Nitric Oxide Modulates Postharvest Physiology to Maintain Abelmoschus esculentus Quality Under Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Treatments
2.2. Weight Loss Measurement
2.3. Texture Quality Assessment
2.4. Determination of Cellulose and Lignin Contents
2.5. Determination of Chlorophyll Content
2.6. Determination of Soluble Protein Content
2.7. Determination of Soluble Sugar Content
2.8. Determination of MDA Content and Relative Conductivity
2.9. Determination of Antioxidant Enzyme Activities
2.10. Data Analysis
3. Results
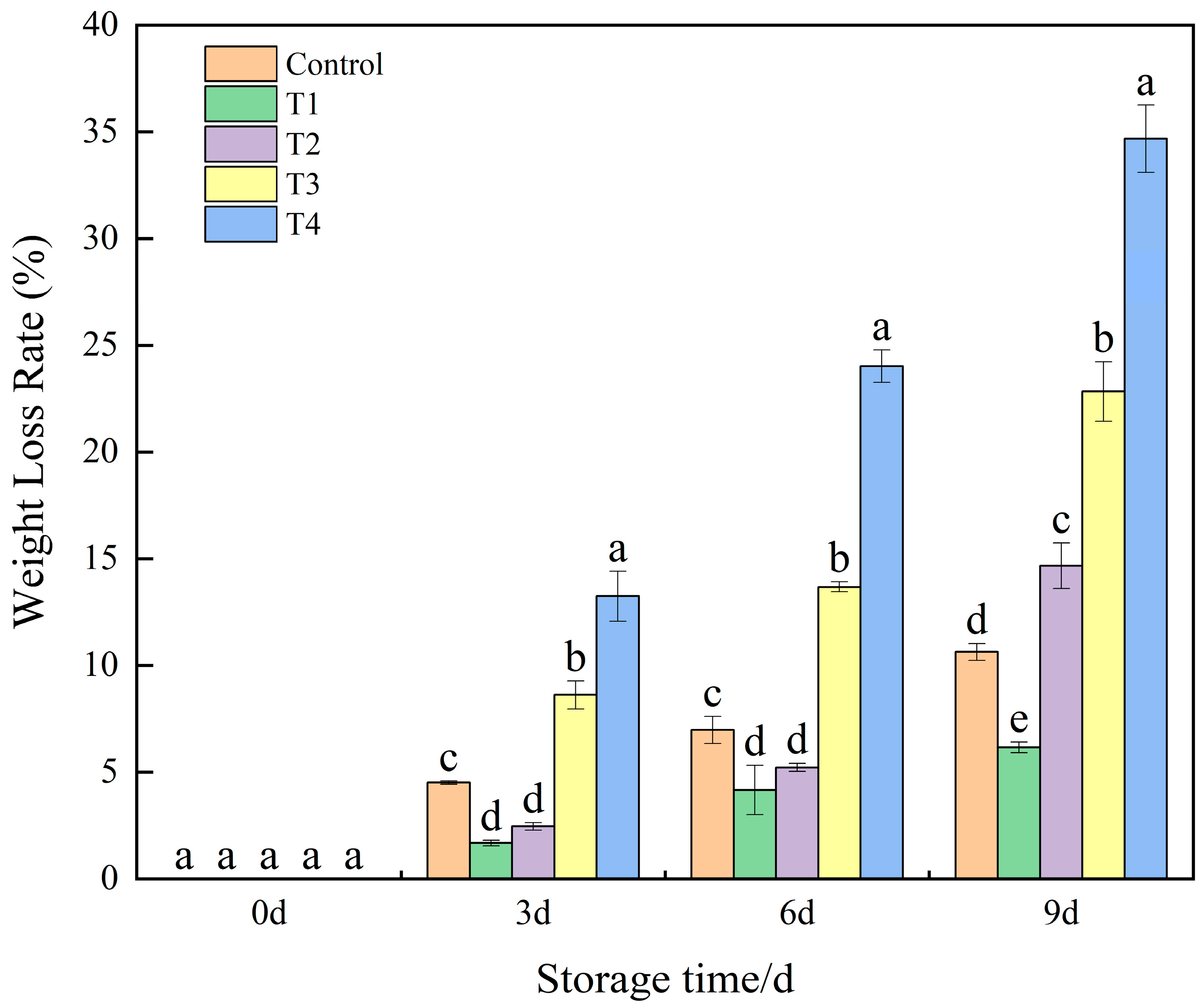
3.1. Effects of Different Concentrations of NO on the Weight Loss Rate of Okra Under Low-Temperature Storage
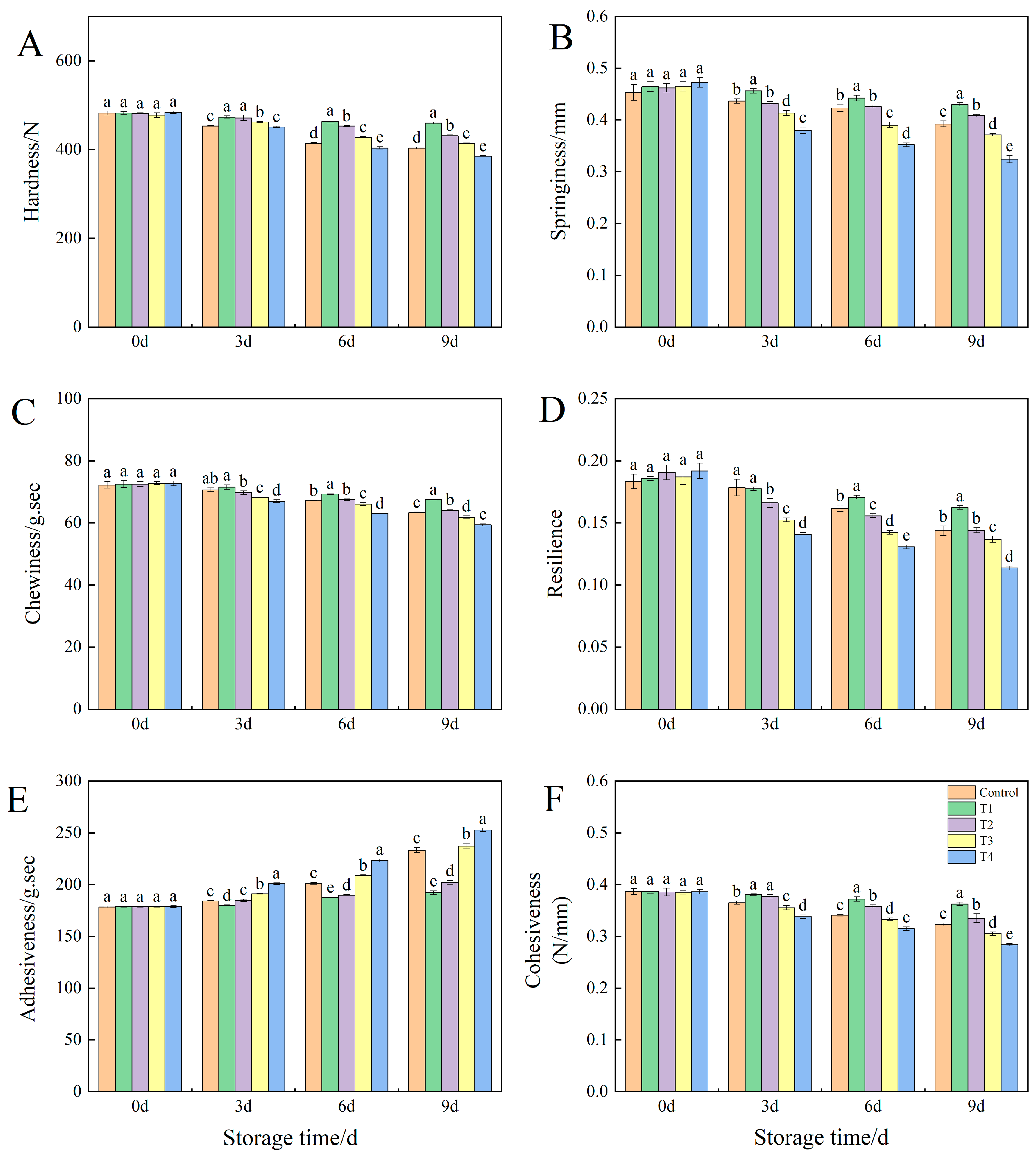
3.2. Effects of Different NO Concentrations on the Texture Profile Analysis (TPA) of Okra Fruit Under Low-Temperature Storage
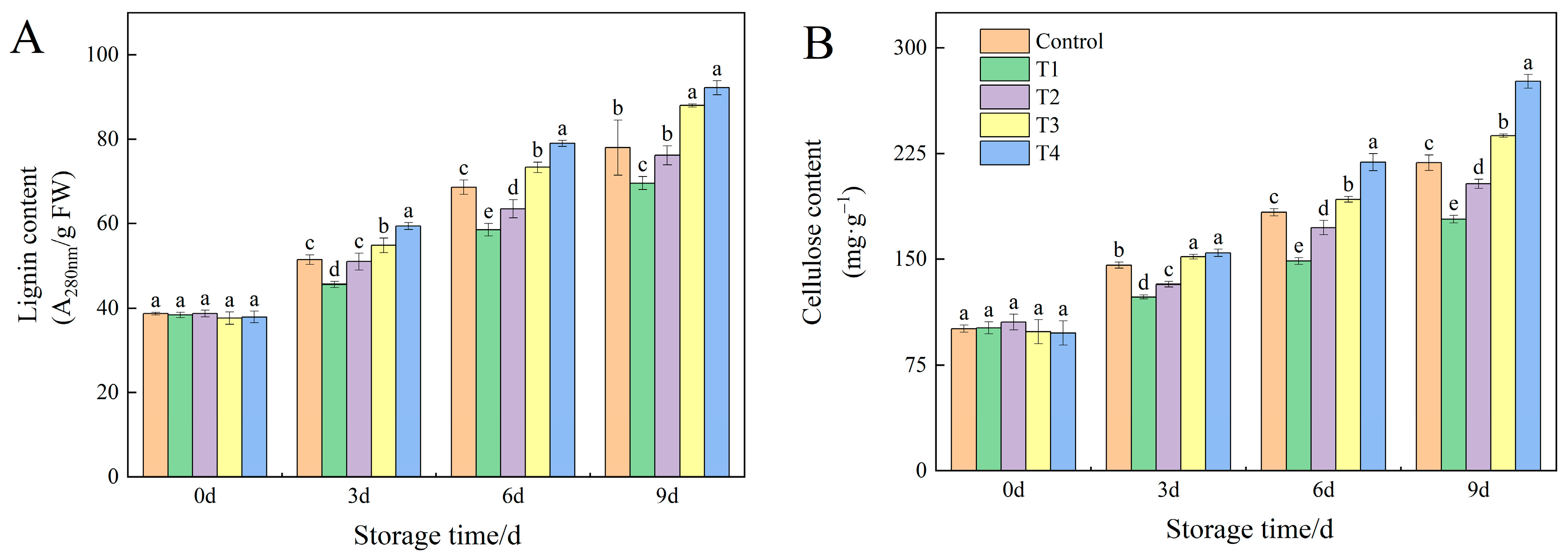
3.3. Effects of Different NO Concentrations on Lignin and Cellulose Contents of Okra Fruit Under Low-Temperature Storage
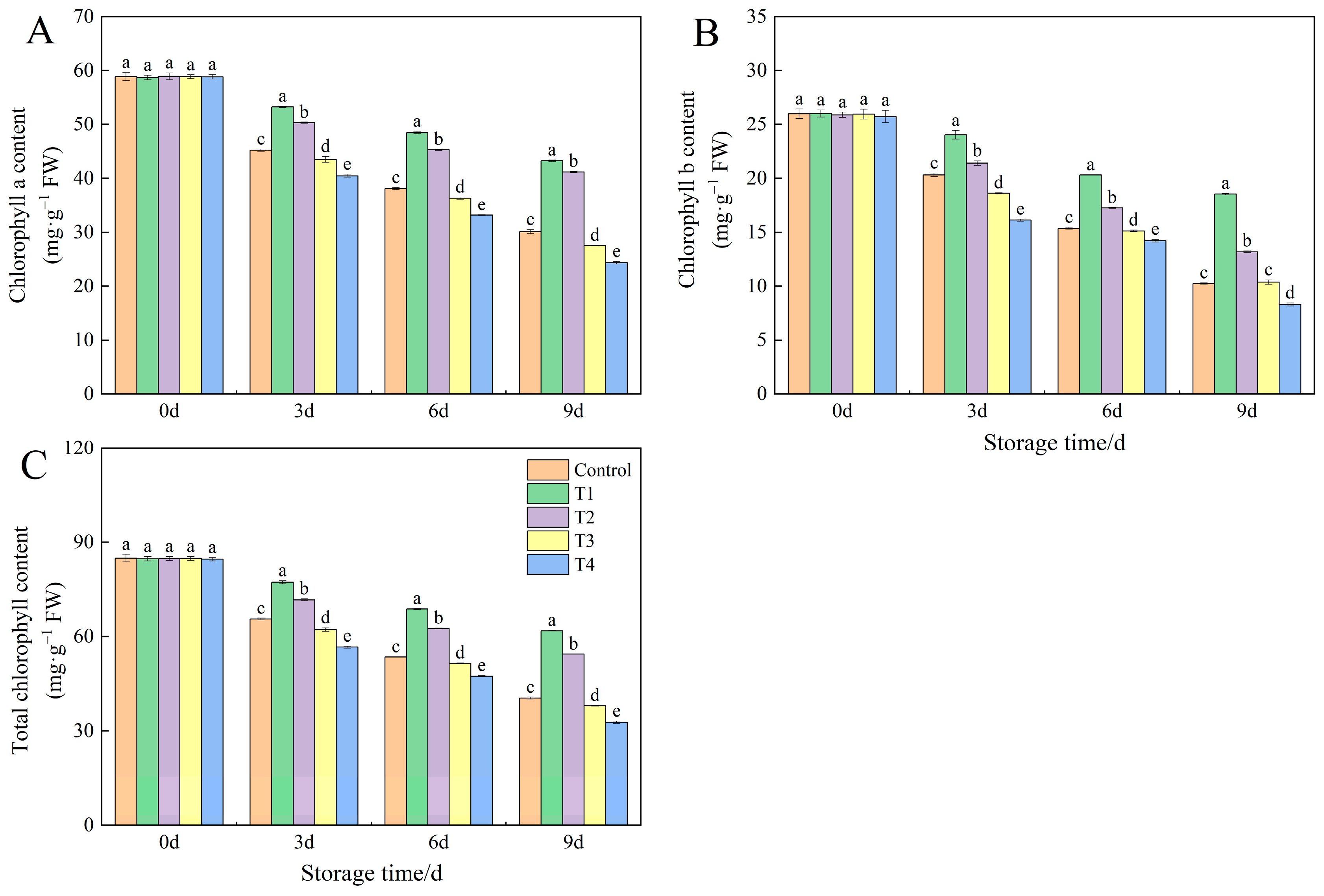
3.4. Effects of Different NO Concentrations on Chlorophyll Content in Okra Fruit During Low-Temperature Storage
3.5. Effects of Different NO Concentrations on Soluble Protein Content in Okra Fruit During Low-Temperature Storage
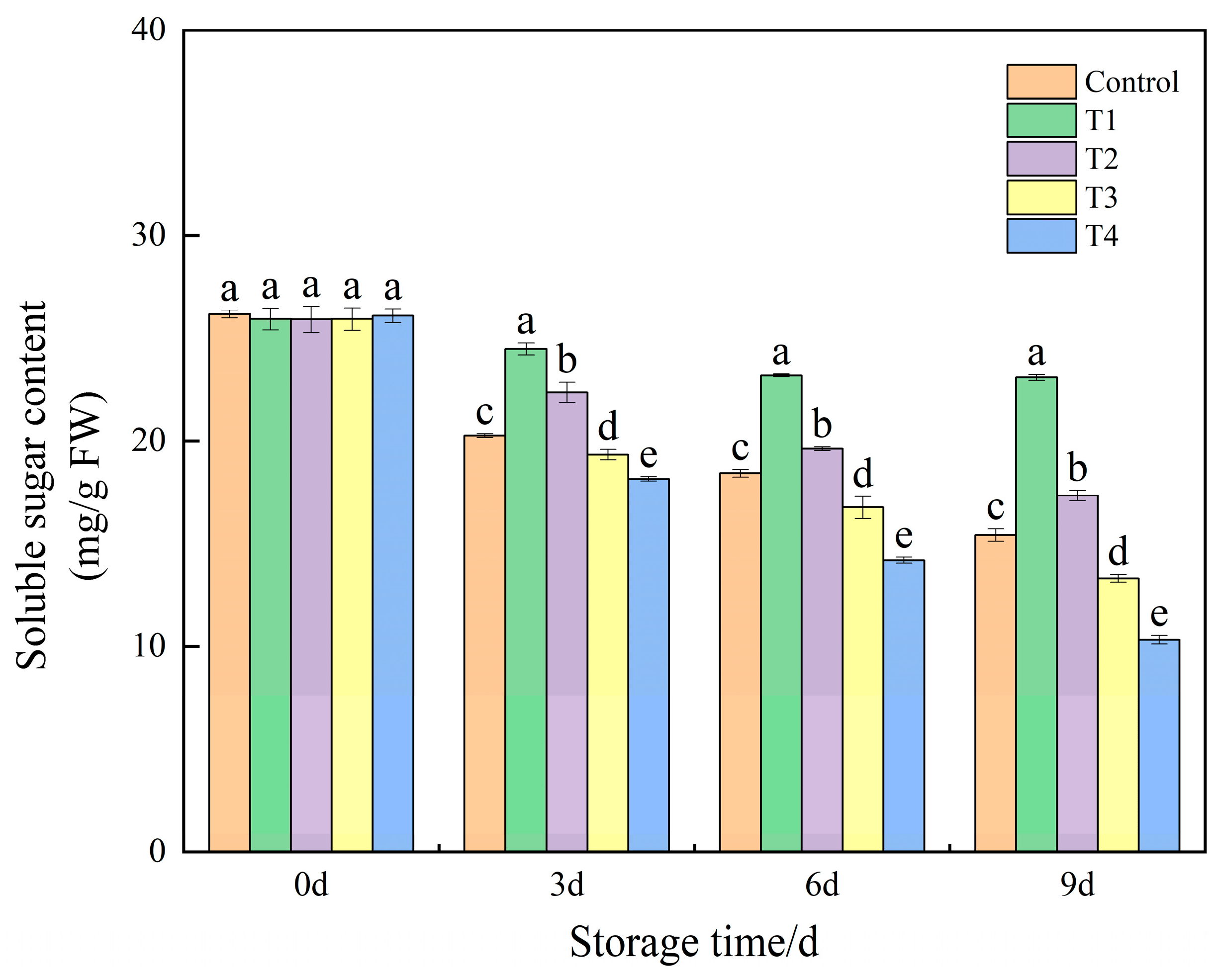
3.6. Effects of Different NO Concentrations on Soluble Sugar Content in Okra Fruit During Low-Temperature Storage
3.7. Effects of Different NO Concentrations on MDA Content and Relative Conductivity in Okra Fruit Under Low-Temperature Storage
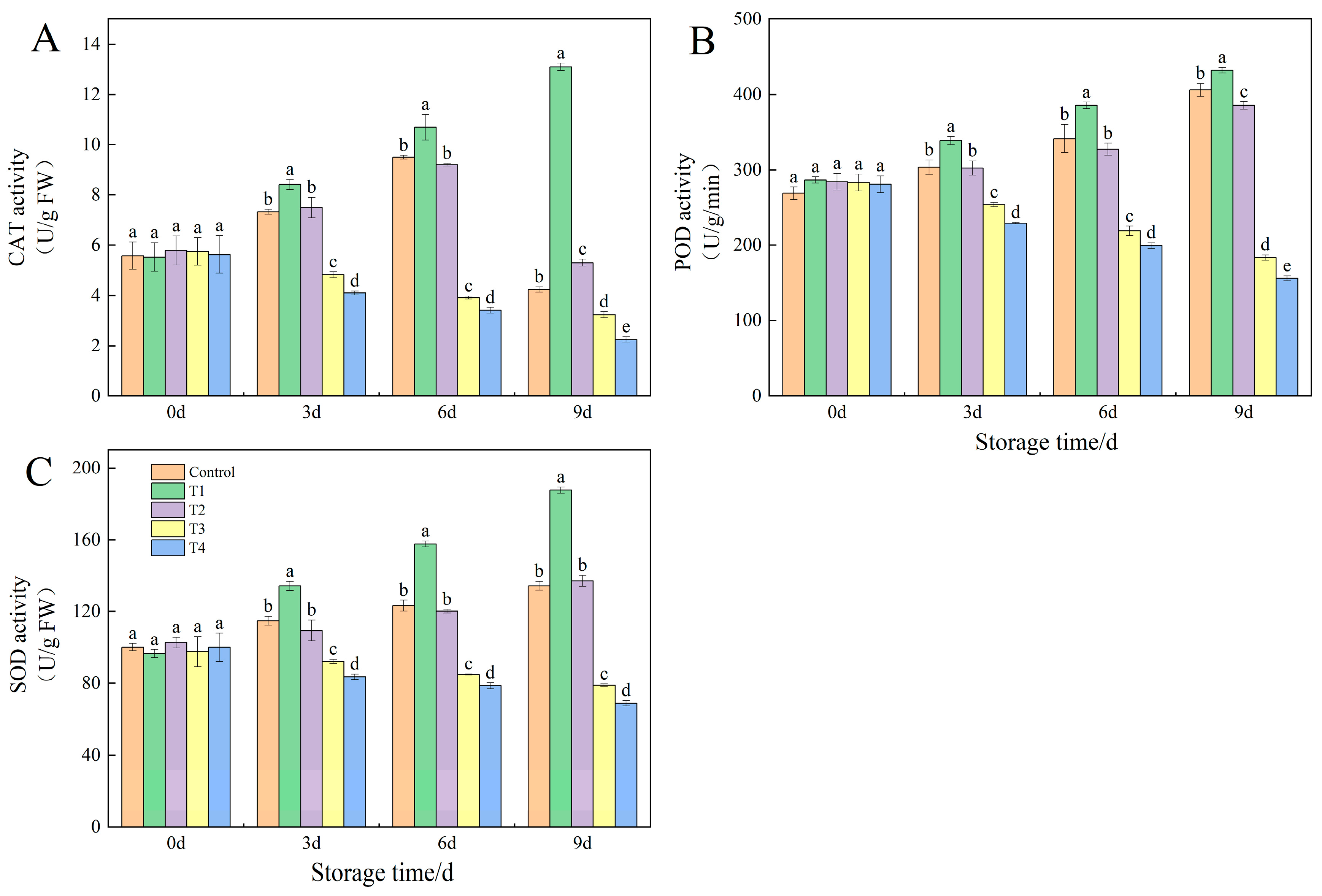
3.8. Effects of Different NO Concentrations on Antioxidant Enzyme Activities in Okra Fruit Under Low-Temperature Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a Potential Functional Food Source of Mucilage and Bioactive Compounds with Technological Applications and Health Benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Shi, L.; Li, S.; Xu, F.; Yang, Z.; Cao, S. Hydrogen-rich water delays fruit softening and prolongs shelf life of postharvest okras. Food Chem. 2023, 399, 133997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, J.; Song, Y.; Miao, M. The Effects of Okra Consumption on Glycemic Parameters and Lipid Profile in Adults: A Systematic Review and Meta-Analysis. Food Sci. Nutr. 2024, 12, 10049–10058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Zhao, Q.; Xie, X.; Li, Y.; Chen, Q.; Cheng, F.; Tian, J.; Gu, H.; Huang, J. Comparative Transcriptome Analysis of the Accumulation of Anthocyanins Revealed the Underlying Metabolic and Molecular Mechanisms of Purple Pod Coloration in Okra (Abelmoschus esculentus L.). Foods 2021, 10, 2180. [Google Scholar] [CrossRef] [PubMed]
- El-Shaieny, A.-H.A.H.; Abd-Elkarim, N.A.A.; Taha, E.M.; Gebril, S. Bio-Stimulants Extend Shelf Life and Maintain Quality of Okra Pods. Agriculture 2022, 12, 1699. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Y. Effect of plant growth regulators on banana fruit and broccoli during storage. Sci. Hortic. 2012, 145, 62–67. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Sci. Hortic. 2013, 156, 73–85. [Google Scholar] [CrossRef]
- Sun, M.; Yang, X.L.; Zhu, Z.P.; Xu, Q.Y.; Wu, K.X.; Kang, Y.J.; Wang, H.; Xiong, A.S. Comparative transcriptome analysis provides insight into nitric oxide suppressing lignin accumulation of postharvest okra (Abelmoschus esculentus L.) during cold storage. Plant Physiol. Biochem. 2021, 167, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.I.; Bokhary, S.U.F.; Xie, B.; Hu, S.; Jin, P.; Zheng, Y. Biochemical and molecular effects of glycine betaine treatment on membrane fatty acid metabolism in cold stored peaches. Postharvest Biol. Technol. 2019, 154, 58–69. [Google Scholar] [CrossRef]
- Charoenphun, N.; Pham, N.H.; Rattanawut, J.; Venkatachalam, K. Exogenous Application of Melatonin on the Preservation of Physicochemical and Enzymatic Qualities of Pepper Fruit from Chilling Injury. Horticulturae 2024, 10, 550. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Ma, L.; Fernie, A.R.; Fu, A.; Bai, C.; Sang, Z.; Guo, S.; Zhang, F.; Wang, Q.; et al. Revealing the Specific Regulations of Nitric Oxide on the Postharvest Ripening and Senescence of Bitter Melon Fruit. aBIOTECH 2024, 5, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.W.; Homa, F.; Ayala-Zavala, J.F.; Singh, D.R.; Irfan, M.; Pal, A.K. Postharvest Nitric Oxide Infiltration Reduces Oxidative Metabolism and Prolongs Shelf-Life of Banana. J. Plant Growth Regul. 2024, 43, 4151–4160. [Google Scholar] [CrossRef]
- Guo, X.; Huang, D.; Jing, G.; Feng, J.; Zhu, S. Nitric Oxide-Mediated DNA Methylation Enhances Cold Resistance in Postharvest Peach Fruit. Food Chem. 2023, 404, 134660. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhao, Y.; Li, A.; Qi, S.; Lin, Q.; Duan, Y. Postharvest Nitric Oxide Treatment Induced the Alternative Oxidase Pathway to Enhance Antioxidant Capacity and Chilling Tolerance in Peach Fruit. Plant Physiol. Biochem. 2021, 167, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Shaarawi, S.A.M.A.; Abdullah, M.A.A.; Ibrahim, H.A.; Mahdy, H.A.A. Influence of Different Concentrations of Nitric Oxide on Fruit Quality of Sweet Pepper and Mango under Mixed Loading Conditions. Not. Sci. Biol. 2023, 15, 4. [Google Scholar] [CrossRef]
- Liu, B.; Xin, Q.; Zhang, M.; Chen, J.; Lu, Q.; Zhou, X.; Li, X.; Zhang, W.; Feng, W.; Pei, H.; et al. Research Progress on Mango Post-Harvest Ripening Physiology and the Regulatory Technologies. Foods 2023, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- González-Gordo, S.; Rodríguez-Ruiz, M.; López-Jaramillo, J.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Nitric Oxide (NO) Differentially Modulates the Ascorbate Peroxidase (APX) Isozymes of Sweet Pepper (Capsicum annuum L.) Fruits. Antioxidants 2022, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, W.; Chen, H.; Niu, B.; Han, Y.; Fang, X.; Chen, H.; Liu, R.; Gao, H. Nitric Oxide Treatment Delays Quality Deterioration and Enzymatic Browning of Agaricus bisporus via Reactive Oxygen Metabolism Regulation. Food Front. 2023, 4, 447–458. [Google Scholar] [CrossRef]
- Dong, J.F.; Zhang, M.; Lu, L.; Sun, L.N.; Xu, M.J. Nitric Oxide Fumigation Stimulates Flavonoid and Phenolic Accumulation and Enhances Antioxidant Activity of Mushroom. Food Chem. 2012, 135, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Freschi, L.; Rodríguez-Ruiz, M.; González-Gordo, S.; Corpas, F.J. Nitric oxide in the physiology and quality of fleshy fruits. J. Exp. Bot. 2019, 70, 4405–4417. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Ji, Z.; Lin, C.; Li, S.; Liu, J.; Kan, J.; Zhang, M.; Jin, C.; Qian, C. Nitric oxide alleviates lignification and softening of water bamboo (Zizania latifolia) shoots during postharvest storage. Food Chem. 2020, 332, 127416. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, J.R.; Gao, M.Y.; Qin, L.; Wang, Y. Decreased cellulose-degrading enzyme activity causes pod hardening of okra (Abelmoschus esculentus L. Moench). Plant Physiol. Biochem. 2021, 162, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, G.; Nie, J.; Lin, Y.; Nie, F.; Zhang, J.; Xu, Y. Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci. Hortic. 2010, 125, 374–379. [Google Scholar] [CrossRef]
- Kotíková, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT-Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Liu, S.; Zhao, Y.; Pang, X.; Li, Y. Autotetraploidization in Ziziphus jujuba Mill. var. spinosa enhances salt tolerance conferred by active, diverse stress responses. Environ. Exp. Bot. 2019, 165, 92–107. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus Esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bao, Z.; Chen, Y.; Lan, Q.; Song, C.; Shi, L.; Chen, W.; Cao, S.; Yang, Z.; Zheng, Q. Exogenous glutathione modulates redox homeostasis in okra (Abelmoschus esculentus) during storage. Postharvest Biol. Technol. 2023, 195, 112145. [Google Scholar] [CrossRef]
- Phornvillay, S.; Pongprasert, N.; Sugaya, S.; Srilaong, V. Low temperature conditioning reduces chilling injury incidence in okra (Abelmoschus esculentus L.) pods. Fruits 2021, 76, 191–200. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, M.; Jiang, X.; Huang, M.; Zhao, J. Nitric Oxide Acts as an Inhibitor of Postharvest Senescence in Horticultural Products. Int. J. Mol. Sci. 2022, 23, 11512. [Google Scholar] [CrossRef] [PubMed]
- Madebo, M.P.; Ayalew, Y.; Zheng, Y.; Jin, P. Nitric Oxide and Its Donor Sodium-Nitroprusside Regulation of the Postharvest Quality and Oxidative Stress on Fruits: A Systematic Review and Meta-Analysis. Food Rev. Int. 2022, 39, 6648–6676. [Google Scholar] [CrossRef]
- Abd Elwahab, S.M.; Abdallatif, A.M.; El-Saeed, S.A.E. Improving the postharvest shelf life of apricot fruits (Prunus armeniaca L. cv. Amal) using preharvest application of spermidine, salicylic acid and sodium nitroprusside. J. Appl. Hortic. 2024, 26, 493–499. [Google Scholar] [CrossRef]
- Elam, E.; Lv, Y.M.; Wang, W.; Thakur, K.; Ma, W.P.; Ni, Z.J.; Wei, Z.J. Effects of nitric oxide on postharvest storage quality of Lycium barbarum fruit. Food Sci. Technol. 2022, 42, e84122. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Homa, F.; Lata, D.; Ahmad, M.S.; Surabhi. Exogenous nitric oxide delays ripening and maintains postharvest quality of pointed gourd during storage. J. Plant Growth Regul. 2021, 40, 2371–2378. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, X.; Gong, X.; Bao, J. Effects of Different Foliar Fertilizer Treatments on Fruit Quality of the Korla Fragrant Pear. Horticulturae 2024, 10, 51. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, O.; Xu, Y.; Huang, J.; Wang, H.; Deng, L.; Yao, S.; Yi, L.; Zeng, K. Glycoside hydrolase PgGal31A inhibits citrus blue mold via modifying cell wall components. Postharvest Biol. Technol. 2025, 225, 113527. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, T.; Hu, C.; Liu, D.; He, J. Exogenous Nitric Oxide-Induced Postharvest Gray Spot Disease Resistance in Loquat Fruit and Its Possible Mechanism of Action. Int. J. Mol. Sci. 2023, 24, 4369. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Shan, Y.; Yao, J.L.; Wang, R.; Xu, S.; Liu, D.; Ye, Z.; Lin, J.; Li, X.; Xue, C.; et al. The transcription factor PbrMYB24 regulates lignin and cellulose biosynthesis in stone cells of pear fruits. Plant Physiol. 2023, 192, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Z.; Han, Z.; Xue, S.; Bi, Y.; Prusky, D. Effects of nitric oxide treatment on lignin biosynthesis and texture properties at wound sites of muskmelons. Food Chem. 2021, 362, 130193. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, Y.; Ding, Z.; Yao, K.; Zhang, J.; Liu, Z.; Hou, X.; Wu, X.; Wang, C.; Liao, W. Nitric oxide delays tomato fruit softening by inhibiting SlNAP2 (NAC-like, activated by apetala3/pistillata2) transcription factor-activated transcription of soften-related genes. Int. J. Biol. Macromol. 2025, 254, 143148. [Google Scholar] [CrossRef] [PubMed]
- Pols, S.; Van de Poel, B.; Hertog, M.L.A.T.M.; Nicolaï, B.M. The regulatory role of nitric oxide and its significance for future postharvest applications. Postharvest Biol. Technol. 2022, 188, 111869. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Guo, Y.; Ma, Y.; Yang, M.; Fu, R.; Sun, Y. Elevated CO2 delayed yellowing by maintaining chlorophyll biosynthesis and inhibiting chlorophyll degradation and carotenoid accumulation of postharvest broccoli. Postharvest Biol. Technol. 2022, 194, 112089. [Google Scholar] [CrossRef]
- Labra, A.; Zoffoli, J.P. Response of Chlorophyllase and Magnesium Dechelatase Enzymes in Yellow- and Green-Fleshed Kiwifruit to Degreening at Different Temperatures. Agronomy 2024, 14, 2481. [Google Scholar] [CrossRef]
- da Veiga, J.C.; Silveira, N.M.; Seabra, A.B.; Pieretti, J.C.; Boza, Y.; Jacomino, A.P.; Filho, J.C.; Campagnoli, V.P.; Cia, P.; Bron, I.U. Spraying with encapsulated nitric oxide donor reduces weight loss and oxidative damage in papaya fruit. Nitric Oxide 2024, 150, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Neuhaus, H.E.; Cheng, J.; Bie, Z. Contributions of sugar transporters to crop yield and fruit quality. J. Exp. Bot. 2022, 73, 2275–2289. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Rodríguez-Ruiz, M.; Muñoz-Vargas, M.A.; González-Gordo, S.; Reiter, R.J.; Palma, J.M. Interactions of melatonin, reactive oxygen species, and nitric oxide during fruit ripening: An update and prospective view. J. Exp. Bot. 2022, 73, 5947–5960. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, L.; Watkins, C.B.; Bai, C.; Ma, L.; Guo, S.; Han, L.; Wang, H.; Wang, Q.; Zuo, J.; et al. Nitric oxide delays the postharvest nutritional quality decline of “Golden Hook” beans. Food Front. 2024, 5, 636–655. [Google Scholar] [CrossRef]
- Bakpa, E.P.; Zhang, J.; Xie, J.; Ma, Y.; Han, K.; Chang, Y. Storage stability of nutritional qualities, enzyme activities, and volatile compounds of “Hangjiao No. 2” chili pepper treated with different concentrations of 1-methyl cyclopropene. Front. Plant Sci. 2022, 13, 838916. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yang, H.; Li, Y. Kinetics of relative electrical conductivity and correlation with gas composition in modified atmosphere packaged bayberries (Myrica rubra Siebold and Zuccarini). LWT-Food Sci. Technol. 2005, 38, 249–254. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Kamrani, A.; Abbasi, Z.; Sadeghi, M. Glycine betaine treatment extends the shelf life and retards cap browning of button mushrooms. Sci. Rep. 2025, 15, 20796. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Changes in antioxidant substances and antioxidant enzyme activities in raspberry fruits at different developmental stages. Sci. Hortic. 2023, 321, 112314. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Mo, F.; Long, Y.; Liu, X.; Jiang, Y.; Zhang, J.; Zhong, C.; Yang, Q.; Liu, H. Nitric Oxide Modulates Postharvest Physiology to Maintain Abelmoschus esculentus Quality Under Cold Storage. Horticulturae 2025, 11, 857. https://doi.org/10.3390/horticulturae11070857
Chen X, Mo F, Long Y, Liu X, Jiang Y, Zhang J, Zhong C, Yang Q, Liu H. Nitric Oxide Modulates Postharvest Physiology to Maintain Abelmoschus esculentus Quality Under Cold Storage. Horticulturae. 2025; 11(7):857. https://doi.org/10.3390/horticulturae11070857
Chicago/Turabian StyleChen, Xianjun, Fenghuang Mo, Ying Long, Xiaofeng Liu, Yao Jiang, Jianwei Zhang, Cheng Zhong, Qin Yang, and Huiying Liu. 2025. "Nitric Oxide Modulates Postharvest Physiology to Maintain Abelmoschus esculentus Quality Under Cold Storage" Horticulturae 11, no. 7: 857. https://doi.org/10.3390/horticulturae11070857
APA StyleChen, X., Mo, F., Long, Y., Liu, X., Jiang, Y., Zhang, J., Zhong, C., Yang, Q., & Liu, H. (2025). Nitric Oxide Modulates Postharvest Physiology to Maintain Abelmoschus esculentus Quality Under Cold Storage. Horticulturae, 11(7), 857. https://doi.org/10.3390/horticulturae11070857





