1. Introduction
Tomato (
Solanum lycopersicum L.) is one of the most economically valuable horticultural crops in the world. According to the Food and Agriculture Organization, it is the second biggest crop in terms of cultivated area, accounting for 14% of total vegetable production (excluding potatoes) in 2023 [
1]. Since the advent of modern plant breeding, programs have prioritized the development of varieties with high yields and disease resistance, as well as a longer postharvest life. This has resulted in a genetic bottleneck and a significant loss of crop and variety diversity [
2]. The recovery of traditional varieties is a promising strategy for increasing agricultural biodiversity, particularly in the Mediterranean basin, a secondary center of tomato diversification [
3]. In addition to reducing genetic erosion, many of these varieties are notable for their flavor and fruit quality, attributes that are gaining increasing importance among consumers [
4,
5]. The flavor-related quality is determined by primary metabolites, such as sugars and organic acids, while nutritional quality is associated with secondary metabolites, such as carotenoids, phenolic compounds, and vitamins [
6,
7]. The flavor profile is influenced by the interaction and balance between organic acids, such as malic, citric, and glutamic acids, and soluble sugars, including fructose and glucose [
8]. Secondary metabolites are bioactive compounds that have been shown to promote health. Lycopene, for instance, has been associated with a reduced risk of cardiovascular disease, macular degeneration, and cancer [
9]. Despite their outstanding quality, traditional varieties are limited by their susceptibility to viral diseases, which are among the leading causes of economic losses in tomato cultivation [
10]. The tomato mosaic virus (ToMV), the tomato spotted wilt virus (TSWV), and the tomato yellow curl virus (TYLCV) are three viral agents that cause significant economic losses in crops. Integrated pest management can reduce populations of thrips and whiteflies, the main vectors of TSWV and TYLCV, to levels that limit direct damage, but it is often insufficient to prevent virus transmission. In the case of ToMV, mechanical spread further complicates disease control in greenhouse systems [
10]. The development of breeding programs to introduce resistance genes is considered to be an effective and sustainable strategy, particularly in the Mediterranean basin, where intensive agriculture contributes to high virosis pressure that compromises tomato production [
11]. The dominant genes that confer resistance to ToMV and TSWV, as well as tolerance to TYLCV, are
Tm-2a,
Sw-5, and
Ty-1, respectively. These genes were isolated and characterized in previous studies. These genes have been identified in wild tomatoes, specifically S.
peruvianum (
Tm-2a and
Sw-5) and S.
chilense (Dunal) accession LA1969 (
Ty-1) [
12,
13,
14]. Due to the high incidence of mixed infections, breeding programs have focused on introducing multiple genes using technologies such as marker-assisted selection (MAS), which accelerates the selection process [
11].
The Muchamiel tomato variety, a traditional cultivar with significant value in local markets in southeastern Spain, is limited by its susceptibility to virosis [
15]. Like other traditional varieties, its cultivation has declined despite being highly appreciated for its flavor, due to its replacement by commercially available varieties that are resistant to viruses, particularly virosis [
10]. In order to address this issue, in 1998, the CIAGRO-UMH plant breeding group initiated a program intending to enhance traditional varieties by introducing virus-resistance genes into local varieties from southeastern Spain. These varieties include the Muchamiel variety, as well as others such as the De la Pera and Moruno varieties. Breeding lines incorporating the
Tm-2a,
Sw-5, and
Ty-1 resistance genes in homozygosis have been developed and officially registered within the Spanish Plant Variety Office since 2011 [
11]. However, the introgression of resistance genes from wild species has been shown to have a negative impact on fruit quality and yield. This can be attributed to either the genes themselves or to linkage drag. A decline in yield and fruit quality has been observed in the processing of tomatoes resistant to ToMV [
16], as well as in tobacco plants that possess the
N gene from the
Nicotiana glutinosa L. species, which confers resistance to TMV [
17]. As described by Verlaan et al., the recombination of the
Ty-1 gene was restricted in the S.
chilense region [
18]. This was attributed to the presence of two chromosomal inversions in S.
chilense LA1969 and
S. lycopersicum. According to Alonso et al., the introgression of
Ty-1 had a negative effect on yield and quality traits in De la Pera breeding lines [
19]. In the Muchamiel and De la Pera breeding lines, yield was reduced by up to 50% due to the presence of
Ty-1 and in the absence of TYLCV [
15,
20]. Rubio et al., also reported that
Ty-1 homozygosity significantly compromised most of the measured parameters in near-isogenic lines (NILs), with homozygosity for one, two or all of the three introgressed genes (
Tm-2a,
Sw-5, and
Ty-1), resulting in a 40–50% yield decrease [
21].
Although the Muchamiel-type breeding lines developed by the CIAGRO-UMH carry resistance genes targeting the major viruses affecting tomatoes, improving quality and productivity remain a challenge. Therefore, alternative strategies must be explored to preserve their exceptional fruit quality. One promising approach is to use resistance genes in the heterozygous state, as this maintains resistance through dominant inheritance while reducing yield and quality losses [
16]. A hybrid resulting from a cross between a Muchamiel breeding line and a US heirloom variety was evaluated in a previous study [
22]. While a reduction in the negative impact of gene introgression on yield and quality was observed, this hybrid could not be categorized as Muchamiel-type, since one of its parents belonged to a different cultivar. In this context, the CIAGRO-UMH and the IMIDA have developed new Muchamiel tomato hybrids using varietal-type Muchamiel parents carrying resistance genes in the homozygous state for ToMV, TSWV, and TYLCV [
15,
23], as well as traditional varieties that were previously selected for their high quality yet remain sensitive to these viruses [
24,
25,
26]. The present study aims to evaluate the impact of introducing the
Tm-2a,
Sw-5, and
Ty-1 genes into Muchamiel-type hybrids on their agronomic performance, fruit morphology, and metabolic composition. This approach seeks to increase the available biodiversity for tomato cultivars and to provide viable alternatives without compromising the distinctive traits that are particularly valued by consumers.
4. Discussion
The recovery of traditional varieties is limited by their susceptibility to viruses, such as ToMV, TYLCV, and TSWV, which leads to a substantial reduction in profitability for farmers [
10]. To address this issue and maintain genetic diversity, in 1998, the CIAGRO-UMH plant breeding group initiated a program to introduce three dominant genes (
Tm-2a,
Sw-5, and
Ty-1), previously described as conferring resistance to these viruses into traditional varieties from southeastern Spain, including the Muchamiel type [
11]. However, the breeding lines with the
Ty-1 gene in a homozygous state obtained through this program exhibited a reduced yield and, in certain instances, an inferior fruit quality compared to the original variety. These losses are attributed to the introgression of linked genes that cannot be eliminated during the backcrossing process [
18]. One strategy to minimize these adverse effects is to use resistance genes in a heterozygous state [
16]. This study sought to evaluate the impact of introducing the
Tm-2a,
Sw-5, and
Ty-1 genes on the agronomic performance, fruit morphology, and metabolic composition of Muchamiel-type hybrids. This approach aimed at enhancing the biodiversity available for tomato cultivars and providing viable alternatives without compromising the distinctive traits that are particularly valued by consumers.
Although the introduction of the
Ty-1 gene has been reported to negatively affect yield [
18,
19], no comparable effects were detected in the present study, either in the breeding lines or the hybrids. These results are consistent with those obtained by [
22], who also found no yield differences between the UMH1200 and UMH1139 breeding lines used in the present study. However, it is essential to note that these breeding lines do not originate from identical Muchamiel cultivars, which could result in minor genetic variations masking the negative impact of
Ty-1 on yield. Indeed, a study previously carried out by the same research group observed a 48% reduction in yield in Muchamiel NILs that were homozygous for
Ty-1 compared to susceptible lines [
21]. In contrast, the hybrids that were developed in this study had a higher yield than their parents, even though this was only significant for UMH1200×MC1 and UMH1200×MC2. This increase could be attributed to the vigor of the hybrid, albeit moderated by the reduced genetic distance between the parental lines, which are both of the Muchamiel type [
33]. The yield of the evaluated hybrids (3 to 4.4 kg per plant) is within the range reported for traditional Muchamiel varieties, with values ranging from 2 to 2.8 kg [
21] and up to 4.5 kg per plant [
23]. Resistance genes were found to influence fruit morphology, as determined by longitudinal and equatorial diameters and parameters directly correlated with fruit weight. The breeding lines exhibited the smallest diameters, consistent with prior research indicating a potential adverse effect of these genes on fruit size [
19,
21,
22]. However, the hybrids that were heterozygous for the resistance genes recovered the longitudinal and equatorial diameter values of their traditional parents. This confirms that the introduction of virus-resistance genes in a heterozygous state can help to avoid the negative effects observed in homozygous lines [
22].
Consumer preferences shape the commercial value of tomatoes, as fruits with a balance between acidity and sweetness are often preferred [
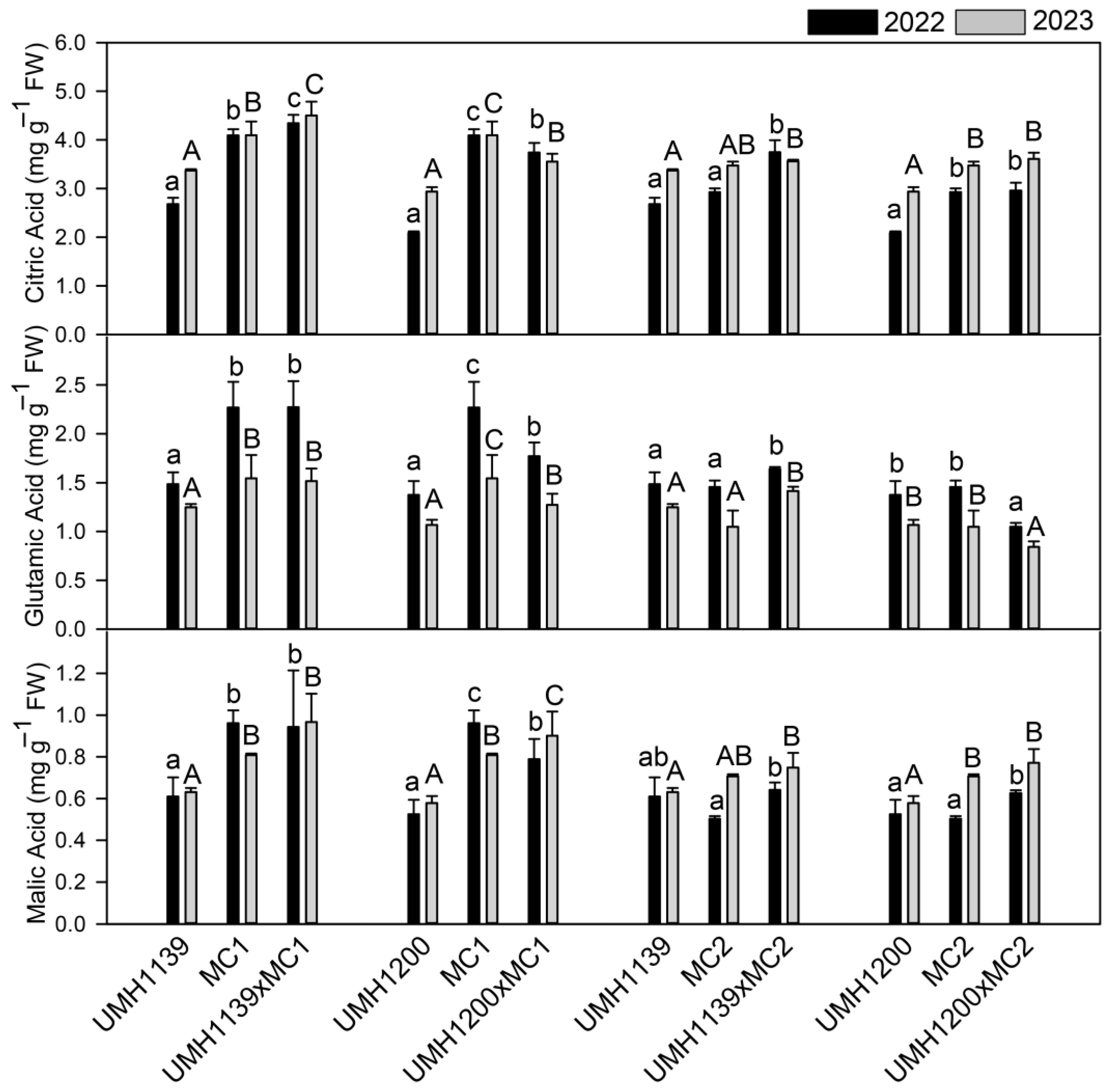
34]. Primary metabolites play a key role in determining the flavor. The Muchamiel variety is characterized by intermediate concentrations of glucose, fructose, and citric acid, as well as high concentrations of malic acid and glutamic acid. The latter is closely linked to the “umami” flavor, which is important for the sensory quality of the fruit [
25,
26]. Whereas the impact of resistance genes’ introductions on the primary metabolite profile of tomatoes remains largely unexplored, the literature does offer insights into their influence on parameters such as titratable acidity and total soluble solids, which are primarily determined by organic acids and soluble sugars, respectively. Rubio et al. observed that the presence of the
Ty-1 gene in homozygosis in NILs of the traditional Muchamiel and De la Pera varieties reduced titratable acidity without affecting the total soluble solids [
21]. This effect was subsequently confirmed in [
22,
35]. The
Ty-1 locus from
S. chilense is located within a non-recombinant genomic region of approximately 35.5 Mb on chromosome 6, where two chromosomal inversions suppress recombination. As a result, this large introgressed segment may retain wild tomato genes that negatively affect acidity [
18]. The results of this study are consistent with these findings. Hybrids derived from the UMH1139 line, in which the
Ty-1 gene is not present, were found to maintain or increase their organic acid concentration compared to the traditional parent line. In contrast, hybrids derived from the UMH1200 line, which carries the
Ty-1 gene, exhibited reductions in all of the three major organic acids (UMH1200×MC1) or the glutamic acid only (UMH1200×MC2). However, in the UMH1200×MC1 hybrid, this decrease did not reach the levels observed in the breeding line, instead presenting an intermediate concentration of the three acids, possibly due to the presence of a single copy of the
Ty-1 gene [
16,
22]. No effect that could be attributed to the introduction of resistance genes was detected with regard to soluble sugars, but it was noted that hybrids consistently outperformed their traditional parents (MC2 and MC1), with an average increase in glucose and fructose of 18% and 22%, respectively, over MC2 and MC1, and variations depending on the hybrid and year.
In recent years, consumer interest in the functional value of agri-food products, primarily driven by the secondary metabolites found in tomatoes, has increased due to the health benefits offered by these metabolites [
36]. The concentrations of different secondary metabolites in the cultivars examined in this study were consistent with those reported in previous studies on Muchamiel-type cultivars [
25,
26,
37]. However, the metabolic profiles of these breeding lines and their hybrids remain to be characterized. The adverse effect of the introduction of resistance genes, particularly
Ty-1, observed on organic acid concentrations, was not detected in the profile of secondary metabolites. It was reported that the
Ty-1 introgression in De la Pera breeding lines did not affect the antioxidant activity of the fruit [
35]. In this study, the cultivars carrying resistance genes (both the homozygous breeding lines and the heterozygous hybrids) showed higher concentrations of rutin, kaempherol-3-O-rutinoside, naringenin, and phloretin than the susceptible cultivars (the traditional parents). The breeding lines showed the highest concentrations, whereas the hybrids exhibited intermediate values. This suggests a direct relationship between the number of copies of the resistance genes and the accumulation of these antioxidant compounds. A higher basal antioxidant level, induced by increased flavonoid synthesis, can enable a quick response to the virus without causing significant oxidative stress. Modulating ROS accumulation during viral infection enables a rapid defense response, such as a hypersensitive reaction, while preventing excessive oxidative damage to surrounding tissues [
38]. Differences in the metabolome of virus-susceptible and virus-resistant tomato cultivars have been observed. For example, a 14.17-fold increase in naringenin in resistant cultivars following TSWV infection has been observed, compared to 2.81- and 1.57-fold increases in susceptible lines [
39]. Similarly, higher concentrations of secondary metabolites were reported in TYLCV-resistant cultivars prior to infection, which suggests that these metabolites play a role in conferring virus tolerance [
40]. Further studies with near-isogenic lines are recommended to confirm the effect of resistance genes on flavonoid accumulation, as these lines are genetically identical, except for the single introgressed region. This enables a more accurate evaluation of the impact of these introgressed regions, particularly the resistance genes, in this context [
21].
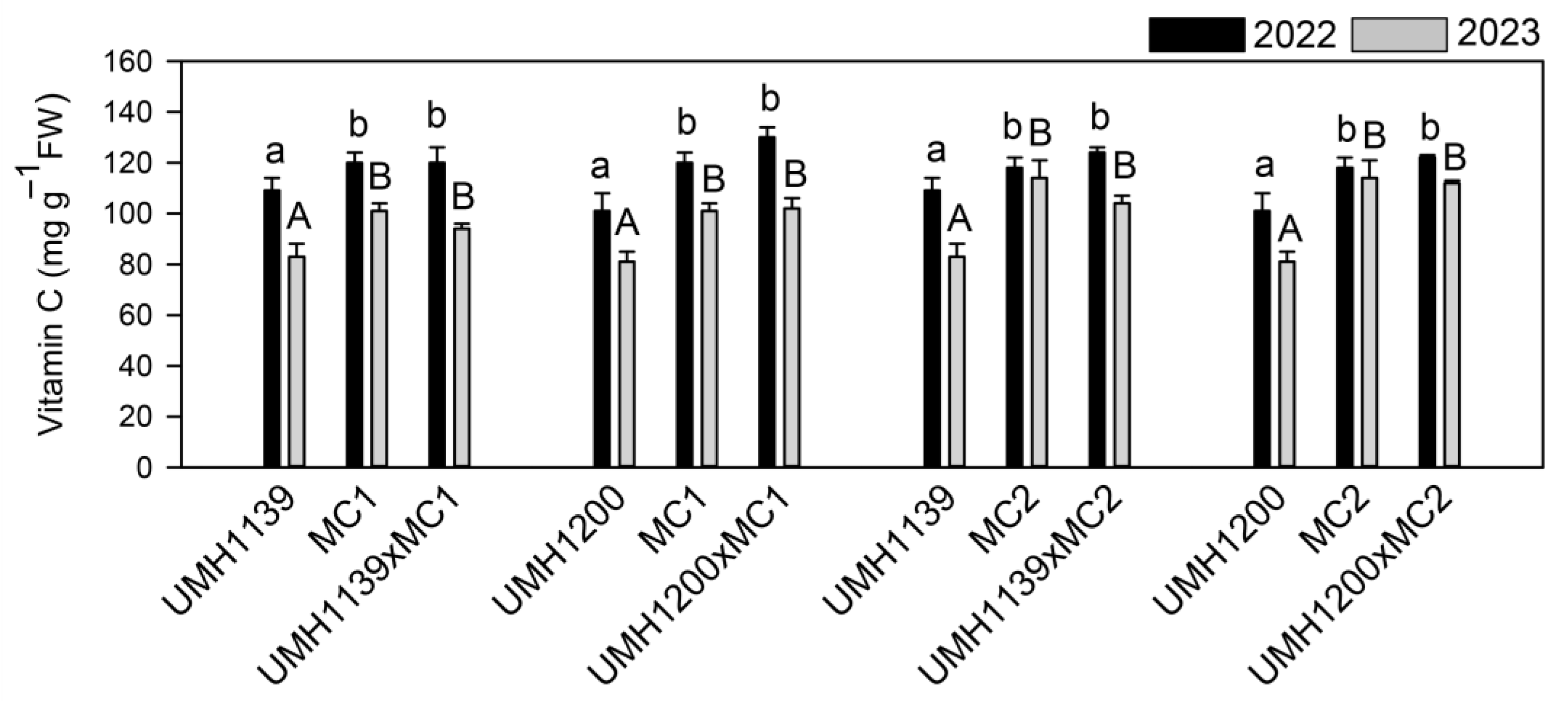
Maintaining an outstanding functional quality is crucial for Muchamiel cultivars. Therefore, it is important to note that hybrids have conserved or improved the functional characteristics of their parents. Depending on the compound, they present a higher concentration of each secondary metabolite in either the breeding line or the traditional parent. Previous studies have suggested that the predominant gene action for traits such as secondary metabolite content is additive [
25,
33], which is consistent with these results. From a nutritional perspective, vitamin C is recognized for its potent antioxidant properties and its ability to donate electrons, thereby protecting DNA from oxidative damage [
41]. The traditional parents were notable for their high vitamin C concentration, a trait that the hybrids have retained. Due to their antioxidant properties, carotenoids such as lycopene,
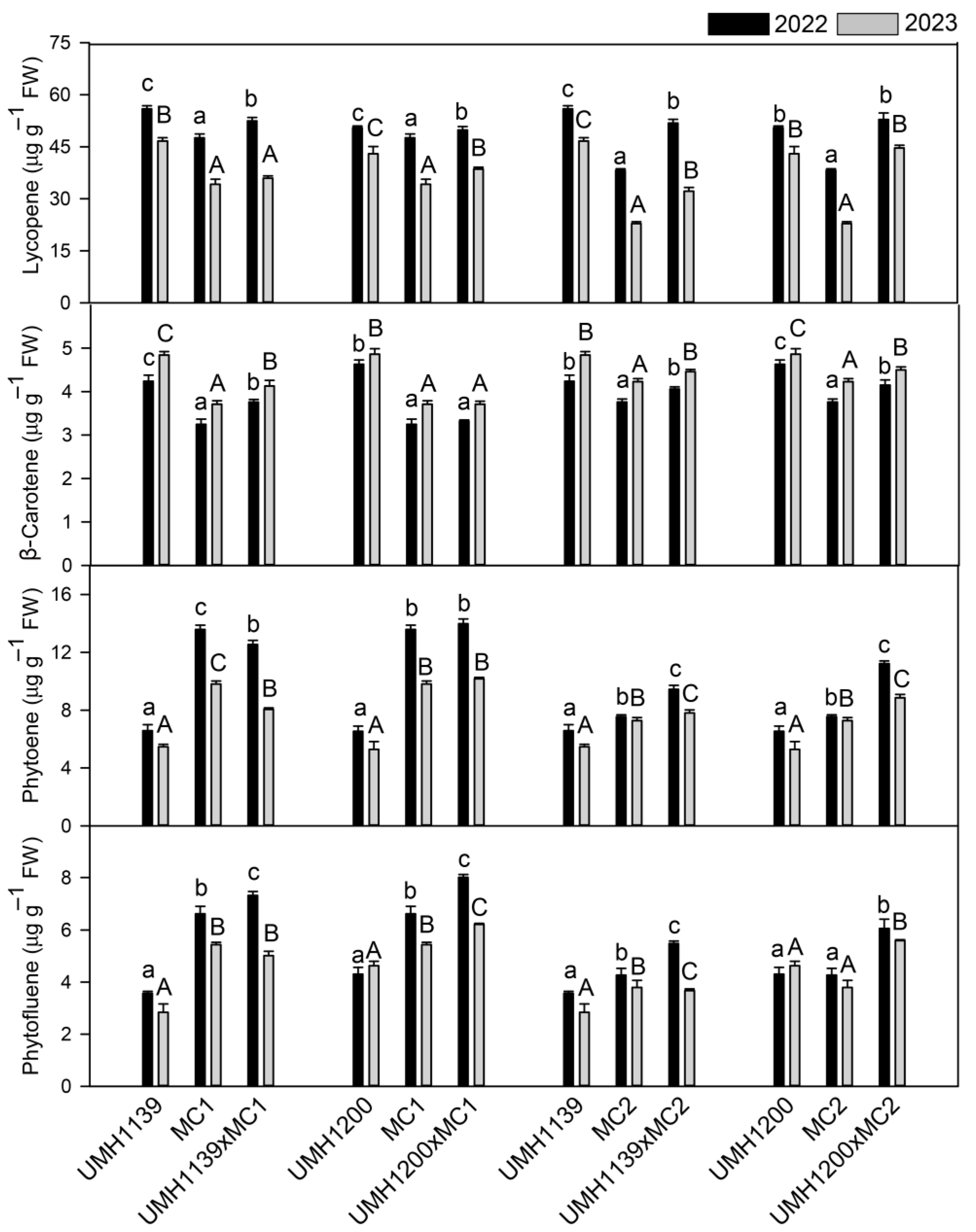
β-carotene, and lutein play a crucial role in preventing chronic diseases, including cancer, cardiovascular disease, and macular degeneration [
9]. Despite their limited bioavailability, xanthophylls are believed to bolster cellular defense and potentially reduce the risk of certain cancers and skin damage [
42]. Breeding lines (UMH1139 and UMH1200) exhibited the highest concentrations of lycopene and
β-carotene, consistent with their intense color. They also showed elevated levels of neoxanthin and violaxanthin, whereas traditional parents had higher amounts of lutein, phytoene, and phytofluene. Hybrids showed intermediate values, even though some equaled their parents in terms of lycopene,
β-carotene, and neoxanthin content. Additionally, they outperformed traditional parents in phytofluene and, in the case of those with MC2 as a parent, in phytoene. Phenolic acids have been associated with anticancer effects and protection against inflammation-induced diabetic nephropathy [
43]. Naringenin, the primary flavanone in tomatoes, has been shown to play a role in preventing cancer and other diseases [
6]. Flavonols have been extensively studied for their pharmacological properties, including anticancer, analgesic, and anti-arthritic effects, as well as their benefits to various bodily systems [
44]. All of the hybrids maintained the high concentration of chlorogenic acid (the main phenolic acid found in tomatoes) characteristic of their traditional parents. Most of the hybrids outperformed their parents in terms of concentrations of caffeic and cryptochlorogenic compounds, except for UMH1200×MC1, which matched them. The ferulic acid concentration of UMH1139×MC1 is similar to that of MC1. Among the flavonols and flavanones, the breeding lines exhibited the highest concentrations, the traditional parents, the lowest, and the hybrids, intermediate values.
Finally, as most traits are quantitative and sensitive to environmental factors, analyzing the effect of the year of culture on the development of new varieties is essential. Semi-arid zones are characterized by elevated levels of solar radiation and high summer temperatures, which can impact crop productivity and quality [
45]. The yield of hybrids remained stable in both years, indicating a high productive stability. The only significant yield reduction observed in UMH1200×MC2 in 2023 is within the range of interannual variation previously reported [
23]. Given the added value of this hybrid, such a reduction can be considered acceptable from the perspective of the farmer. However, visual (morphology and color), flavor-related (primary metabolites), and nutritional (secondary metabolites) quality parameters exhibited interannual variability, attributed to environmental differences between 2022 and 2023. Increased temperatures and cumulative irradiance during the fruiting period in 2023 compared to 2022 could have triggered variations in the composition of the studied fruit varieties (
Supplementary Figure S1). Such conditions have been reported to favor an increase in sugars, as well as variations in organic acids [
45,
46,
47]. Regarding the secondary metabolites, most compounds showed a reduction in 2023, which was consistent with previous studies [
47], although
β-carotene increased in all of the cultivars [
48]. Other metabolites, such as lutein and violaxanthin in certain cases, chlorogenic acid in some cultivars, and caffeic acid, naringenin, and its derivatives under certain conditions, remained stable between years. The interannual response of these compounds varied according to the analyzed cultivar and metabolite, explaining the diversity of patterns that were observed [
49]. Despite these variations, differences between parents and hybrids were maintained, indicating that the observed variability depended mainly on the genetic component, with annual environmental conditions having a minor effect.