Delayed Starch Degradation Triggers Chromoplast Structural Aberration to Inhibit Carotenoid Cleavage: A Novel Mechanism for Flower Color Deepening in Osmanthus fragrans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Genome Resequencing
2.3. Observation of Ultrastructure
2.4. Carotenoid Extraction and Determination
2.5. Determination of Volatile Components
2.6. Transcriptome Sequencing
2.7. Data Analysis
3. Results
3.1. Identification of a Bud Mutation Cultivar of O. fragrans
3.2. Ultrastructural Analysis of Plastids in O. fragrans Petals
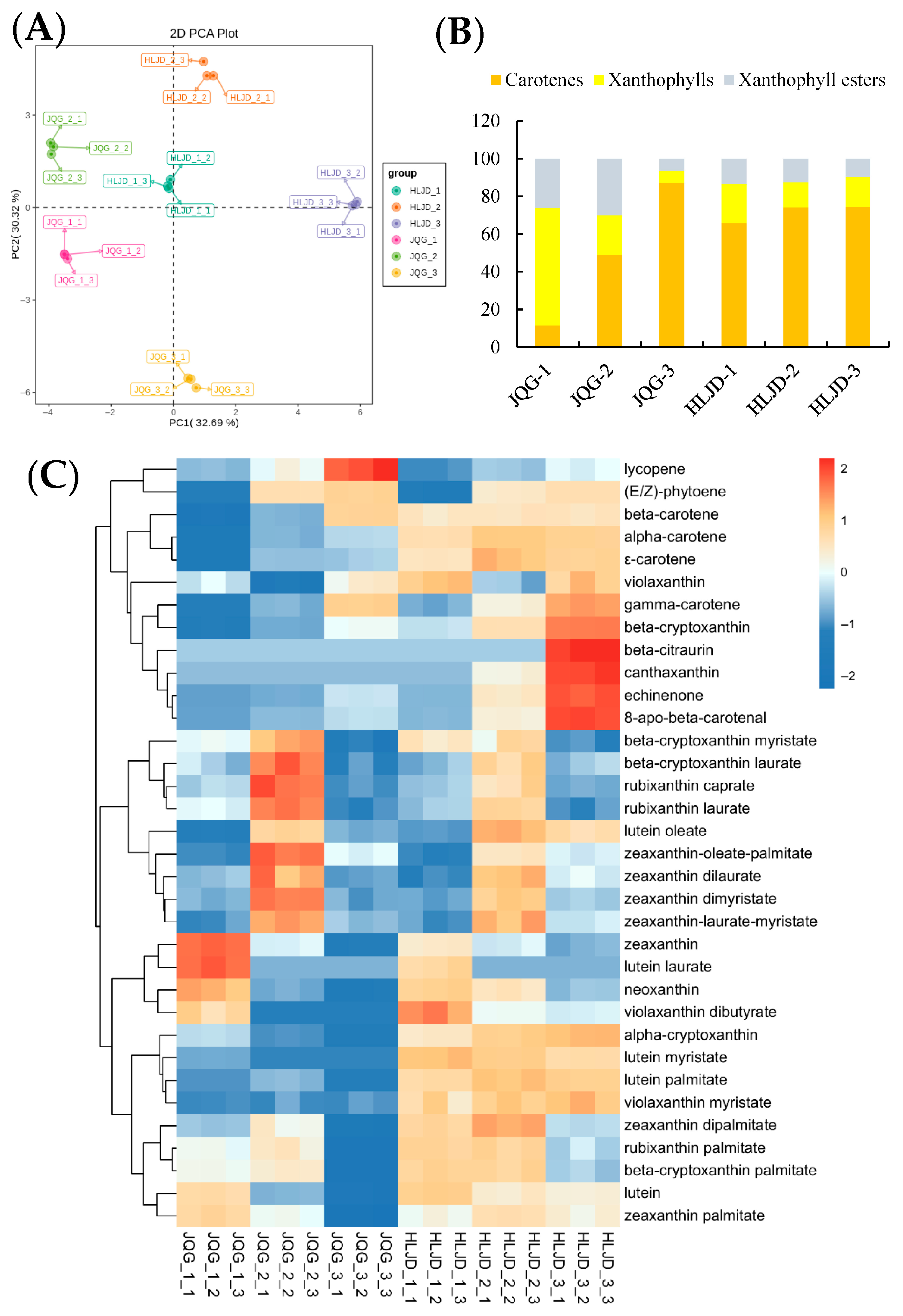
3.3. Quantitative Evaluation of Carotenoids in O. fragrans Petals
3.4. Transcriptome Sequencing and DEGs Analysis Between JQG and HLJD Petals of O. fragrans
3.5. Association Analysis Between Metabolome and Transcriptome of the Important Genes in O. fragrans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Zeng, X.; Yang, J.; Cai, X.; Shi, Y.; Zheng, R.; Wang, Z.; Liu, J.; Yi, X.; Xiao, S.; et al. Whole-genome resequencing of Osmanthus fragrans provides insights into flower color evolution. Hortic. Res. 2021, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Cai, X.; Yang, J.; Zeng, X.; Liu, D.; Huang, S.; Chen, X.; Yang, Q.; Wang, C.; Chen, H. DNA hypomethylation mediates flower opening and senescence in sweet osmanthus through auxin and ethylene responsive pathways. Postharvest Biol. Technol. 2023, 198, 112250. [Google Scholar] [CrossRef]
- Wei, S.; Wu, J.; Yu, P.; Tan, Y.; He, Q.; Yang, J.; Cai, X.; Zou, J.; Chen, H.; Zeng, X. Metabolomic and Transcriptomic Analysis of Unique Floral Coloration in Osmanthus fragrans Cultivars. Horticulturae 2024, 10, 801. [Google Scholar] [CrossRef]
- Cai, X.; Mai, R.; Zou, J.; Zhang, H.; Zeng, X.; Zheng, R.; Wang, C. Analysis of aroma-active compounds in three sweet osmanthus (Osmanthus fragrans) cultivars by GC-olfactometry and GC-MS. J. Zhejiang Univ.-Sci. B Biomed. Biotechnol. 2014, 15, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, C.; Zheng, R.; Cai, X.; Luo, J.; Zou, J.; Wang, C. Emission and accumulation of monoterpene and the key terpene synthase (TPS) associated with monoterpene biosynthesis in Osmanthus fragrans Lour. Front. Plant Sci. 2016, 6, 1232. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P.; et al. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Genet. 2016, 48, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Villwock, S.S.; Li, L.; Jannink, J.L. Carotenoid-carbohydrate crosstalk: Evidence for genetic and physiological interactions in storage tissues across crop species. New Phytol. 2024, 244, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Rao, S.; Wrightstone, E.; Sun, T.; Lui, A.C.W.; Welsch, R.; Li, L. Phytoene synthase: The key rate-limiting enzyme of carotenoid biosynthesis in plants. Front. Plant Sci. 2022, 13, 884720. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; He, Z.; Wei, R.; Ye, J.; Chai, L.; Cheng, Y.; Xu, Q.; Deng, X. Red peel regulator 1 links ethylene response factor 25 and β-citraurin biosynthetic genes to regulate ethylene-induced peel reddening in citrus. Plant Cell 2024, 37, 10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Mi, J.; Balakrishna, A.; Liew, K.X.; Ablazov, A.; Sougrat, R.; Al-Babili, S. Gardenia carotenoid cleavage dioxygenase 4a is an efficient tool for biotechnological production of crocins in green and non-green plant tissues. Plant Biotechnol. J. 2022, 20, 2202–2216. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, S.; Kato, M.; Kurosawa, M.; Kurobayashi, Y.; Fujita, A.; Fleischmann, P.; Watanabe, N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010, 61, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Effroy, D.; Lefebvre, V.; Seo, M.; Perreau, F.; Berger, A.; Sechet, J.; To, A.; North, H.M.; Marion-Poll, A. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012, 70, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Gavassi, M.A.; Silva, G.S.; Silva, C.M.S.; Thompson, A.J.; Macleod, K.; Oliveira, P.M.R.; Cavalheiro, M.F.; Domingues, D.S.; Habermann, G. NCED expression is related to increased ABA biosynthesis and stomatal closure under aluminum stress. Environ. Exp. Bot. 2021, 185, 104404. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Sadali, N.M.; Soufi, Z.; Zhou, Y.; Huang, B.; Zeng, Y.; Rodriguez-Concepcion, M.; Jarvis, R.P. The chloroplast-associated protein degradation pathway controls chromoplast development and fruit ripening in tomato. Nat. Plants 2021, 7, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Fu, X.; Lu, P.; Grierson, D.; Xu, W. Molecular mechanisms determining the differential accumulation of carotenoids in plant species and varieties. Crit. Rev. Plant Sci. 2020, 39, 125–139. [Google Scholar] [CrossRef]
- Hermanns, A.; Zhou, X.; Xu, Q.; Tadmor, Y.; Li, L. Carotenoid pigment accumulation in horticultural plants. Hortic. Plant J. 2020, 6, 343–360. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Dong, B.; Fu, J.; Hu, S.; Zhao, H. Carotenoid Accumulation and Its Contribution to Flower Coloration of Osmanthus fragrans. Front. Plant Sci. 2018, 9, 1499. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Liu, Y. An Illustrated Monograph of the Sweet Osmanthus Cultivars in China; Zhejiang Science and Technology Press: Hangzhou, China, 2008. [Google Scholar]
- Yang, K. Chinese Osmanthus; China Forestry Publishing House: Beijing, China, 2020. [Google Scholar]
- Wang, Y.; Luo, Y.; Zhang, C.; Fu, J.; Hu, S.; Zhao, H. Flower Color and Pigment Composition in the Petals of Bud Mutation and its Stock Plant of Osmanthus fragrans ‘Jingui’. Acta Hortic. Sin. 2017, 44, 528–536. [Google Scholar]
- Simon, A. FastQC: A Quality Control Tool for High Throughput Sequence Data; Version 0.10.1; Babraham Bioinformatics: Cambridge, UK, 2010. [Google Scholar]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Drapal, M.; Gerrish, C.; Fraser, P.D. Changes in carbon allocation and subplastidal amyloplast structures of specialised Ipomoea batatas (sweet potato) storage root phenotypes. Phytochemistry 2022, 203, 113409. [Google Scholar] [CrossRef] [PubMed]
- Gutschker, S.; Ruescher, D.; Rabbi, I.Y.; Rosado-Souza, L.; Pommerrenig, B.; Pauly, M.; Robertz, S.; van Doorn, A.M.; Schlereth, A.; Neuhaus, H.E.; et al. Carbon usage in yellow-fleshed Manihot esculenta storage roots shifts from starch biosynthesis to cell wall and raffinose biosynthesis via the myo-inositol pathway. Plant J. 2024, 119, 2045–2062. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Mcquinn, R.; Fei, Z.; Wolters, A.A.; van Eck, J.; Brown, C.; Giovannoni, J.J.; Li, L. Regulatory control of high levels of carotenoid accumulation in potato tubers. Plant Cell Environ. 2011, 34, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Du, J.; Wang, L.; Pan, Z.; Xu, Q.; Xiao, S.; Deng, X. A comprehensive analysis ofchromoplastdifferentiationrevealscomplexproteinchangesassociated with plastoglobule biogenesis and remodeling of protein systems in sweet orange flesh. Plant Physiol. 2015, 168, 1648–1665. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Lin, J.; Zeng, Z.; Deng, Z.; Tan, J.; Chen, X.; Ding, G.; Zhu, M.; Xu, B.; Atkinson, R.G.; et al. The kiwifruit amyloplast proteome (kfALP): A resource to better understand the mechanisms underlying amyloplast biogenesis and differentiation. Plant J. 2024, 118, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, K.; Du, X.; Peng, Y.; Qin, J.; Zeng, L. Correlation analysis of petal morphology and pigment content of Rosa ‘Angela’. J. Beijing Univ. Agric. 2022, 37, 1–10. [Google Scholar]
- Yuan, Y.; Li, X.; Yao, X.; Fu, X.; Cheng, J.; Shan, H.; Yin, X.; Kong, H. Mechanisms underlying the formation of complex color patterns on Nigella orientalis (Ranunculaceae) petals. New Phytol. 2023, 237, 2450–2466. [Google Scholar] [CrossRef] [PubMed]
- May, T.S.Y.; Edwige, M. Under the rainbow: Novel insights on the mechanisms driving the development and evolution of petal pigmentation. Curr. Opin. Plant Biol. 2025, 86, 102743. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wu, B.; Zhang, H.; Wang, C.; Li, J.; Yang, B.; Li, S. Characterization of volatile compounds in flowers from four groups of sweet osmanthus (Osmanthus fragrans) cultivars. Can. J. Plant Sci. 2013, 93, 923–931. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Chen, W.; Dong, M.; Yuan, W.; Liu, X.; Shang, F. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans. Tree Genet. Genomes 2014, 10, 329–338. [Google Scholar] [CrossRef]
- Han, Y.; Lu, M.; Yue, S.; Li, K.; Dong, M.; Liu, L.; Wang, H.; Shang, F. Comparative methylomics and chromatin accessibility analysis in Osmanthus fragrans uncovers regulation of genic transcription and mechanisms of key floral scent production. Hortic. Res. 2022, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Y.; Xiao, Q.; Huang, X.; Li, C.; Gao, X.; Wang, Q.; Xiang, X.; Zhu, Y.; Wang, J.; et al. Carotenoids modulate kernel texture in maize by influencing amyloplast envelope integrity. Nat. Commun. 2020, 11, 5346. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Tan, Y.; Wen, X.; He, Q.; Wu, H.; Zou, J.; Yang, J.; Cai, X.; Chen, H. Delayed Starch Degradation Triggers Chromoplast Structural Aberration to Inhibit Carotenoid Cleavage: A Novel Mechanism for Flower Color Deepening in Osmanthus fragrans. Horticulturae 2025, 11, 864. https://doi.org/10.3390/horticulturae11070864
Zeng X, Tan Y, Wen X, He Q, Wu H, Zou J, Yang J, Cai X, Chen H. Delayed Starch Degradation Triggers Chromoplast Structural Aberration to Inhibit Carotenoid Cleavage: A Novel Mechanism for Flower Color Deepening in Osmanthus fragrans. Horticulturae. 2025; 11(7):864. https://doi.org/10.3390/horticulturae11070864
Chicago/Turabian StyleZeng, Xiangling, Yunfei Tan, Xin Wen, Qiang He, Hui Wu, Jingjing Zou, Jie Yang, Xuan Cai, and Hongguo Chen. 2025. "Delayed Starch Degradation Triggers Chromoplast Structural Aberration to Inhibit Carotenoid Cleavage: A Novel Mechanism for Flower Color Deepening in Osmanthus fragrans" Horticulturae 11, no. 7: 864. https://doi.org/10.3390/horticulturae11070864
APA StyleZeng, X., Tan, Y., Wen, X., He, Q., Wu, H., Zou, J., Yang, J., Cai, X., & Chen, H. (2025). Delayed Starch Degradation Triggers Chromoplast Structural Aberration to Inhibit Carotenoid Cleavage: A Novel Mechanism for Flower Color Deepening in Osmanthus fragrans. Horticulturae, 11(7), 864. https://doi.org/10.3390/horticulturae11070864





