Effects of Crop Load Management on Berry and Wine Composition of Marselan Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Experimental Design
2.1.2. Reagent
2.2. Instruments and Equipment
2.3. Methods
2.3.1. Grape Sampling
2.3.2. Winemaking
2.3.3. Basic Physicochemical Analyses of Grapes
2.3.4. Determination of Organic Acids
2.3.5. Determination of Individual Phenolics
2.3.6. Determination of Anthocyanins
2.3.7. Determination of Volatile Compounds
2.3.8. Sensory Assessment
2.4. Data Analysis
3. Results
3.1. Effect of the Crop Load on the Yield of Marselan
3.2. Effects of Crop Load on Wine Chemical Composition
3.2.1. Basic Physicochemical Parameters
3.2.2. Analysis of Organic Acids
3.2.3. Analysis of Phenolic Compounds
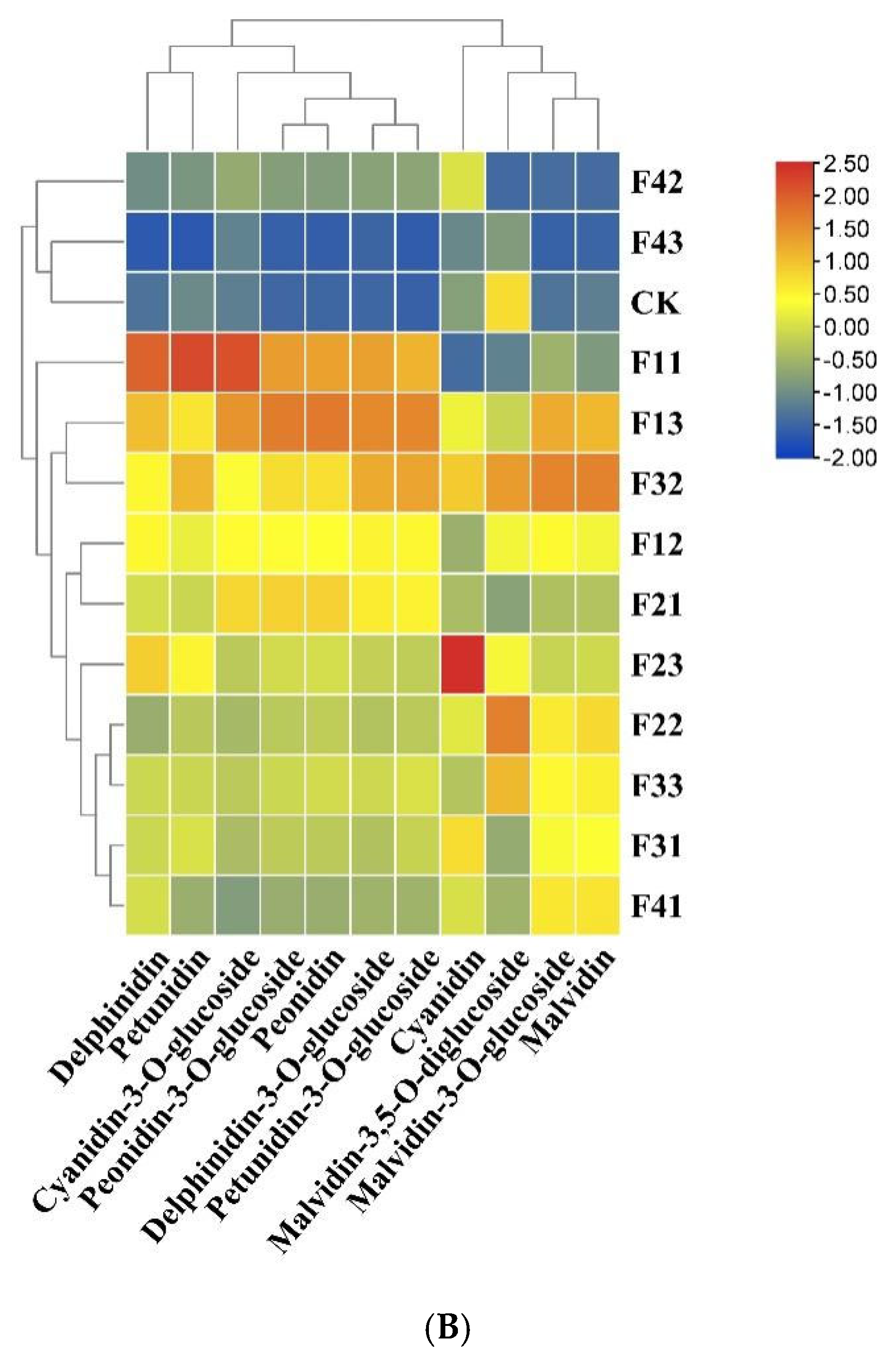
3.2.4. Anthocyanin Composition Analysis
3.2.5. Analysis of Aroma Components
3.2.6. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sivilotti, P.; Falchi, R.; Vanderweide, J.; Sabbatini, P.; Bubola, M.; Vanzo, A.; Lisjak, K.; Peterlunger, E.; Herrera, J.C. Yield reduction through cluster or selective berry thinning similarly modulates anthocyanins and proanthocyanidins composition in Refosco dal peduncolo rosso (Vitis vinifera L.) grapes. Sci. Hortic. 2020, 264, 109166. [Google Scholar] [CrossRef]
- Wei, M.Y. Nutritional Analysis and Application of Thinned Unripe Table Grape. Master’s Thesis, Northwest A&F University, Yangling, China, 2022. [Google Scholar]
- Xi, X.; Zha, Q.; He, Y.; Tian, Y.; Jiang, A. Influence of cluster thinning and girdling on aroma composition in ‘Jumeigui’ table grape. Sci. Rep. 2020, 10, 6877. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Esteruelas, M.; González, E.; Kontoudakis, N.; Jiménez, J.; Fort, F.; Canals, J.M.; Hermosín-Gutiérrez, I.; Zamora, F. Effect of two different treatments for reducing grape yield in Vitis vinifera cv Syrah on wine composition and quality: Berry thinning versus cluster thinning. J. Agric. Food Chem. 2013, 61, 4968–4978. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, Y.; Quan, G.; Wang, X.; Xi, Z. Thinning of cluster improves berry composition and sugar accumulation in Syrah grapes. Sci. Hortic. 2022, 297, 110966. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.-N.; He, L.; He, F.; Chen, W.; Duan, C.-Q.; Wang, J. Changes in global aroma profiles of Cabernet Sauvignon in response to cluster thinning. Food Res. Int. 2019, 122, 56–65. [Google Scholar] [CrossRef] [PubMed]
- VanderWeide, J.; Nasrollahiazar, E.; Schultze, S.; Sabbatini, P.; Castellarin, S.D.; Parker, A. Impact of Cluster Thinning on Wine Grape Yield and Fruit Composition: A Review and Meta-Analysis. Aust. J. Grape Wine Res. 2024, 2024, 20. [Google Scholar] [CrossRef]
- Matteo, M.; Zoffoli, J.P.; Ayala, M. Calcium sprays and crop load reduction increase fruit quality and postharvest storage in sweet cherry (Prunus avium L.). Agronomy 2022, 12, 829. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Roberto, S.R.; Borges, W.F.S.; Colombo, R.C.; Koyama, R.; Hussain, I.; de Souza, R.T. Berry-cluster thinning to prevent bunch compactness of ‘BRS Vitoria’, a new black seedless grape. Sci. Hortic. 2015, 197, 297–303. [Google Scholar] [CrossRef]
- Xiao, L.Z.; Lu, H.L.; Jia, Y.; Yang, R.H.; Ye, W.J.; Zhu, L.; Wang, M.J. Effects of flower thinning on fruit qualities and yield of ‘Summer Black’ grape. Sino-Overseas Grapevine Wine 2021, 5, 45–48,54. [Google Scholar]
- Kovalenko, Y.; VanderWeide, J.; Tindjau, R.; Zandberg, W.F.; Castellarin, S.D. Timing and severity of crop load management influences technological maturity, terpene synthase expression and free terpene accumulation in Gewürztraminer grapes (Vitis vinifera L.). Aust. J. Grape Wine Res. 2022, 28, 638–650. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Aroma compounds in Ontario Vidal and Riesling icewines. II. Effects of crop level. Food Res. Int. 2015, 76, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhang, Y.K.; Yan, Y.; Kai, J.R.; Zhou, X.M.; Jiang, W.G.; Li, X.H.; Chang, T.W.; Yi, Z.C.; Sun, X.Y.; et al. Effect of crop load on the quality of Cabernet Sauvignon grapes and wines at different harvest times. Food Sci. 2025, 46, 128–137. [Google Scholar]
- Glišić, M.; Matijašević, S.; Bešlić, Z.; Ranković-Vasić, Z.; Glišić, I.; Ćirković, D. Effect of early berry thinning and girdling on grape quality of cv.victoria. In Annals of the University of Craiova—Agriculture Montanology Cadastre Series; University of Craiova: Craiova, Romania, 2023. [Google Scholar]
- Wei, M.; Ma, T.; Cao, M.; Wei, B.; Li, C.; Li, C.; Zhang, K.; Fang, Y.; Sun, X. Biomass estimation and characterization of the nutrient components of thinned unripe grapes in China and the global grape industries. Food Chem. X 2022, 15, 100363. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.7-2016; National Food Safety Standard—Determination of Reducing Sugar in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016. (In Chinese)
- Li, C.H.; Wang, Y.; Lv, Y.; Qiu, X.Y.; Wu, B.L.; Ma, T.T.; Fang, Y.L.; Sun, X.Y. Physicochemical characterization and antioxidant capacity analysis of commercial Marselan wines from Ningxia and Hebei regions based on OPLS-DA. Food Ferment. Ind. 2023, 49, 283–292. (In Chinese) [Google Scholar]
- T/NAIA 084—2021; Determination of Hydroxycinnamic Acids in Wine—High Performance Liquid Chromatography. Ningxia Association for Chemical Analysis and Testing: Yinchuan, China, 2021. (In Chinese)
- T/NAIA 085—2021; Determination of Flavanols in Wine—High Performance Liquid Chromatography. Ningxia Association for Chemical Analysis and Testing: Yinchuan, China, 2021. (In Chinese)
- T/NAIA 082—2021; Determination of Water-Soluble Humic Acid Content in Organic Fertilizers—Volumetric Method. Ningxia Association for Chemical Analysis and Testing: Yinchuan, China, 2021. (In Chinese)
- Wang, Y.; Ge, Q.; Zhang, J.; Ma, T.T.; Fang, Y.L.; Li, C.H.; Sun, X.Y. Analysis of characteristics and differences between commercial Marselan wines from three Chinese origins based on of intelligent sensory and GC-MS. Food Ferment. Ind. 2024, 50, 253–264. [Google Scholar]
- Issa-Issa, H.; Guclu, G.; Noguera-Artiaga, L.; López-Lluch, D.; Poveda, R.; Kelebek, H.; Selli, S.; Carbonell-Barrachina, Á.A. Aromaactive compounds, sensory profile, and phenolic composition of Fondillón. Food Chem. 2020, 316, 126353. [Google Scholar] [CrossRef] [PubMed]
- GB/T 15038-2006; Analytical methods of wine and fruit wine. China National Standardization Administration Committee: Beijing, China, 2021. (In Chinese)
- Previtali, P.; Dokoozlian, N.K.; Pan, B.S.; Wilkinson, K.L.; Ford, C.M. Crop load and plant water status influence the ripening rate and aroma development in berries of grapevine (Vitis vinifera L.) cv. Cabernet Sauvignon. J. Agric. Food Chem. 2021, 69, 7709–7724. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, T.; Bao, S.; Yin, D.; Ge, Q.; Li, C.; Fang, Y.; Sun, X. Suitable crop loading: An effective method to improve “Shine Muscat” grape quality. Food Chem. 2023, 424, 136451. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. The effects of bagging and debagging on external fruit quality, metabolites, and the expression of anthocyanin biosynthetic genes in ‘Jonagold’ apple (Malus domestica Borkh.). Sci. Hortic. 2014, 165, 123–131. [Google Scholar] [CrossRef]
- Yue, X.; Yu, Y.; Ju, Y.; Zhao, Y.; Xi, Z.; Zhang, Z. Effects of Different Capacity on Fruit Quality of ‘Red Globe’ Grape. North. Hortic. 2018, 8, 29–34. [Google Scholar]
- Wang, X.; De Bei, R.; Fuentes, S.; Collins, C. Influence of canopy management practices on canopy architecture and reproductive performance of semillon and shiraz grapevines in a hot climate. Am. J. Enol. Vitic. 2019, 70, 360–372. [Google Scholar] [CrossRef]
- Lan, T.; Wang, J.; Yuan, Q.; Lei, Y.; Peng, W.; Zhang, M.; Li, X.; Sun, X.; Ma, T. Evaluation of the color and aroma characteristics of commercially available Chinese kiwi wines via intelligent sensory technologies and gas chromatography-mass spectrometry. Food Chem. X 2022, 15, 100427. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y. Study on Maturity Index System of Wine Grape in Tuha Region of Xinjiang. Master’s Thesis, Northwest A&F University, Yangling, China, 2019. [Google Scholar]
- Kliewer, M.W.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar] [CrossRef]
- Keller, M. Deficit Irrigation and Vine Mineral Nutrition. Am. J. Enol. Vitic. 2005, 56, 267–283. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Regulated deficit irrigation effects on growth, yield, grape quality and individual anthocyanin composition in Vitis vinifera L. cv. ‘Tempranillo’. Agric. Water Manag. 2011, 98, 1171–1179. [Google Scholar] [CrossRef]
- Tian, M.B.; Hu, R.Q.; Liu, Z.L.; Shi, N.; Lu, H.C.; Duan, C.Q.; Wang, J.; Sun, Y.F.; Kong, Q.S.; He, F. The pH adjustment of Vitis amurensis dry red wine revealed the evolution of organic acids, volatomics, and sensory quality during winemaking. Food Chem. 2024, 436, 137730. [Google Scholar] [CrossRef] [PubMed]
- PPoni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A.; et al. Grapevine quality: A multiple choice issue. Sci. Hortic. 2017, 234, 445–462. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Sparacio, A.; Giglio, D.M.L.; Sparla, S.; Verzera, A. Effects of Cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.). Eur. Food Res. Technol. 2016, 242, 1719–1726. [Google Scholar] [CrossRef]
- Prajitna, A.; Dami, I.E.; Steiner, T.E.; Ferree, D.C.; Scheerens, J.C.; Schwartz, S.J. Influence of cluster thinning onphenolic composition, resveratrol, and antioxidant capacity in Chambourcin wine. Am. J. Enol. Vitic. 2007, 58, 346–350. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.; González-Manzano, S.; Escribano-Bailón, M.T.; Heredia, F.J.; Santos-Buelga, C. Influence of different phenolic copigments on the colorofmalvidin3-glucoside. J. Agric. Food Chem. 2006, 54, 5422–5429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhao, Y.M.; Wang, Y.T.; Duan, B.B.; Zhang, Z.W. Effect of potassium dihydrogen phosphate onflavonoids of Vitisvinifera Cabernet sauvignon grape. J. North West For. Univ. 2020, 35, 118–123. (In Chinese) [Google Scholar]
- Zeng, G.H.; Gao, F.F.; Hui, Z.M.; Zhang, Z.W. Impact of Cluster Thinning on the Contents of Flavonoids in Cabernet Sauvignon Wine. J. North West For. Univ. 2021, 36, 112–117. (In Chinese) [Google Scholar]
- Šarac, D.P.; Torović, L.; Orčić, D.; Mimica-Dukić, N.; Đorđević, T.; Lesjak, M. Comprehensive study of phenolic profile and biochemical activity of monovarietal red and white wines from Fruška Gora region, Serbia. Food Chem. 2024, 448, 139099. [Google Scholar] [CrossRef] [PubMed]
- Song, C.Z.; Wang, C.; Xie, S.; Zhang, Z.W. Effects of leaf removal and cluster thinning on berry quality of Vitisvinifera cultivars In the region of Weibei Dryland in China. J. Integr. Agric. 2018, 17, 1620–1630. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, A.X.; Peng, B.Z. Research progress on anthocyanins and their regulation of uric acid metabolism. J. Chin. Inst. Food Sci. Technol. 2023, 23, 365–375. [Google Scholar]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Garrido-Bauelos, G.; Buica, A.; Toit, W.D. Relationship between anthocyanins, proanthocyanidins, and cell wall polysaccharides in grapes and red wines. A current state-of-art review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7743–7759. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cheng, G.; Gao, Y.; Wang, Y.; Dong, X.; Chen, W.; Duan, C.; Wang, J. Effects of Cluster Thinning on Berry Size and Anthocyanins of Cabernet Sauvignon. Acta Agric. Boreali-Occident. Sin. 2015, 24, 111–121. (In Chinese) [Google Scholar]
- Mota, R.V.D.; Souza, C.R.D.; Silva, C.P.C.; Freitas, G.D.F.; Shiga, T.M.; Purgatto, E.; Lajolo, F.M.; Regina, M.D.A. Biochemical and agronomical responses of grapevines to alteration of source—Sink ratio by cluster thinning and shoot trimming. Bragantia 2010, 69, 17–25. [Google Scholar] [CrossRef]
- Favre, G.; Hermosín-Gutiérrez, I.; Piccardo, D.; Gómez-Alonso, S.; González-Neves, G. Selectivity of pigments extraction from grapes and their partial retention in the pomace during red-winemaking. Food Chem. 2019, 277, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Optimization of the Microdomain Environment of Wine Grapes and Its Effect on Wine Quality; Yanshan University: Qinghuangdao, China, 2024. [Google Scholar]
- Sun, Q.; Sacks, G.; Lerch, S.; Vanden Heuvel, J.E. Impact of shoot thinning and harvest date on yield components, fruit composition, and wine quality of marechal foch. Am. J. Enol. Vitic. 2011, 62, 32–41. [Google Scholar] [CrossRef]






| Group | Targeted Shoot Density (Shoots/Meter of Canopy) | Targeted Number of Clusters per Shoot (Number of Clusters/Shoot) | Targeted Total Number of Clusters | Yield (t/ha) |
|---|---|---|---|---|
| F11 | 10 | 1.0 | 10 | 7.512 |
| F12 | 10 | 1.5 | 15 | 8.328 |
| F13 | 10 | 2.0 | 20 | 7.176 |
| F21 | 15 | 1.0 | 15 | 8.940 |
| F22 | 15 | 1.5 | 22.5 | 10.488 |
| F23 | 15 | 2.0 | 30 | 11.028 |
| F31 | 20 | 1.0 | 20 | 10.980 |
| F32 | 20 | 1.5 | 30 | 10.566 |
| F33 | 20 | 2.0 | 40 | 11.928 |
| F41 | 25 | 1.0 | 25 | 9.696 |
| F42 | 25 | 1.5 | 37.5 | 11.028 |
| F43 | 25 | 2.0 | 50 | 11.130 |
| CK | 28 | 2.0 | 56 | 11.098 |
| 26 July | 7 August | 16 August | 26 August | 2 September | 12 September | 19 September | |
|---|---|---|---|---|---|---|---|
| CK | 0.693 ± 0.005 hE | 0.906 ± 0.003 deBC | 1.015 ± 0.023 bA | 0.937 ± 0.025 eB | 0.867 ± 0.054 cdC | 0.774 ± 0.054 gD | 0.864 ± 0.021 abC |
| F11 | 1.029 ± 0.019 bB | 1.116 ± 0.058 aAB | 1.149 ± 0.019 aAB | 1.055 ± 0.036 aB | 1.201 ± 0.144 aA | 1.036 ± 0.026 aB | 1.051 ± 0.016 abB |
| F12 | 1.109 ± 0.019 aA | 1.074 ± 0.067 abAB | 1.057 ± 0.033 bAB | 0.943 ± 0.029 deC | 0.964 ± 0.022 bcC | 0.974 ± 0.044 abcdC | 1.021 ± 0.013 bB |
| F13 | 0.898 ± 0.016 eC | 1.074 ± 0.038 abA | 1.024 ± 0.025 bB | 0.987 ± 0.035 bcdB | 0.891 ± 0.012 bcdC | 0.911 ± 0.063 deC | 0.999 ± 0.018 cdeB |
| F21 | 0.924 ± 0.008 dB | 1.026 ± 0.060 bcA | 1.051 ± 0.052 bA | 1.023 ± 0.013 abA | 1.001 ± 0.023 bA | 1.015 ± 0.032 abA | 0.987 ± 0.026 deA |
| F22 | 0.949 ± 0.022 cBC | 0.986 ± 0.043 cdB | 0.971 ± 0.015 cB | 0.998 ± 0.017 bcAB | 0.977 ± 0.053 bcB | 0.916 ± 0.003 cdeC | 1.037 ± 0.018 bA |
| F23 | 0.888 ± 0.016 eC | 0.950 ± 0.017 cdB | 1.025 ± 0.028 bA | 0.915 ± 0.056 eBC | 0.899 ± 0.024 bcdC | 0.956 ± 0.026 bcdB | 0.955 ± 0.027 efB |
| F31 | 0.960 ± 0.003 cA | 0.967 ± 0.048 dA | 0.822 ± 0.025 eBC | 0.803 ± 0.018 fC | 0.841 ± 0.017 dBC | 0.862 ± 0.033 eB | 0.764 ± 0.028 hD |
| F32 | 0.963 ± 0.014 cCD | 0.923 ± 0.052 deD | 1.138 ± 0.034 aA | 0.955 ± 0.019 cdeCD | 1.005 ± 0.061 bC | 0.988 ± 0.030 abcC | 1.086 ± 0.056 aB |
| F33 | 0.807 ± 0.009 fD | 0.972 ± 0.039 cdB | 1.037 ± 0.023 bA | 0.928 ± 0.017 eBC | 0.894 ± 0.026 bcdC | 1.043 ± 0.041 aA | 0.933 ± 0.026 fBC |
| F41 | 0.805 ± 0.005 fE | 0.868 ± 0.042 eD | 1.111 ± 0.042 aA | 1.021 ± 0.028 abB | 0.997 ± 0.031 bBC | 0.952 ± 0.053 bcdC | 1.019 ± 0.041 bB |
| F42 | 0.802 ± 0.005 fC | 0.991 ± 0.010 cdA | 0.953 ± 0.025 cdAB | 0.907 ± 0.060 eAB | 0.912 ± 0.102 bcdAB | 0.923 ± 0.020 cdeAB | 0.881 ± 0.021 gB |
| F43 | 0.702 ± 0.005 hD | 0.942 ± 0.030 cdC | 0.921 ± 0.020 dC | 0.933 ± 0.041 eC | 0.978 ± 0.068 bcB | 1.024 ± 0.032 abB | 0.983 ± 0.044 aB |
| 26 July | 7 August | 16 August | 26 August | 2 September | 12 September | 19 September | |
|---|---|---|---|---|---|---|---|
| CK | 13.9 ± 1.1 cdE | 17.9 ± 0.6 cD | 19.8 ± 0.5 bcCD | 21.5 ± 1.1 bBC | 24.6 ± 2.1 abcA | 23.4 ± 1.0 abcAB | 23.7 ± 0.4 abA |
| F11 | 20.3 ± 0.4 aD | 21.8 ± 1.4 aCD | 24.1 ± 2.0 aABC | 26.0 ± 1.4 aAB | 27.0 ± 2.6 aA | 23.7 ± 1.0 abcBC | 24.6 ± 1.5 abABC |
| F12 | 17.8 ± 1.2 abD | 20.9 ± 0.8 abC | 22.3 ± 1.2 abBC | 24.1 ± 1.1 abAB | 26.2 ± 2.3 abA | 20.5 ± 0.8 cC | 24.3 ± 0.4 abAB |
| F13 | 18.7 ± 1.8 abD | 21.2 ± 1.5 abCD | 22.2 ± 0.6 abC | 25.7 ± 2.3 aAB | 26.1 ± 1.0 abA | 23.0 ± 1.4 bcBC | 23.4 ± 1.7 abBC |
| F21 | 18.6 ± 1.7 abC | 19.3 ± 1.4 abcBC | 22.1 ± 1.9 abAB | 24.5 ± 1.5 abA | 23.4 ± 2.0 abcA | 22.7 ± 2.0 bcA | 25.1 ± 1.3 abA |
| F22 | 16.9 ± 1.5 bcC | 20.2 ± 0.4 abcB | 21.5 ± 0.6 abB | 25.5 ± 1.9 aA | 22.1 ± 1.1 abcB | 24.5 ± 1.3 abA | 24.6 ± 1.1 abA |
| F23 | 18.0 ± 0.8 abD | 19.9 ± 0.5 abcCD | 22.9 ± 1.4 abB | 20.1 ± 2.0 bCD | 24.3 ± 0.0 abcB | 26.7 ± 2.0 aA | 22.2 ± 1.2 abBC |
| F31 | 15.2 ± 1.6 bcdC | 18.8 ± 0.9 abcB | 20.7 ± 1.3 abcAB | 20.4 ± 1.7 bAB | 23.6 ± 1.2 abcA | 21.9 ± 1.5 bcAB | 23.5 ± 2.9 abA |
| F32 | 15.8 ± 1.6 bcdC | 17.2 ± 1.8 cC | 21.7 ± 0.4 abAB | 20.4 ± 1.2 bB | 23.0 ± 1.8 abcA | 22.8 ± 0.3 bcA | 23.1 ± 0.4 abA |
| F33 | 15.4 ± 0.8 bcdD | 19.4 ± 1.4 abcC | 22.1 ± 1.3 abAB | 20.4 ± 0.8 bBC | 22.6 ± 1.8 abcAB | 24.4 ± 2.1 abA | 22.6 ± 1.4 abAB |
| F41 | 16.1 ± 1.3 bcdE | 18.6 ± 1.8 abcD | 20.9 ± 1.9 abcCD | 21.4 ± 1.0 bBC | 20.6 ± 1.1 cCD | 23.8 ± 0.9 abcB | 26.3 ± 1.6 aA |
| F42 | 15.8 ± 0.8 bcdC | 18.3 ± 1.4 bcBC | 19.6 ± 1.6 bcB | 21.1 ± 1.4 bB | 24.1 ± 2.3 abcA | 24.7 ± 1.2 abA | 24.6 ± 2.0 abA |
| F43 | 13.4 ± 1.0 dC | 17.2 ± 0.8 cB | 18.1 ± 0.5 cB | 21.5 ± 2.4 bA | 21.5 ± 1.0 bcA | 22.5 ± 0.4 bcA | 23.3 ± 0.6 abA |
| 26 July | 7 August | 16 August | 26 August | 2 September | 12 September | 19 September | |
|---|---|---|---|---|---|---|---|
| CK | 120.3 ± 4.8 cD | 128.8 ± 11.2 bD | 162.6 ± 12.9 bcdeC | 175.1 ± 10.9 dC | 238.9 ± 8.6 aA | 180.8 ± 8.3 cdC | 206.9 ± 13.6 abB |
| F11 | 169.2 ± 15.0 aD | 159.8 ± 11.1 aD | 183.1 ± 14.6 bcdCD | 219.7 ± 17.4 abB | 262.6 ± 14.6 aA | 205.8 ± 10.3 bcBC | 184.2 ± 16.9 bCD |
| F12 | 159.4 ± 12.5 abC | 140.3 ± 10.9 abC | 137.8 ± 15.9 eC | 207.0 ± 10.9 bcB | 270.3 ± 8.1 aA | 250.8 ± 20.1 aA | 205.3 ± 18.8 abB |
| F13 | 171.4 ± 15.7 aBC | 141.5 ± 7.5 abC | 178.1 ± 5.3 bcdB | 242.3 ± 19.8 aA | 260 ± 29.9 aA | 167.5 ± 13.1 dBC | 193.7 ± 10.2 bB |
| F21 | 163.6 ± 8.5 abD | 136.2 ± 12.2 abE | 185.0 ± 5.5 bcCD | 207.4 ± 7.4 bcC | 270.1 ± 5.4 aA | 168.6 ± 10.5 dD | 246.5 ± 28.4 aB |
| F22 | 153.1 ± 7.0 abD | 149.5 ± 10.8 abD | 161.2 ± 7.4 bcdeD | 186.6 ± 11.4 cdC | 250.8 ± 20.5 aA | 171.7 ± 10.7 dCD | 223.1 ± 9.7 abB |
| F23 | 161.9 ± 15.4 abCD | 144.7 ± 5.8 abD | 178.4 ± 13.9 bcdBC | 147.1 ± 10.6 eD | 273.4 ± 7.2 aA | 190.6 ± 9.9 bcdB | 184.1 ± 14.6 bBC |
| F31 | 141.3 ± 9.9 abcC | 123.0 ± 11.7 bC | 165.8 ± 7.6 bcdeB | 177.8 ± 7.1 dB | 230.9 ± 10.1 aA | 185.1 ± 14.8 cdB | 230.8 ± 20.1 abA |
| F32 | 153.3 ± 11.1 abE | 136.5 ± 3.6 abF | 177.3 ± 12.4 bcdCD | 162.3 ± 1.6 deDE | 267.9 ± 9.7 aA | 192.7 ± 8.8 bcdC | 209.7 ± 12.7 abB |
| F33 | 134.4 ± 4.6 bcEF | 120.8 ± 8.7 bF | 152.0 ± 4.0 deD | 145.7 ± 11.0 eDE | 243.4 ± 2.4 aA | 178.5 ± 7.8 cdC | 210.2 ± 11.7 abB |
| F41 | 159.9 ± 8.5 abD | 139.4 ± 18.4 abD | 192.6 ± 14.5 abC | 242.3 ± 15.1 aAB | 257.1 ± 18.0 aA | 218.7 ± 20.9 bBC | 203.8 ± 19.4 abC |
| F42 | 150.4 ± 13.8 abCD | 138.1 ± 6.3 abD | 210 ± 16.4 aB | 174.2 ± 4.6 dC | 251.4 ± 26.2 aA | 159.7 ± 5.7 dCD | 206.7 ± 2.1 abB |
| F43 | 118.2 ± 7.7 cE | 100.2 ± 6.0 cE | 154.0 ± 9.2 cdeD | 190.7 ± 15.3 cdC | 244.5 ± 13.6 aA | 163.1 ± 13.0 dD | 218.8 ± 18.7 abB |
| 26 July | 7 August | 16 August | 26 August | 2 September | 12 September | 19 September | |
|---|---|---|---|---|---|---|---|
| CK | 0.91 ± 0.055 bcA | 0.97 ± 0.085 abA | 0.57 ± 0.049 cdefB | 0.44 ± 0.049 cCD | 0.53 ± 0.047 dBC | 0.46 ± 0.024 abCD | 0.39 ± 0.010 cD |
| F11 | 0.98 ± 0.090 abA | 0.98 ± 0.094 aA | 0.48 ± 0.024 efB | 0.45 ± 0.039 cB | 0.41 ± 0.0041 eB | 0.39 ± 0.020 cdB | 0.36 ± 0.012 cB |
| F12 | 0.93 ± 0.043 abcA | 0.93 ± 0.025 abA | 0.50 ± 0.015 defC | 0.46 ± 0.051 cC | 0.63 ± 0.060 abcdB | 0.52 ± 0.010 aC | 0.39 ± 0.014 cD |
| F13 | 0.95 ± 0.066 abcA | 0.79 ± 0.067 cB | 0.75 ± 0.061 aB | 0.67 ± 0.052 aB | 0.68 ± 0.031 abB | 0.49 ± 0.047 abC | 0.39 ± 0.018 cD |
| F21 | 0.97 ± 0.070 abA | 0.84 ± 0.015 bcB | 0.60 ± 0.024 bcdeC | 0.45 ± 0.016 cD | 0.58 ± 0.027 bcdC | 0.38 ± 0.027 cdeD | 0.45 ± 0.043 bcD |
| F22 | 0.90 ± 0.080 bcdA | 0.98 ± 0.052 aA | 0.68 ± 0.080 abcB | 0.49 ± 0.045 cC | 0.66 ± 0.060 abcB | 0.44 ± 0.038 bcC | 0.41 ± 0.015 cC |
| F23 | 0.80 ± 0.040 dA | 0.87 ± 0.046 abcA | 0.54 ± 0.056 defC | 0.51 ± 0.020 bcC | 0.69 ± 0.038 abB | 0.43 ± 0.057 bcD | 0.39 ± 0.028 cD |
| F31 | 0.84 ± 0.075 cdA | 0.89 ± 0.096 abcA | 0.53 ± 0.040 defBC | 0.42 ± 0.037 cC | 0.62 ± 0.022 abcdB | 0.44 ± 0.019 bcC | 0.50 ± 0.053 bC |
| F32 | 0.96 ± 0.029 abA | 0.88 ± 0.063 abcB | 0.69 ± 0.042 abC | 0.44 ± 0.012 cD | 0.72 ± 0.019 aC | 0.48 ± 0.013 abD | 0.49 ± 0.030 bD |
| F33 | 0.95 ± 0.041 abcA | 0.95 ± 0.057 abA | 0.47 ± 0.043 fC | 0.51 ± 0.018 bcC | 0.66 ± 0.046 abcB | 0.36 ± 0.031 deD | 0.37 ± 0.017 cD |
| F41 | 0.97 ± 0.044 abA | 0.91 ± 0.072 abcA | 0.61 ± 0.053 bcdB | 0.59 ± 0.045 abB | 0.64 ± 0.055 abcB | 0.47 ± 0.054 abD | 0.57 ± 0.060 aC |
| F42 | 0.93 ± 0.034 abcA | 0.97 ± 0.078 abA | 0.73 ± 0.032 aB | 0.45 ± 0.025 cD | 0.60 ± 0.043 bcdC | 0.43 ± 0.0074 bcD | 0.45 ± 0.043 bcD |
| F43 | 1.03 ± 0.047 aA | 0.95 ± 0.083 abA | 0.57 ± 0.057 cdefB | 0.66 ± 0.078 aB | 0.56 ± 0.037 cdB | 0.33 ± 0.023 eC | 0.40 ± 0.035 cC |
| 26 July | 7 August | 16 August | 26 August | 2 September | 12 September | 19 September | |
|---|---|---|---|---|---|---|---|
| CK | 2.95 ± 0.051 aB | 2.97 ± 0.22 aB | 3.40 ± 0.12 aA | 3.43 ± 0.29 aA | 3.66 ± 0.097 aA | 3.72 ± 0.26 aA | 3.66 ± 0.17 aA |
| F11 | 2.89 ± 0.15 aB | 2.92 ± 0.18 aB | 3.40 ± 0.32 aAB | 3.51 ± 0.25 aA | 3.66 ± 0.26 aA | 3.81 ± 0.20 aA | 3.73 ± 0.46 aA |
| F12 | 2.80 ± 0.24 aB | 2.87 ± 0.20 aB | 3.32 ± 0.20 aA | 3.44 ± 0.091 aA | 3.56 ± 0.28 aA | 3.60 ± 0.24 aA | 3.71 ± 0.23 aA |
| F13 | 2.89 ± 0.10 aC | 2.98 ± 0.26 aBC | 3.25 ± 0.26 aABC | 3.37 ± 0.38 aAB | 3.55 ± 0.16 aA | 3.55 ± 0.18 aA | 3.60 ± 0.095 aA |
| F21 | 2.91 ± 0.23 aB | 2.99 ± 0.23 aB | 3.39 ± 0.15 aA | 3.50 ± 0.093 aA | 3.62 ± 0.31 aA | 3.70 ± 0.29 aA | 3.77 ± 0.10 aA |
| F22 | 2.84 ± 0.18 aB | 2.87 ± 0.13 aB | 3.26 ± 0.29 aAB | 3.43 ± 0.12 aA | 3.55 ± 0.34 aA | 3.65 ± 0.33 aA | 3.64 ± 0.30 aA |
| F23 | 2.99 ± 0.11 aB | 3.05 ± 0.11 aB | 3.35 ± 0.10 aA | 3.43 ± 0.17 aA | 3.58 ± 0.036 aA | 3.62 ± 0.33 aA | 3.74 ± 0.099 aA |
| F31 | 2.92 ± 0.32 aB | 3.00 ± 0.15 aB | 3.43 ± 0.25 aAB | 3.52 ± 0.070 aAB | 3.63 ± 0.26 aA | 3.72 ± 0.44 aA | 3.79 ± 0.20 aA |
| F32 | 2.95 ± 0.21 aB | 2.99 ± 0.29 aB | 3.24 ± 0.43 aAB | 3.41 ± 0.15 aAB | 3.58 ± 0.072 aA | 3.61 ± 0.25 aA | 3.60 ± 0.32 aA |
| F33 | 2.84 ± 0.24 aB | 2.89 ± 0.058 aB | 3.36 ± 0.15 aA | 3.43 ± 0.30 aA | 3.56 ± 0.18 aA | 3.70 ± 0.23 aA | 3.56 ± 0.16 aA |
| F41 | 2.88 ± 0.14 aB | 2.95 ± 0.26 aB | 3.30 ± 0.25 aA | 3.47 ± 0.24 aA | 3.57 ± 0.20 aA | 3.67 ± 0.29 aA | 3.65 ± 0.36 aA |
| F42 | 2.85 ± 0.25 aB | 2.91 ± 0.058 aB | 3.27 ± 0.17 aAB | 3.43 ± 0.27 aA | 3.59 ± 0.44 aA | 3.54 ± 0.094 aA | 3.59 ± 0.095 aA |
| F43 | 2.83 ± 0.057 aB | 2.91 ± 0.20 aB | 3.30 ± 0.41 aA | 3.43 ± 0.12 aA | 3.52 ± 0.15 aA | 3.56 ± 0.16 aA | 3.63 ± 0.26 aA |
| Group | Ethanol Content/% | pH | TA Concentration/(g/L) | Residual Sugar Content/(g/L) |
|---|---|---|---|---|
| F11 | 16.32 ± 1.02 a | 3.58 ± 0.16 a | 7.54 ± 0.54 a | 2.30 ± 0.18 defg |
| F12 | 14.75 ± 1.21 ab | 3.73 ± 0.33 a | 6.60 ± 0.17 ab | 2.60 ± 0.069 cde |
| F13 | 15.48 ± 1.35 ab | 3.55 ± 0.25 a | 7.38 ± 0.45 a | 2.90 ± 0.34 bc |
| F21 | 15.51 ± 1.50 ab | 3.66 ± 0.35 a | 6.74 ± 0.47 ab | 2.50 ± 0.17 cdef |
| F22 | 15.14 ± 1.32 ab | 3.79 ± 0.29 a | 6.3 ± 0.39 ab | 2.40 ± 0.12 defg |
| F23 | 14.60 ± 0.15 ab | 3.66 ± 0.30 a | 6.72 ± 0.59 ab | 2.71 ± 0.054 bcd |
| F31 | 14.93 ± 1.05 ab | 3.70 ± 0.26 a | 6.38 ± 0.17 ab | 2.20 ± 0.079 efg |
| F32 | 15.01 ± 1.20 ab | 3.70 ± 0.32 a | 6.76 ± 0.41 ab | 2.50 ± 0.11 cdef |
| F33 | 14.62 ± 0.64 ab | 3.81 ± 0.29 a | 6.05 ± 0.22 b | 2.20 ± 0.20 efg |
| F41 | 13.49 ± 1.46 ab | 3.68 ± 0.29 a | 6.49 ± 0.49 ab | 3.00 ± 0.26 b |
| F42 | 15.15 ± 1.06 ab | 3.65 ± 0.32 a | 6.36 ± 0.57 ab | 2.10 ± 0.13 fg |
| F43 | 12.85 ± 0.68 b | 3.78 ± 0.076 a | 5.81 ± 0.59 b | 2.00 ± 0.04 g |
| CK | 13.17 ± 0.60 ab | 3.54 ± 0.36 a | 6.51 ± 0.46 ab | 3.61 ± 0.22 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kai, J.; Zhang, J.; Wang, C.; Wang, F.; Sun, X.; Ma, T.; Ge, Q.; Xu, Z. Effects of Crop Load Management on Berry and Wine Composition of Marselan Grapes. Horticulturae 2025, 11, 851. https://doi.org/10.3390/horticulturae11070851
Kai J, Zhang J, Wang C, Wang F, Sun X, Ma T, Ge Q, Xu Z. Effects of Crop Load Management on Berry and Wine Composition of Marselan Grapes. Horticulturae. 2025; 11(7):851. https://doi.org/10.3390/horticulturae11070851
Chicago/Turabian StyleKai, Jianrong, Jing Zhang, Caiyan Wang, Fang Wang, Xiangyu Sun, Tingting Ma, Qian Ge, and Zehua Xu. 2025. "Effects of Crop Load Management on Berry and Wine Composition of Marselan Grapes" Horticulturae 11, no. 7: 851. https://doi.org/10.3390/horticulturae11070851
APA StyleKai, J., Zhang, J., Wang, C., Wang, F., Sun, X., Ma, T., Ge, Q., & Xu, Z. (2025). Effects of Crop Load Management on Berry and Wine Composition of Marselan Grapes. Horticulturae, 11(7), 851. https://doi.org/10.3390/horticulturae11070851








