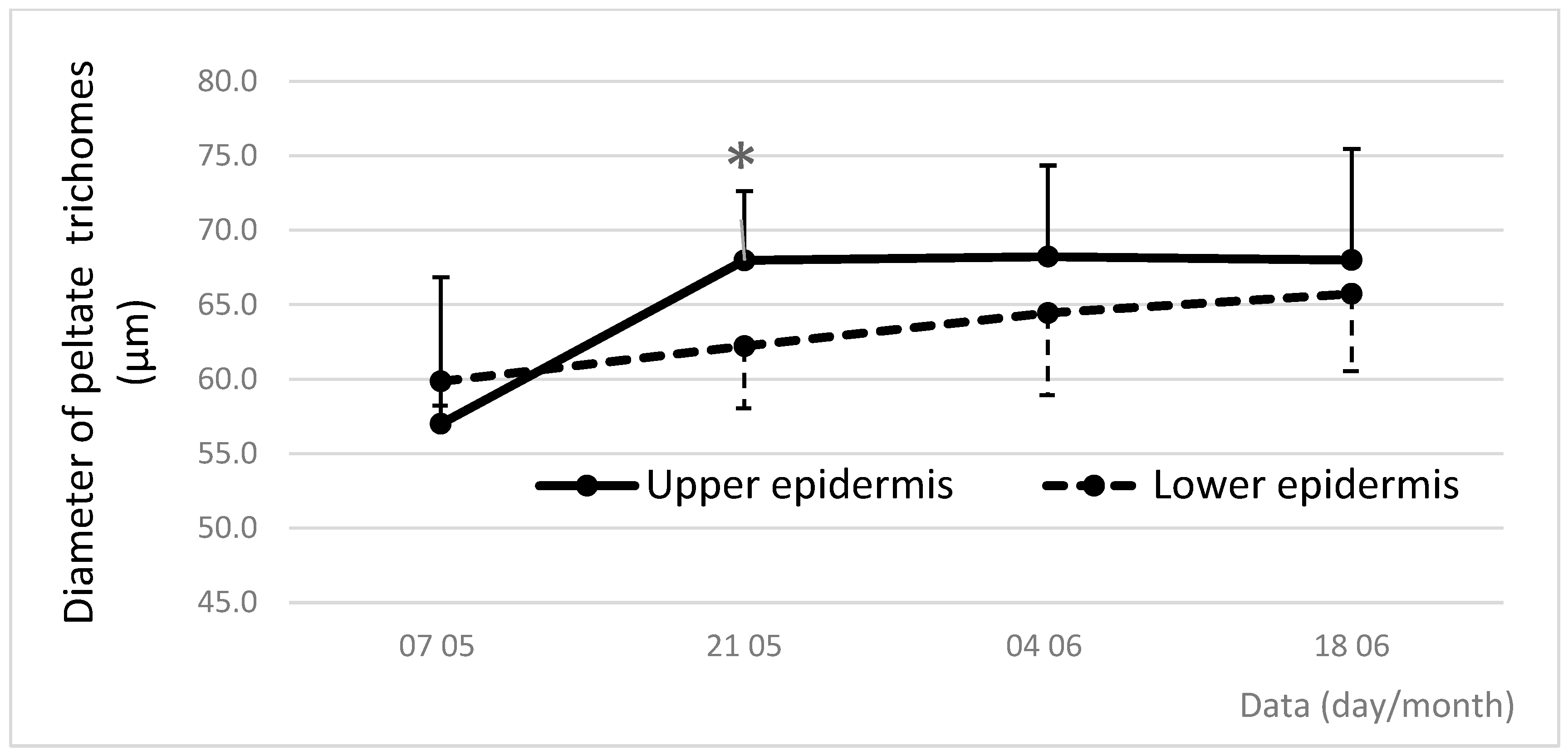
| No. | Locality | Coordinates WGS-84 | Class of Vegetation | Relief | Inclination, ° | Exposition | Lightning, % | Total Herb Cover, % | Thymus pulegioides ** | Thymus pulegioides *** |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Vilnius dist., Uosininkai | 54.714975, 25.587978 | M-A | wavy | – | – | 95 | 90 | 1 | 4 |
| 2 | Vilnius dist., Dunojai | 54.620109, 25.708344 | T-G | wavy | – | – | 80 | 80 | 2 | 6 |
| 3 | Vilnius dist., Vindžiūnai | 54.563248, 25.703089 | F-B | wavy | – | – | 80 | 80 | 1 | 5 |
| 4 | Vilnius dist., Grikieniai | 54.777020, 25.114203 | T-G | slope | 30 | S | 100 | 98 | 2 | 7 |
| 5 | Širvintos dist., Kernavė | 54.879947, 24.846071 | T-G | plane | – | – | 100 | 85 | + | 7 |
| 6 | Vilnius dist., Trečiokiškės | 54.833401, 24.996916 | M-A | wavy | – | – | 98 | 90 | 2 | 5 |
| 7 | Vilnius, Visoriai | 54.753797, 25.261059 | M-A | wavy | – | – | 98 | 90 | 3 | 5 |
| 8 | Elektrėnai mun., Paaliosė | 54.795416, 24.885738 | F-B | slope | 10 | S | 90 | 80 | 1 | 6 |
| 9 | Trakai dist., Meiriškės | 54.725080, 24.878361 | F-B | slope | 20 | S | 100 | 95 | 1 | 7 |
| 10 | Trakai dist., Maušiškės | 54.605374, 24.869349 | M-A | wavy | – | – | 100 | 95 | + | 5 |
| 11 | Trakai dist., Onuškis | 54.522103, 24.616383 | M-A | slope | 20 | SE | 100 | 85 | + | 5 |
| 12 | Trakai dist., Rūdiškės | 54.512941, 24.783850 | T-G | plane | – | – | 100 | 90 | 1 | 5 |
| 13 | Vilnius dist., Nemenčinė | 54.908972, 25.322706 | M-A | slope | 30 | S | 100 | 95 | + | 5 |
| 14 | Vilnius dist., Paberžė | 55.016576, 25.401064 | M-A | wavy | – | – | 100 | 90 | 1 | 5 |
| 15 | Molėtai dist., Dubingiai | 55.091495, 25.432930 | M-A | slope | 15 | SW | 100 | 90 | 1 | 5 |
| 16 | Vilnius, Mažieji Gulbinai | 54.777692, 25.290565 | T-G | plane | – | – | 100 | 90 | 1 | 5 |
| 17 | Vilnius dist., Minkeliai | 54.947612, 25.489782 | M-A | slope | 10 | SE | 100 | 85 | + | 5 |
| 18 | Vilnius dist., Bratoniškės | 54.853805, 25.377456 | * | plane | – | – | 60 | 75 | 1 | 5 |
| 19 | Šilalė dist., Rubinavas | 55.285243, 22.811440 | F-B | slope | 10 | SE | 100 | 85 | 2 | 6 |
| 20 | Šilalė dist., Medvėgalis | 55.374361, 22231996 | T-G | slope | 30 | SW | 100 | 95 | + | 6 |
| 21 | Šilalė dist., Kaltinėnai | 55.340161, 22.260123 | T-G | slope | 10 | S | 100 | 70 | + | 5 |
| 22 | Telšiai dist., Rainiai | 55.574721, 22.174702 | M-A | plane | – | – | 100 | 80 | 1 | 5 |
| 23 | Tauragė dist., Skaudvilė | 55.406632, 22.598877 | T-G | slope | 20 | S | 100 | 85 | 1 | 5 |
| No. | Locality | pHKCl | Phosphorus, mg/kg | Potassium, mg/kg | Manganese, mg/kg | Iron, mg/kg | Calcium, mg/kg | Magnesium, mg/kg | Sulphur, mg/kg |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Vilnius dist., Uosininkai | 7.2 | 76 | 72 | 116 | 544 | 8696 | 768 | 3.0 |
| 2 | Vilnius dist., Dunojai | 6.0 | 92 | 61 | 54.4 | 369 | 856 | 93 | 3.5 |
| 3 | Vilnius dist., Vindžiūnai | 6.0 | 115 | 78 | 72.7 | 477 | 1116 | 179 | 4.1 |
| 4 | Vilnius dist., Grikieniai | 6.7 | 57 | 122 | 176 | 1092 | 4776 | 1076 | 4.3 |
| 5 | Širvintos dist., Kernavė | 4.8 | 174 | 102 | 85.8 | 743 | 644 | 86 | 2.2 |
| 6 | Vilnius dist., Trečiokiškės | 7.1 | 116 | 78 | 122 | 547 | 6212 | 1096 | 7.9 |
| 7 | Vilnius, Visoriai | 7.2 | 119 | 86 | 139 | 777 | 12,768 | 1704 | 5.1 |
| 8 | Elektrėnai mun., Paaliosė | 6.8 | 132 | 52 | 101 | 785 | 1153 | 241 | 1.6 |
| 9 | Trakai dist., Meiriškės | 7.4 | 115 | 82 | 84.9 | 436 | 20,880 | 1252 | 2.2 |
| 10 | Trakai dist., Maušiškės | 7.3 | 310 | 63 | 96.1 | 501 | 9396 | 1412 | 5.0 |
| 11 | Trakai dist., Onuškis | 7.5 | 387 | 92 | 73.6 | 794 | 16,784 | 1624 | 3.1 |
| 12 | Trakai dist., Rūdiškės | 6.3 | 278 | 96 | 142 | 2560 | 4382 | 333 | 2.5 |
| 13 | Vilnius dist., Nemenčinė | 6.1 | 82 | 51 | 50.4 | 440 | 974 | 148 | 3.4 |
| 14 | Vilnius dist., Paberžė | 6.7 | 134 | 118 | 102 | 767 | 3588 | 1072 | 1.8 |
| 15 | Molėtai dist., Dubingiai | 7.0 | 115 | 92 | 122 | 703 | 3026 | 746 | 3.2 |
| 16 | Vilnius, Mažieji Gulbinai | 5.0 | 212 | 105 | 88.2 | 998 | 782 | 108 | 2.0 |
| 17 | Vilnius dist., Minkeliai | 7.6 | 141 | 58 | 103 | 766 | 8864 | 1792 | 3.3 |
| 18 | Vilnius dist., Bratoniškės | 6.5 | 202 | 79 | 105 | 700 | 1445 | 222 | 5.5 |
| 19 | Šilalė dist., Rubinavas | 7.0 | 180 | 53 | 142 | 808 | 4336 | 255 | 6.7 |
| 20 | Šilalė dist., Medvėgalis | 7.5 | 179 | 61 | 95.9 | 611 | 12,672 | 412 | 6.8 |
| 21 | Šilalė dist., Kaltinėnai | 5.1 | 91 | 78 | 59.5 | 611 | 661 | 91 | 5.2 |
| 22 | Telšiai dist., Rainiai | 7.4 | 110 | 101 | 87.2 | 937 | 19,900 | 1084 | 4.8 |
| 23 | Tauragė dist., Skaudvilė | 6.1 | 28 | 85 | 51.8 | 270 | 1657 | 148 | 5.4 |
| Parameter | Chemotypes | Sexes | |||
|---|---|---|---|---|---|
| Phenolic (N = 79) | Non-Phenolic (N = 45) | Females (N = 80) | Hermaphrodites (N = 44) | ||
| Essential oil, % | Means ± SD | 0.90 ± 0.32 a | 0.67 ± 0.31 b | 0.85 ± 0.34 | 0.76 ± 0.3 |
| Min | 0.18 | 0.12 | 0.12 | 0.21 | |
| Max | 1.68 | 1.54 | 1.68 | 1.54 | |
| Density of peltate glandular trichomes in upper epidermis (in mm2) | Means ± SD | 7.0 ± 1.8 | 7.2 ± 1.6 | 7.2 ± 1.6 | 7.4 ± 1.9 |
| Min | 3.3 | 4.3 | 3.3 | 4.1 | |
| Max | 10.2 | 11.7 | 10.9 | 11.9 | |
| Density of peltate glandular trichomes in lower epidermis (in mm2) | Means ± SD | 8.0 ± 2.1 | 8.1 ± 1.7 | 8.0 ± 2.1 | 7.5 ± 2.0 |
| Min | 4.3 | 4.4 | 3.7 | 3.4 | |
| Max | 13.2 | 10.7 | 13.2 | 11.4 | |
| Diameter of peltate glandular trichomes in upper epidermis, µm | Means ± SD | 64.7 ± 3.6 | 63.3 ± 4.5 | 64.1 ± 4.1 | 64.2 ± 3.9 |
| Min | 52.2 | 51.1 | 51.1 | 53.3 | |
| Max | 73.7 | 74.2 | 73.7 | 74.2 | |
| Diameter of peltate glandular trichomes in lower epidermis, µm | Means ± SD | 61.0 ± 5.5 | 59.6 ± 4.1 | 59.9 ± 3.4 | 61.6 ± 7.0 |
| Min | 47.5 | 48.9 | 47.5 | 48.9 | |
| Max | 101.0 | 69.9 | 67.3 | 101.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ložienė, K. Peltate Glandular Trichomes in Relation to Their Parameters, Essential Oil Amount, Chemotype, Plant Sex and Habitat Characteristics in Thymus pulegioides. Horticulturae 2025, 11, 871. https://doi.org/10.3390/horticulturae11080871
Ložienė K. Peltate Glandular Trichomes in Relation to Their Parameters, Essential Oil Amount, Chemotype, Plant Sex and Habitat Characteristics in Thymus pulegioides. Horticulturae. 2025; 11(8):871. https://doi.org/10.3390/horticulturae11080871
Chicago/Turabian StyleLožienė, Kristina. 2025. "Peltate Glandular Trichomes in Relation to Their Parameters, Essential Oil Amount, Chemotype, Plant Sex and Habitat Characteristics in Thymus pulegioides" Horticulturae 11, no. 8: 871. https://doi.org/10.3390/horticulturae11080871
APA StyleLožienė, K. (2025). Peltate Glandular Trichomes in Relation to Their Parameters, Essential Oil Amount, Chemotype, Plant Sex and Habitat Characteristics in Thymus pulegioides. Horticulturae, 11(8), 871. https://doi.org/10.3390/horticulturae11080871







