Difference Analysis of Non-Volatile and Volatile Components in Kujingcha (Ilex dabieshanensis) Compared with Green Tea (Camellia sinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Chemicals and Reagents
2.3. Experiment Methods
2.3.1. Sensory Evaluation and Quantitative Descriptive Analysis (QDA)
2.3.2. Determination of Main Conventional Biochemical Components
2.3.3. Determination of Flavonoid Content
2.3.4. Determination of Catechins and Caffeine Content
2.3.5. Determination of Amino Acid Content
2.3.6. Determination of Volatile Content
2.3.7. Data Analysis
3. Results
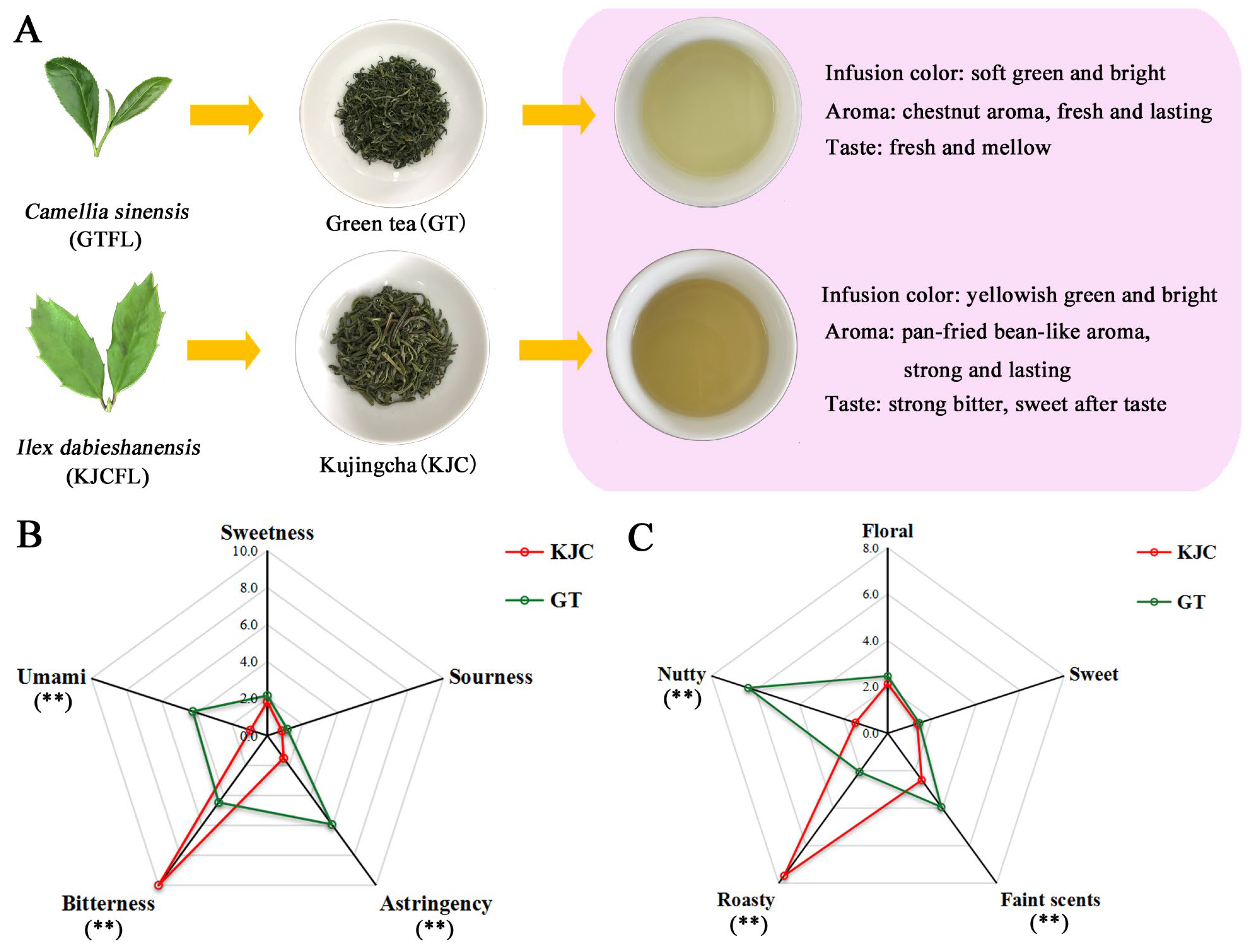
3.1. Sensory Evaluation of KJC and GT
3.2. Analysis of Non-Volatile Component Content in Tea Samples
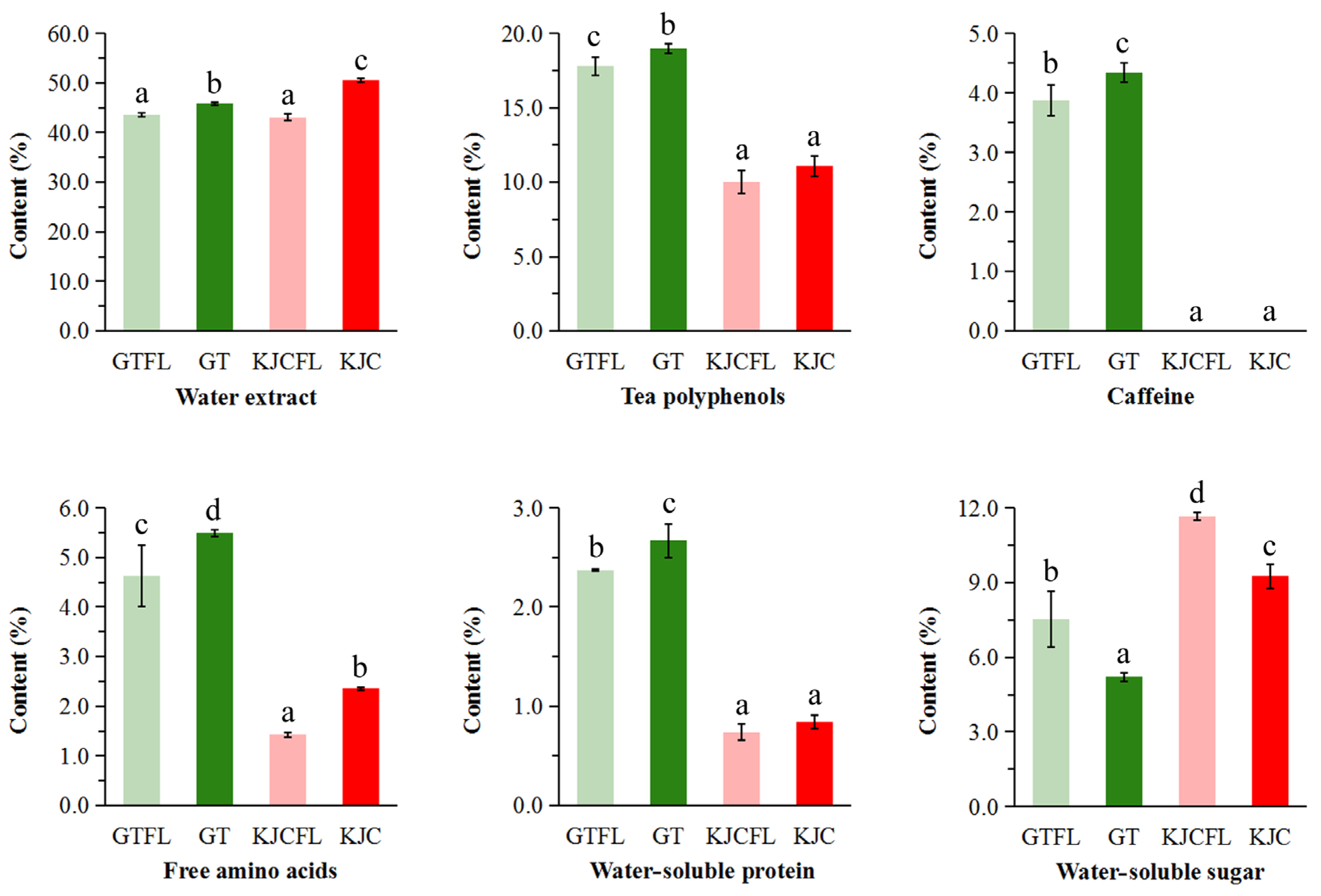
3.2.1. Main Conventional Biochemical Components Analysis
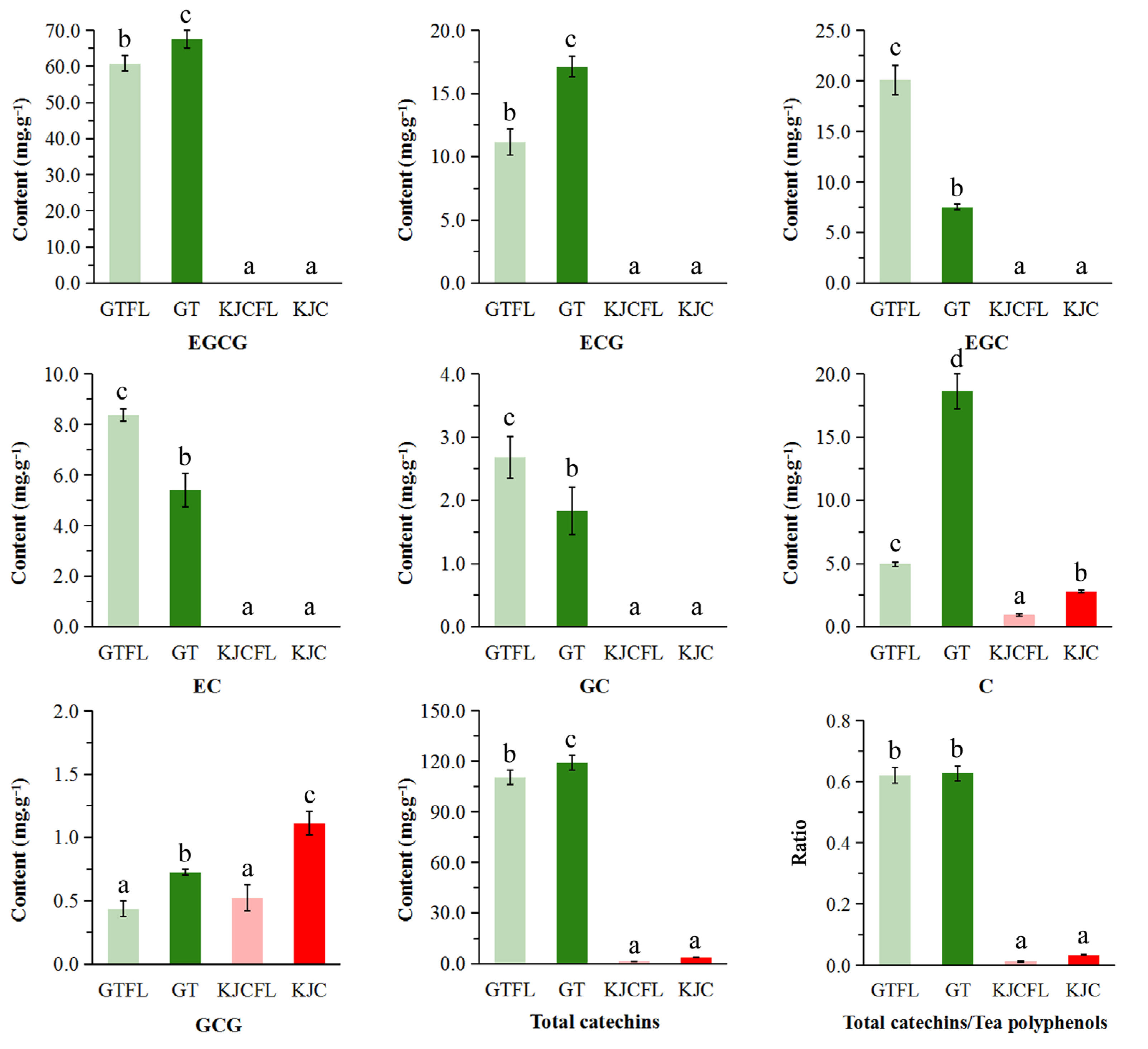
3.2.2. Catechin Analysis
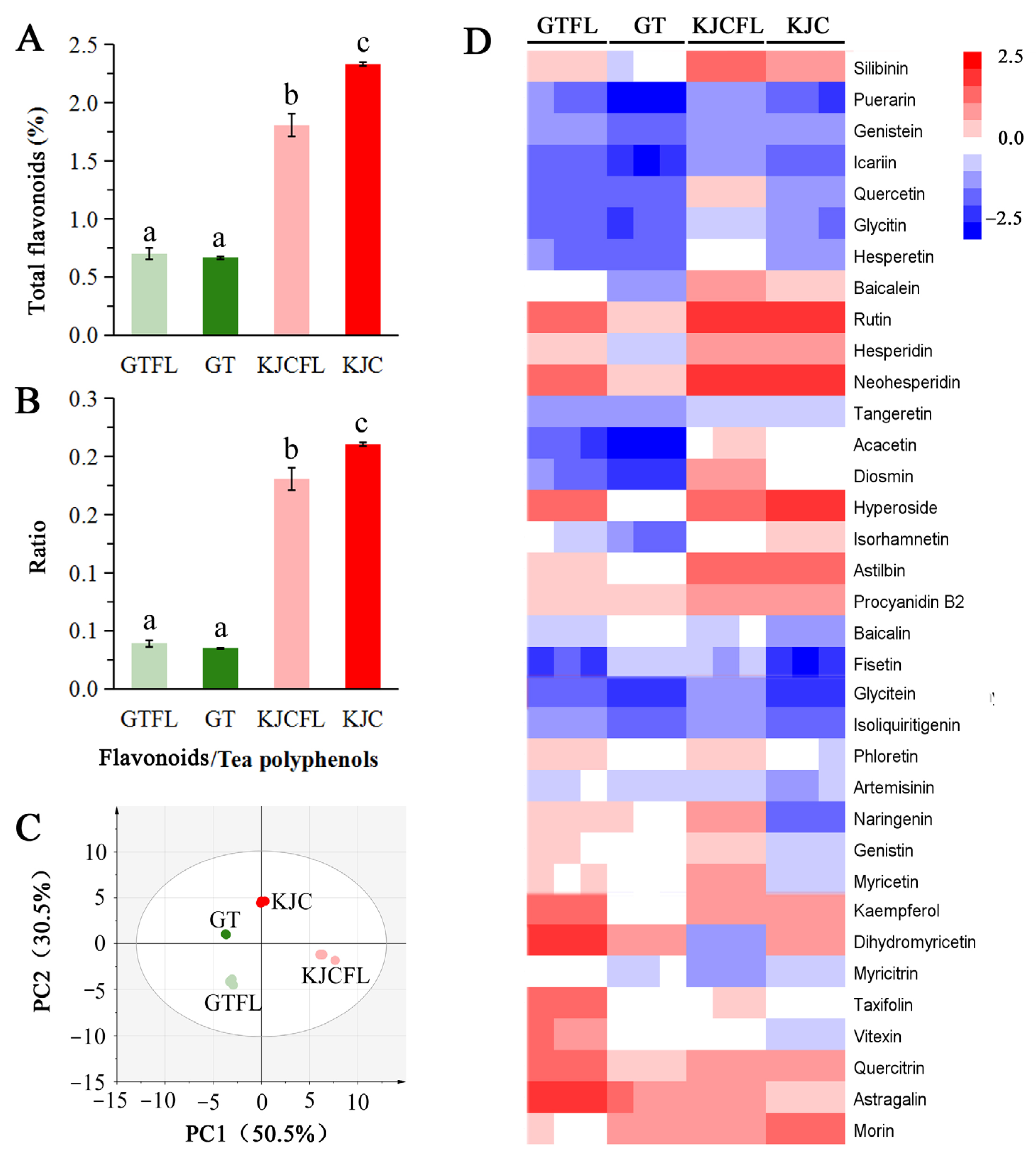
3.2.3. Flavonoid Analysis
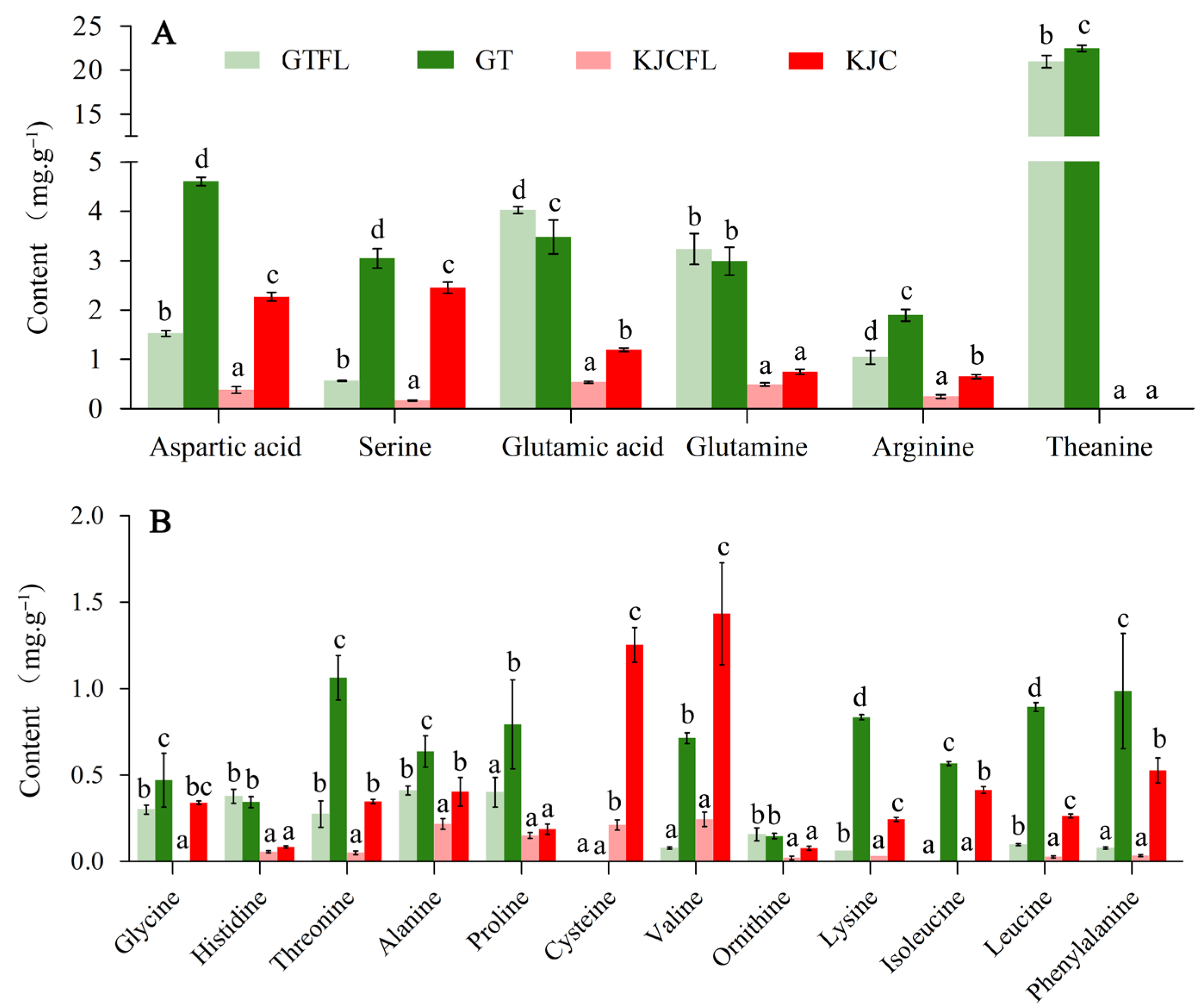
3.2.4. Amino Acid Analysis
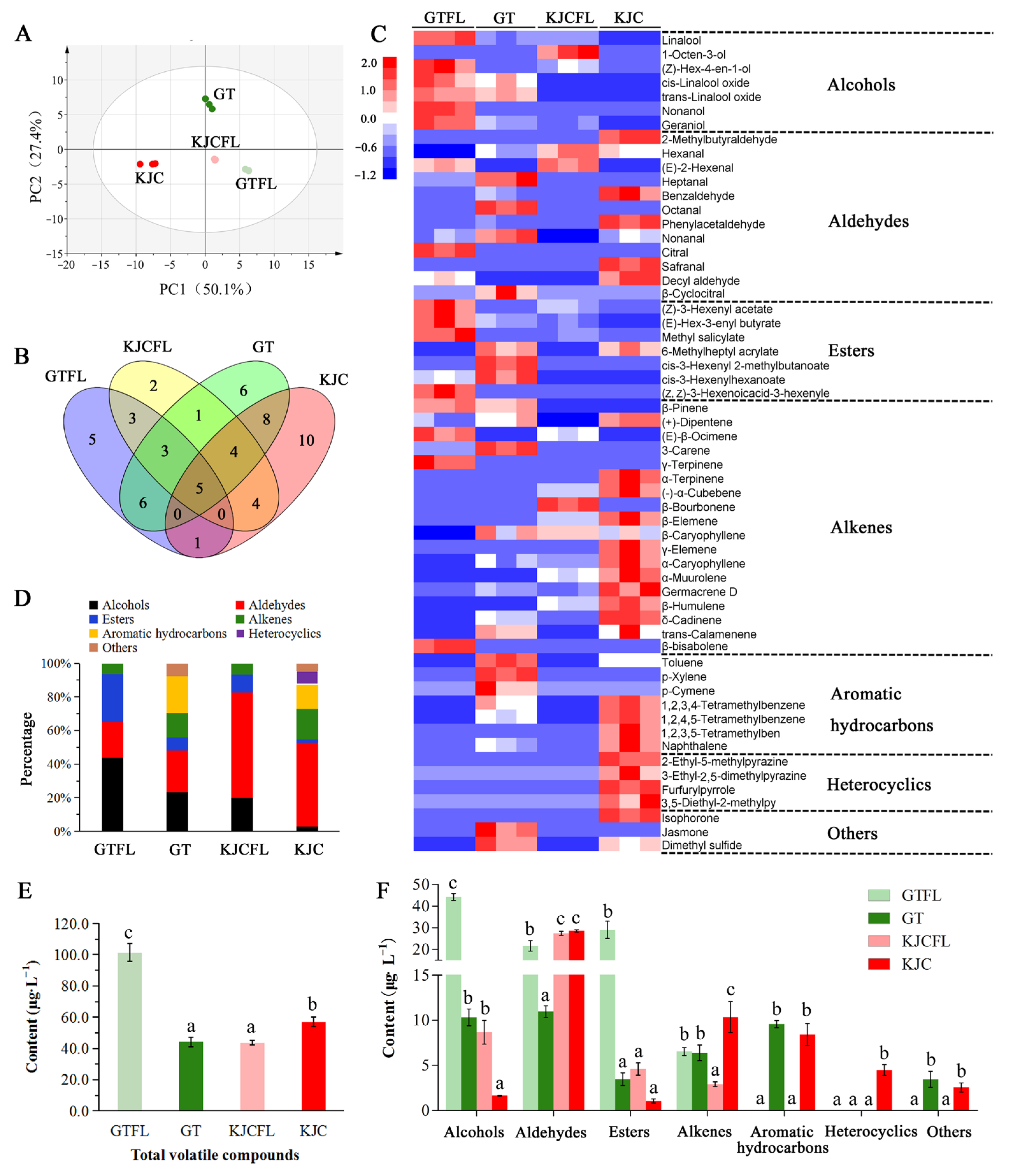
3.3. Analysis of Volatile Component Content in Tea Samples
4. Discussion
4.1. Flavonoids Are Significantly Associated with the Overall Flavor and Health Benefits of KJC
4.2. Umami and Sweet Components Are Important Supplements to Regulate the Overall Taste of KJC
4.3. Heterocyclics Contribute to the Formation of the Bean-Like Aroma of KJC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wüpper, S.; Lüersen, K.; Rimbach, G. Chemical composition, bioactivity and safety aspects of Kuding tea-from beverage to herbal extract. Nutrients 2020, 12, 2796. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, L.; Ma, G.; Dong, Y.; Peng, Y.; Xiao, P. The large-leaved kudingcha (Ilex latifolia Thunb and Ilex kudingcha C.J. Tseng): A traditional Chinese tea with plentiful secondary metabolites and potential biological activities. J. Nat. Med. 2013, 67, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, Y.; Ma, G.; He, C.; Feng, Y.; Lei, Q.; Xiao, P. Quantitative analysis of five kudinosides in the large-leaved Kudingcha and related species from the genus Ilex by UPLC-ELSD. Phytochem. Anal. 2012, 23, 677–683. [Google Scholar] [CrossRef]
- Gan, R.; Zhang, D.; Wang, M.; Corke, H. Health benefits of bioactive compounds from the genus Ilex, a source of traditional caffeinated beverages. Nutrients 2018, 10, 1682. [Google Scholar] [CrossRef]
- Lei, G.; Wu, Z.; Xu, Y.; Bao, Y. Effects of different processing methods on the contents of polyphenols in Kujingcha tea. Lishizhen Med. Mater. Med. Res. 2003, 14, 345–346. [Google Scholar]
- Zhang, F.; Lu, X.; Chu, D.; Yin, R.; Wang, C.; Zhou, Y.; Li, Y.; Li, N.; Cai, X.; Chen, H. A New Ilex dabieshanensis Cultivar “Ilex dabieshanensis No.1”. Acta Hortic. Sin. 2018, 45, 2827–2828. [Google Scholar]
- Jiang, A.; Wang, X.; Xiang, J.; Huang, W. Research on instant Kujing tea. Sci. Technol. Food Ind. 2012, 33, 280–282. [Google Scholar]
- Wan, X. Tea Biochemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Zhang, L.; Cao, Q.; Granato, D.; Xu, Y.; Ho, C. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Ma, L.; Cao, D.; Liu, Y.; Gong, Z.; Liu, P.; Jin, X. A comparative analysis of the volatile components of green tea produced from various tea cultivars in China. Turk. J. Agric. For. 2019, 43, 451–463. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.; Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhou, H.; Wang, Y.; Bian, J.; Sun, Y.; Du, X. Utilizing nontargeted metabolomics integrated with quality component quantitation via differential analysis to reveal the taste differences between and metabolite characteristics of hawk black tea and hawk white tea. Food Res. Int. 2024, 197, 115216. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Y.; Cao, D.; Liu, Y.; Gong, Z.; Ercisli, S.; Luo, Z.; Jin, X. Analysis of the biochemical and volatile components of Qianlincha and Qiandingcha prepared from Eurya alata Kobuski and Camellia cuspidate. Agronomy 2021, 11, 657. [Google Scholar] [CrossRef]
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. Standardization Administration of China: Beijing, China, 2018.
- GB/T 8314-2013; Tea—Determination of Free Amino Acids Content. Standardization Administration of China: Beijing, China, 2013.
- GB/T 8313-2018; Determination of Total Polyphenols and Catechins Content in Tea. Standardization Administration of China: Beijing, China, 2018.
- GB/T 8305-2013; Tea—Determination of Water Extracts Content. Standardization Administration of China: Beijing, China, 2013.
- Li, Y. Comprehensive Experiment of Tea Science; China Light Industry Press: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Xue, J.; Liu, P.; Xu, Y.; Gui, A.; Wang, X.; Ye, F.; Feng, L.; Wang, S.; Teng, J.; Gao, S.; et al. Effect of cultivar and process on the astringency of matcha based on flavonoids-targeted metabolomic analysis. Food Res. Int. 2025, 204, 115954. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wei, X.; Liu, H.; Li, H.; Xia, Y.; Wu, D.; Zhang, P.; Gandhi, G.; Li, H.; Gan, R. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Atmani, D.; Chaher, N.; Atmani, D.; Berboucha, M.; Debbache, N.; Boudaoud, H. Flavonoids in human health: From structure to biological activity. Curr. Nutr. Food Sci. 2009, 5, 225–237. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, C.; Yan, Y.; Chen, Q.; Luo, F.; Zhu, X.; Li, X.; Chen, K. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chem. 2012, 135, 1471–1478. [Google Scholar] [CrossRef]
- Cao, J.; Tang, C.; Gao, M.; Rui, Y.; Zhang, J.; Wang, L.; Wang, Y.; Xu, B.; Yan, B. Hyperoside alleviates epilepsy-induced neuronal damage by enhancing antioxidant levels and reducing autophagy. J. Ethnopharmacol. 2020, 257, 112884. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.; Semwal, R.; Semwal, D. Health benefits and limitations of rutin-A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar]
- Xia, H.; Lv, C.; Lu, Y.; Zeng, C.; Qin, S.; Shi, M. Natural deep eutectic ready to use extract of astilbin: Super high in vitro bioaccessibility, α-amylase and α-glucosidase enzyme inhibition kinetics. Food Res. Int. 2023, 173, 113368. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, L.; Wang, M.; Zhang, Y. Inhibitory Mechanism of Morin on Pancreatic Lipase. Food Sci. 2025, 46, 40–48. [Google Scholar]
- Rouseff, R.; Martin, S.; Youtsey, C. Quantitative survey of narirutin, naringin, hesperidin, and neohesperidin in citrus. J. Agric. Food Chem. 1987, 35, 1027–1030. [Google Scholar] [CrossRef]
- Qi, D.; Dai, W.; Tan, L.; Peng, Q.; Zhang, Y.; Lin, Z. Study on the effects of the fixation methods on the chemical components and taste quality of summer green tea. J. Tea Sci. 2016, 36, 18–26. [Google Scholar]
- Shi, Z. Chaye Shenping Yu Jianyan; China Agricultural Press: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Li, Y.; Han, Z.; Wang, M.; Yan, Y.; Ma, R.; Wang, H.; Deng, W. Metabolomics and sensory evaluation reveal the influence of four albino tea cultivars on the quality of processed green tea. Food Res. Int. 2025, 209, 116180. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Chen, R.; Sun, L.; Lai, X.; Li, Q.; Hao, M.; Zhang, S.; Li, Q.; Sun, S.; et al. Metabolomics-based analysis of the effects of differences in soluble sugars on the sweetness quality of six major tea types in China. Food Funct. 2025, 16, 3707–3720. [Google Scholar]
- Lu, T.; Dong, X.; Lan, W.; Zhou, B.; Teng, J.; Xia, N.; Khan, M.; Huang, L. From microbial perspective: Manufacturing process, chemical composition and health benefis of Liupao tea-A comprehensive review. Food Res. Int. 2025, 206, 116088. [Google Scholar] [CrossRef]
- Xiao, J.; Jiang, H. A review on the structure-function relationship aspect of polysaccharides from tea materials. Crit. Rev. Food Sci. Nutr. 2015, 55, 930–938. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Van Boekel, M. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Song, C.; Ho, C.; Wan, X. Contribution of L-theanine to the formation of 2,5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chem. 2018, 263, 18–28. [Google Scholar] [PubMed]
- Ho, C.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Pei, Z.; He, G.; Liu, Y.; Hu, Y.; Li, M.; Wan, X.; Zhai, X. Characterization of the key aroma-active compounds responsible for the rice cruse-like aroma of large-leafed yellow tea (Camellia sinensis). Food Sci. 2023, 44, 289–297. [Google Scholar]
- Yang, P.; Yu, M.; Song, H.; Xu, Y.; Lin, Y.; Granvogl, M. Characterization of key aroma-active compounds in rough and moderate fire rougui wuyi rock tea (Camellia sinensis) by sensory-directed flavor analysis and elucidation of the influences of roasting on aroma. J. Agric. Food Chem. 2022, 70, 267–278. [Google Scholar]
- Zhu, Y.; Lv, H.; Shao, C.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.; Tan, J.; Peng, Q.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Peng, Y.; Cao, D.; Fan, P.; Wang, L.; Feng, G.; Lei, A.; Hu, B.; Liu, Y.; Liu, Y.; et al. Difference Analysis of Non-Volatile and Volatile Components in Kujingcha (Ilex dabieshanensis) Compared with Green Tea (Camellia sinensis). Horticulturae 2025, 11, 804. https://doi.org/10.3390/horticulturae11070804
Ma L, Peng Y, Cao D, Fan P, Wang L, Feng G, Lei A, Hu B, Liu Y, Liu Y, et al. Difference Analysis of Non-Volatile and Volatile Components in Kujingcha (Ilex dabieshanensis) Compared with Green Tea (Camellia sinensis). Horticulturae. 2025; 11(7):804. https://doi.org/10.3390/horticulturae11070804
Chicago/Turabian StyleMa, Linlong, Yanan Peng, Dan Cao, Ping Fan, Lingyi Wang, Guobiao Feng, Aimin Lei, Baisong Hu, Yijin Liu, Yanli Liu, and et al. 2025. "Difference Analysis of Non-Volatile and Volatile Components in Kujingcha (Ilex dabieshanensis) Compared with Green Tea (Camellia sinensis)" Horticulturae 11, no. 7: 804. https://doi.org/10.3390/horticulturae11070804
APA StyleMa, L., Peng, Y., Cao, D., Fan, P., Wang, L., Feng, G., Lei, A., Hu, B., Liu, Y., Liu, Y., & Jin, X. (2025). Difference Analysis of Non-Volatile and Volatile Components in Kujingcha (Ilex dabieshanensis) Compared with Green Tea (Camellia sinensis). Horticulturae, 11(7), 804. https://doi.org/10.3390/horticulturae11070804






