Abstract
The establishment of a transient expression system in petals is significant for elucidating gene functions in flowering trees characterized by a prolonged juvenile phase. Genetic improvements in Camellia japonica have been hindered due to the absence of a functional validation platform. In this study, we explored an Agrobacterium-mediated and readily observable transient expression system in camellia petals to systematically optimize four critical factors affecting transformation efficiency. As a result, the bud stage, ‘Banliuxiang’ genotype, OD600 of 1.0, and 1-day co-cultivation achieved the highest intensity of transient expression, and overexpression of the Ruby1 reporter gene induced substantial anthocyanin synthesis, manifested as distinct red pigmentation. Furthermore, the optimized transient expression system revealed that the R2R3-MYB transcription factor CjMYB5, which interacted with CjGL3, promoted anthocyanin biosynthesis in camellia petals by transactivating key DFR structural genes. This transient expression platform not only advances functional genomics studies in ornamental woody species but also lays a foundation for molecular breeding programs in C. japonica.
1. Introduction
The plant transient transformation system provides rapid functional characterization of genes, particularly in woody plants characterized by challenging genetic transformation and prolonged juvenile phases [1]. Agrobacterium-mediated transient expression systems have been employed to functionally validate target genes across a variety of plant species. Simple and efficient transient expression platforms have been established in several herbaceous models, including tobacco (Nicotiana benthamiana) leaves [2], spinach (Spinacia oleracea) foliage [3], and strawberry (Fragaria × ananassa) fruits [4]. Notable progress has also been documented in woody species, with successful implementations in rose (Rosa hybrida) leaves [5], Torreya grandis cones [1], and apple (Malus domestica) fruits [6]. However, transient expression systems utilizing petal tissues from flowering trees have not been developed. Thus, there is a need to establish readily observable transient expression platforms in floral organs of ornamental woody plants.
The efficiency of transient expression systems is affected by several parameters, including explant type, the Agrobacterium strain, and optimizing the bacterial concentration [7]. An efficient virus-induced gene silencing system was established in peach (Prunus persica) leaf tissues by systematically optimizing the infiltration method, temperature regime, and bacterial suspension density [8]. In rose (Rosa hybrida) petals, a comprehensive evaluation of genotype-dependent responses, co-cultivation temperatures, and floral developmental stages revealed significant effects on transformation efficiency [9]. Tian et al. [7] investigated critical determinants, such as cultivar specificity, compatibility of the Agrobacterium strain, and floral development stages in Prunus mume using β-glucuronidase (GUS) as the reporter gene, and demonstrated stage-dependent expression patterns. Notably, temporal regulation of infection duration combined with OD600 optimization successfully modified floral pigmentation in Phalaenopsis orchids, establishing a visually detectable transient expression platform in petals [10].
Camellia japonica (Family Theaceae, genus Camellia, section Camellia) is a flowering tree renowned for its high ornamental value and is widely utilized in urban landscapes [11]. Recent completion of its high-quality genome has laid the foundation for deciphering the molecular mechanisms underlying floral pigmentation, patterning, and chronology [12]. Our previous investigations screened candidate genes associated with petal coloration, floral architecture, and the cold stress response in camellia [13,14,15]. Functional characterization predominantly relies on heterologous expression systems due to the absence of robust genetic transformation protocols. These limitations emphasize the need for establishing functional validation platforms in ornamental woody plants.
The MYB family represents one of the largest transcription factor groups in plants, playing crucial roles as transcriptional regulators in the flavonoid biosynthetic pathway [16]. MYB proteins have been classified into 1R-MYB, R2R3-MYB, and 3R-MYB subfamilies based on variations in the repeat domain [17]. Notably, some R2R3-MYB members serve as activators of anthocyanin biosynthesis. Ruby1 has been employed as a molecular marker in transgenic studies [18]. In contrast, MYB factors also demonstrate repressive functions in regulating anthocyanins, including AtMYB4 in Arabidopsis thaliana [19] and MdMYB16 in Malus domestica [20]. Functional diversification of MYB5 homologs exhibits species-specific characteristics: Fragaria × ananassa FaMYB5 interacts with FaEGL3 and FaLWD1 to form the MBW activation complex, which enhances anthocyanin production [4]. Other studies suggest that CsMYB5 is involved in proanthocyanidin biosynthesis without affecting the accumulation of anthocyanins [21]. Interestingly, MdMYB5 is functionally specialized in secondary cell wall biosynthesis in Malus domestica [22]. The molecular mechanisms underlying MYB-mediated anthocyanin regulation in C. japonica remain poorly characterized. Although CjMYB114 has been identified as a positive regulator of floral pigmentation [23], the function of its paralog CjMYB5 is unknown.
In this study, we established a high-efficiency Agrobacterium-mediated transient expression platform in camellia petals. Employing the green fluorescent protein (GFP) and the anthocyanin-activator Ruby1 as dual reporter systems, we systematically optimized the transformation parameters, including the cultivar genotype, floral developmental stages, bacterial optical density (OD600), and co-culture time. The optimized platform was used to investigate the regulatory role of CjMYB5 in anthocyanin biosynthesis during petal pigmentation.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The five C. japonica cultivars (‘Nanjixing’, ‘Queen Diana’, ‘One Alone’, ‘CangwuHuanjing’, and ‘Banliuxiang’) used in this study were cultivated at the Camellia Germplasm Resource Center of Research, Institute of Subtropical Forestry, Chinese Academy of Forestry, Hangzhou, Zhejiang, China (30.06° N, 119.96° E). This region is characterized by an annual rainfall of approximately 1500 mm, a frost-free period of 320 days, and soil pH values of 5.5–6.5. Petal samples were collected during the flower bud stage (December), the balloon stage (February), and the blooming stage (March).
2.2. RNA Extraction, Gene Cloning, and Plasmid Construction
Total RNA was isolated from camellia petals using the RN38 RNA Extraction Kit (Aidlab, Beijing, China), yielding high-quality RNA samples (concentration > 200 ng·μL−1). First-strand cDNA was synthesized with the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) according to the manufacturer’s protocol. The anthocyanin-activating transcription factor Ruby1 was amplified using the Phanta Max Master Mix (Vazyme) with gene-specific primers to optimize the transient expression platform (Supplementary Table S1). The full-length coding sequence was directionally cloned into the Pcambia1300-GFP binary vector via in-fusion cloning to generate the Ruby1-GFP dual-reporter construct. To investigate the regulatory function of CjMYB5, the following expression vectors were engineered: (1) The CjMYB5 coding sequence was fused with GFP under the CaMV 35S promoter for subcellular localization studies. (2) A dual-luciferase reporter system was created by inserting the CjMYB5 coding sequence into the pGreenII 62-SK effector vector and cloning the DFR promoter (2000 bp upstream ATG) into the pGreenII 0800-LUC reporter vector (MYB5-sk/DFRpro-LUC). All constructs were sequence-verified by Tsingke Biotechnology.
2.3. Sequence Alignment and Phylogenetic Analysis
The CjMYB5 and AtMYB amino acid sequences were aligned using MAFFT version 7.525 [24] with the L-INS-I option. TRIMAL version 1.5.0 [25] (with “-automated 1” option) was used to remove poorly aligned regions. The phylogenetic tree for these genes was constructed based on the maximum-likelihood method using IQ-TREE 2.2.0 with 5000 ultrafast bootstrap replicates [26], and the best model was selected using ModelFinder [27].
2.4. Preparation and Infiltration of the Agrobacterium Culture
The GV3101 (pSoup-p19) Agrobacterium tumefaciens strain was procured from Weidi Biotechnology (Shanghai, China). The recombinant plasmids Pcambia1300-Ruby1-GFP or Pcambia1300-MYB5-GFP were introduced into the strain via heat-shock transformation. The transformed bacterial suspensions were mixed with an equal volume of 50% (v/v) glycerol and stored at −80 °C for subsequent experiments.
Transformed Agrobacterium colonies were inoculated in YEB liquid medium supplemented with 25 μg/mL rifampicin and 100 μg/mL kanamycin, then cultured at 28 °C with 200 rpm agitation until the OD600 was 0.6–0.8. Bacterial cells were pelleted by centrifugation at 5000× g for 10 min at room temperature, and the supernatant was removed. The pellet was suspended in infiltration buffer containing MS basal salts (pH 5.7–5.8, adjusted with 0.1 M NaOH), 10 mM MgCl2, 10 mM MES, and 200 μM AS. The bacterial suspensions were adjusted to OD600 = 0.6, 0.8, and 1.0, and incubated in the dark at room temperature for 3 h before infiltration.
Petal discs (0.8 cm diameter) from distinct developmental stages were immersed in 20 mL infiltration buffer in a vacuum desiccator. Vacuum infiltration was performed under cyclic pressure (−1 to 1 MPa) until the tissue was completely saturated. The petal discs were rinsed 5–10 times with sterile deionized water to remove residual bacteria. The treated tissues were initially maintained in the dark at 18 °C for 24 h, then transferred to light for phenotypic observations over 48–120 h.
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
SYBR® Green Master Mix (Takara Bio, Kusatsu, Japan) was used to detect the gene expression levels on an ABI 7500 Fast Real-Time PCR System (ABI, Foster City, CA, USA). The amplification program consisted of initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. GAPDH was used as the internal reference gene, and relative expression levels were calculated using the 2−ΔΔCt method. Three biological replicates were included for each sample. Primer information is provided in Supplementary Table S1.
2.6. Quantitative Analysis of Total Anthocyanin and Proanthocyanin Contents
The extract and the structure of anthocyanin were analyzed according to our previous study using an ultra-high-performance liquid chromatography-quantitative time-of-flight mass spectrometry system (Waters Corp., Manchester, UK) [11]. A cyanidin-3-0-β-glucoside standard (Sigma, St. Louis, MO, USA) was used to calculate anthocyanin content. The proanthocyanidin content was determined according to the instructions with the Micro Plant Proanthocyanidins Assay Kit (Solarbio Technology, Beijing, China) [28].
2.7. Bimolecular Fluorescent Complementation (BiFC) Assay
The BiFC assays were performed as described previously [29]. The CjMYB5 and CjGL3 coding sequences without the stop codon were subcloned into vectors containing the C- and N-terminal fragments of GFP. Recombinant constructs and negative controls (empty vectors) were introduced into Agrobacterium and co-infiltrated into leaves of N. benthamiana at a ratio of 1:1 for interacting pairs. The GFP fluorescence signals were visualized 72 h post-infiltration using a laser scanning confocal microscope.
2.8. Dual-Luciferase Reporter Assay
Dual-luciferase assays were performed according to the protocol described by Su et al. [5]. Promoters of the anthocyanin biosynthetic gene DFR were cloned into the pGreenII 0800-LUC vector, and the CjMYB5 and CjbHLH1 coding sequences were inserted into the pGreenII 0029 62-SK vector. All recombinant constructs were introduced into the Agrobacterium strain GV3101 and co-infiltrated into N. benthamiana leaves at a ratio of 2:1 (effector: promoter construct). The empty pGreenII 0029 62-SK vector (SK) served as a negative control, with at least five biological replicates included. Three days post-infiltration, firefly luciferase (LUC) and Renilla luciferase (REN) activities were quantified using the Dual-Luciferase Reporter Assay Kit (Promega, Madison, WI, USA), and the transcriptional activation effects were calculated as the ratio of LUC to REN activity.
2.9. Statistical Analysis
Data are expressed as mean ± standard deviation from three independent experiments. Statistical analysis was performed with Student’s t-test and one-way ANOVA using SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA). The least significant difference was used to compare treatment means, and p ≤ 0.05 was considered significant.
3. Results
3.1. Effects of Genotype and Flower Developmental Stage on Transient Overexpression
Under the same Agrobacterium-mediated infiltration conditions (OD600 = 1.0, 24 h co-cultivation), systematic phenotypic changes and GFP signals were compared across the flower bud, balloon, and bloom stages. The results revealed stronger GFP signals in flower bud stage petals than in the other developmental stages (Figure 1c), accompanied by significant anthocyanin accumulation that conferred a distinct red color (Figure 1a,b). In contrast, bloom stage petals exhibited lower transient expression efficiency with no observable color change and only minor tissue maceration. Thus, transient expression efficiency progressively declined as the flowers opened.

Figure 1.
Effects of flower developmental phase and genotype on the transient assays. (a) Schematic representation of the transient assay procedure. ➀ Agrobacterium was cultured on YEB medium. ➁ Enriched by centrifugation. ➂ Agrobacterium suspensions were injected into the petals. ➃ The transformed petals were cultured in light for 3 days. (b) Bud stage: calyx not swollen, balloon stage: calyx swollen but petals not unfurled, blooming stage: petals fully expanded. G1: Nanjixing, G2: One Alone, G3: Queen Diana, G4: CangwuHuanjing, G5: Banliuxiang. Scale bar = 1 cm. (c) GFP fluorescence images of the two genotypes. Scale bar = 20 μm. (d) Transformation efficiency of the petals was evaluated across more than 20 biological replicates, and red indicates high efficiency.
We evaluated the effect of genotype on transient expression efficiency. The G5 genotype with white-pink petals exhibited superior transient expression efficiency with concomitant anthocyanin hyperaccumulation that conferred a distinct red color. In contrast, the other camellia cultivars accumulated no significant anthocyanins and retained their original white color. These results demonstrate that the genotype and flower developmental stage exert substantial effects on transient transformation efficiency and anthocyanin biosynthesis.
3.2. Optimizing the Experimental Conditions for Petals at the Flower Bud Stage
A previous study revealed that strain density and co-cultivation time affect transient expression efficiency. Thus, we conducted optimization assays using G5 flower bud stage petals. Petals infiltrated at OD600 values of 1.0 and 0.6 were not phenotypically different; both demonstrated high transient expression efficiency with substantial anthocyanin accumulation and conferred a distinct red color (Figure 2a). In contrast, OD600 = 0.2 failed to induce visible anthocyanin accumulation. In addition, petals subjected to 1-day and 3-day co-cultivation exhibited superior transient expression efficiency compared to those after 5 days, with marked anthocyanin accumulation resulting in a red phenotype (Figure 2c). These results indicate that establishing an efficient Agrobacterium-mediated transient transformation system in camellia petals enabled rapid validation of gene function.
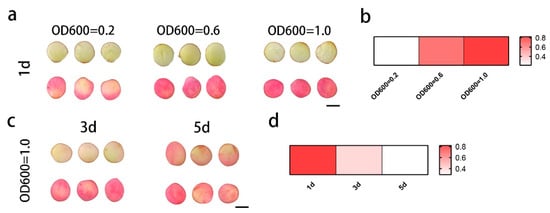
Figure 2.
Effects of different strain densities and co-cultivation times on the transient assay. (a) Phenotypic changes in petals with varying Agrobacterium concentrations, with co-cultivation time set to 1 day. (c) Phenotypic changes in petals with various co-cultivation times. The OD600 value was 1.0. (b,d) Transformation efficiency of the petals was evaluated; red indicates high efficiency.
3.3. CjMYB5 Encodes an R2R3-MYB Transcription Factor
To validate the utility of the transient expression system developed in this study, we selected the candidate gene CjMYB5, which was identified through previously published transcriptome analyses [13] for further molecular investigation. The R2R3-CjMYB5 CDS sequence was 882 bp in length and encoded a protein of 294 aa. Motif analysis revealed that CjMYB5 contained the conserved R2R3-MYB motif and the bHLH-interaction domain but lacked the anthocyanin characteristic motifs (ANDV and KPRPRS/TF) (Figure 3a). Phylogenetic analysis demonstrated CjMYB5 as the orthologue of AtMYB5 within subgroup 5 of the MYB family. This is the group containing established regulators of proanthocyanidin biosynthesis (Figure S1).

Figure 3.
CjMYB5 is an R2R3-MYB transcription factor expressed in floral organs. (a) Comparison of the R2R3 domains. (b) Subcellular localization of CjMYB5; the nuclear marker was 35S: mCherry. Scale bars represent 20 μm. (c) The four stages of flower bud development, S1: early bud stage, petals unpigmented, S2: initial anthocyanin deposition stage, pigments patchily deposited in petal tissues, S3: calyx swollen but petals not unfurled, significant anthocyanin accumulation observed, S4: petals fully expanded. (d) The four flower tissues. SE: sepal, Pe: petal, St: stamen, CA: carpel. (e,f) CjMYB5 expression profiles during flower developmental stages and different tissue; significant differences (p < 0.05) are indicated by different letters; error bars indicate ± SEs.
Subcellular localization analysis showed that CjMYB5 was located in the nucleus (Figure 3b). qRT-PCR analysis demonstrated that the relative expression of CjMYB5 in petals was significantly higher than that in other flower tissues (Figure 3d,f), and anthocyanin accumulation was upregulated in parallel during flower development (Figure 3c,e). These results suggest that CjMYB5 may be involved in anthocyanin accumulation.
3.4. Transient Transformation of CjMYB5 Promotes Proanthocyanin and Anthocyanin Accumulation in Camellia Petals
As expected, overexpressing CjMYB5 in camellia petals resulted in significant pigment accumulation, leading to a distinct color transition from white to red (Figure 4a). The high-performance liquid chromatography analysis demonstrated a substantial accumulation of cyanidin-based anthocyanins, increasing to 128.21 μg/g fresh weight (FW) (Figure 4c), and proanthocyanin content increased significantly from 11.76 to 20.03 mg/g FW (Figure 4b). The qRT-PCR analysis confirmed transcriptional activation of the majority of structural genes after overexpressing CjMYB5 (Figure 4e), with upregulated expression patterns mirroring the progressive accumulation of anthocyanins. Furthermore, CjMYB5 was overexpressed in tobacco. As shown in Figure 4d, transgenic tobacco flowers changed to a purple color. Collectively, these results indicate that CjMYB5 functions as a positive regulator coordinating anthocyanin and proanthocyanidin biosynthesis in camellia.
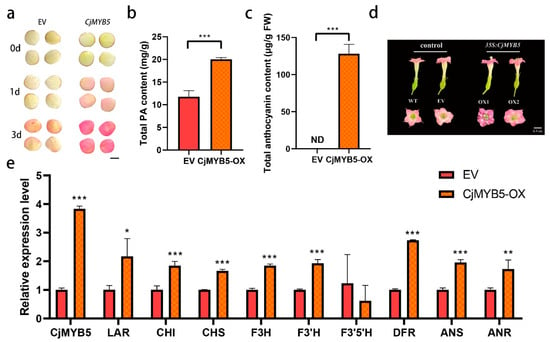
Figure 4.
Overexpressing CjMYB5 in camellia petals and tobacco. (a) Camellia petals transformed with the empty vector (EV) or a vector containing CjMYB5. Images were digitally extracted for comparison. (b,c) Proanthocyanin and anthocyanin contents in camellia petals transforming EV or CjMYB5. (d) Phenotypic changes in transgenic tobacco flowers overexpressing CjMYB5. (e) Relative expression level of CjMYB5 and anthocyanin biosynthesis structural genes in camellia petals. LAR: leucoanthocyanidin reductase, CHI: chalcone isomerase, CHS: chalcone synthase, F3H: flavanone 3-hydroxylase, F3′H: flavonoid 3′-hydroxylase, F3′5′H: flavonoid 3′,5′-hydroxylase, DFR: dihydroflavonol 4-reductase, ANS: anthocyanidin synthase, ANR: anthocyanidin reductase. * indicate p < 0.05, ** indicate p < 0.01, *** indicate p < 0.001.
3.5. CjMYB5 Interacts with CjGL3 to Activate DFR Expression
Previous studies have demonstrated that AtGL3, encoding a bHLH transcription factor, physically interacts with MYB proteins to regulate anthocyanin biosynthesis [30]. CjGL3 was identified as the AtGL3 camellia orthologue. The BIFC system demonstrated an interaction between CjMYB5 and CjGL3, as evidenced by a reconstituted YFP signal. In contrast, co-expression of CjMYB5 with negative control nYFP fragments failed to produce detectable fluorescence (Figure 5a,c). We tested whether CjMYB5 directly activated structural genes in the anthocyanin biosynthetic pathway. A dual-luciferase reporter system demonstrated that CjMYB5 transactivated the DFR promoter, while co-transfection with CjGL3 synergistically enhanced promoter activity compared to CjMYB5 alone (Figure 5b,d,e).

Figure 5.
Analyses of the interactions between CjMYB5 and CjGL3, promoters of the structural gene (DFR). (a) Schematic diagram of the bimolecular fluorescence complementation (BiFC) vectors. (b) Schematic diagram of the modified dual-luciferase transient expression vectors. (c) BiFC assays demonstrating an interaction between CjMYB5 and CjGL3 in N. benthamiana leaves. (d,e) LUC assays verified that CjMYB5 and CjGL3 activated the DFR gene. Each assay measured at least five samples, and significant differences (p < 0.05) are indicated by different letters; error bars indicate ± SEs.
4. Discussion
Genetic transformation in woody ornamental plants remains technically challenging due to low transformation efficiency, and a prolonged juvenile phase impedes rapid functional gene validation, thereby constraining molecular breeding applications. In contrast, transient expression systems enable direct phenotypic assessments in floral organs and fruits through Agrobacterium-mediated infiltration, bypassing the protracted juvenile period. The transient transformation platform has been successfully implemented in fruit tree species, such as M. domestica [6] and T. grandis [1], but its application in petal tissues of flowering trees remains largely unexplored. In this study, we established a highly efficient transient expression system in camellia petals and functionally characterized CjMYB5 as a master regulator of anthocyanin biosynthesis using this platform.
The genotype-specific susceptibility to Agrobacterium-mediated transformation highlights the essential role of genetic background in optimizing transient expression systems. S. oleracea ‘Sp75’ exhibited higher susceptibility to transient transformation compared to ‘Sp73’, which is a phenotypic disparity probably associated with enlarged intercellular spaces observed in leaves [3]. The structural integrity of floral tissues affects transfection success in P. mume [7]. In this study, five genetically distinct C. japonica cultivars were subjected to Agrobacterium-mediated infiltration for comparative analysis of petal phenotype and the GFP expression signal. C. japonica ‘Banliuxiang’ exhibited superior transformation performance compared to the other genotypes. This enhanced transient expression likely originated from their compact petal morphology and cellular viability.
Transient expression efficiency is affected by developmental status. In P. mume, GUS transient expression efficiency peaks at the flower bud stage, demonstrating an inverse correlation between developmental maturity and transformation competence [7]. Consistent with previous reports, we observed peak GFP signal intensity concomitant with significant anthocyanin accumulation in camellia petals at the floral bud stage. In contrast, minimal GFP expression was detected in petals at the bloom stage with negligible anthocyanin contents, corresponding to their white phenotype. We postulate that DNA methylation-mediated epigenetic silencing of anthocyanin structural genes may have inhibited anthocyanin accumulation, so further functional validation is needed. In addition, anthocyanin accumulated preferentially at the wound periphery of petal discs compared to central regions, indicating that wounding may facilitate Agrobacterium-mediated transformation efficiency. In Arabidopsis transient expression assays, transformation efficiency is affected by strain-specific concentration [31]. Our systematic optimization revealed that OD600 values also exhibited a significant effect on transient efficiency in camellia petals. Notably, the floral developmental stage emerged as the predominant determinant. Beyond the parameters evaluated in this study, future optimization efforts should prioritize C. japonica ‘Banliuxiang’ as a model system to assess critical variables, including the Agrobacterium strain and the transformation method [1]. The high-efficiency transient expression platform established in this study enables spatiotemporal-resolved functional gene validation in flowering trees, with particular efficacy in dissecting anthocyanin biosynthesis regulatory networks.
CjMYB5 encodes an R2R3-MYB transcription factor that has been functionally characterized and is involved in proanthocyanidin biosynthesis [4], anthocyanin accumulation [32], and the regulation of secondary cell wall deposition through hierarchical transcriptional networks [22]. Utilizing our established high-efficiency transient expression platform, we demonstrated that CjMYB5 overexpression drives anthocyanin and proanthocyanin accumulation in camellia petals. This result differs from previous reports documenting exclusive proanthocyanidin regulation, yet aligns with functional evidence where MYB5 homologs enhance anthocyanin biosynthesis in S. melongena [33] and V. vinifera fruits [34]. Notably, MYB5 shows lineage-specific functional divergence during core eudicot evolution. Furthermore, a previous report in angiosperms established that MYB transcription factors orchestrate anthocyanin biosynthesis through conserved protein–protein interactions with bHLH co-regulators [4,23]. Integrating in vivo assays, we first demonstrated that CjMYB5 interacts with CjGL3 to transactivate the DFR promoter.
5. Conclusions
In this study, we developed a robust transient expression system for camellia petals and systematically evaluated various factors affecting transformation efficiency. The system demonstrated that CjMYB5 significantly enhances anthocyanin accumulation in camellia petals by activating transcription of the DFR gene. A practical framework was established for developing transient expression systems in floral organs of ornamental woody plants.
Supplementary Materials
The following Supporting Information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11070839/s1, Table S1: Quantitative Real-time PCR primer; Figure S1: The phylogenetic tree of CjMYB5 and AtMYBs.
Author Contributions
Conceptualization, M.F. and H.J.; methodology, M.F.; software, H.J.; validation, Z.S., S.W. and Y.Z.; resources, X.L.; writing—original draft preparation, M.F.; writing—review and editing, M.F.; supervision, Y.W.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the cooperation project of Zhejiang province and Chinese academy of forestry for forestry science and technology (2025SY04).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge Guoren He and Feng Ming (College of Life Sciences, Shanghai Normal University, China) for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Wang, S.Y.; Liu, X.; Wang, R.M.; Chen, W.J.; Suo, J.W.; Yan, J.W.; Wu, J.S. Agrobacterium-mediated transient expression in Torreya grandis cones: A simple and rapid tool for gene expression and functional gene assay. Sci. Hortic. 2024, 338, 113664. [Google Scholar] [CrossRef]
- Norkunas, K.; Harding, R.; Dale, J.; Dugdale, B. Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana. Plant Methods 2018, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiu, L.; Zhang, Y.; Wang, Y.; Fu, C.; Dai, S.; Sun, M. A high-efficiency transient expression system mediated by Agrobacterium tumefaciens in Spinacia oleracea leaves. Plant Methods 2024, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yue, M.; Liu, Y.; Zhang, N.; Lin, Y.; Zhang, Y.; Wang, Y.; Li, M.; Luo, Y.; Zhang, Y.; et al. A novel R2R3-MYB transcription factor FaMYB5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria × ananassa). Plant Biotechnol. J. 2023, 21, 1140–1158. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, Y.C.; Yu, S.; Geng, L.F.; Lin, S.; Ouyang, L.; Jiang, X.Q. RcbHLH59-RcPRs module enhances salinity stress tolerance by balancing Na+/K+ through callose deposition in rose (Rosa chinensis). Hortic. Res. 2023, 10, uhac291. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Zhang, X.W.; You, C.X.; Bi, S.Q.; Wang, X.F.; Hao, Y.J. MdWRKY40 promotes wounding-induced anthocyanin biosynthesis in association with MdMYB1 and undergoes MdBT2-mediated degradation. New Phytol. 2019, 224, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Fang, C.X.; Liu, Y.T.; Wang, Y.T.; Xue, L.L.; Shi, J.X.; Ren, T.F.; Zhang, J.W.; Bao, M.Z.; Zhang, J.; et al. Construction of Transient Transformation System of Petals in Prunus mume. Acta Hortic. Sin. 2025, 54, 4. [Google Scholar]
- Pan, J.J.; Zhang, D.M.; Meng, J.; Gao, S.N.; Zhu, K.J.; Liu, J.W.; Li, G.H. Optimization and Validation of PpPDS Gene Silencing Induced by Prunus Necrotic Ring Spot Virus in Prunus persica. Acta Hortic. Sin. 2023, 50, 1587–1600. [Google Scholar]
- Yasmin, A.; Debener, T. Transient gene expression in rose petals via Agrobacterium infiltration. Plant Cell Tissue Organ. Plant Cell Tissue Organ Cult. 2010, 102, 245–250. [Google Scholar] [CrossRef]
- Meng, N.; Liu, Y.L.; Dou, X.X.; Liu, H.L.; Li, F.Y. Establishment of a transient transformation system for petals of Phalaenopsis. Northwest J. Bot. 2018, 38, 1017–1023. (In Chinese) [Google Scholar]
- Fan, M.; Zhang, Y.; Yang, M.; Wu, S.; Yin, H.; Li, J.; Li, X. Transcriptomic and Chemical Analyses Reveal the Hub Regulators of Flower Color Variation from Camellia japonica Bud Sport. Horticulturae 2022, 8, 129. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, Z.; Li, S.; Wang, M.; Huang, M.; Ma, X.; Liu, W.; Wang, Y.; Yu, Y.; Li, Y.; et al. Genomics insights into flowering and floral pattern formation: Regional duplication and seasonal pattern of gene expression in Camellia. BMC Biol. 2024, 22, 50. [Google Scholar] [PubMed]
- Fan, M.; Yang, K.; Zhou, R.; Liu, Q.; Guo, X.; Sun, Y. Temporal transcriptome profiling reveals candidate genes involved in cold acclimation of Camellia japonica (Naidong). Plant Physiol. Biochem. 2021, 167, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, X.; Zhang, Y.; Wu, S.; Song, Z.; Yin, H.; Liu, W.; Fan, Z.; Li, J. Floral organ transcriptome in Camellia sasanqua provided insight into stamen petaloid. BMC Plant Biol. 2022, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, X.; Zhang, Y.; Yang, M.; Wu, S.; Yin, H.; Liu, W.; Fan, Z.; Li, J. Novel insight into anthocyanin metabolism and molecular characterization of its key regulators in Camellia sasanqua. Plant Mol. Biol. 2023, 111, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, R.; Jiang, S.; Wang, H.; Ming, F. The MYB transcription factor RcMYB1 plays a central role in rose anthocyanin biosynthesis. Hortic. Res. 2023, 10, uhad080. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, D.; Fu, J.; Zhang, Z.; Qin, Y.; Hu, G.; Zhao, J. Agrobacterium rhizogenes-mediated hairy root transformation as an efficient system for gene function analysis in Litchi chinensis. Plant Methods 2021, 17, 103. [Google Scholar] [CrossRef]
- Wang, X.C.; Wu, J.; Guan, M.L.; Zhao, C.H.; Geng, P.; Zhao, Q. Arabidopsis MYB4 plays dual roles in flavonoid biosynthesis. Plant J. 2020, 101, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, N.; Liu, J.; Qu, C.; Wang, Y.; Jiang, S.; Lu, N.; Wang, D.; Zhang, Z.; Chen, X. The molecular mechanism under lying anthocyanin metabolism in apple using the MdMYB16 and MdbHLH33 genes. Plant Mol. Biol. 2017, 94, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, K.; Zheng, G.; Hou, H.; Wang, P.; Jiang, H.; Zhao, X.; Li, M.; Zhang, S.; Liu, Y.; et al. CsMYB5a and CsMYB5e from Camellia sinensis differentially regulate anthocyanin and proanthocyanidin biosynthesis. Plant Sci. 2018, 270, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tang, X.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. Functional identification of MdMYB5 involved in secondary cell wall formation in apple. Fruit Res. 2021, 1, 6. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Liu, X.; Ma, H.P.; Liu, X.H.; Huang, Y.; Lu, X.; Cheng, Y.W. R2R3-MYB transcription factor CjMYB114 interacts with CjbHLH1 to jointly regulate anthocyanins in Camellia japonica. L ‘Fendan’. Sci. Hortic. 2024, 328, 112897. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutierrez, S.; Silla-Martınez, J.M.; Gabaldon, T. TRIMAL: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, S.; Xie, J.; Tan, P.; Han, L. Discontinuous low temperature stress and plant growth regulators during the germination period promote roots growth in alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2023, 197, 107624. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Kopperud, K.; Chakrabarty, R.; Banerjee, R.; Brooks, R.; Goodin, M.M. Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J. 2009, 59, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Baek, K.; Park, C.M. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009, 28, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zuo, Q.; Sadeghnezhad, E.; Zheng, T.; Chen, X.; Dong, T.; Fang, J. HDAC19 recruits ERF4 to the MYB5a promoter and diminishes anthocyanin accumulation during grape ripening. Plant J. 2023, 113, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, Y.; Li, D.; Shi, S.; Zhao, N.; Liao, J.; Liu, Y.; Chen, H. Eggplant transcription factor SmMYB5 integrates jasmonate and light signaling during anthocyanin biosynthesis. Plant Physiol. 2024, 194, 1139–1165. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).