Male Date Palm Chlorotype Selection Based on Fertility, Metaxenia, and Transcription Aspects
Abstract
1. Introduction
2. Results and Discussion
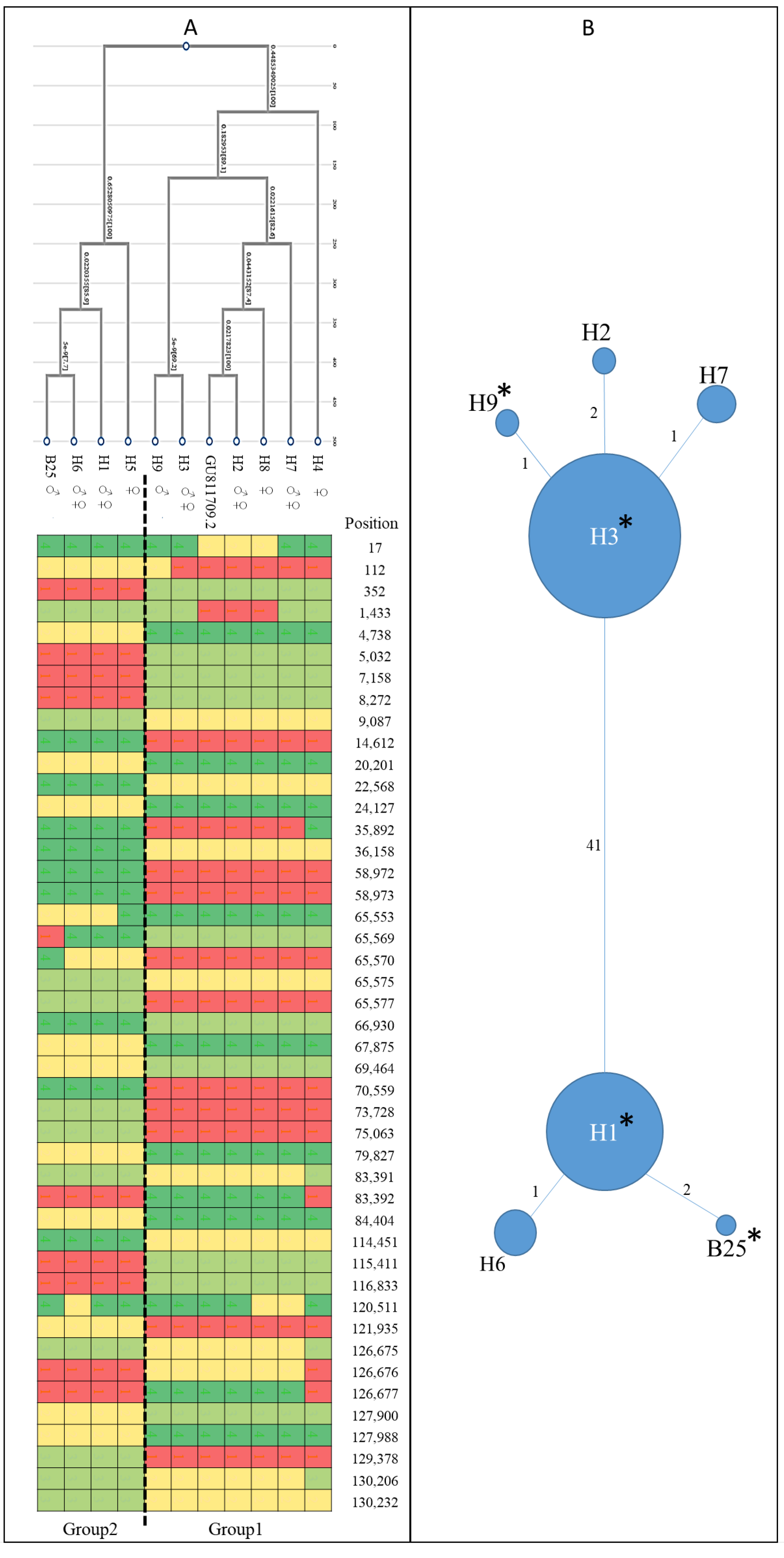
2.1. Chlorotypes Analysis of the Selected Male Cultivars
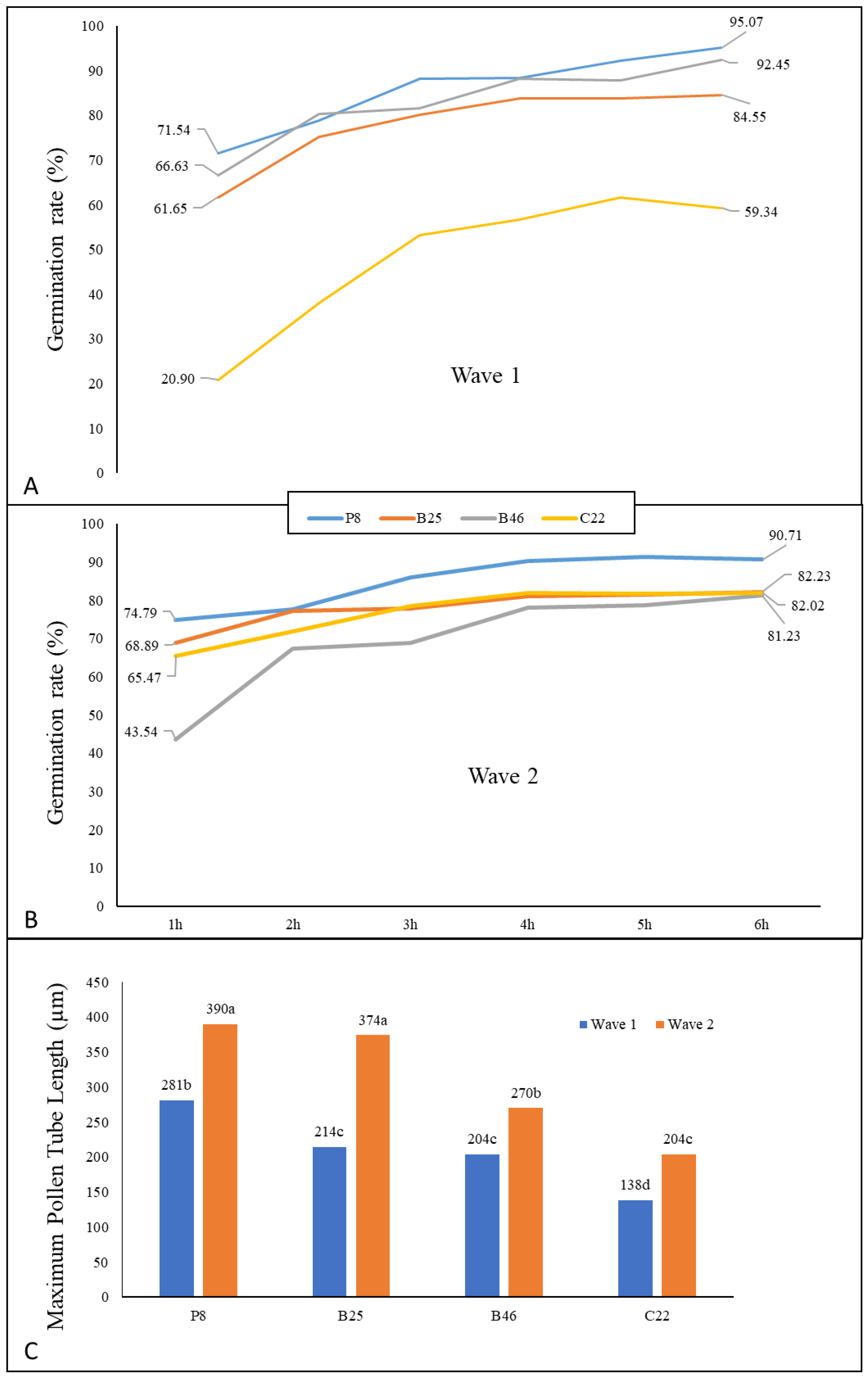
2.2. In Vitro Analysis of Pollen Germination and Tube Elongation
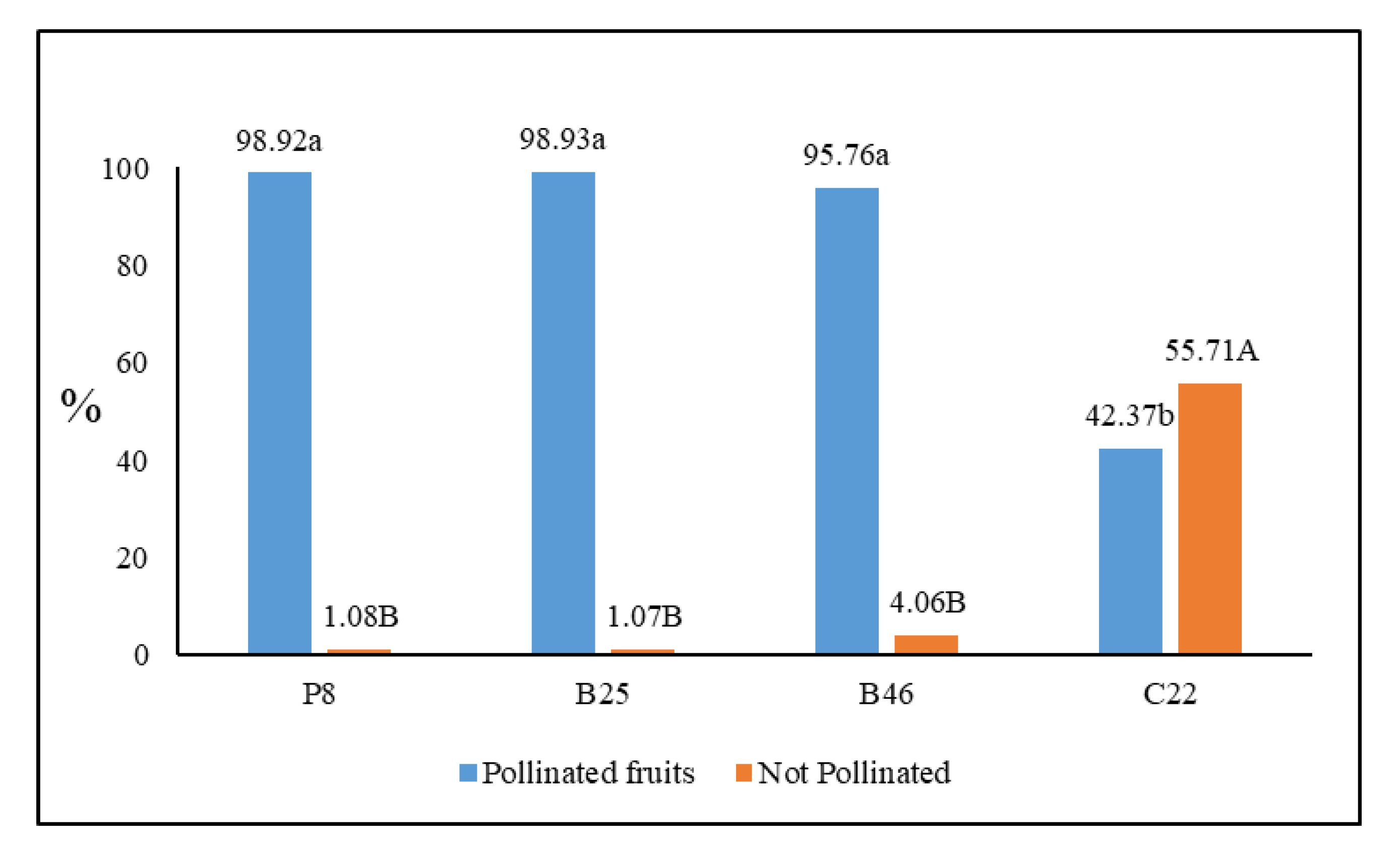
2.3. Pollination Efficiency
2.4. Morphological and Phytochemical Characterization
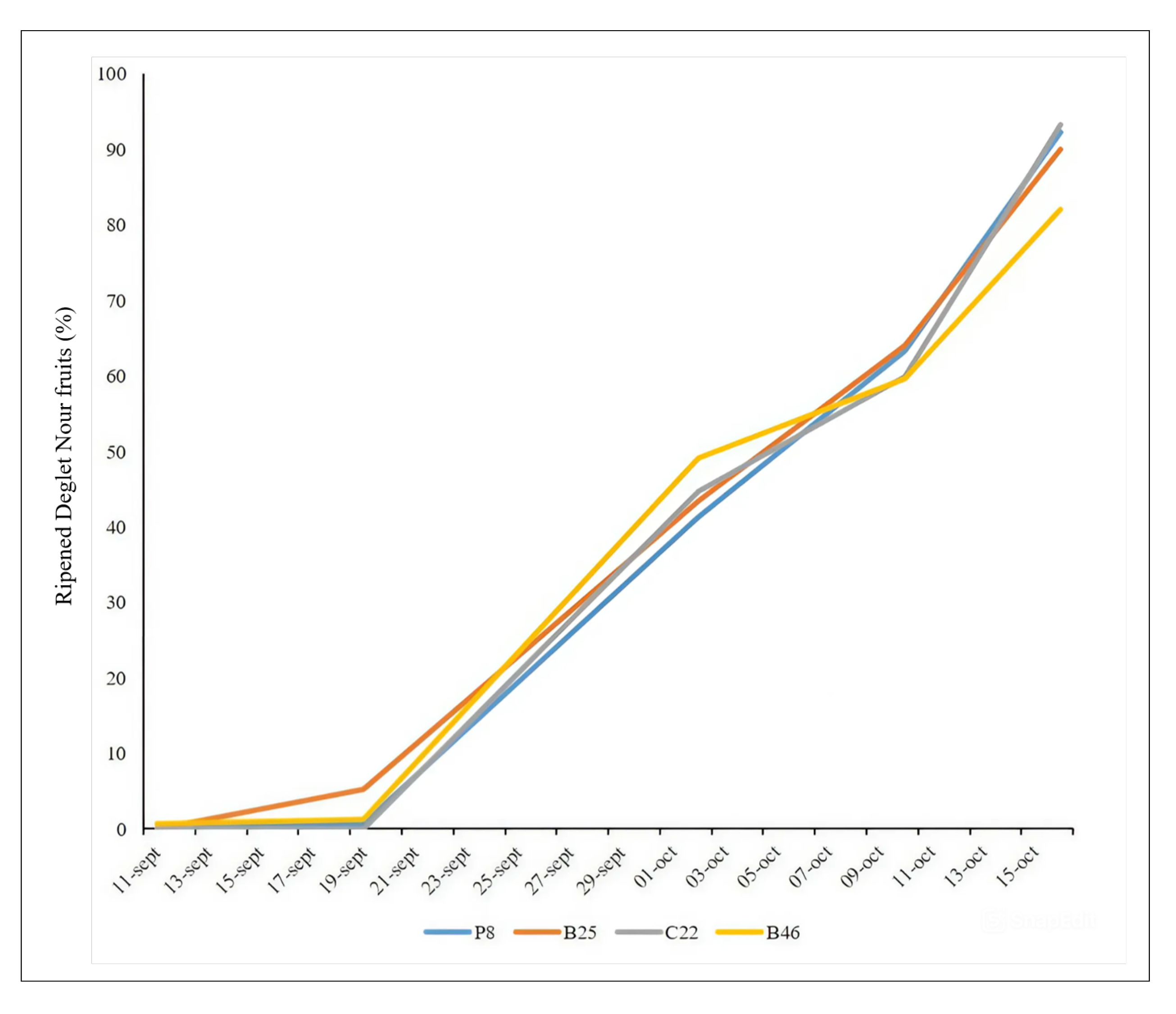
2.5. Maturation Dynamics
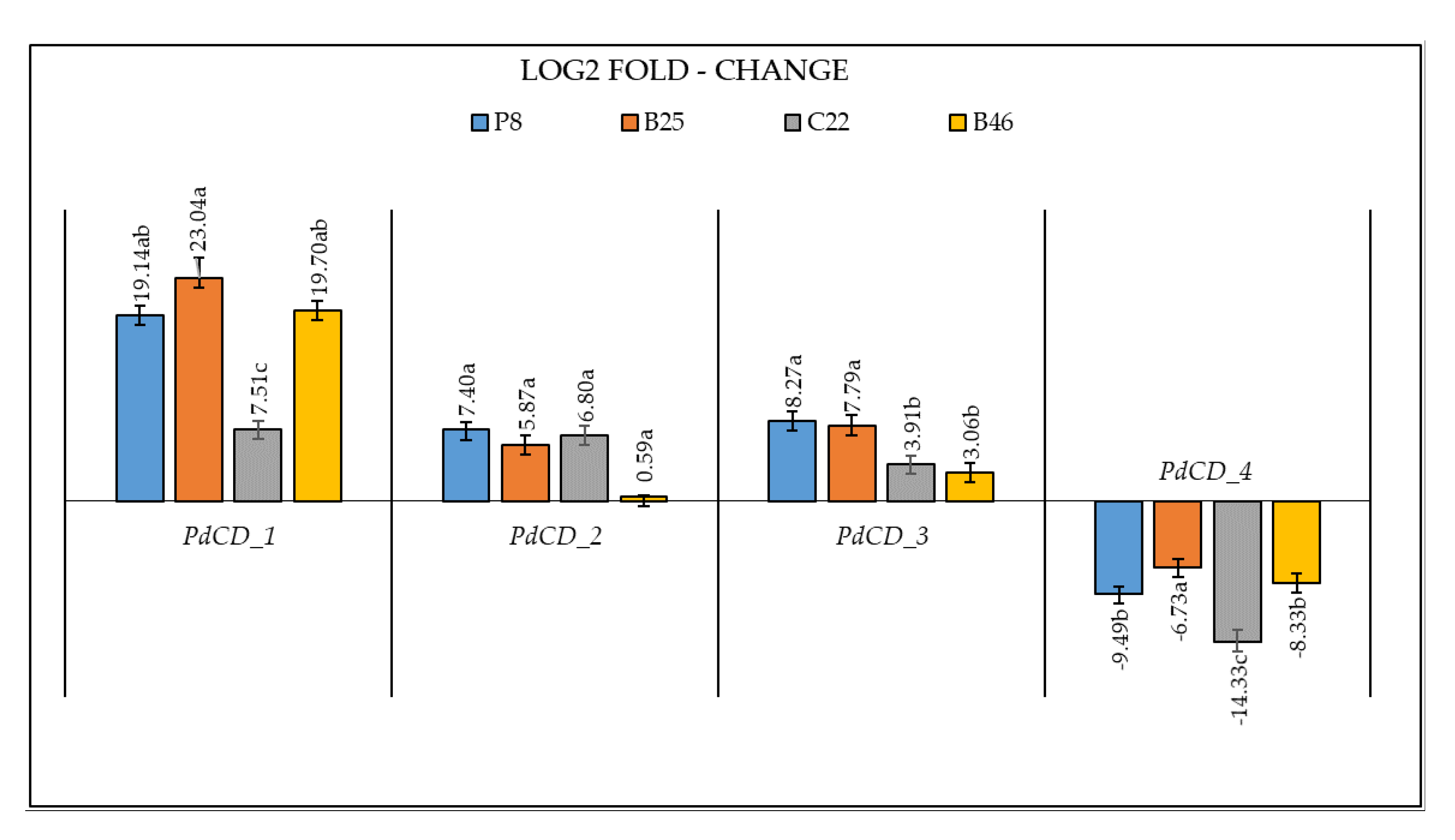
2.6. Effect of Pollination on Gene Expression of Cell Division Control Genes
3. Materials and Methods
3.1. Male Cultivar and Chlorotypes Analysis
3.2. Pollen Quality Assessment
3.3. Pollination and Pollination Efficiency
3.4. Morphological and Phytochemical Characterization
3.5. RNA Extraction and cDNA Synthesis
3.6. Selection of Reference Genes and Primers Design
3.7. qPCR and Relative Quantification of the Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| cpDNA | Chloroplast DNA |
| qPCR | Quantitative PCR |
| SNP | Single-nucleotide polymorphism |
References
- Sedra, M.H.; Lashermes, P.; Trouslot, P.; Combes, M.; Hamon, S. Identification and genetic diversity analysis of date palm (Phoenix dactylifera L.) varieties from Morocco using RAPD markers. Euphytica 1998, 103, 75–82. [Google Scholar] [CrossRef]
- Hachef, A.; Bourguiba, H.; Cherif, E.; Ivorra, S.; Terral, J.-F.; Zehdi-Azouzi, S. Agro-morphological traits assessment of Tunisian male date palms (Phœnix dactylifera L.) for preservation and sustainable utilization of local germplasm. Saudi J. Biol. Sci. 2023, 30, 103574. [Google Scholar] [CrossRef]
- Elmeer, K.; Mattat, I. Marker-assisted sex differentiation in date palm using simple sequence repeats. 3 Biotech 2012, 2, 241–247. [Google Scholar] [CrossRef]
- Abdelouahhab, Z.; Arias-Jimenez, E.J. Date palm cultivation, Food and Agriculture Organization of the United Nations (FAO) Agricultural Services. Bulletin 2002, 156. [Google Scholar]
- Djerbi, M. Disease of the Date Palm (Phoenix dactylifera L.). F.A.O Regional Project for Palm and Dates Research Center in the Near East and North Africa; FAO: Baghdad, Iraq, 1983; p. 127. [Google Scholar]
- Shafique, M.; Khan, A.S.; Malik, A.U.; Shahid, M.; Rajwana, I.A.; Saleem, V.A.; Ahmad, I. Influence of pollen source and pollination frequency on fruit drop, yield and quality of date palm (Phoenix dactylifera L.) cv. Dhakki. Pak. J. Bot. 2011, 43, 831–839. [Google Scholar]
- Pintaud, J.-C.; Ludena, B.; Zehdi, S.; Gros-Balthazard, M.; Ivorra, S.; Terral, J.-F.; Newton, C.; Tengberg, M.; Santoni, S.; Boughedoura, N. Biogeography of the date palm (Phoenix dactylifera L., Arecaceae): Insights on the origin and on the structure of modern diversity. ISHS Acta Hortic. 2013, 994, 19–38. [Google Scholar] [CrossRef]
- Hazzouri, K.M.; Gros-Balthazard, M.; Flowers, J.M.; Copetti, D.; Lemansour, A.; Lebrun, M.; Purugganan, M.D. Ge-Nome-Wide Association Mapping of Date Palm Fruit Traits. Nat. Commun. 2020, 11, 2464. [Google Scholar]
- Hamza, H.; Villa, S.; Torre, S.; Marchesini, A.; Benabderrahim, M.A.; Rejili, M.; Sebastiani, F. Whole mitochondrial and chloroplast genome sequencing of Tunisian date palm cultivars: Diversity and evolutionary relationships. BMC Genom. 2023, 24, 772. [Google Scholar] [CrossRef]
- Munier, P. Le Palmier-Dattier; GP Maisonneuve et Larose: Paris, France, 1973. [Google Scholar]
- Salomon-Torres, R.; Ortiz-Uribe, N.; Villa-Angulo, R.; Villa-Angulo, C.; Norzagaray-Plasencia, S.; García-Verdugo, C.D. Effect of pollenizers on production and fruit characteristics of date palm (Phoenix dactylifera L.) cultivar Medjool in Mexico. Turk. J. Agric. For. 2017, 41, 338–347. [Google Scholar] [CrossRef]
- Kadri, K.; Jemni, M.; Mesnoua, M.; Sharma, S.S.; Malik, A.A.; Makhlouf, S.; Elsafy, M. Study on the effects of pollen sources on the agronomic, biochemical, mineral, and pomological traits of date palm (Phoenix dactylifera L.) cv ‘Deglet Nour’ fruits in Degache Oases (Tunisia). Genet. Resour. Crop. Evol. 2024, 71, 3721–3733. [Google Scholar] [CrossRef]
- El-Kassaby, Y.A.; Klápště, J.; Guy, R.D. Breeding without Breeding: Selection Using the Genomic Best Linear Unbiased Predictor Method (GBLUP). New For. 2012, 43, 631–637. [Google Scholar] [CrossRef]
- Gros-Balthazard, M.; Galimberti, M.; Kousathanas, A.; Newton, C.; Ivorra, S.; Paradis, L.; Vigouroux, Y.; Carter, R.; Tengberg, M.; Battesti, V.; et al. The Discovery of Wild Date Palms in Oman Reveals a Complex Domestication History Involving Centers in the Middle East and Africa. Curr. Biol. 2017, 27, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Hazzouri, K.M.; Flowers, J.M.; Nelson, D.; Lemansour, A.; Masmoudi, K.; Amiri, K.M.A. Prospects for the Study and Improvement of Abiotic Stress Tolerance in Date Palms in the Post-Genomics Era. Front. Plant Sci. 2020, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, X.; Liu, G.; Yin, Y.; Chen, K.; Yun, Q.; Zhao, D.; Al-Mssallem, I.S.; Yu, J. The complete chloroplast genome sequence of date palm (Phoenix dactylifera L.). PLoS ONE 2010, 5, e12762. [Google Scholar] [CrossRef]
- Al-Khalifah, N.S.; Askari, E. Morphological and Molecular Characterization of Date Palm (Phoenix dactylifera L.) Cultivars in Saudi Arabia. J. Hortic. Sci. Biotechnol. 2003, 78, 392–398. [Google Scholar]
- Fu, Y. The actin cytoskeleton and signaling network during pollen tube tip growth. J. Integr. Plant Biol. 2010, 52, 131–137. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Liu, X.; Kasahara, R.D. Mechanics of Pollen Tube Elongation: A Perspective. Front. Plant Sci. 2020, 11, 589712. [Google Scholar] [CrossRef]
- Chao, C.C.T.; Krueger, R.R. The date palm (Phoenix dactylifera L.): Overview of biology, uses, and cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef]
- Ouonkap, S.V.Y.; Palaniappan, M.; Pryze, K.; Jong, E.; Ali, M.F.; Styler, B.; Almasaud, R.A.; Harkey, A.F.; Reid, R.W.; Loraine, A.E.; et al. Enhanced pollen tube performance at high temperature contributes to thermotolerant fruit and seed production in tomato. Curr. Biol. 2024, 34, 5319–5333. [Google Scholar] [CrossRef]
- Ferri, A.; Giordani, E.; Padula, G.; Bellini, E. Viability and in vitro germinability of pollen grains of olive cultivars and advanced selections obtained in Italy. Adv. Hort. Sci. 2008, 22, 116–122. [Google Scholar]
- Zargari, M.; Sadeghi, M.; Aghdaie, F. Effect of pollen source on fruit set, yield, and quality of date palm (Phoenix dactylifera L.). Hort. Sci. Technol. 2021, 39, 512–523. [Google Scholar]
- Barreveld, W.H. Date Palm Products. In FAO Bulletin Services Agricole No 101; Food and Agriculture Organization of the United Nations: Rome, Italy, 1993. [Google Scholar]
- Sawaya, W.N.; Khalil, J.K.; Safi, W.J. Chemical Composition and Nutritional Quality of Date Seeds. J. Food Sci. 1984, 49, 617–619. [Google Scholar] [CrossRef]
- Al-Shahib, W.; Marshall, R.J. The fruit of the date palm: Its possible use as the best food for the future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.; Woolf, A. Avocado (Persea americana Mill.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 125–186. [Google Scholar]
- Rygg, G.L. Date development, handling and packing in the United States. In Agricultural Handbook No. 482, 1st ed.; Agricultural Research Service, US Department of Agriculture Date Development: Washington, DC, USA, 1975. [Google Scholar]
- Hammouda, H.d.; Chérif, J.K.; Trabelsi-Ayadi, M.; Baron, A.; Guyot, S. Detailed polyphenol and tannin composition and its variability in Tunisian dates (Phoenix dactylifera L.) at different maturity stages. J. Agric. Food Chem. 2013, 61, 3252–3263. [Google Scholar] [CrossRef] [PubMed]
- Al-Turki, S.; Shahba, M.A.; Stushnoff, C. Diversity of antioxidant properties and phenolic content of date palm (Phoenix dactylifera L.) fruits as affected by cultivar and location. J. Food Agric. Environ. 2010, 8, 253–260. [Google Scholar]
- Omar, A. Effect of amount of pollen on anatomy and quality of Zagloul date palm fruit (Phoenix dactylifera, L.). In ARAB PALM Conference, Proceedings of the First International Scientific Conference for the Development of Date Palm and Dates Sector in the Arab World, Riyadh, Saudi Arabia, 4–7 December 2011; National Centre for Agriculture Technologies: Riyadh, Saudi Arabia, 2011; Volume 1, pp. 121–131. [Google Scholar]
- Elmeer, K.; Mattat, I. Genetic diversity of Qatari date palm using SSR markers. Genet. Mol. Res. 2015, 14, 1624–1635. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.-P.; Bouzayen, M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Ozga, J.A.; Reinecke, D.M. Hormonal interactions in fruit development. J. Plant Growth Regul. 2003, 22, 73–81. [Google Scholar] [CrossRef]
- Patankar, H.V.; Assaha, D.V.M.; Al-Yahyai, R.; Sunkar, R.; Yaish, M.W. Identification of Reference Genes for Quantitative Real-Time PCR in Date Palm (Phoenix dactylifera L.) Subjected to Drought and Salinity. PLoS ONE 2016, 11, e0166216. [Google Scholar] [CrossRef]
| Parameters | P8 | B25 | C22 | B46 | |
|---|---|---|---|---|---|
| Fruit | Length (cm) | 3.83 (±0.01) b | 3.69 (±0.09) c | 3.99 (±0.02) a | 3.94 (±0.23) a |
| Width (cm) | 1.94 (±0.05) a | 1.87 (±0.09) b | 2.01 (±0.04) a | 1.98 (±0.09) a | |
| Weight (g) | 9.17 (±0.20) b | 8.15 (±0.55) c | 10.74 (±0.53) a | 9.54 (±2.27) b | |
| Flesh (cm) | 0.63 (±0.07) bc | 0.59 (±0.50) c | 0.72 (±0.03) a | 0.64 (±0.10) b | |
| Seed | Length (cm) | 2.53 (±0.04) b | 2.48 (±0.07) b | 2.65 (±0.03) a | 2.7 (±0.13) a |
| Width (cm) | 0.84 (±0.00) a | 0.85 (±0.01) a | 0.83 (±0.01) a | 0.86 (±0.02) a | |
| Weight (g) | 1.40 (±0.37) a | 1.20 (±0.05) a | 1.19 (±0.00) a | 1.31 (±0.03) a | |
| Ratio (%) | Seed weight/Fruit weight | 15.22 (±4.30) a | 14.91 (±1.45) a | 11.18 (±0.97) a | 13.89 (±15.10) a |
| Photos of the pollinated fruits |  |  |  |  | |
| Water Content (%) | Brix (°) | Total Sugar Content (%) | Phenol Content (mg GAE/100 g) | |
|---|---|---|---|---|
| P8 | 82.18 (±0.01) a | 2.00 (±0.05) a | 5.41 (±0.05) b | 1.14 (±0.20) b |
| B25 | 80.68 (±0.09) b | 2.15 (±0.09) a | 6.98 (±0.09) a | 0.81 (±0.55) c |
| C22 | 81.78 (±0.02) a | 1.98 (±0.04) a | 5.78 (±0.04) b | 1.02 (±0.53) b |
| B46 | 81.78 (±0.23) a | 1.75 (±0.09) a | 5.91 (±0.09) b | 1.31 (±2.27) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, H.; Benabderrahim, M.A.; Boualleg, A.; Sebastiani, F.; Haouala, F.; Rejili, M. Male Date Palm Chlorotype Selection Based on Fertility, Metaxenia, and Transcription Aspects. Horticulturae 2025, 11, 865. https://doi.org/10.3390/horticulturae11070865
Hamza H, Benabderrahim MA, Boualleg A, Sebastiani F, Haouala F, Rejili M. Male Date Palm Chlorotype Selection Based on Fertility, Metaxenia, and Transcription Aspects. Horticulturae. 2025; 11(7):865. https://doi.org/10.3390/horticulturae11070865
Chicago/Turabian StyleHamza, Hammadi, Mohamed Ali Benabderrahim, Achwak Boualleg, Federico Sebastiani, Faouzi Haouala, and Mokhtar Rejili. 2025. "Male Date Palm Chlorotype Selection Based on Fertility, Metaxenia, and Transcription Aspects" Horticulturae 11, no. 7: 865. https://doi.org/10.3390/horticulturae11070865
APA StyleHamza, H., Benabderrahim, M. A., Boualleg, A., Sebastiani, F., Haouala, F., & Rejili, M. (2025). Male Date Palm Chlorotype Selection Based on Fertility, Metaxenia, and Transcription Aspects. Horticulturae, 11(7), 865. https://doi.org/10.3390/horticulturae11070865








