Abstract
The nutrient requirements of coffee plants vary according to their phenological stages, with each nutrient playing specific roles in different structures and developmental phases. This study evaluated dry matter accumulation and the concentrations of N, P, K, Ca, Mg, S, Fe, Mn, Cu, Zn, and B in the leaves, branches, and reproductive organs of five Coffea canephora genotypes during three phenophases: flowering, fruit development, and fruit ripening. This work aimed to evaluate the distribution of nutrients in three phenophases in Coffeea canephora genotypes. Significant differences were observed among genotypes and phenophases. During flowering, leaves accumulated the highest amount of dry matter, but this pattern reversed in later stages, with greater accumulation in the fruits, especially during fruit ripening. The Verdim TA genotype showed the lowest dry matter accumulation in the branches across all phenophases. Genotypes A1 and Clementino presented the highest mean concentrations of P, Ca, Mg, Fe, Cu, and Zn in the leaves during the fruit development phase, while Verdim TA showed the lowest concentrations of P, K, Ca, Mn, Zn, and B. Future studies may include additional phenological stages and quantify nutrient remobilization efficiency in each genotype, contributing to improved management recommendation.
1. Introduction
After water, coffee is the most widely consumed beverage worldwide. It is predominantly cultivated in tropical regions in various countries and often serves as a primary source of income for rural communities. Although the genus Coffea comprises 130 species, only Coffea arabica L. (Arabica coffee) and C. canephora Pierre ex Froehner (Conilon/Robusta coffee) play a significant role in the global economy [1,2]. The cultivation of Conilon/Robusta coffee is a key agricultural activity in Brazil, Vietnam, Indonesia, Uganda, and India [2,3]. In these countries, the climatic and topographical conditions are favorable for coffee production, especially in regions up to 500 m asl with temperatures between 22 and 26 °C [4].
Coffee is a perennial C3 metabolism plant, with a biennial phenological cycle, which is divided into two phases: the plant growth phase (first phenological year) and the reproductive phase (second phenological year) [5]. On the same C. canephora plants, plagiotropic branches can be observed in both the plant growth and reproductive stages of development [6]. This results in varying nutritional demands, nutrient concentrations, and dry matter production in each phenological phase [7].
Fertilizer recommendations for coffee plants are based on the quantity of nutrients removed in the beans at harvest, the expected yield for the subsequent season, and the nutrients removed by roots and shoot biomass. However, this process involves several variables, including soil, climatic conditions, irrigation, planting density, productivity, phenological phase, and genetic factors related to the genotype [8,9]. Nutritional needs are unsteady, since each nutrient plays a specific role at certain phases of plant development, and the demand is also influenced by the genetic diversity of C. canephora, its allogamous reproduction, and the physiological processes within the plant [10].
A sufficient supply of mineral nutrition to plants is essential, as the nutrient availability of most soils is naturally lower, and there are factors that limit nutrient uptake [11]. During the vegetative phase, branch growth is intensified, and floral buds develop and mature, which may vary among genotypes with different maturation cycles [8,12]. In this stage, the coffee plant requires nitrogen, which is essential for leaf and branch formation [13]. In the reproductive phase, flowering occurs after floral bud differentiation, followed by fruit expansion, development, and ripening [14]. Nutrients are redistributed according to the plant’s demands. During the early stages of grain formation (pinhead phase), calcium, magnesium, iron, zinc, and boron are heavily accumulated, as they are crucial for cellular division, membrane stabilization, and the respiratory activities of fruits [15,16]. During fruit development, potassium becomes the most required nutrient, as it facilitates the translocation of sugars and organic acids, regulates stomatal closure, and influences cell water potential [17].
Throughout the phenological cycle, certain nutrients are redistributed among organs [18]. For instance, nitrogen and potassium levels decrease in leaves during the reproductive stage of Conilon coffee, whereas phosphorus concentrations are higher in leaves compared to fruits [8]. In fertigated coffee plantations, micronutrient concentrations are higher during the plant growth stage and anthesis, decrease during fruit formation, and increase again as fruits begin to mature [19]. Therefore, understanding the nutritional status of coffee genotypes in the different phenophases can help optimize fertilization.
This study hypothesized that the dry mass and concentration of nutrients in the organs of C. canephora plants is influenced by both genotype and phenophase. To test this hypothesis, the dry mass and nutrient distribution in leaves, branches, and reproductive organs were evaluated in three phenophases of five C. canephora genotypes. This work aimed to evaluate the distribution of nutrients in three phenophases in Coffeea canephora genotypes.
2. Materials and Methods
2.1. Characterization of the Experimental Area and Plant Material
The experiment was carried out on an experimental farm of the Federal University of Espírito Santo (UFES), Brazil (18°42′58″ S, 39°51′32″ W; altitude 36 m a.s.l.). The regional climate is tropical Aw, with dry winters and wet summers [20]. The soil at the experimental site was classified as Abruptic Dystrophic Yellow Argisol (Ultisol) [21].
The genotype seedlings were planted in June 2018, at a spacing of 2 m between rows and 1 m between plants (5000 plants ha−1). The maturation cycles of the five sampled genotypes differed: Pirata, A1, and Verdim TA are early/medium-maturing, while Clementino and K61 are medium-maturing [22]. The genotypes were selected based on their reproductive characteristics and their ranking among the most commonly cultivated in the state of Espírito Santo, Brazil. The experiment was arranged in a randomized block design with three replications.
The plantation was established with two stems per plant (10,000 stems ha−1) under full sunlight. Irrigation hoses were installed along the planting rows at a distance of 5 cm from the plant stems, with emitters placed 0.5–0.10 m apart. Irrigation was applied as needed, based on the soil water balance method, by drip irrigation. Reference evapotranspiration was calculated by the Penman–Monteith method [23].
Management practices such as preventive phytosanitary control, weed management with herbicides and brush cutters, liming, and fertilization were applied according to the crop-specific technical recommendations. Approximately 1.5 t ha−1 of lime was applied, based on soil analysis recommendations, using a base saturation percentage (BSP), which is the total of four exchangeable base cations (Ca2+, Mg2+, K+, and Na+) relative to cation exchange capacity (CEC). Fertilization was applied according to soil analysis and yield data, as described by Paye et al. [24]. The nutrient requirements of the plants and phenological stage were also considered, and a total of 500 kg N ha−1, 80 kg P2 O5 ha−1, and 400 kg K2O ha−1 were applied in four rates: two before and two after flowering. Ca and Mg were provided via liming application, and micronutrient levels were adjusted with annual applications of 2.0 kg ha−1 of Zn, 1.0 kg ha−1 of B, 2.0 kg ha−1 of Cu, and 10 kg ha−1 of Mn.
2.2. Identification and Sampling of Plagiotropic Branches
After harvesting and pruning, 36 plagiotropic branches were randomly selected from nine plants of each genotype, 4 plagiotropic branches in each plant, resulting in a total of 180 branches (5 genotypes × 36 branches). Each plagiotropic branch was considered an experimental unit, and each plagiotropic branch contained 13 to 16 productive metamers. The selected branches were marked with ribbons and identification tags. The branches were collected in similar conditions, considering the position on the plant to minimize sampling bias. Sampling began on 16 August 2021, immediately after floral anthesis, and continued until the fruits were fully mature.
Three randomly selected plants per block were sampled (two branches per block), i.e., six plagiotropic branches per genotype. These branches were previously marked for each treatment and were completely detached from the orthotropic branch. The first sampling occurred immediately after the main anthesis, during flowering. The second sampling, during fruit development, was performed 170 days after floral anthesis. The third sampling, representing fruit ripening, was performed when 80–90% of the fruits were at the cherry stage, ready for harvest. All leaves, branches, flowers, and fruits of each sampled plagiotropic branch were separated and individually stored in labeled paper bags.
2.3. Nutritional Analysis
The collected and duly identified plant material was dried to constant weight in a forced air circulation oven at 65 °C. The samples (leaves, branches, flowers, and fruits) were separately ground in a Wiley-type mill (Pinhalense®, Espírito Santo do Pinhal, Brazil), sieved through a mesh of 0.841 mm, and stored in plastic bags for nutrient analysis. To determine the concentration of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), zinc (Zn), copper (Cu), manganese (Mn), and boron (B), we used the methodology of Silva et al. [25]. The nutrient contents in plant tissues were determined using standard analytical methods: nitrogen (N) by the Kjeldahl method; phosphorus (P) by colorimetry following nitric–perchloric acid digestion; potassium (K), calcium (Ca), and magnesium (Mg) by flame atomic absorption spectrophotometry; and micronutrients such as iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu) by atomic absorption spectrophotometry after digestion.
2.4. Statistical Analysis
Data of nutrient concentrations and dry matter of plant organs were subjected to the Shapiro–Wilk normality test, followed by two-way analysis of variance (ANOVA). When significant differences among genotypes were detected by ANOVA, means were compared by Tukey’s test. All tests were conducted at 5% significance using the software Statistical Analysis System Version 9.2 [26].
3. Results
3.1. Dry Matter Percentage and Accumulation in Leaves, Branches, and Reproductive Organs
Throughout the evaluations, dry matter accumulation in the different organs varied according to the phenophases (Figure 1). During flowering, the five genotypes had already accumulated a mean of more than 55% leaf dry matter, while flowers accounted for 21% and branches for 20%. During fruit development and ripening, fruit dry matter increased gradually, accompanied by a percentage reduction in branches and leaves. At this phenophase, fruit production was intensified while plant growth decreased, and leaf drop was observed. On average, the fruits accounted for 60 and 85%, respectively, during development and ripening, when the branches consisted almost entirely of fruits. On the other hand, branches and leaves represented a total of only 15% of the entire plagiotropic branch.
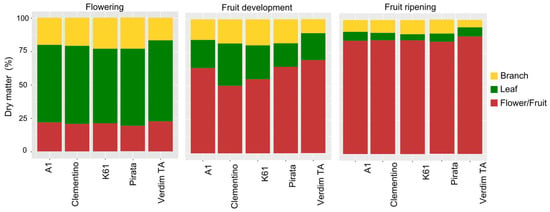
Figure 1.
Percentage of dry matter distribution in leaves, branches, and reproductive organs of five C. canephora genotypes in three phenophases.
Throughout the three phenophases (Figure 1), leaf and branch dry matter decreased proportionally in relation to the other plant parts. In contrast, the representativeness of dry matter of reproductive organs (flowers/fruits) increased during the sampled phenophases.
Significant differences among genotypes and phenophases were observed in mean dry matter accumulation (Table 1). Comparing genotypes, Verdim TA consistently had the lowest mean dry matter in branches across all three phenophases. During flowering, dry matter of genotype Clementino in flowers was the highest and the lowest in Verdim TA. For branches, no differences were observed among the genotypes A1, Clementino, K61, and Pirata in the first two phenophases (flowering and fruit development).

Table 1.
Comparison of estimated means of dry matter (g) in leaves, branches, and reproductive organs (flowers/fruits) among five C. canephora genotypes in three phenophases.
As shown in Table 1, leaf dry matter decreased throughout the phenophases, with a reduction of 50.6 to 66.0% from the first to the last phenophase (maturation). Conversely, dry matter in reproductive organs and branches increased throughout the phenophase. Branch dry matter increased by 1.45 to 2.17 times during ripening, compared to flowering. For fruits, dry matter increased 2.22 to 3.8 times during ripening compared to fruit development. The increase in fruit and branch dry matter became more evident during ripening, reflecting the plant’s productivity.
3.2. Nutrient Concentrations in Leaves, Branches, and Reproductive Organs in Three Phenophases
Significant differences regarding leaf nutrient concentrations were detected among genotypes across the three phenophases evaluated (Table 2). However, no significant differences were observed for Mg, Fe, and B during flowering; N and K in fruit development; and Fe and Cu during ripening.

Table 2.
Comparison of estimated means of leaf nutrient concentrations for nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), and boron (B) among five C. canephora genotypes in three phenophases.
As shown in Table 2, during flowering, genotype K61 had a higher leaf N concentration than A1, which in turn had higher P and Cu concentrations. Genotype Verdim TA had the lowest P concentration and K61 the lowest Cu concentration. In genotype Pirata, K and S concentrations were higher than in the other genotypes. Additionally, genotype Clementino had a higher Zn concentration than K61, Pirata, and Verdim TA. During fruit development (Table 2), the genotypes A1, Clementino, and Verdim TA stood out by exhibiting the highest foliar concentrations of Ca and Mg compared to the other genotypes. On the other hand, during fruit maturation, the genotype Verdim TA showed the lowest concentrations of P, K, Ca, Mn, Zn, and B.
Significant differences were detected among genotypes for the most nutrient concentrations in branches across the three phenophases evaluated. Only P and K did not differ significantly in any phase (Table 3).

Table 3.
Comparison of estimated means of branch nutrient concentrations for nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), and boron (B) among five C. canephora genotypes in three phenophases.
During flowering and fruit development (Table 3), the genotype Pirata had a higher S concentration in branches than A1, Clementino, and K61. During fruit development, genotype A1 had a higher Fe concentration than Clementino, K61, and Verdim TA. Genotype K61 had a higher Mn concentration than A1, Clementino, and Verdim TA. During ripening, genotypes Verdim TA and K61 had the highest S and Mn concentrations, respectively, compared to the other genotypes. Unlike what was observed for the leaves, the Verdim TA genotype showed higher concentrations of Mg, S, Cu, Zn, and B, standing out among the genotypes.
Significant differences were also observed among genotypes for nutrient concentrations in flowers/fruits across the three phenophases (F-test, p < 0.05), except for Fe, which did not vary significantly at any stage (Table 4). As shown in Table 4, during flowering, the Pirata genotype stood out with the highest sulfur concentration in the flowers. During fruit development, the Clementino genotype exhibited the highest concentrations of P, Mg, Cu, and Zn, whereas Verdim TA showed the lowest concentrations of P, Mg, S, Mn, and Zn in the fruits. Additionally, during fruit maturation, there was no significant difference in nutritional content between the genotypes, except for N and Zn; in these cases, the Verdim TA genotype presented higher N content and Clementino higher Zn content.

Table 4.
Comparison of estimated means of flower/fruit nutrient concentrations for nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), and boron (B) among five C. canephora genotypes in three phenophases.
4. Discussion
This study evaluated the dry matter and macro- and micronutrients in the different organs of coffee plants at various phenological stages, as well as compared the results across different genotypes. Thus, it contributes to the understanding of nutrient assimilation capacity in coffee plants and reveals the dynamic nature of this process across different plant organs, developmental stages, and genetic compositions.
The highest percentage of dry matter in the leaves was recorded during flowering (Figure 1). This result can be attributed to the fact that this phase occurs after the period of the highest growth rates for leaves and plagiotropic branches [8]. On the other hand, the lowest percentage of leaf dry matter was observed during fruit ripening (Figure 1). These results were already expected, as in this phase the plant transfers a large portion of the photosynthesis products to the fruits [12,27]. Furthermore, during the fruit ripening phase, leaf senescence occurs, which coincides with the dry season typical of the main coffee-producing regions in Brazil, resulting in a decrease in leaf dry mass [28]. As reported by Leon-Burgos et al. [27], there is a negative correlation between the allocation of dry matter to leaves and branches and the accumulation of dry matter in the fruits.
The variations in dry matter observed among genotypes (Table 1) indicate that such differences may be attributed to the genetic characteristics of each genotype. However, studies with a greater number of repetitions over time and in different locations are necessary to confirm this hypothesis. The Verdim TA genotype presented the lowest dry matter in the leaves across the three phenophases, while Clementino stood out with the highest leaf dry matter during flowering. In addition to similarities in dry mass of leaves, branches, and flowers/fruits observed for A1, Clementino, and Pirata, these genotypes are also characterized as plants with large internodes and wider and longer leaves compared to the others [22]. According to Colodetti et al. [29], the leaf area of the plants during flowering can influence final productivity, as plants with a small leaf area tend to show lower yield and grain classification based on sieve size.
Nutrient availability at the beginning of the production period is crucial for high leaf areas and, consequently, for ensuring proper fruit development. This study evaluated nutrient concentrations in leaves, branches, and flowers/fruits across three phenophases (flowering, fruit development, and fruit ripening), showing variations among genotypes in the different phenophases (Table 2, Table 3 and Table 4). The work by Rodrigues et al. [7], which evaluated nutrient concentration in fruits of twenty C. canephora genotypes, showed genotypic variations, with heritability indices above 80% for Ca, Mg, Cu, and Mn. Similarly, the study by Alberto et al. [30], which evaluated nutrient concentrations in beans, husks, fruits, and leaves of different C. arabica cultivars, showed that the cultivars formed only two distinct groups (cophenetic correlation 0.83), confirming the narrow genetic variability of this species. It is worth noting that Ca, Mg, Cu, and Mn were also the nutrients that most contributed to the differentiation of C. arabica cultivars, with cumulative values ranging from 91 to 99.98% [30]. The high heritability reported by these studies, along with the moderate coefficients of variation observed in this work, supports the hypothesis that nutrient uptake, in general, has heritable traits that can be exploited in breeding programs aimed at improving nutrient use efficiency.
Leaf nutrient analysis is a fundamental tool for assessing the plants’ nutritional status at a given moment and allows for the detection of nutrient deficiency, sufficiency, or toxicity [31]. It also helps determine the most appropriate times for soil or foliar nutrient applications, contributing to higher yield [32]. Genotypes A1 and Clementino showed the highest average values for P, Ca, Mg, Fe, Cu, and Zn concentrations in the leaves during the fruit development phenophase, whereas Verdim TA showed the lowest concentrations of P, K, Ca, Mn, Zn, and B. During the fruit development phase, some nutrients are particularly important, such as N, P, K, Mg, Ca, and B, as they directly influence bean formation, size, and quality, in addition to supporting plant metabolism during this high-demand period [11,33]. Based on this, it is possible that beans from genotypes A1 and Clementino may develop better than those from Verdim TA. However, it is necessary to evaluate whether there is a correlation between leaf nutrient concentrations during fruit development and coffee bean size/quality.
As the fruits mature, there is an increase in dry matter, as well as in the size and thickness of the branches, and mobile nutrients are often translocated to prioritize fruit development [34,35]. Since the Verdim TA genotype showed the highest concentrations of all nutrients, except for Mn, in the branches during the fruit ripening phase, rather than during the development phase, it is possible that these branches also function as strong metabolic sinks. If this is the case, that is, if the branches indeed act as major metabolic sinks during maturation, the plant may not prioritize nutrient translocation exclusively to the fruits. This condition could result in significant losses, as in Coffea canephora (cv. Conilon), where the branches that have fruited are removed after harvest because, over time, they become unproductive [36].
Regarding nutrient concentrations in flowers/fruits, there were few significant differences among genotypes during the fruit ripening phase, limited to N, Mn, and Zn. The fruit is the part of the plant intended for commercialization and processing. Therefore, it is desirable that it contains the highest concentration of nutrients. Deficiencies in N, Mn, and Zn in fruits can result in decreased beverage quality [37] and may even affect the human diet, since Mn, for example, is involved in several biological processes, such as brain development, acting as an enzymatic cofactor in the synthesis of metabolites like amino acids and in enzymatic defense mechanisms against oxidative stress [38,39].
Research shows that flower analysis is an efficient method for early diagnosis of the nutritional status of coffee plants, compared to leaf analysis [32,40]. The association of the nutritional information of leaf and flower can help in nutritional diagnosis and even help in the recommended dosage of fertilizers for plants. However, the present study showed that, in absolute values, only the concentration of S in leaves and flowers was similar during the flowering period. Still, in absolute terms, the concentrations of N, P, K, and Zn were higher in the flowers, whereas the concentrations of Ca, Mg, Fe, Cu, Mn, and B were higher in the leaves during this phase. Therefore, for more effective nutritional monitoring, it is recommended that both floral and foliar analyses be carried out. Furthermore, future studies could establish reference values for nutritional analysis of coffee plants based on tissue analyses of flowers and leaves during flowering. Considering that mineral fertilizers represent a considerable portion of production costs, these analyses could make coffee cultivation even more economically viable. In addition to the hypotheses raised throughout this work, future studies could include additional phenological stages and quantify nutrient remobilization efficiency in each genotype, contributing to improved management recommendations.
5. Conclusions
Dry matter and nutrient concentrations varied among the C. canephora genotypes across different phenophases. During flowering, the leaves accumulated the highest amount of dry matter, but this pattern reversed throughout the phenological stages, with a significant increase in dry matter in the reproductive organs (flowers and fruits) during development and especially during fruit ripening. Genotypes A1 and Clementino showed the highest average values for the concentrations of P, Ca, Mg, Fe, Cu, and Zn in the leaves during the fruit development phenophase, while Verdim TA exhibited the lowest concentrations of P, K, Ca, Mn, Zn, and B. Future studies may include additional phenological stages and quantify the efficiency of nutrient remobilization in each genotype, contributing to improved management recommendations. This knowledge can contribute to understanding the nutrient demand in different parts of the plant and therefore help in recommending fertilization, considering the quantity and fertilizer distribution according to genotypes and phenophases.
Author Contributions
Conceptualization, M.J.L.R. and F.L.P.; validation, F.L.P.; formal analysis, A.P.V.; investigation, M.J.L.R.; data curation, M.J.L.R.; writing—original draft preparation, M.J.L.R.; writing—review and editing, L.O.E.S., I.G., H.D.V., M.R. and F.L.P.; visualization, L.O.E.S., I.G., H.D.V., A.P.V. and M.R.; supervision, F.L.P.; project administration, F.L.P.; funding acquisition, F.L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa e Inovação do Espírito Santo-FAPES (Proc. 2022-WTZQP and 2024-9H43M for F.L.P.) and Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Proc. 309.535/2021-2 for F.L.P.).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank the Núcleo de Excelência de Pesquisa em Café conilon for logistical support during the experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northen Madagascar. Kew Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- ICO—International Coffee Organization. Trade Statistics. 2024. Available online: https://ico.org/resources/about-economics-and-statistics/ (accessed on 19 July 2024).
- Salvador, H.P.; Berilli, A.P.C.G.; Rodrigues, W.P.; Mazzafera, P.; Partelli, F.L. A climate change perspective on the selection, development, and management of Coffea canephora genotypes. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Bunn, C.; Laderach, P.; Ovalle Rivera, O.; Kirschke, D. A bitter cup: Climate change profile of global production of Arabica and Robusta coffee. Clim. Change 2015, 129, 89–101. [Google Scholar] [CrossRef]
- Camargo, ÂP.D.; Camargo, M.B.P. Definição e esquematização das fases fenológicas do cafeeiro arábica nas condições tropicais do Brasil. Bragantia 2001, 60, 65–68. [Google Scholar] [CrossRef]
- Rakocevic, M.; Matsunaga, F.T.; Baroni, D.F.; Campostrini, E.; Costes, E. Multiscale analyses of growth and berry distributions along four branching orders and vertical profile of Coffea arabica L. cultivated under high-density planting systems. Sci. Hortic. 2021, 281, 109934. [Google Scholar] [CrossRef]
- Rodrigues, M.J.L.; Silva, C.A.; Braun, H.; Partelli, F.L. Nutritional balance and genetic diversity of Coffea canephora genotypes. Plants 2023, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Oliosi, G.; Partelli, F.L.; Silva, C.A.; Dubberstein, D.; Gontijo, I.; Tomaz, M.A. Seasonal variation in leaf nutrient concentration of conilon coffee genotypes. J. Plant Nutr. 2020, 44, 74–85. [Google Scholar] [CrossRef]
- Ramirez-Builes, V.H.; Küsters, J.; Thiele, E.; Leal-Varon, L.A. Boron nutrition in coffee improves drought stress resistance and, together with calcium, improves long-term productivity and seed composition. Agronomy 2024, 14, 474. [Google Scholar] [CrossRef]
- Martins, L.D.; Machado, L.D.S.; Tomaz, M.A.; Amaral, J.F.T. The nutritional efficiency of Coffea spp. A review. Afr. J. Biotec 2015, 14, 728–734. [Google Scholar] [CrossRef]
- Laviola, B.G.; Martinez, H.E.P.; de Souza, R.B.; Salomão, L.C.C.; Cruz, C.D. Macronutrient accumulation in coffee fruits at Brazilian Zona da Mata conditions. J. Plant Nutr. 2009, 32, 980–995. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of coffee growth and production. Braz. J. Plant Physiol. 2009, 19, 485–510. [Google Scholar] [CrossRef]
- Carréra, J.C.; Resende, T.B.; Vicente Campos, A.A.; de Souza, R.R.; Oliveira, I.M.M.; Alves Ribeiro, C.; Gavilanes, M.L.; Guimarães, R.J.; Mori, F.A. Anatomic characteristics of branches related to the vegetative growth of coffee tree (Coffea arabica L., Rubiaceae) under nutritional variation. J. Plant Nutr. 2023, 46, 4594–4605. [Google Scholar] [CrossRef]
- López, M.E.; Santos, I.S.; de Oliveira, R.R.; Lima, A.A.; Cardon, C.H.; Chalfun-Junior, A. An overview of the endogenous and environmental factors related to the Coffea arabica flowering process. Bev. Plant Res. 2021, 1, 13. [Google Scholar] [CrossRef]
- Poltronieri, Y.; Martinez, H.E.; Cecon, P.R. Effect of zinc and its form of supply on production and quality of coffee beans. J. Sci. Food Agric. 2011, 91, 2431–2436. [Google Scholar] [CrossRef] [PubMed]
- Dubberstein, D.; Partelli, F.L.; Espindula, M.C.; Dias, J.R.M. Concentration and accumulation of micronutrients in robust coffee. Acta Sci. Agron. 2019, 41, e42685. [Google Scholar] [CrossRef]
- Clemente, J.M.; Martinez, H.E.P.; Alves, L.C.; Finger, F.L.; Cecon, P.R. Effects of nitrogen and potassium on the chemical composition of coffee beans and on beverage quality. Acta Sci. Agron. 2015, 37, 297–305. [Google Scholar] [CrossRef]
- Silva, C.D.; Silva, L.F.V.; Barbosa, G.M.D.; Franco, M.F.S.; Delgado, E.U.A.; Fariña, P.R.V.; Aquino, L.A. Diagnosis of the nutritional status of coffee tree according to fruit phenology. Res. Soc. Dev. 2022, 11, e1311628591. [Google Scholar] [CrossRef]
- Neto, A.P.; Favarin, J.L.; Almeida, R.E.M.; Santos Dias, C.T.; Tezotto, T.; Alves, A.L.G.; Moraes, M.F. Changes of nutritional status during a phenological cycle of coffee under high nitrogen supply by fertigation. Commun. Soil. Sci. Plant Anal. 2011, 42, 2414–2425. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Lima, H.N.; Marques, F.A.; et al. Brazilian Soil Classification System, 6th ed.; Embrapa: Brasília, Brazil, 2025; p. 393. [Google Scholar]
- Partelli, F.L.; Oliveira, H.F.; Gomes, W.S.; Oliosi, G.; Salvador, H.P. Registro fotográfico e Caracterização de 41 Genótipos de Café Conilon; Khas Editora: São Matheus, Brazil, 2022. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; Irrigation and Drainage Paper; United Nations Food and Agriculture Organization, FAO: Rome, Italy, 1998; p. 300. [Google Scholar]
- Paye, H.S.; Partelli, F.L.; Martins, A.G.; Siebeneichler, E.A. Recomendação de adubação e calagem. In Café Conilon: Conhecimento Para Superar Desafios; Partelli, F.L., Espindula, M.C., Eds.; CAUFES: Alegre, ES, Brazil, 2019; pp. 75–98. [Google Scholar]
- Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Rio de Janeiro, Brazil; Embrapa Solos: Brasília, Brazil, 2009; p. 627. [Google Scholar]
- SAS Institute. SAS/STAT: User’s Guide, Version 9.2.; SAS Institute: Cary, CA, USA, 2009. [Google Scholar]
- León-Burgos, A.F.; Sáenz, J.R.R.; Quinchua, L.C.I.; Toro-Herrera, M.A.; Unigarro, C.A.; Osorio, V.; Balaguera-López, H.E. Increased fruit load influences vegetative growth, dry mass partitioning, and bean quality attributes in full-sun coffee cultivation. Front. Sustain. Food Syst. 2024, 8, 1379207. [Google Scholar] [CrossRef]
- Bote, A.D.; Jan, V. Branch growth dynamics, photosynthesis, yield and bean size distribution in response to fruit load manipulation in coffee trees. Trees 2016, 30, 1275–1285. [Google Scholar] [CrossRef]
- Colodetti, T.V.; Rodrigues, W.N.; Martins, L.D.; Tomaz, M.A. Differential tolerance between genotypes of conilon coffee (‘Coffea canephora’) to low availability of nitrogen in the soil. Aust. J. Crop Sci. 2014, 8, 1648–1657. [Google Scholar]
- Alberto, N.J.; Ramalho, J.C.; Ribeiro-Barros, A.I.; Viana, A.P.; Krohling, C.A.; Moiane, S.S.; Alberto, Z.; Rodrigues, W.P.; Partelli, F.L. Diversity in Coffea arabica cultivars in the Mountains of Gorongosa National Park, Mozambique, regarding bean and leaf nutrient accumulation and physical fruit traits. Agronomy 2023, 13, 1162. [Google Scholar] [CrossRef]
- Sousa, J.S.; Neves, J.C.L.; Martinez, H.E.P.; Alvarez, V.H.V. Relationship between coffee leaf analysis and soil chemical analysis. Rev. Bras. Ciênc Solo 2018, 42, e0170109. [Google Scholar] [CrossRef]
- Zabini, A.V.; Martinez, H.E.P.; Neves, J.C.L.; Cruz, C.D.; Valadares, S.V. Chemical analyses of flowers and leaves for nutritional diagnoses of coffee trees. Ciênc. Rural. 2021, 51, e20190796. [Google Scholar] [CrossRef]
- Torres, J.D.; de Araújo, L.F.B.; Espindula, M.C.; Campanharo, M.; Rocha, R.B. Export of macronutrients for coffee fruits submitted to different doses of formulation 20-00-20. J. Plant Nutr. 2022, 45, 2737–2747. [Google Scholar] [CrossRef]
- Bragança, S.M.; Martinez, H.E.P.; Leite, H.G.; Santos, L.P.; Lane, J.A.; Sediyama, C.S.; Venegas, V.H.A. Accumulation of macronutrients for the Conilon coffee tree. J. Plant Nutr. 2007, 31, 103–120. [Google Scholar] [CrossRef]
- Khalajabadi, S.S.; Poveda, V.C.D.; Sáenz, J.R.R. Coffee productive branch growth, development and nutrient accumulation from flowering to harvest under Colombian conditions. Coffee Sci. 2025, 20, e202274. [Google Scholar] [CrossRef]
- Verdin Filho, A.C.; Tomaz, M.A.; Ferrão, R.G.; Ferrão, M.A.G.; Fonseca, A.F.A.D.; Rodrigues, W.N. Conilon coffee yield using the programmed pruning cycle and different cultivation densities. Coffee Sci. 2014, 9, 489–494. [Google Scholar]
- Lacerda, J.S.; Martinez, H.E.; Pedrosa, A.W.; Clemente, J.M.; Santos, R.H.; Oliveira, G.L.; Jifon, J.L. Importance of zinc for arabica coffee and its effects on the chemical composition of raw grain and beverage quality. Crop Sci. 2018, 58, 1360–1370. [Google Scholar] [CrossRef]
- Martins, A.C.; Krum, B.N.; Queirós, L.; Tinkov, A.A.; Skalny, A.V.; Bowman, A.B.; Aschner, M. Manganese in the diet: Bioaccessibility, adequate intake, and neurotoxicological effects. J. Agric. Food Chem. 2020, 68, 12893–12903. [Google Scholar] [CrossRef] [PubMed]
- Espinelli Junior, J.B.D.S.; Wesz, I.S.; Araujo, I.D.S.; Furlong, E.B.; Carapelli, R. Chemical fractionation of manganese in commercial coffee samples from conventional and organic cultivation systems. Anal. Lett. 2025, 58, 1479–1494. [Google Scholar] [CrossRef]
- Martinez, H.E.P.; Souza, R.B.; Bayona, J.A.; Venegas, V.H.A.; Sanz, M. Coffee-tree floral analysis as a mean of nutritional diagnosis. J. Plant Nutr. 2003, 26, 1467–1482. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).