Drought Stress Drives Sex-Specific Physiological and Biochemical Differences in Female and Male Litsea cubeba
Abstract
1. Introduction
2. Materials and Methods
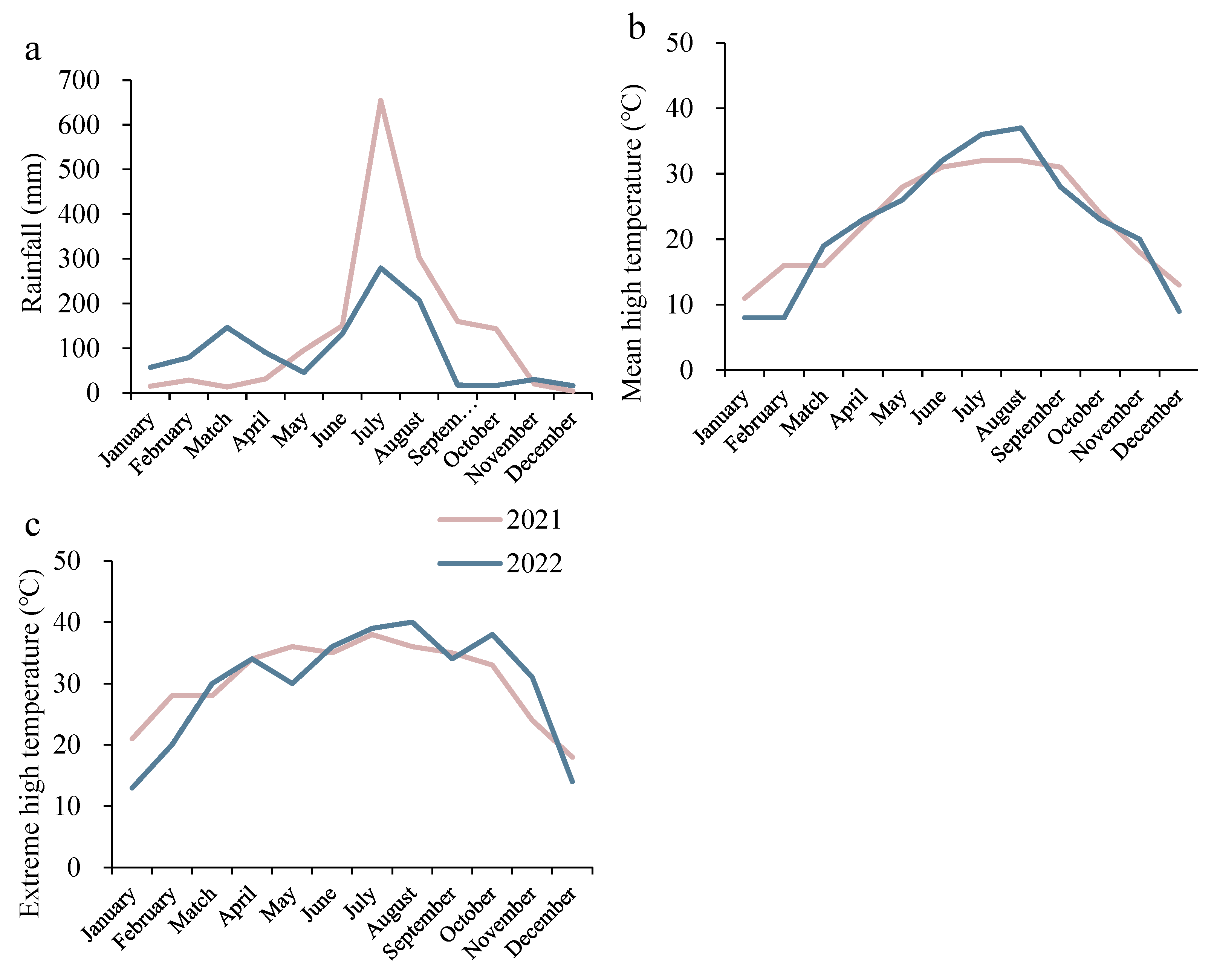
2.1. Description of Site
2.2. Plant Material
2.3. Determination of Indicators of Oxidative Metabolism
2.4. Determination of Proline and Soluble Sugar
2.5. Analysis of Photosynthetic Pigments
2.6. Phytohormone Content Quantification
2.7. Dehydrogenase Activity Measurement
2.8. Statistical Analyses
3. Results
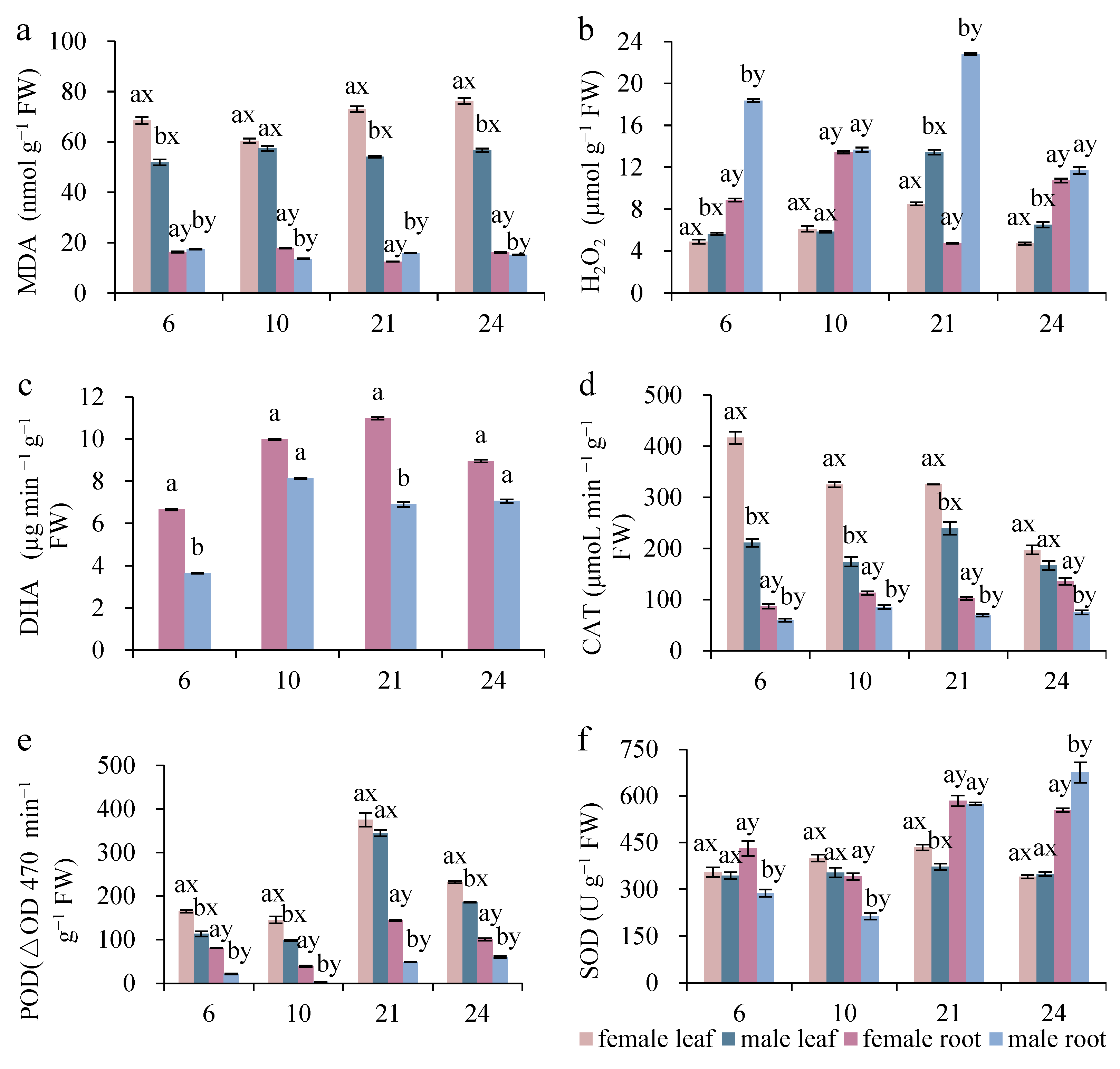
3.1. Sexual Differences in Oxidative Stress and Antioxidants
3.2. Sexual Differences in Photosynthetic Pigments
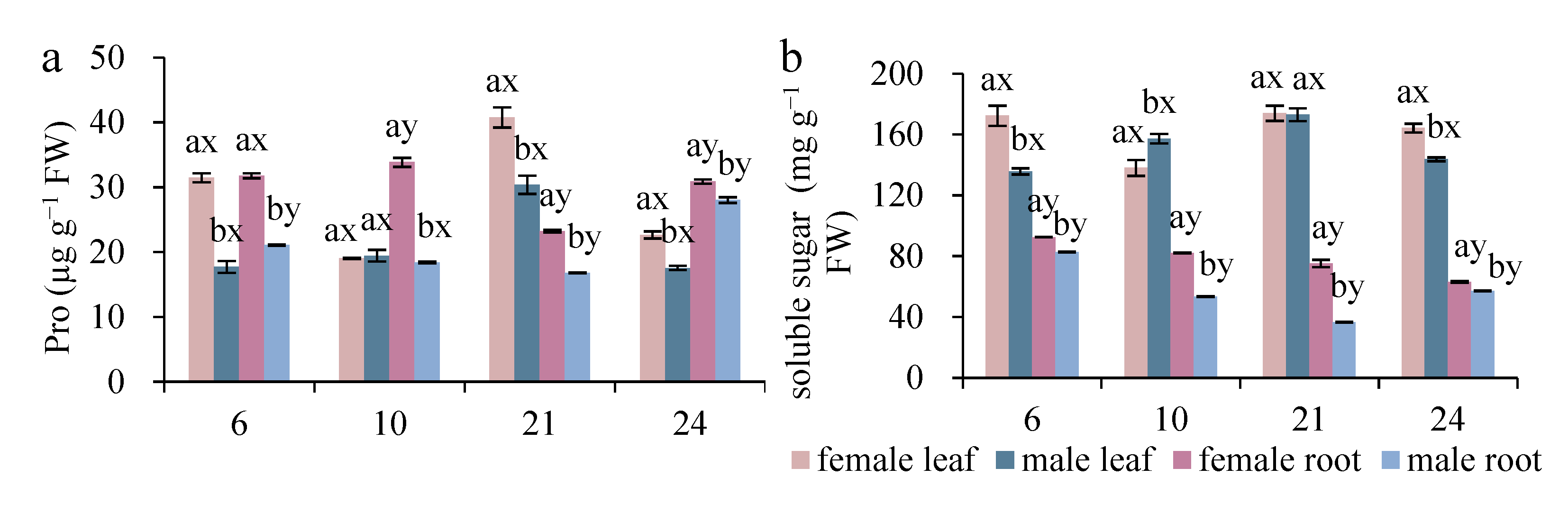
3.3. Sexual Differences in Proline and Soluble Sugar Contents
3.4. Sexual Differences in ABA and JA
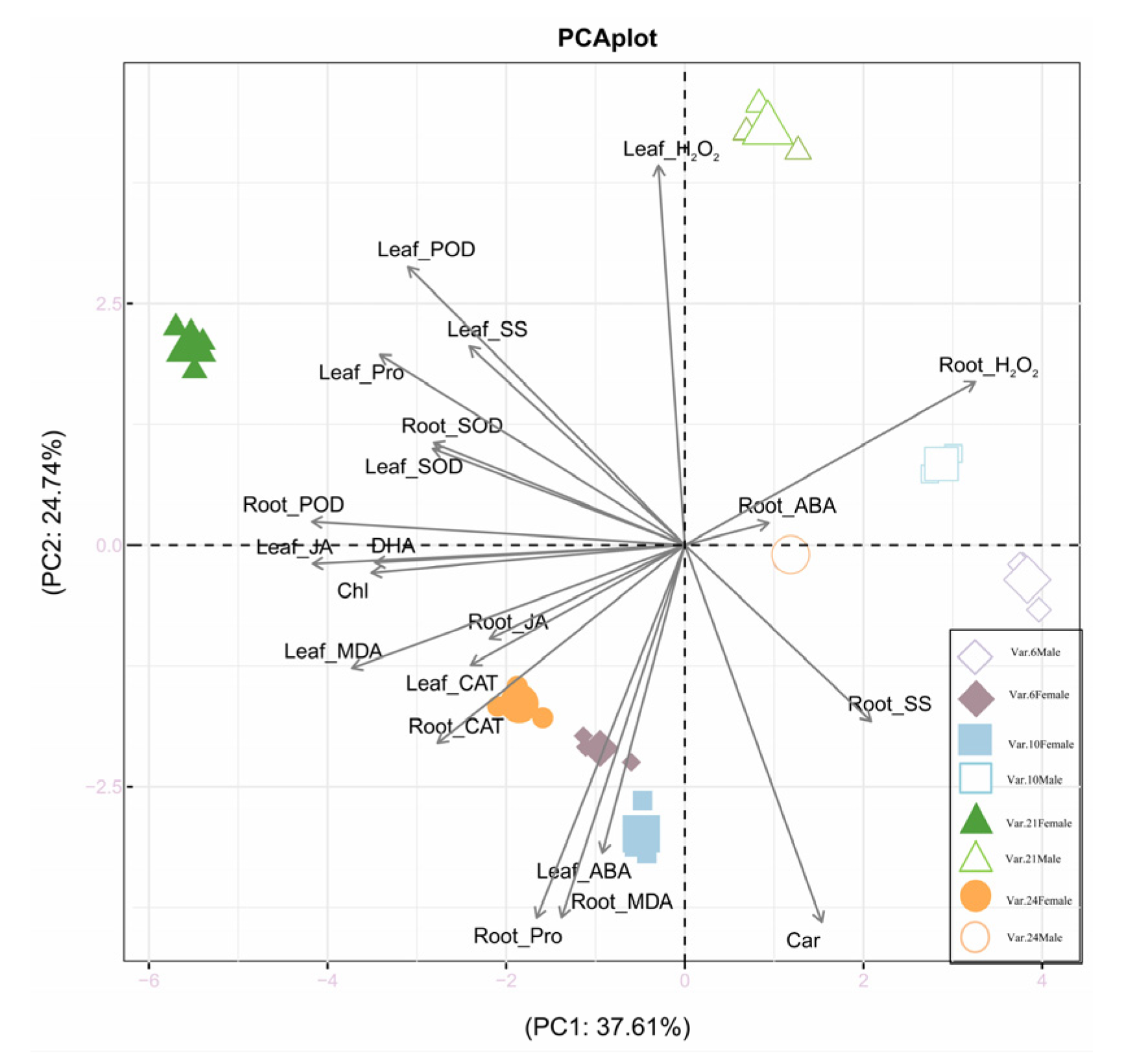
3.5. Relationships Among All Traits in Each Sex Under Drought Stress
4. Discussion
4.1. Drought Stress Significantly Affects Sexual Dimorphism in Physiological Responses of L. cubeba
4.2. Females Exhibited More Effective Protective Mechanisms than Males Under Drought Condition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Zhao, Y.; Wang, Y.; Korpelainen, H.; Li, C. Stem xylem traits and wood formation affect sex-specific responses to drought and rewatering in Populus cathayana. Tree Physiol. 2022, 42, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Change 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A. Forest resilience to drought varies across biomes. Glob. Change Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, B.; Wang, H.; Zhang, D. Record-breaking summer-autumn drought in southern China in 2022: Roles of tropical sea surface temperature and Eurasian warming. Sci. China Earth Sci. 2024, 67, 420–431. [Google Scholar] [CrossRef]
- Pan, S.; Yin, Z.; Duan, M.; Han, T.; Fan, Y.; Huang, Y.; Wang, H. Seasonal prediction of extreme high-temperature days over the Yangtze River basin. Sci. China Earth Sci. 2024, 67, 2137–2147. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, B.; Duan, M.; Chen, H.; Wang, H. Climate extremes become increasingly fierce in China. Innovation 2023, 4, 100406. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef][Green Version]
- He, F.; Wu, Z.; Zhao, Z.; Chen, G.; Wang, X.; Cui, X.; Zhu, T.; Chen, L.; Yang, P.; Bi, L.; et al. Drought stress drives sex-specific differences in plant resistance against herbivores between male and female poplars through changes in transcriptional and metabolic profiles. Sci. Total Environ. 2022, 845, 157171. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Liu, T.; Chen, H.; Tang, M. Sex-related responses of Populus cathayana shoots and roots to AM fungi and drought stress. PLoS ONE 2015, 10, e0128841. [Google Scholar]
- Lin, T.; Tang, J.; Li, S.; Li, S.; Han, S.; Liu, Y.; Yang, C.; Chen, G.; Chen, L.; Zhu, T. Drought stress-mediated differences in phyllosphere microbiome and associated pathogen resistance between male and female poplars. Plant J. 2023, 115, 1100–1113. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, Z.; Tang, S.; Korpelainen, H.; Li, C. Populus euphratica males exhibit stronger drought and salt stress resistance than females. Environ. Exp. Bot. 2023, 205, 105114. [Google Scholar] [CrossRef]
- Chaffiai, R. Plant Adaptation to Abiotic Stress: From Signaling Pathways and Microbiomes to Molecular Mechanisms; Springer Nature: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Shahid, M.; Gaur, R. Molecular Dynamics of Plant Stress and Its Management; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Wang, B.; Zhang, J.; Pei, D.; Yu, L. Combined effects of water stress and salinity on growth, physiological, and biochemical traits in two walnut genotypes. Physiol. Plant 2021, 172, 176–187. [Google Scholar] [CrossRef] [PubMed]
- de Ollas, C.; Arbona, V.; Gómez-Cadenas, A. Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ. 2015, 38, 2157–2170. [Google Scholar] [CrossRef]
- Sun, Z.; Feng, Z.; Ding, Y.; Qi, Y.; Jiang, S.; Li, Z.; Wang, Y.; Qi, J.; Song, C.; Yang, S.; et al. RAF22, ABI1 and OST1 form a dynamic interactive network that optimizes plant growth and responses to drought stress in Arabidopsis. Mol. Plant 2022, 15, 1192–1210. [Google Scholar] [CrossRef]
- Singh, A.P.; Mani, B.; Giri, J. OsJAZ9 is involved in water-deficit stress tolerance by regulating leaf width and stomatal density in rice. Plant Physiol. Biochem. 2021, 162, 161–170. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Exogenous application of acetic acid enhances drought tolerance by influencing the MAPK signaling pathway induced by ABA and JA in apple plants. Tree Physiol. 2022, 42, 1827–1840. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Jin, Y.; Wang, C.; Yang, J.; Qi, H. Drought-induced ABA, H₂O₂ and JA positively regulate CmCAD genes and lignin synthesis in melon stems. BMC Plant Biol. 2021, 21, 83. [Google Scholar] [CrossRef]
- Kong, L.; Song, Q.; Wei, H.; Wang, Y.; Lin, M.; Sun, K.; Zhang, Y.; Yang, J.; Li, C.; Luo, K. The AP2/ERF transcription factor PtoERF15 confers drought tolerance via JA-mediated signaling in Populus. New Phytol. 2023, 240, 1848–1867. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Chen, Y.; Zhao, Y.; Wang, Y. Sex-specific physiological and biochemical responses of Litsea cubeba under waterlogging stress. Environ. Exp. Bot. 2022, 202, 105018. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Xu, X.; Fowler, J.C.; Miller, T.E.X.; Dong, T. Effect of summer warming on growth, photosynthesis and water status in female and male Populus cathayana: Implications for sex-specific drought and heat tolerances. Tree Physiol. 2020, 40, 1178–1191. [Google Scholar] [CrossRef]
- Silva, J.L.S.; Cruz-Neto, O.; Rito, K.F.; Arnan, X.; Leal, I.R.; Peres, C.A.; Tabarelli, M.; Lopes, A.V. Divergent responses of plant reproductive strategies to chronic anthropogenic disturbance and aridity in the Caatinga dry forest. Sci. Total Environ. 2020, 704, 135240. [Google Scholar] [CrossRef]
- Xia, Z.; He, Y.; Yu, L.; Lv, R.; Korpelainen, H.; Li, C. Sex-specific strategies of phosphorus (P) acquisition in Populus cathayana as affected by soil P availability and distribution. New Phytol. 2020, 225, 782–792. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Liu, G.; Qin, F.; Yang, S.; Xu, X. Effects of enhanced UV-B radiation on morphology, physiology, biomass, leaf anatomy and ultrastructure in male and female mulberry (Morus alba) saplings. Environ. Exp. Bot. 2016, 129, 85–93. [Google Scholar] [CrossRef]
- Xia, Z.; He, Y.; Zhou, B.; Korpelainen, H.; Li, C. Sex-related responses in rhizosphere processes of dioecious Populus cathayana exposed to drought and low phosphorus stress. Environ. Exp. Bot. 2020, 175, 104049. [Google Scholar] [CrossRef]
- Randriamanana, T.R.; Nissinen, K.; Moilanen, J.; Nybakken, L.; Julkunen-Tiitto, R. Long-term UV-B and temperature enhancements suggest that females of Salix myrsinifolia plants are more tolerant to UV-B than males. Environ. Exp. Bot. 2015, 109, 296–305. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Lei, Y.; Xu, G.; Zhang, D. Alternative Growth and Defensive Strategies Reveal Potential and Gender Specific Trade-Offs in Dioecious Plants Salix paraplesia to Nutrient Availability. Front. Plant Sci. 2016, 7, 1064. [Google Scholar] [CrossRef]
- Fu, M.; Liao, J.; Liu, X.; Li, M.; Zhang, S. Artificial warming affects sugar signals and flavonoid accumulation to improve female willows’ growth faster than males. Tree Physiol. 2023, 43, 1584–1602. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.; Wang, J.; Jiang, L.; Fan, Y.; Li, G. Foliar water uptake and its influencing factors differ between female and male Populus euphratica. Environ. Exp. Bot. 2023, 213, 105419. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Gao, M.; Wang, Y. Alcohol dehydrogenases regulated by a MYB44 transcription factor underlie Lauraceae citral biosynthesis. Plant Physiol. 2024, 194, 1674–1691. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yin, H.; Chen, Y.; Gao, M.; Wu, L.; Wang, Y. Ectopic expression of Litsea cubeba LcMADS20 modifies silique architecture. G3 Genes Genomes Genet. 2019, 9, 4139–4147. [Google Scholar] [CrossRef]
- Gao, M.; Lin, L.; Chen, Y.; Wang, Y. Digital gene expression profiling to explore differentially expressed genes associated with terpenoid biosynthesis during fruit development in Litsea cubeba. Molecules 2016, 21, 1251. [Google Scholar] [CrossRef]
- Özcan, M.; Gökbulak, F.; Hizal, A. Exclosure effects on recovery of selected soil properties in a mixed broadleaf forest recreation site. Land Degrad. Dev. 2013, 24, 266–276. [Google Scholar] [CrossRef]
- Metzner, H.; Rau, H.; Senger, H. Studies on synchronization of some pigment-deficient Chlorella mutants. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Liu, P.; Bao, X.; Hou, X.; Yang, M.; Zhen, W. Rehydration compensation of winter wheat is mediated by hormone metabolism and de-peroxidative activities under field conditions. Front. Plant Sci. 2022, 13, 823846. [Google Scholar] [CrossRef]
- Yang, R.; Yang, T.; Zhang, H.; Qi, Y.; Xing, Y.; Zhang, N.; Li, R.; Weeda, S.; Ren, S.; Ouyang, B. Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol. Biochem. 2014, 77, 23–34. [Google Scholar] [CrossRef]
- Yao, Y.; Xia, L.; Yang, L.; Liu, R.; Zhang, S. Drought responses and carbon allocation strategies of poplar with different leaf maturity. Physiol. Plant. 2024, 176, e14224. [Google Scholar] [CrossRef]
- Puixeu, G.; Pickup, M.; Field, D.L.; Barrett, S.C.H. Variation in sexual dimorphism in a wind-pollinated plant: The influence of geographical context and life-cycle dynamics. New Phytol. 2019, 224, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H.; Hough, J. Sexual dimorphism in flowering plants. J. Exp. Bot. 2012, 64, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Juvany, M.; Munné-Bosch, S. Sex-related differences in stress tolerance in dioecious plants: A critical appraisal in a physiological context. J. Exp. Bot. 2015, 66, 6083–6092. [Google Scholar] [CrossRef]
- Olano, J.M.; González-Muñoz, N.; Arzac, A.; Rozas, V.; von Arx, G.; Delzon, S.; García-Cervigón, A.I. Sex determines xylem anatomy in a dioecious conifer: Hydraulic consequences in a drier world. Tree Physiol. 2017, 37, 1493–1502. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Zhao, Y.; Korpelainen, H.; Li, C. Sex-specific nitrogen allocation tradeoffs in the leaves of Populus cathayana cuttings under salt and drought stress. Plant Physiol. Biochem. 2022, 172, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Thammavong, H.T.; Berry, L.G.; Huang, C.H.; Park, D.S. Sex-dependent phenological responses to climate vary across species’ ranges. Proc. Natl. Acad. Sci. USA 2023, 120, e2306723120. [Google Scholar] [CrossRef]
- Tang, S.; Lin, X.; Li, W.; Guo, C.; Han, J.; Yu, L. Nutrient resorption responses of female and male Populus cathayana to drought and shade stress. Physiol. Plant. 2023, 175, e13980. [Google Scholar] [CrossRef]
- Chen, J.; Duan, B.; Li, W.; Mao, L.; Korpelainen, H.; Li, C. Intra- and inter-sexual competition of Populus cathayana under different watering regimes. Funct. Ecol. 2014, 28, 124–136. [Google Scholar] [CrossRef]
- Liu, E.; Mei, X.; Gong, D.; Yan, C.; Zhuang, Y. Effects of drought on N absorption and utilization in winter wheat at different developmental stages. Chin. J. Plant Ecol. 2010, 34, 555–562. [Google Scholar]
- Wang, C.; Tu, Q.; Ye, Z.; Shi, Y.; Xiao, M.; Fang, Y.; Lu, Y.; You, R. Active constituents, encapsulation technology, bioactivities and applications in food industry by essential oils of Litsea cubeba (Lour) Pers: A review. Trends Food Sci. Technol. 2024, 153, 104728. [Google Scholar] [CrossRef]
- Ma, S.; Liu, G.; Wang, L.; Liu, G.; Xu, X. Salix gordejevii females exhibit more resistance against wind erosion than males under aeolian environment. Front. Plant Sci. 2022, 13, 1053741. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Jiang, Y.; Chen, K.; Duan, B.; Zhang, S.; Korpelainen, H.; Niinemets, Ü.; Li, C. Reproductive investments driven by sex and altitude in sympatric Populus and Salix trees. Tree Physiol. 2017, 37, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bi, J.; Liu, X.; Kang, J.; Korpelainen, H.; Niinemets, Ü.; Li, C. Microstructural and physiological responses to cadmium stress under different nitrogen levels in Populus cathayana females and males. Tree Physiol. 2020, 40, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Hussain, I.; Shehzad, M.A.; Akhtar, G.; Ahmad, K.S.; Mubeen, K.; Hassan, W.; Faried, H.N.; Ahmad, S.; Aziz, M.; Yasin, S.; et al. Supplemental sodium nitroprusside and spermidine regulate water balance and chlorophyll pigments to improve sunflower yield under terminal drought. ACS Omega 2024, 9, 30478–30491. [Google Scholar] [CrossRef]
- Arndt, S.K.; Livesley, S.J.; Merchant, A.; Bleby, T.M.; Grierson, P.F. Quercitol and osmotic adaptation of field-grown Eucalyptus under seasonal drought stress. Plant Cell Environ. 2010, 31, 915–924. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, C.; Yang, N.; Cao, W.; Li, Y.; Peng, Y.; Wei, X.; Ma, B.; Ma, F.; Ruan, Y.-L.; et al. Apple vacuolar sugar transporters regulated by MdDREB2A enhance drought resistance by promoting accumulation of soluble sugars and activating ABA signaling. Hortic. Res. 2024, 11, uhae251. [Google Scholar] [CrossRef]
- Li, W.; de Ollas, C.; Dodd, I.C. Long-distance ABA transport can mediate distal tissue responses by affecting local ABA concentrations. J Integr Plant Biol. 2018, 60, 16–33. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]



| Litsea cubeba Selections Used in This Study | Origin |
|---|---|
| 6 | Bijie county, Guizhou Province, China |
| 10 | Lingyun county, Guangxi Province, China |
| 21 | Yuexi county, Anhui Province, China |
| 24 | Jianou county, Fujian Province, China |
| Plant Sex | Soil Layer Depth (cm) | SWC (%) | |
|---|---|---|---|
| Under Drought Stress (16 August 2022) | Non-Stressed Levels (20 August 2021) | ||
| Female | >0~10 | 13.4 ± 0.2 | 20.4 ± 0.3 |
| >10~30 | 15.2 ± 0.2 | 22.2 ± 0.4 | |
| >30~60 | 17.3 ± 0.3 | 24.5 ± 0.2 | |
| Male | >0~10 | 13.2 ± 0.1 | 21.1 ± 0.2 |
| >10~30 | 15.6 ± 0.3 | 22.6 ± 0.1 | |
| >30~60 | 17.4 ± 0.4 | 24.8 ± 0.3 | |
| Mean | 15.35 ± 0.2 | 22.6 ± 0.2 | |
| Variable | Sex (S) | Variety (V) | Organ (O) | S × V × O | S × V | V × O | S × O |
|---|---|---|---|---|---|---|---|
| MDA | 518.680 *** | 17.012 *** | 15711.770 *** | 36.475 *** | 19.945 *** | 28.511 *** | 264.042 *** |
| H2O2 | 2391.170 *** | 325.167 *** | 4240.718 *** | 333.294 *** | 736.063 *** | 175.849 *** | 789.521 *** |
| SOD | 77.755 *** | 136.095 *** | 152.126 *** | 8.986 *** | 2.422 *** | 130.586 *** | 24.882 *** |
| CAT | 513.311 *** | 39.791 *** | 2344.433 *** | 43.532 *** | 21.162 | 104.328 *** | 139.786 *** |
| POD | 377.157 *** | 690.778 *** | 3043.903 *** | 11.217 *** | 4.204 * | 233.945 *** | 7.389 * |
| JA | 6606.920 *** | 712.558 *** | 1114.566 *** | 1030.721 *** | 371.936 *** | 542.481 *** | 346.581 *** |
| ABA | 1244.458 *** | 910.037 *** | 10909.375 *** | 214.865 *** | 360.426 *** | 941.893 *** | 1304.941 *** |
| SS | 6.727 *** | 18.884 *** | 3573.972 *** | 43.561 *** | 35.228 *** | 53.506 *** | 12.840 ** |
| Pro | 518.735 *** | 35.815 *** | 3.142 *** | 45.615 *** | 23.108 *** | 254.150 *** | 5.417 * |
| Variable | Sex (S) | Variety (V) | S × V |
|---|---|---|---|
| DHA | 3784.008 *** | 1712.571 *** | 144.415 *** |
| Total chlorophyll | 4548.496 *** | 1041.385 *** | 528.176 *** |
| Carotenoids | 5509.921 *** | 1370.861 *** | 523.607 *** |
| Variables | Total Chl (mg g−1 FW) | Carotenoid (µg g−1 FW) | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| 6 | 2.97 ± 0.02 a | 2.54 ± 0.02 b | 342.68 ± 2.68 a | 202.40 ± 1.25 b |
| 10 | 2.90 ± 0.01 a | 1.81 ± 0.01 b | 376.71 ± 1.19 a | 214.67 ± 1.46 b |
| 21 | 3.46 ± 0.02 a | 2.56 ± 0.02 b | 417.94 ± 3.11 a | 348.39 ± 1.47 b |
| 24 | 2.49 ± 0.01 a | 2.35 ± 0.01 b | 301.37 ± 0.45 a | 272.45 ± 2.20 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Zhao, Y.; Chen, Y.; Wang, Y. Drought Stress Drives Sex-Specific Physiological and Biochemical Differences in Female and Male Litsea cubeba. Horticulturae 2025, 11, 594. https://doi.org/10.3390/horticulturae11060594
Gao M, Zhao Y, Chen Y, Wang Y. Drought Stress Drives Sex-Specific Physiological and Biochemical Differences in Female and Male Litsea cubeba. Horticulturae. 2025; 11(6):594. https://doi.org/10.3390/horticulturae11060594
Chicago/Turabian StyleGao, Ming, Yunxiao Zhao, Yicun Chen, and Yangdong Wang. 2025. "Drought Stress Drives Sex-Specific Physiological and Biochemical Differences in Female and Male Litsea cubeba" Horticulturae 11, no. 6: 594. https://doi.org/10.3390/horticulturae11060594
APA StyleGao, M., Zhao, Y., Chen, Y., & Wang, Y. (2025). Drought Stress Drives Sex-Specific Physiological and Biochemical Differences in Female and Male Litsea cubeba. Horticulturae, 11(6), 594. https://doi.org/10.3390/horticulturae11060594




