Morphological Analysis, Bud Differentiation, and Regulation of “Bud Jumping” Phenomenon in Oncidium Using Plant Growth Regulators
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Materials
2.2.1. Paraffin Section Observation
2.2.2. Transcribed Plant Material
2.2.3. Plant Growth Regulator Spraying Experiment
2.3. Methodologies
2.3.1. Paraffin Sectioning
2.3.2. Total RNA Extraction and Library Construction, Sequencing, and Data Processing
2.3.3. Screening, Functional Annotation and Classification of Differentially Expressed Genes (DEGs)
2.3.4. qRT-PCR Validation
2.3.5. Application Test of Plant Growth Regulators
- (1)
- Test setup of plant growth regulators
- (2)
- Experimental methods and observational indicators
2.4. Data Analysis
3. Results
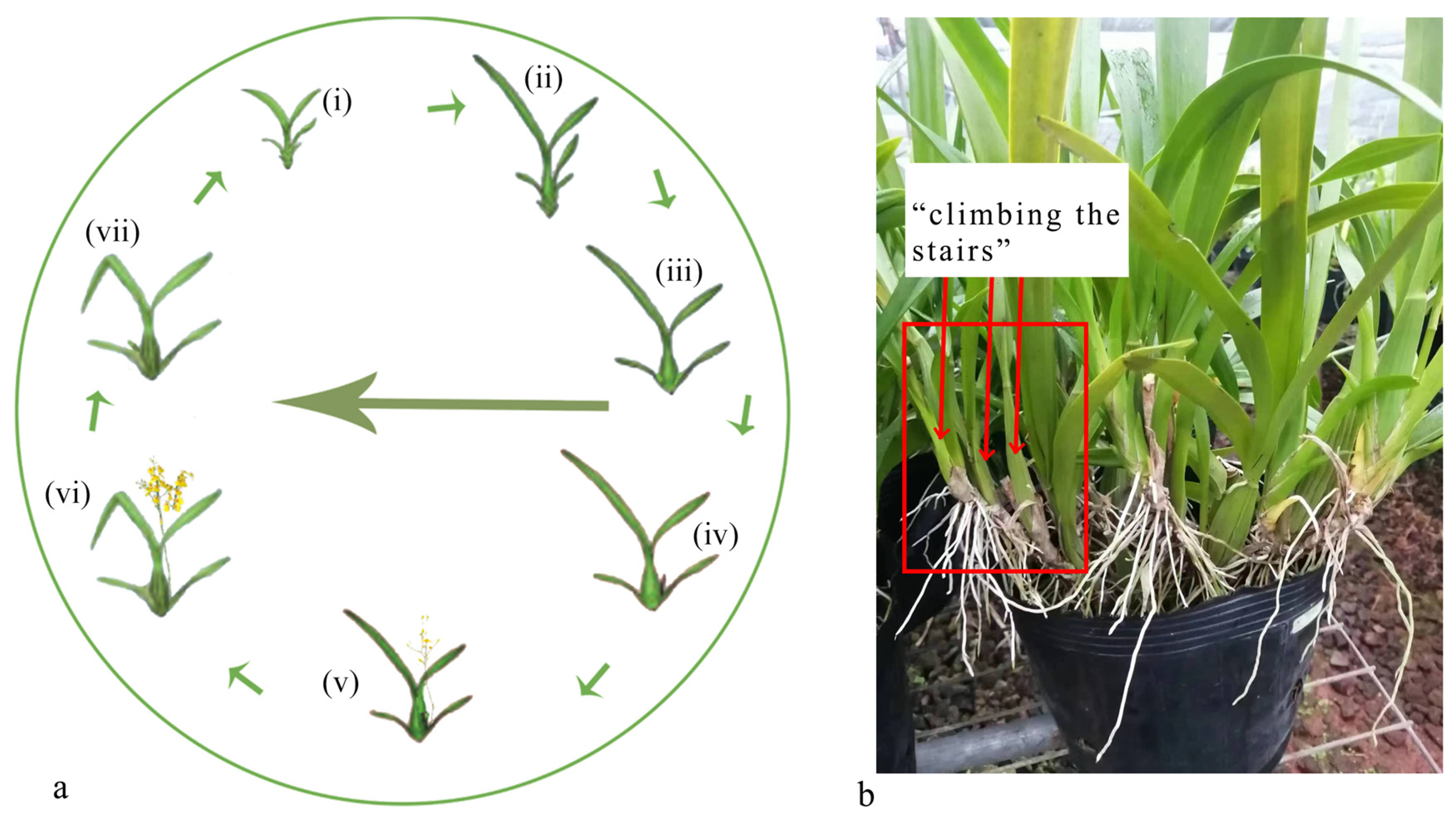
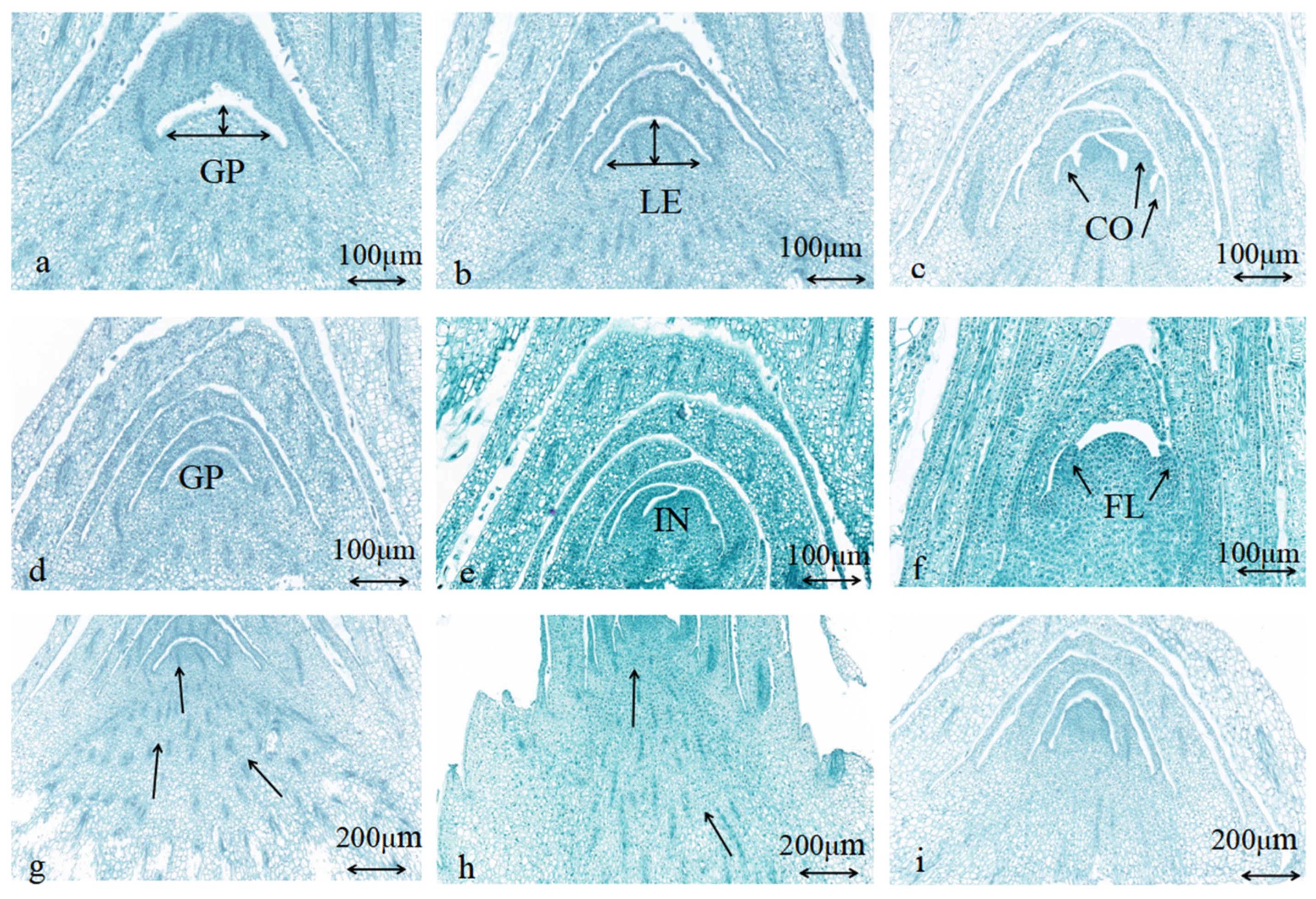
3.1. Morphological Characteristics of Adventitious Bud and Leaf/Flower Bud Differentiation in Oncidium “Honey Angel”
| Periods | Cellular Level | External Circumstances |
|---|---|---|
| Initial stage of leaf bud differentiation | The growth cone located at the stem tip has a mountain cone-like shape, where its width is broader than its height. The cells within its apical meristematic tissue are tightly packed, whereas the cells situated at its lower base are more loosely arranged and exhibit a tendency to converge and develop towards the stem tip (Figure 2a). | A new round of bud differentiation in Oncidium was observed in mid-July (Northern Hemisphere summer) at the experimental site buds emanating from the bases of the four axils of the pseudobulb “lead”, which are very small and are adventitious buds for which it is uncertain which one is the pilot bud at this time (Figure 3a) |
| Leaf primordial differentiation stage | Significant elongation at the tip of the growth cone in the meristematic tissue at the stem tip, with rounded, expanded, and loosely arranged cells and a tendency to develop a convex protrusion (Figure 2b). | Adventitious buds on the “lead” continued to grow and develop, and the pioneer buds have not yet exposed their leaf sheaths and need to be plucked off the pseudobulb cotyledons to be visible (Figure 3b) |
| Late leaf bud differentiation (visually distinguishable) | The meristematic tissue at the stem end had differentiated into the first and second cotyledon morphology (Figure 2c). | The first and second axillary buds from the “lead” stop growing, while the third or fourth axillary buds develop into vegetative buds. At this stage, the cotyledons of the pseudobulb can only be observed by gently separating them, revealing flattened leaf buds that are beginning to emerge (Figure 3d) |
| Initial stage of bud differentiation | The cellular morphology and external phenotype observed in the initial stages of flower bud differentiation were similar to those seen in leaf bud differentiation, characterized by closely positioned axillary leaves (Figure 2d). | Therefore, these features could not be used to distinguish between flower and vegetative buds (Figure 3a) |
| Differentiation stage of inflorescence primordium | The meristematic tissue at the tip of the stem began to differentiate into reproductive tissue, exhibiting a notable protrusion at the apex of the growth cone. The basal cells became rounded, and there was a tendency for finger-like projections to develop at both ends of the growth cone later (Figure 2e). | |
| Late stage of flower bud differentiation | The middle rounded stem end continues to grow, and its ends develop into angular protuberances that differentiate into bud primordia (Figure 2f). Subsequently, the flower organs, including calyx and petal primordia, gradually differentiate, and each floret begins to develop on both sides, alongside the differentiation of the inflorescence primordium initiated (Figure 3e). | At this point, when the leaves are plucked, the round-tipped buds can be seen to appear (Figure 3c) |
3.2. Relationship Between Bud Differentiation and Pseudobulb Development in Oncidium “Honey Angel”
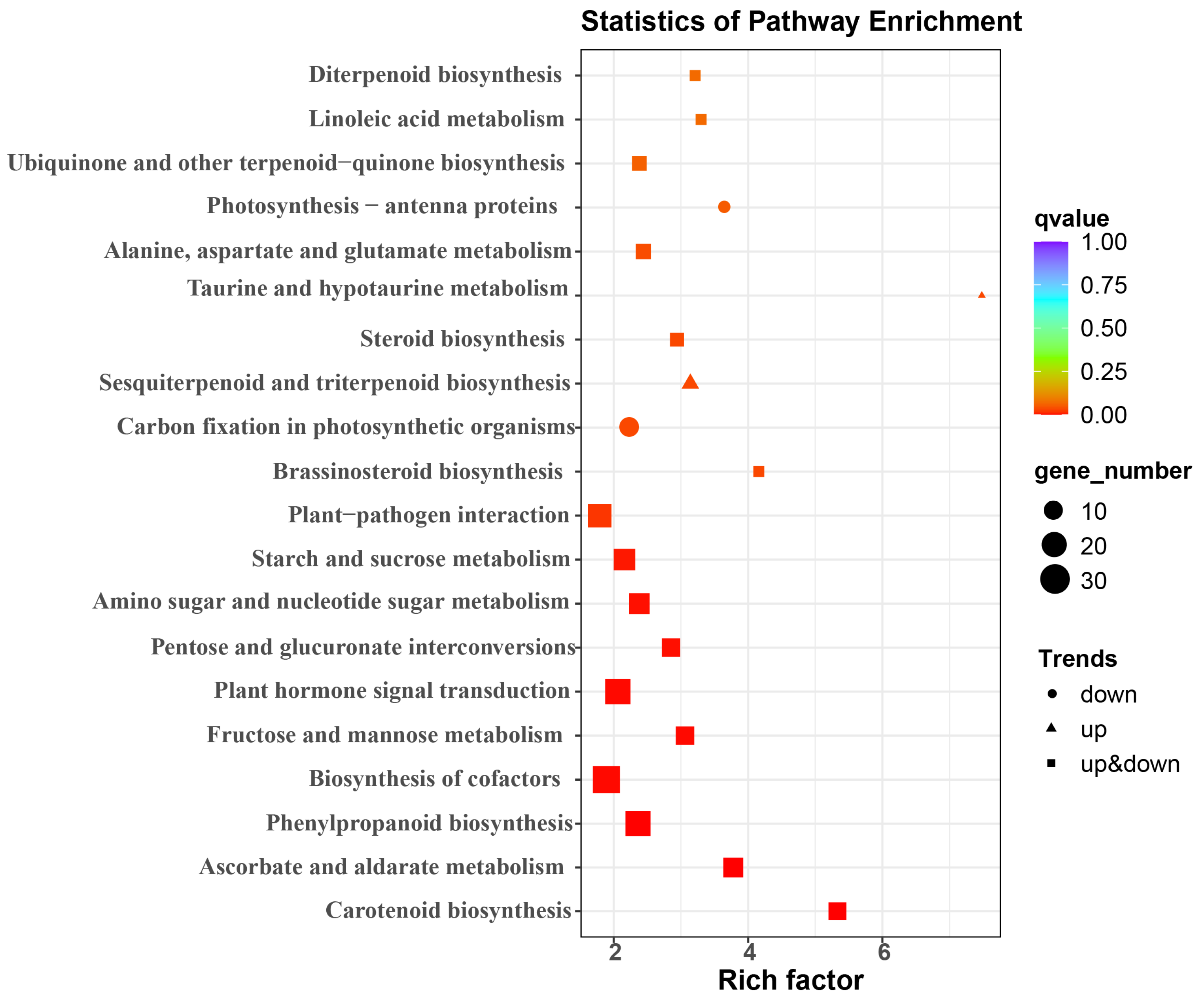
3.3. RNA Sequencing, Quality Control, and Functional Annotation and Classification
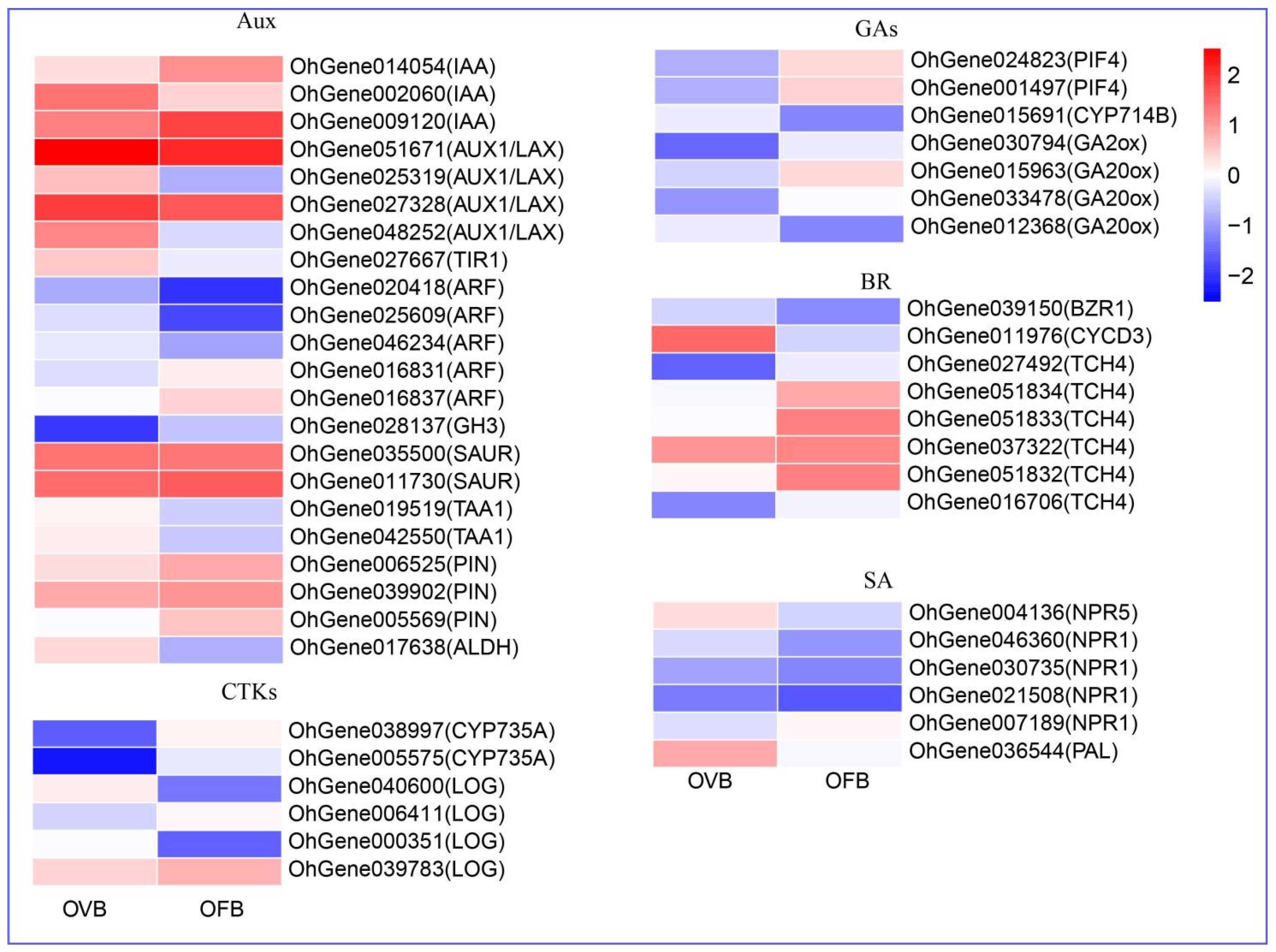
3.4. Phytohormone Signaling Regulation
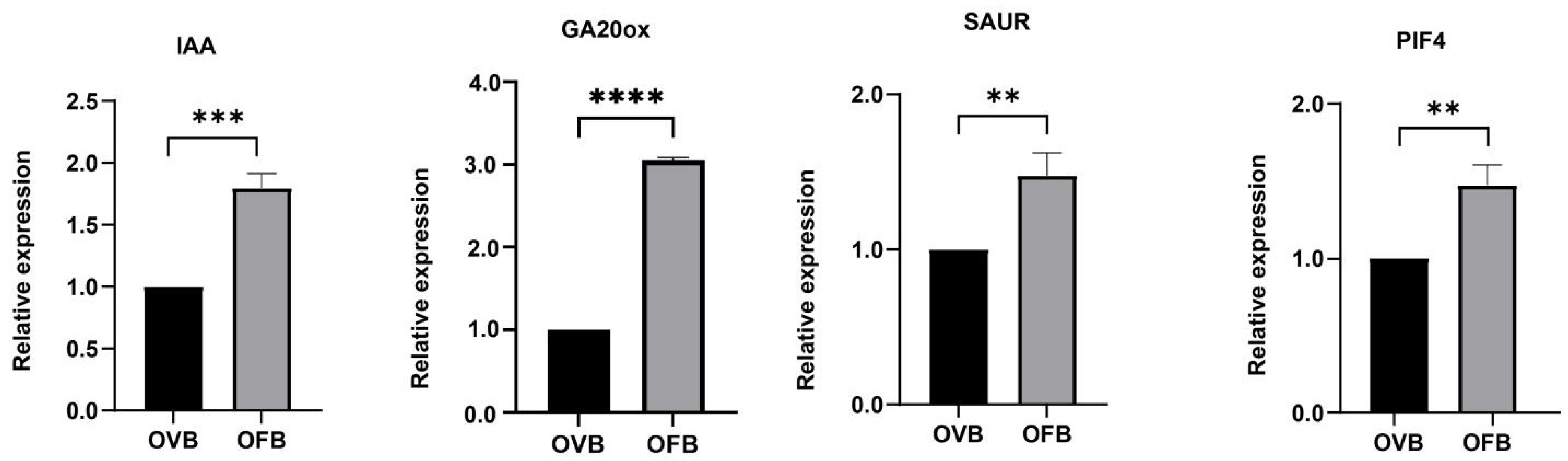
3.5. qRT-PCR Quantitative Analysis
3.6. Foliar Application Test of Plant Growth Regulators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chin, D.C.; Hsieh, C.C.; Lin, H.Y.; Yeh, K.W. A Low glutathione redox state couples with a decreased ascorbate redox ratio to accelerate flowering in Oncidium orchid. Plant Cell Physiol. 2016, 57, 423–436. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Yin, H. Separate propagation and flower control-treated culture of Oncidium. China Flower Hortic. 2003, 12, 26–27. [Google Scholar]
- Pan, R.S. Plant Physiology; Higher Education Press: Beijing, China, 2004. [Google Scholar]
- Qu, B.; Zhang, W.; Chen, X.H.; Li, N.; Cui, I.N.; Li, T.L. Research progress of flower bud differentiation in plants. Chin. Agric. Sci. Bull. 2010, 26, 109–114. [Google Scholar]
- Clemens, J.; Henriod, R.E.; Bailey, D.G.; Jameson, P.E. Vegetative phase change in metrosideros: Shoot and root restrictiopn. Plant Growth Regul. 1999, 28, 207–214. [Google Scholar] [CrossRef]
- Wang, Y.H.; Fan, C.H.; Shen, X.; Qu, G.; Shi, J. Changes in endogenous hormones during the flower bud differentiation of sweet cherry. Northwest J. Agric. 2002, 11, 64–67. [Google Scholar]
- Zeng, X.P.; Liu, Z.C.; Su, M.H.; Chen, C. Changes of endogenous hormones and polyamines in leaves of Phalaenopsis amabilis during flowering. Subtrop. Plant Sci. 2008, 3, 1–5. [Google Scholar]
- Ahmad, S.; Lu, C.; Gao, J.; Wei, Y.; Xie, Q.; Jin, J.; Zhu, G.; Yang, F. Integrated proteomic, transcriptomic, and metabolomic profiling reveals that the gibberellin–abscisic acid hub runs flower development in the Chinese orchid Cymbidium sinense. Hortic. Res. 2024, 11, uhae073. [Google Scholar] [CrossRef]
- Wen, Z.; Guo, W.; Li, J.; Lin, H.; He, C.; Liu, Y.; Zhang, Q.; Liu, W. Comparative transcriptomic analysis of vernalization and cytokinin induced floral transition in Dendrobium nobile. Sci. Rep. 2017, 7, 45748. [Google Scholar] [CrossRef]
- Gao, X.; Hao, Y.; Yang, X.; Shi, Y.; Zhang, L.; Yan, R. Transcriptomics and metabolomics analysis reveal the mechanism of hormones regulation of the flower bud formation and development in Phalaenopsis Orchid. J. Plant Growth Regul. 2025, 1–17. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Nie, C.R.; Guo, W.J.; Zhang, J.W.; Lyu, Y.M.; Ding, P.S.; Sun, J.X. Exploring flowering genes in Phalaenopsis through transcriptome analysis and critical gene validation of hormone signal transduction pathway. Russ. J. Plant Physiol. 2023, 70, 25. [Google Scholar] [CrossRef]
- Su, W.R.; Chen, W.S.; Koshioka, M.; Mander, L.N.; Hung, L.S.; Chen, W.H.; Fu, Y.M.; Huang, K.L. Changes in gibberellin levels in the flowering shoot of Phalaenopsis hybrida under high temperature conditionswhen flower development is blocked. Plant Physiol. Biochem. 2002, 39, 45–50. [Google Scholar] [CrossRef]
- Luo, S.; Sun, M.; Liang, W. Morphological and physiological investigations reveal the regulatory effect of exogenous paclobutrazol on flowering promotion by winter warming in Chaenomeles speciosa ‘Changshouguan’. Sci. Rep. 2024, 14, 17694. [Google Scholar] [CrossRef]
- Gao, X.F.; Shi, Y.P.; Zhang, L. Effects of exogenous hormones on photosynthetic physiology and flower bud differentiation of Phalaenopsis amabilis. North. Hortic. 2024, 13, 57–64. [Google Scholar]
- Tanaka, M.; Yamada, S.; Goi, M. Morphological observation on vegetative growth and flower bud formation in Oncidium Boissiense. Sci. Hortic. 1986, 28, 133–146. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022, 50, D27–D38. [CrossRef]
- The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinform. 2021, 19, 578–583. [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Yamaya, T.; Sakakibara, H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-Zeatin. J. Biol. Chem. 2004, 279, 41866–41872. [Google Scholar] [CrossRef]
- Rieu, I.; Ruiz-Rivero, O.; Fernandez-Garcia, N.; Griffiths, J.; Powers, S.J.; Gong, F.; Linhartova, T.; Eriksson, S.; Nilsson, O.; Thomas, S.G.; et al. The gibberellin biosynthetic genes AtGA20ox1 and AtCA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2008, 53, 488–504. [Google Scholar] [CrossRef]
- Hew, C.S.; Yong, J.W.H. Physiology of Tropical Orchids in Relation to the Industry; World Scientifc Publishing: Singapore, 1997. [Google Scholar]
- Peng, F.; Tian, M.; Wang, C.; Wei, P.X. Morphological and anatomical characteristics of bud differentiation of Oncidium and dynamic changes of vegetatives. J. Zhejiang A F Univ. 2012, 29, 7–11. [Google Scholar]
- Sato, A.; Yamamoto, K.T. Overexpression of the non-canonical AUX/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol Plant. 2008, 133, 397–405. [Google Scholar] [CrossRef]
- Korasick, D.A.; Westfall, C.S.; Lee, S.G.; Nanao, M.H.; Dumas, R.; Hagen, G.; Guilfoyle, T.J.; Jez, J.M.; Strader, L.C. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc. Natl. Acad. Sci. USA 2014, 111, 5427–5432. [Google Scholar] [CrossRef]
- Xie, X.; Qin, G.; Si, P.; Luo, Z.; Gao, J.; Chen, X.; Zhang, J.; Wei, P.; Xia, Q.; Lin, F.; et al. Analysis of nicotiana tabacum PIN genes identifies NtPIN4 as a key regulator of axillary bud growth. Physiol. Plant. 2017, 160, 222–239. [Google Scholar] [CrossRef]
- Krogan, N.T.; Marcos, D.; Weiner, A.I.; Berleth, T. The auxin response factor MONOPTEROS controls meristem function and organogenesis in both the shoot and root through the direct regulation of PIN genes. New Phytol. 2016, 212, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Gao, Z.P.; Zhu, Z.Q. DELLA-PIF modules: Old dogs learn new tricks. Trends Plant Sci. 2016, 21, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmulling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Chandler, J.W. The hormonal regulation of flower development. J. Plant Growth Regul. 2011, 30, 242–254. [Google Scholar] [CrossRef]
- Swarup, R.; Parry, G.; Graham, N.; Allen, T.; Bennett, M. Auxin cross-talk: Integration of signalling pathways to control plant development. Plant Mol. Biol. 2002, 49, 411–426. [Google Scholar] [CrossRef]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef]
- Shi, Y.B. Comparative Transcriptome Analysis and Cloning of Genes Related to Flowering in the Process of Agapanthus Praecox ssp. Orientalis Flower Bud Differentiation. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2014. [Google Scholar]
- Sun, C.; Li, J. Biosynthesis, catabolism, and signal transduction of brassinosteroids. Plant Physiol. J. 2017, 53, 291–307. [Google Scholar]
- Lv, Z.; Yu, L.; Zhan, H.; Li, J.; Wang, C.; Huang, L.; Wang, S. Shoot differentiation from Dendrocalamus brandisii callus and the related physiological roles of sugar and hormones during shoot differentiation. Tree Physiol. 2023, 43, 1159–1186. [Google Scholar] [CrossRef]
- Guan, Y.R.; Xue, J.Q.; Xue, Y.Q.; Yang, R.W.; Wang, S.L.; Zhang, X.X. Effect of exogenous GA3 on flowering quality, endogenous hormones, and hormone-and flowering-associated gene expression in forcing-cultured tree peony (Paeonia suffruticosa). J. Integr. Agric. 2019, 18, 1295–1311. [Google Scholar] [CrossRef]
- Weiss, D.; Ori, N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 2017, 144, 1240–1246. [Google Scholar] [CrossRef]
- Grochowska, J.; Karaszewska, A.; Jankowska, B.; Maksymiuk, J. Dormant pruning influence on auxin, gibberellin, and cytokinin levels in apple trees. J Amer Soc Hort Sci. 1984, 109, 312–318. [Google Scholar] [CrossRef]
- Lee, H.B.; Im, N.H.; Aa, S.K.; Kim, K.S. Changes of growth and inflorescence initiation by exogenous gibberellic acid3 and 6-benzylaminopurine application in Phalaenopsis orchids. Agronomy 2021, 11, 196. [Google Scholar] [CrossRef]
- Azizi, P.; Rafii, M.Y.; Maziah, M.; Abdullah, S.N.; Hanafi, M.M.; Latif, M.A.; Rashid, A.A.; Sahebi, M. Understanding the shoot apical meristem regulation: A study of the phytohormones, auxin and cytokinin, in rice. Mech. Dev. 2015, 135, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.C. Apical Dominance. HortScience 1987, 22, 824–833. [Google Scholar] [CrossRef]
- Tanaka, M.; Takei, K.; Kojima, M.; Kojima, M.; Sakakibara, H.; MoriAuxin, H. control-treateds local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006, 45, 1028–1036. [Google Scholar] [CrossRef] [PubMed]

| Period of Bud Differentiation | Length of Buds (mm) | Length of Pseudobulbs (mm) | Thickness of Pseudobulbs (mm) | Width of Pseudobulbs (mm) |
|---|---|---|---|---|
| Initial stage of bud differentiation | 6.37 ± 0.2 c | 49.40 ± 0.5 b | 7.04 ± 0.1 b | 10.83 ± 0.9 c |
| Leaf primordium differentiation stage | 8.73 ± 0.9 b | 54.07 ± 1.3 b | 13.45 ± 3.8 a | 15.21 ± 3.9 b |
| Late stage of bud differentiation | 11.76 ± 1.7 a | 60.54 ± 1.8 a | 12.84 ± 0.7 a | 19.22 ± 1.8 a |
| Initial stage of flower bud differentiation | 6.40 ± 0.0 c | 49.83 ± 0.5 b | 7.05 ± 0.1 b | 11.53 ± 0.6 c |
| Differentiation stage of inflorescence primordium | 9.54 ± 0.4 b | 50.20 ± 1.1 b | 10.81 ± 1.2 a | 16.14 ± 1.0 ab |
| Late stage of flower bud differentiation | 11.46 ± 1.0 a | 61.47 ± 2.1 a | 13.95 ± 0.1 a | 19.07 ± 0.3 a |
| Samples | Raw Reads | Clean Reads | GC Content (%) | % ≥ Q30 | Mapped Reads | Gene Transcripts |
|---|---|---|---|---|---|---|
| OFB1 | 42,848,732 | 20,441,097 | 45.21 | 95.16% | 33,594,880 (82.17%) | 12,954 |
| OFB2 | 41,237,108 | 19,801,580 | 45.29 | 95.05% | 32,618,072 (82.36%) | 12,979 |
| OFB3 | 46,854,894 | 22,485,145 | 45.22 | 94.76% | 36,864,547 (81.98%) | 13,168 |
| OVB1 | 42,178,172 | 20,269,341 | 44.88 | 94.98% | 33,099,228 (81.65%) | 13,253 |
| OVB2 | 44,292,884 | 21,251,957 | 44.81 | 94.86% | 34,730,115 (81.71%) | 13,114 |
| OVB3 | 40,630,874 | 19,481,320 | 45.58 | 95.47% | 31,975,871 (82.07%) | 13,000 |
| Treatment Group | Flower Bud Rate% | Vegetative Bud Rate% | Adventitious Bud Rate% |
|---|---|---|---|
| mock | 50.00 ± 0 d | 38.89 ± 9.6 abc | 11.11 ± 9.6 ab |
| IAA (25 mg/L) | 77.78 ± 9.6 abc | 5.56 ± 9.6 d | 16.67 ± 0 ab |
| IAA (50 mg/L) | 66.67 ± 16.7 abcd | 11.11 ± 9.6 d | 22.22 ± 9.6 a |
| IAA (100 mg/L) | 66.67 ± 16.7 abcd | 16.67 ± 16.7 d | 16.67 ± 0 ab |
| 6-BA (10 mg/L) | 83.33 ± 16.7 ab | 5.56 ± 9.6 d | 11.11 ± 9.6 ab |
| 6-BA (25 mg/L) | 78.89 ± 7.7 abc | 21.11 ± 7.7 cd | 0 b |
| 6-BA (50 mg/L) | 57.94 ± 8.4 cd | 42.06 ± 8.4 ab | 0 b |
| GA3 (50 mg/L) | 64.44 ± 17.1 abcd | 12.22 ± 10.7 d | 23.33 ± 25.2 a |
| GA3 (75 mg/L) | 61.11 ± 9.6 bcd | 22.22 ± 19.3 bcd | 16.67 ± 16.7 ab |
| GA3 (100 mg/L) | 88.89 ± 9.6 a | 0 d | 11.11 ± 9.6 ab |
| GA3 (100 mg/L) + 6-BA (10 mg/L) | 88.89 ± 19.3 a | 5.56 ± 9.6 d | 5.56 ± 9.6 ab |
| GA3 (100 mg/L) + 6-BA (25 mg/L) | 77.78 ± 9.6 abc | 16.67 ± 16.7 d | 5.56 ± 9.6 ab |
| GA3 (100 mg/L) + 6-BA (50 mg/L) | 55.56 ± 9.6 cd | 44.44 ± 9.6 a | 0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, H.; Liu, L.; Li, W.; Hao, D.; Lin, S.; Ye, B.; Tang, M.; Ling, P. Morphological Analysis, Bud Differentiation, and Regulation of “Bud Jumping” Phenomenon in Oncidium Using Plant Growth Regulators. Horticulturae 2025, 11, 852. https://doi.org/10.3390/horticulturae11070852
Lan H, Liu L, Li W, Hao D, Lin S, Ye B, Tang M, Ling P. Morphological Analysis, Bud Differentiation, and Regulation of “Bud Jumping” Phenomenon in Oncidium Using Plant Growth Regulators. Horticulturae. 2025; 11(7):852. https://doi.org/10.3390/horticulturae11070852
Chicago/Turabian StyleLan, Hanqiao, Le Liu, Weishi Li, Daicheng Hao, Shanzhi Lin, Beilei Ye, Minqiang Tang, and Peng Ling. 2025. "Morphological Analysis, Bud Differentiation, and Regulation of “Bud Jumping” Phenomenon in Oncidium Using Plant Growth Regulators" Horticulturae 11, no. 7: 852. https://doi.org/10.3390/horticulturae11070852
APA StyleLan, H., Liu, L., Li, W., Hao, D., Lin, S., Ye, B., Tang, M., & Ling, P. (2025). Morphological Analysis, Bud Differentiation, and Regulation of “Bud Jumping” Phenomenon in Oncidium Using Plant Growth Regulators. Horticulturae, 11(7), 852. https://doi.org/10.3390/horticulturae11070852






