Bionanocomposite Coating Film Technologies for Disease Management in Fruits and Vegetables
Abstract
1. Introduction
2. Postharvest Diseases in Fruits and Vegetables
- Impact of fruit ripening on postharvest control
- b.
- The process of postharvest pathogen infection
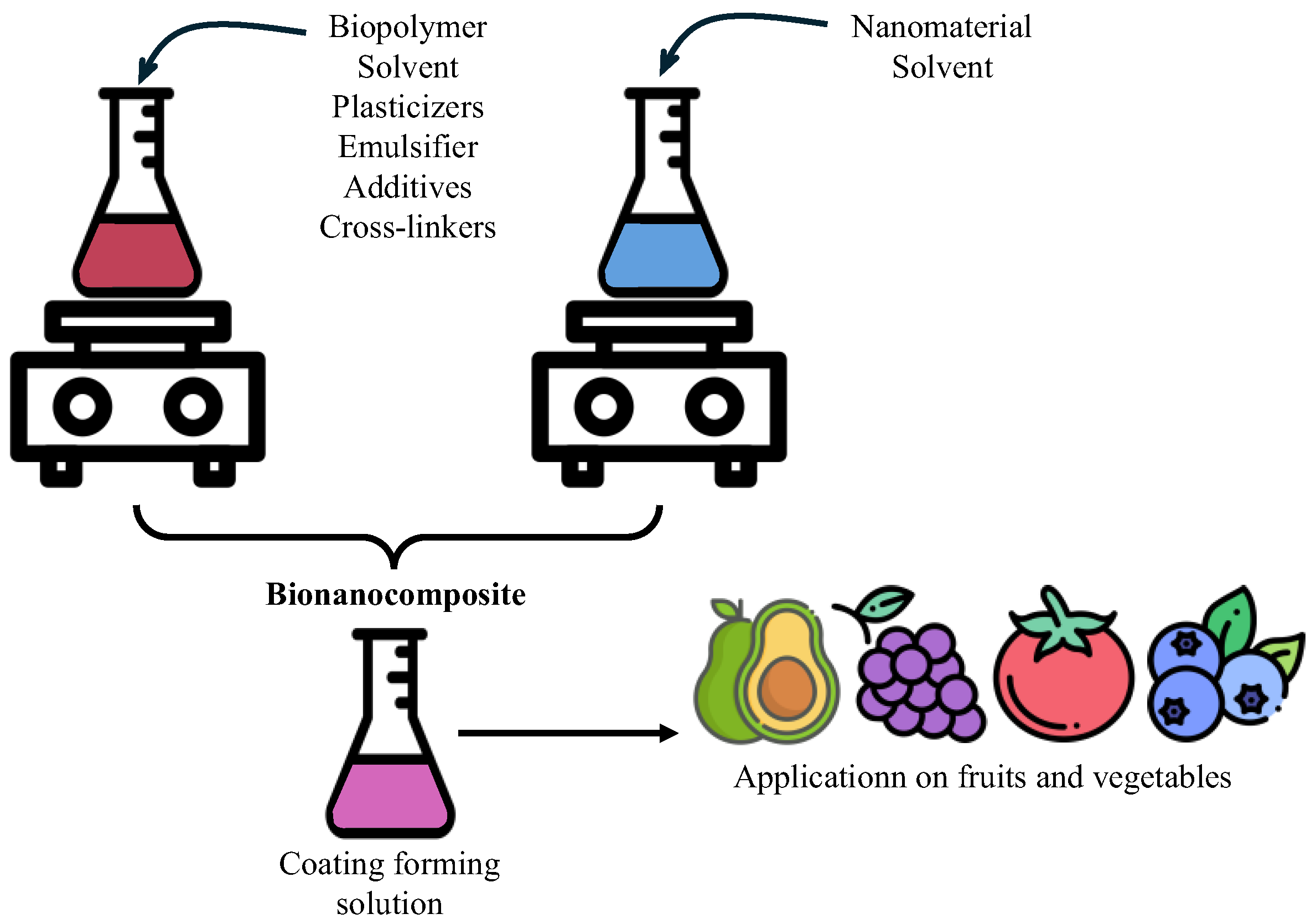
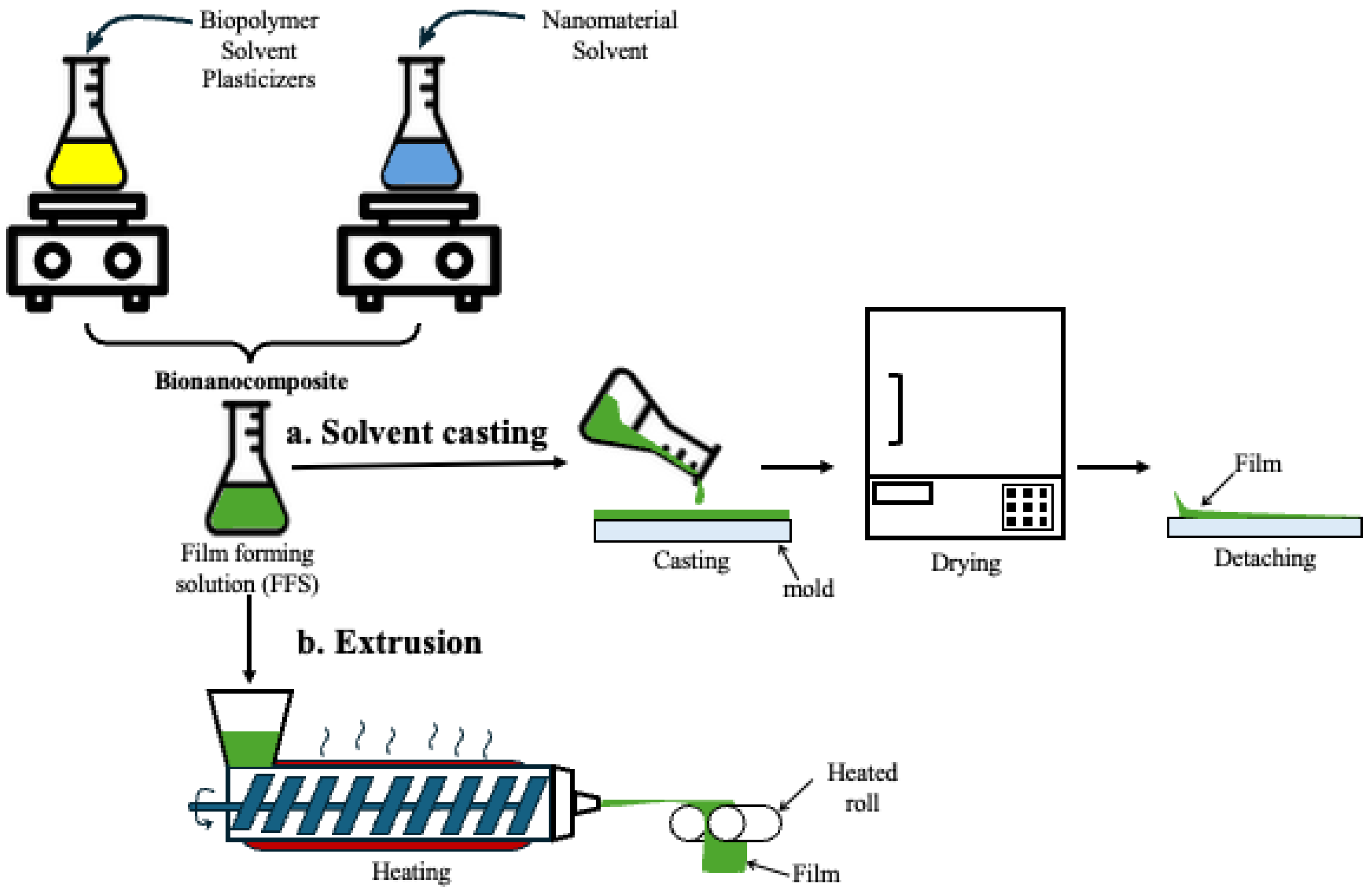
3. Bionanocomposites
- Coatings and edible films
- b.
- In vitro effectiveness
- c.
- Application on fruits and vegetables (in vivo studies)
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent Developments in Gum Edible Coating Applications for Fruits and Vegetables Preservation: A Review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Radoor, S.; Kim, J.T.; Rhim, J.W.; Nandi, D.; Parameswaranpillai, J.; Siengchin, S. Recent Innovations in Bionanocomposites-Based Food Packaging Films—A Comprehensive Review. Food Packag. Shelf Life 2022, 33, 100877. [Google Scholar] [CrossRef]
- Rameez, M.; Khan, N.; Ahmad, S.; Ahmad, M.M. Bionanocomposites: A New Approach for Fungal Disease Management. Biocatal. Agric. Biotechnol. 2024, 57, 103115. [Google Scholar] [CrossRef]
- PostHarvest Technologies. The Top Food Loss and Waste Statistics of 2022. Available online: https://www.postharvest.com/blog/top-food-waste-statistics-of-2021/ (accessed on 19 May 2025).
- Bano, A.; Gupta, A.; Prusty, M.R.; Kumar, M. Elicitation of Fruit Fungi Infection and Its Protective Response to Improve the Postharvest Quality of Fruits. Stresses 2023, 3, 231–255. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Wu, P.; Grierson, D.; Gao, L. Ripening and Rot: How Ripening Processes Influence Disease Susceptibility in Fleshy Fruits. J. Integr. Plant Biol. 2024, 66, 1831–1863. [Google Scholar] [CrossRef]
- Khan, R.; Anwar, F.; Ghazali, F.M. A Comprehensive Review of Mycotoxins: Toxicology, Detection, and Effective Mitigation Approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef]
- Shakeel, Q.; Shaheen, M.R.; Ali, S.; Ahmad, A.; Raheel, M.; Bajwa, R.T. Chapter 1—Postharvest Management of Fruits and Vegetables. In Applications of Biosurfactant in Agriculture; Inamuddin, Adetunji, C.O., Eds.; Academic Press: New York, NY, USA, 2022; pp. 1–16. ISBN 978-0-12-822921-7. [Google Scholar]
- Duncan, T. V Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid. Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M. Bionanocomposites. In Kirk-Othmer Encyclopedia of Chemical Technology; Publisher: John Wiley & Sons, 2008; pp. 1–28. ISBN 9780471238966. [Google Scholar]
- Raghuvanshi, S.; Khan, H.; Saroha, V.; Sharma, H.; Gupta, H.S.; Kadam, A.; Dutt, D. Recent Advances in Biomacromolecule-Based Nanocomposite Films for Intelligent Food Packaging—A Review. Int. J. Biol. Macromol. 2023, 253, 127420. [Google Scholar] [CrossRef]
- Barbhuiya, R.I.; Natalia Nevarez, T.; Saipriya, R.; Abdallah, E.; Jayasankar, S.; Winny, R.; Singh, A. A Review of Nanoparticle Synthesis and Application in the Suppression of Diseases in Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2024, 64, 4477–4499. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carrageenan-Based Antimicrobial Bionanocomposite Films Incorporated with ZnO Nanoparticles Stabilized by Melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Risyon, N.P.; Othman, S.H.; Basha, R.K.; Talib, R.A. Characterization of Polylactic Acid/Halloysite Nanotubes Bionanocomposite Films for Food Packaging. Food Packag. Shelf Life 2020, 23, 100450. [Google Scholar] [CrossRef]
- Reis, L.C.B.; de Souza, C.O.; da Silva, J.B.A.; Martins, A.C.; Nunes, I.L.; Druzian, J.I. Active Biocomposites of Cassava Starch: The Effect of Yerba Mate Extract and Mango Pulp as Antioxidant Additives on the Properties and the Stability of a Packaged Product. Food Bioprod. Process. 2015, 94, 382–391. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Nazrin, A.; Sherwani, S.F.K.; Khalina, A. Antimicrobial Activities of Starch-Based Biopolymers and Biocomposites Incorporated with Plant Essential Oils: A Review. Polymers 2020, 12, 2403. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Niaounakis, M. Chapter 12—Manufacture of Biocomposites. In Biopolymers: Processing and Products; Niaounakis, M., Ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 411–430. ISBN 978-0-323-26698-7. [Google Scholar]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Syakir, M.I.; Fazita, M.R.N.; Bhat, A.; Banerjee, A.; Fizree, H.M.; Rizal, S.; Tahir, P.M. 13—Barrier Properties of Biocomposites/Hybrid Films. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Jawaid, M., Thariq, M., Saba, N., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 241–258. ISBN 978-0-08-102292-4. [Google Scholar]
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying Household Waste of Fresh Fruit and Vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef]
- Chaboud, G.; Daviron, B. Food Losses and Waste: Navigating the Inconsistencies. Glob. Food Sec 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Kitinoja, L.; Tokala, V.Y.; Brondy, A. Challenges and Opportunities for Improved Postharvest Loss Measurements in Plant-Based Food Crops. J. Postharvest Technol. 2018, 6, 16–34. [Google Scholar]
- Yahia, E.M.; Fonseca, J.M.; Kitinoja, L. Chapter 2—Postharvest Losses and Waste. In Postharvest Technology of Perishable Horticultural Commodities; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 43–69. ISBN 978-0-12-813276-0. [Google Scholar]
- Singh, D.; Sharma, R.R. Chapter 1—Postharvest Diseases of Fruits and Vegetables and Their Management. In Postharvest Disinfection of Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: New York, NY, USA, 2018; pp. 1–52. ISBN 978-0-12-812698-1. [Google Scholar]
- Shahbazi, F.; Shahbazi, S.; Nadimi, M.; Paliwal, J. Losses in Agricultural Produce: A Review of Causes and Solutions, with a Specific Focus on Grain Crops. J. Stored Prod. Res. 2025, 111, 102547. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Z.; Pan, X.; Yang, T.; An, C.; Wang, Y.; Li, L.; Liao, W.; Wang, C. Effects of Reactive Oxygen Species on Fruit Ripening and Postharvest Fruit Quality. Plant Sci. 2025, 352, 112391. [Google Scholar] [CrossRef]
- Kapoor, L.; Simkin, A.J.; George Priya Doss, C.; Siva, R. Fruit Ripening: Dynamics and Integrated Analysis of Carotenoids and Anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Cherian, S.; Figueroa, C.R.; Nair, H. ‘Movers and Shakers’ in the Regulation of Fruit Ripening: A Cross-Dissection of Climacteric versus Non-Climacteric Fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef]
- Su, G.; Lin, Y.; Wang, C.; Lu, J.; Liu, Z.; He, Z.; Shu, X.; Chen, W.; Wu, R.; Li, B.; et al. Expansin SlExp1 and Endoglucanase SlCel2 Synergistically Promote Fruit Softening and Cell Wall Disassembly in Tomato. Plant Cell 2024, 36, 709–726. [Google Scholar] [CrossRef]
- Forlani, S.; Masiero, S.; Mizzotti, C. Fruit Ripening: The Role of Hormones, Cell Wall Modifications, and Their Relationship with Pathogens. J. Exp. Bot. 2019, 70, 2993–3006. [Google Scholar] [CrossRef]
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked Regulatory Loops of ABA Catabolism and Biosynthesis Coordinate Fruit Growth and Ripening in Woodland Strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, E11542–E11550. [Google Scholar] [CrossRef]
- Yan, T.; Ren, Y.; Zhang, R.; Li, K.; Yang, B.; Tong, M.; He, J. Biodegradable Chitosan-Based Films Decorated with Biosynthetic Copper Oxide Nanoparticle for Post-Harvest Tomato Preservation. Int. J. Biol. Macromol. 2025, 295, 139595. [Google Scholar] [CrossRef]
- El-Basiouny, N.M.; Soliman, S.M.A.; Khalil, N.M.; Abd El-Ghany, M.N. Chitosan and Alginate/Aspergillus Flavus-Mediated Nanocomposite Films for Preservation of Postharvest Tomatoes. Int. J. Biol. Macromol. 2025, 297, 139559. [Google Scholar] [CrossRef]
- Sun, S.; Wang, N.; Ali, E.; Qiao, L.; Guo, Q.; Lu, L. Glucose-Responsive Carboxymethyl Chitosan/ Sodium Alginate Film Protects against Mechanical Wound on Postharvest Blueberry. Food Hydrocoll. 2025, 159, 110648. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Chen, C.; Liu, J.; Liu, H.; Pei, Y. Defense Responses of Salicylic Acid in Mango Fruit Against Postharvest Anthracnose, Caused by Colletotrichum gloeosporioides and Its Possible Mechanism. J. Food Saf. 2017, 37, e12294. [Google Scholar] [CrossRef]
- Xuan, S.; Shen, P.; Ren, Y.; Li, S.; Jin, P.; Zheng, Y.; Wu, Z. Modified SiO2@cinnamaldehyde/Nanocellulose Coating Film for Loquat Preservation. Int. J. Biol. Macromol. 2024, 278, 134862. [Google Scholar] [CrossRef]
- Bally, I.; Hofman, P.J.; Irving, D.E.; Coates, L.; Dann, E. The Effects of Nitrogen on Postharvest Disease in Mango (Mangifera indica L. ’Keitt’). Acta Hortic. 2009, 820, 365–370. [Google Scholar] [CrossRef]
- He, M.; Pan, J.; Hong, M.; Shen, Y.; Zhang, H.; Jiang, Y.; Gong, L. Fabrication of Antimicrobial Packaging Based on Polyaminopropyl Biguanide Incorporated Pectin/Polyvinyl Alcohol Films for Fruit Preservation. Food Chem. 2024, 457, 140106. [Google Scholar] [CrossRef]
- Nair, U.K.A.; Periyar Selvam, S.; Dharini, V.; Nambiar, R.B.; Anand Babu, P.; Sadiku, E.R.; Jayaramudu, J. Development of Antifungal Biocomposite Film against Postharvest Pathogens Colletotrichum gloeosporioides and Lasiodiplodia theobromae. Mater. Today Proc. 2021, 38, 1113–1120. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.; Li, J.; Li, M.; Xing, D.; An, H.; Wu, X.; Wu, Y. A Chitosan Composite Film Sprayed before Pathogen Infection Effectively Controls Postharvest Soft Rot in Kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef]
- García, M.; Ventosa, M.; Diaz, R.; Silvia, F.; Casariego, A. Effects of Aloe Vera Coating on Postharvest Quality of Tomato. Fruits 2014, 69, 117–126. [Google Scholar] [CrossRef]
- Aswani, R.; Das, S.; Sebastian, K.S.; Mathew, J.; Radhakrishnan, E.K. Development of Biocomposite Films Incorporated with the Extract from Pitcher Associated Bacteria for the Postharvest Protection from Fungi. J. Food Sci. Technol. 2024, 61, 2157–2165. [Google Scholar] [CrossRef]
- Du, H.; Min, T.; Sun, X.; Bian, X.; Zhu, Z.; Wen, Y. Multifunctional Film Based on Gelatin with Titanium Dioxide and Thymol@β-Cyclodextrins for Fresh-Keeping Packaging. Food Biosci. 2022, 50, 102168. [Google Scholar] [CrossRef]
- Zhang, R.; Cui, Y.; Cheng, M.; Guo, Y.; Wang, X.; Wang, J. Antifungal Activity and Mechanism of Cinnamon Essential Oil Loaded into Mesoporous Silica Nanoparticles. Ind. Crops Prod. 2021, 171, 113846. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, M.; Cao, Y.; Mi, Q.; Liang, S.; Feng, J.; Wang, Y. Postharvest Sclerotinia Rot Control in Carrot by the Natural Product Hinokitiol and the Potential Mechanisms Involved. Int. J. Food Microbiol. 2022, 383, 109939. [Google Scholar] [CrossRef]
- Osman Mohamed Ali, E.; Shakil, N.A.; Rana, V.S.; Sarkar, D.J.; Majumder, S.; Kaushik, P.; Singh, B.B.; Kumar, J. Antifungal Activity of Nano Emulsions of Neem and Citronella Oils against Phytopathogenic Fungi, Rhizoctonia solani and Sclerotium rolfsii. Ind. Crops Prod. 2017, 108, 379–387. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Encapsulation of Thyme Essential Oil Using Electrospun Zein Fiber for Strawberry Preservation. Chem. Biol. Technol. Agric. 2022, 9, 2. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, H.; Chen, K.; Cui, R.; Cao, J.; Wang, Z.; Zhang, Z.-H.; Soteyome, T. Effects of Chitosan/Eugenol-Loaded IRMOF-3 Nanoparticles Composite Films on Reactive Oxygen Species Metabolism and Microbial Community Dynamics in Postharvest Strawberries. Food Biosci. 2025, 63, 105652. [Google Scholar] [CrossRef]
- Filgueiras, C.T.; Fakhouri, F.M.; Garcia, V.A.d.S.; Velasco, J.I.; Nogueira, G.F.; da Silva, L.R.; de Oliveira, R.A. Effect of Adding Red Propolis to Edible Biodegradable Protein Films for Coating Grapes: Shelf Life and Sensory Analysis. Polymers 2024, 16, 888. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, W.G.; Rosa, G.B.; Ferreira, A.L.A.; da Rosa, C.G.; Beling, P.C.; Xavier, L.O.; Hansen, C.M.; Ferrareze, J.P.; Nunes, M.R.; Barreto, P.L.M.; et al. Bioactive Food Packaging Based on Starch, Citric Pectin and Functionalized with Acca sellowiana Waste by-Product: Characterization and Application in the Postharvest Conservation of Apple. Int. J. Biol. Macromol. 2020, 147, 295–303. [Google Scholar] [CrossRef]
- Men, C.; Wu, C.; Wang, L.; Liu, S.; Ning, C.; Liu, C.; Zheng, L. A Novel LA@Cu-MOF Film with Dual Response to PH and Humidity: Preparation, Antibacterial Activity, and Fruit Preservation. Food Chem. 2025, 475, 143304. [Google Scholar] [CrossRef]
- Alkan, D.; Yemenicioğlu, A. Potential Application of Natural Phenolic Antimicrobials and Edible Film Technology against Bacterial Plant Pathogens. Food Hydrocoll. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- de Ramón-Carbonell, M.; López-Pérez, M.; González-Candelas, L.; Sánchez-Torres, P. PdMFS1 Transporter Contributes to Penicilliun Digitatum Fungicide Resistance and Fungal Virulence during Citrus Fruit Infection. J. Fungi 2019, 5, 100. [Google Scholar] [CrossRef]
- Liu, S.; Du, Y.; Zhang, D.; Yang, F.; He, X.; Long, C. Aluminum Sulfate Inhibits Green Mold by Inducing Chitinase Activity of Penicillium digitatum and Enzyme Activity of Citrus Fruit. Food Control 2022, 136, 108854. [Google Scholar] [CrossRef]
- da Cunha, T.; Ferraz, L.P.; Wehr, P.P.; Kupper, K.C. Antifungal Activity and Action Mechanisms of Yeasts Isolates from Citrus against Penicillium italicum. Int. J. Food Microbiol. 2018, 276, 20–27. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Yi, L.; Zeng, K. Tryptophan Enhances Biocontrol Efficacy of Metschnikowia Citriensis FL01 against Postharvest Fungal Diseases of Citrus Fruit by Increasing Pulcherriminic Acid Production. Int. J. Food Microbiol. 2023, 386, 110013. [Google Scholar] [CrossRef]
- Cruz-Lachica, I.; Márquez-Zequera, I.; García-Estrada, R.S.; Carrillo-Fasio, J.A.; León-Félix, J.; Allende-Molar, R. Identification of Mucoralean Fungi Causing Soft Rot in Papaya (Carica Papaya L.) Fruit in Mexico. Rev. Mex. De. Fitopatol. 2017, 35, 397–417. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; de Souza Coelho, C.C.; Soares, A.G.; Freitas-Silva, O. Current Technologies to Control Fungal Diseases in Postharvest Papaya (Carica Papaya L.). Biocatal. Agric. Biotechnol. 2021, 36, 102128. [Google Scholar] [CrossRef]
- Cruz-Lagunas, B.; Ortega-Acosta, S.Á.; Reyes-García, G.; Toribio-Jiménez, J.; Juárez-López, P.; Guillén-Sánchez, D.; Damián-Nava, A.; Romero-Ramírez, Y.; Palemón-Alberto, F. Colletotrichum gloeosporioides Causes Anthracnose on Grapefruit (Citrus Paradisi) in Mexico. Australas. Plant Dis. Notes 2020, 15, 31. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R.; de Oliveira, A.G. Ultra-Structural Alterations in Botrytis Cinerea—The Causal Agent of Gray Mold—Treated with Salt Solutions. Biomolecules 2019, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, X.; Vargas, M.; Vero, S.; Zapata, N. Indigenous Yeasts for the Biocontrol of Botrytis Cinerea on Table Grapes in Chile. J. Fungi 2023, 9, 557. [Google Scholar] [CrossRef]
- Luciano-Rosario, D.; Keller, N.P.; Jurick II, W.M. Penicillium expansum: Biology, Omics, and Management Tools for a Global Postharvest Pathogen Causing Blue Mould of Pome Fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P.H.J. Latent Postharvest Pathogens of Pome Fruit and Their Management: From Single Measures to a Systems Intervention Approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Mo, J.; Zhao, G.; Li, Q.; Solangi, G.S.; Tang, L.; Guo, T.; Huang, S.; Hsiang, T. Identification and Characterization of Colletotrichum Species Associated with Mango Anthracnose in Guangxi, China. Plant Dis. 2018, 102, 1283–1289. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, S.; Du, J.; Wang, X.; Xu, W.; Luo, C. Monilinia fructicola on Loquat: An Old Pathogen Invading a New Host. J. Integr. Agric. 2021, 20, 2009–2014. [Google Scholar] [CrossRef]
- Yilmaz, A.; Bozkurt, F.; Cicek, P.K.; Dertli, E.; Durak, M.Z.; Yilmaz, M.T. A Novel Antifungal Surface-Coating Application to Limit Postharvest Decay on Coated Apples: Molecular, Thermal and Morphological Properties of Electrospun Zein–Nanofiber Mats Loaded with Curcumin. Innov. Food Sci. Emerg. Technol. 2016, 37, 74–83. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Dilip, D.; Sten, J.; Ravat, V.K.; Bhutia, D.D.; Panja, B.; Saha, J. Antagonistic Yeasts for Biocontrol of the Banana Postharvest Anthracnose Pathogen Colletotrichum musae. J. Phytopathol. 2017, 165, 35–43. [Google Scholar] [CrossRef]
- Kumar, V.; Anal, A.K.D.; Rai, S.; Nath, V. Leaf, Panicle and Fruit Blight of Litchi (Litchi Chinensis) Caused by Alternaria alternata in Bihar State, India. Can. J. Plant Pathol. 2018, 40, 84–89. [Google Scholar] [CrossRef]
- Jianying, F.; Bianyu, Y.; Xin, L.; Dong, T.; Weisong, M. Evaluation on Risks of Sustainable Supply Chain Based on Optimized BP Neural Networks in Fresh Grape Industry. Comput. Electron. Agric. 2021, 183, 105988. [Google Scholar] [CrossRef]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical Treatments to Control Postharvest Diseases of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public. Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Eskola, M.; Gregor, K.; Christopher, T.E.; Jana, H.; Sultan, M.; and Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Xue, H.; Liu, Q.; Yang, Z. Pathogenicity, Mycotoxin Production, and Control of Potato Dry Rot Caused by Fusarium Spp.: A Review. J. Fungi 2023, 9, 843. [Google Scholar] [CrossRef]
- Yellareddygari, S.K.R.; Domfeh, O.; Bittara, F.G.; Gudmestad, N.C. Analysis of Potato Mop-Top Virus Survival Probability in Post-Harvest Storage. Am. J. Potato Res. 2017, 94, 632–637. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current Status and Future Directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Zhao, P.; Jean Pierre, N.; Xiao, L.; Xia, X. Microbial Spoilage of Fruits: A Review on Causes and Prevention Methods. Food Rev. Int. 2022, 38, 225–246. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, R.; Fang, X.; Wu, W.; Han, Y.; Chen, H.; Xu, F.; Gao, H. Botrytis cinerea Infection Affects Wax Composition, Content and Gene Expression in Blueberry Fruit. Postharvest Biol. Technol. 2022, 192, 112020. [Google Scholar] [CrossRef]
- Choquer, M.; Fournier, E.; Kunz, C.; Levis, C.; Pradier, J.-M.; Simon, A.; Viaud, M. Botrytis Cinerea Virulence Factors: New Insights into a Necrotrophic and Polyphageous Pathogen. FEMS Microbiol. Lett. 2007, 277, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van Kan, J.A.L. Licensed to Kill: The Lifestyle of a Necrotrophic Plant Pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest Treatments of Fresh Produce. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130309. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial Biocontrol as an Alternative to Synthetic Fungicides: Boundaries between Pre- and Postharvest Applications on Vegetables and Fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Huang, X.; Ren, J.; Li, P.; Feng, S.; Dong, P.; Ren, M. Potential of Microbial Endophytes to Enhance the Resistance to Postharvest Diseases of Fruit and Vegetables. J. Sci. Food Agric. 2021, 101, 1744–1757. [Google Scholar] [CrossRef]
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A Review of Recent Trends in the Development of the Microbial Safety of Fruits and Vegetables. Trends Food Sci. Technol. 2020, 103, 321–332. [Google Scholar] [CrossRef]
- Montes-Ramírez, P.; Montaño-Leyva, B.; Blancas-Benitez, F.J.; Bautista-Rosales, P.U.; Ruelas-Hernández, N.D.; Martínez-Robinson, K.; González-Estrada, R.R. Active Films and Coatings Based on Commercial Chitosan with Natural Extracts Addition from Coconut By-Products: Physicochemical Characterization and Antifungal Protection on Tomato Fruits. Food Control 2024, 155, 110077. [Google Scholar] [CrossRef]
- García-Bramasco, C.A.; Blancas-Benitez, F.J.; Montaño-Leyva, B.; Medrano-Castellón, L.M.; Gutierrez-Martinez, P.; González-Estrada, R.R. Influence of Marine Yeast Debaryomyces hansenii on Antifungal and Physicochemical Properties of Chitosan-Based Films. J. Fungi 2022, 8, 369. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of Antimicrobial Packaging Film Made from Poly(Lactic Acid) Incorporating Titanium Dioxide and Silver Nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H. Construction and Application of Nano ZnO/Eugenol@yam Starch/Microcrystalline Cellulose Active Antibacterial Film. Int. J. Biol. Macromol. 2023, 239, 124215. [Google Scholar] [CrossRef]
- Mathew, S.; Jayakumar, A.; Kumar, V.P.; Mathew, J.; Radhakrishnan, E.K. One-Step Synthesis of Eco-Friendly Boiled Rice Starch Blended Polyvinyl Alcohol Bionanocomposite Films Decorated with in Situ Generated Silver Nanoparticles for Food Packaging Purpose. Int. J. Biol. Macromol. 2019, 139, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Liu, Y.-K.; Chiu, F.-C. Fabrication of Cellulose Nanocrystal/Silver/Alginate Bionanocomposite Films with Enhanced Mechanical and Barrier Properties for Food Packaging Application. Nanomaterials 2019, 9, 1523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Ren, Y.; Guo, J.; Guo, M.; Yang, X.; Kong, X.; Feng, Y.; Liu, G. Recent Advancements in Crop Straw Cellulose: Sustainable Extraction, Modification, and Active Film Performance Enhancement, and Food Preservation Application. Trends Food Sci. Technol. 2025, 162, 105079. [Google Scholar] [CrossRef]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Oz, F.; Khan, M.H.; Roy, S.; Esatbeyoglu, T.; Pratap-Singh, A. Thermal Properties of Biopolymer Films: Insights for Sustainable Food Packaging Applications. Food Eng. Rev. 2024, 16, 497–512. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M. A Review on Thermomechanical Properties of Polymers and Fibers Reinforced Polymer Composites. J. Ind. Eng. Chem. 2018, 67, 1–11. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benítez, J.J.; Tedeschi, G.; Porras-Vázquez, J.M.; Heredia, A.; Athanassiou, A.; Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-Blocking, and High Barrier Cellulose-Based Bioplastics with Naringin as Active Food Packaging Materials. Int. J. Biol. Macromol. 2022, 209, 1985–1994. [Google Scholar] [CrossRef]
- Duncan, S.E.; Chang, H.-H. Chapter Two—Implications of Light Energy on Food Quality and Packaging Selection. In Advances in Food and Nutrition Research; Henry, J., Ed.; Academic Press: New York, NY, USA, 2012; Volume 67, pp. 25–73. ISBN 1043-4526. [Google Scholar]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Łopusiewicz, Ł. Recent Progress on UV-Light Barrier Food Packaging Films—A Systematic Review. Innov. Food Sci. Emerg. Technol. 2024, 91, 103550. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liu, C. Film Transparency and Opacity Measurements. Food Anal. Methods 2022, 15, 2840–2846. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of Polymeric Food Packaging Materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Muhammad, M.; Ullah, S.; Abdi, G.; Shah, G.M.; Zaman, W.; Ayaz, A. Agriculture and Environmental Management through Nanotechnology: Eco-Friendly Nanomaterial Synthesis for Soil-Plant Systems, Food Safety, and Sustainability. Sci. Total Environ. 2024, 926, 171862. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in Sustainable Agriculture: An Emerging Opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current Applications and Future Scope in Food. Food Front. 2021, 2, 3–22. [Google Scholar] [CrossRef]
- Qiu, M.; Tian, Y.; Qu, W.; Ma, Y.; Zhao, F.; Jiang, Y.; Zhao, Q.; Man, C. Postbiotic-Biosynthesized Silver Nanoparticles Anchored on Covalent Organic Frameworks Integrated into Carboxymethyl Chitosan-Based Film for Enhancing Antibacterial Packaging. Int. J. Biol. Macromol. 2025, 291, 139143. [Google Scholar] [CrossRef]
- Mousavi, S.F.; Arsalani, N.; Ghorbani, M. Preparation of Sodium Alginate and Xanthan Gum Bionanocomposite Films Reinforced with Hybrid Halloysite Nanotubes Containing ZnO and Licorice Root Extract for Wound Dressing Applications. Int. J. Biol. Macromol. 2025, 307, 141974. [Google Scholar] [CrossRef]
- Li, C.; Li, F.; Wang, K.; Xie, D. Green and Facile Fabrication of Multifunctional Cellulose Nanocrystal and Carvacrol Together Reinforced Chitosan Bio-Nanocomposite Coatings for Fruit Preservation. Int. J. Biol. Macromol. 2024, 265, 130651. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Su, S.; Long, F.; Zhong, L.; Hu, J. Multifunctional Bio-Nanocomposite Films Integrated with Essential Oils@metal−phenolic Network Nanocapsules for Durable Fruit Preservation. Int. J. Biol. Macromol. 2024, 278, 134916. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S. Edible Coating for Fresh Fruit: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2619–2626. [Google Scholar] [CrossRef]
- Sharma, H.; Shami, V.; Samsher; Chaudhary, V.; Sunil; Kumar, M. Importance of Edible Coating on Fruits and Vegetables: A Review. J. Pharmacogn. Phytochem. 2019, 8, 4104–4110. [Google Scholar]
- Olivas, G.; Dávila-Aviña, J.; Salazar, N.A.; Molina, F.J. Use of Edible Coatings to Preserve the Quality of Fruits and Vegetables during Storage. Stewart Postharvest Rev. 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Kumar, N. Neeraj Polysaccharide-Based Component and Their Relevance in Edible Film/Coating: A Review. Nutr. Food Sci. 2019, 49, 793–823. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Mohamed Amin Tawakkal, I.S.; Muda Mohamed, M.T. Recent Advance in Edible Coating and Its Effect on Fresh/Fresh-Cut Fruits Quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Dwibedi, V.; Kaur, G.; George, N.; Rana, P.; Ge, Y.; Sun, T. Research Progress in the Preservation and Packaging of Fruits and Vegetables: From Traditional Methods to Innovative Technologies. Food Packag. Shelf Life 2024, 46, 101385. [Google Scholar] [CrossRef]
- Thakur, M.; Majid, I.; Nanda, V. Advances in Edible Coating for Improving the Shelf Life of Fruits. In Emerging Technologies for Shelf-Life Enhancement of Fruits; Dar, N.B., Mir, S.A., Eds.; Apple Academic Press: New York, NY, USA, 2020. [Google Scholar]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Preparing Valuable Renewable Nanocomposite Films Based Exclusively on Oceanic Biomass—Chitin Nanofillers and Chitosan. React. Funct. Polym. 2015, 89, 31–39. [Google Scholar] [CrossRef]
- Gennadios, A. Proteins as Raw Materials for Films and Coatings: Definitions, Current Status, and Opportunities. In Protein-Based Films and Coatings; Gennadios, A., Ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. 18—Edible Films and Coatings from Proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 477–500. ISBN 978-0-08-100722-8. [Google Scholar]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Raghav, P.; Agarwal, N.; Saini, M. Edible Coatings of Fruits and Vegetables: A Review. IJSRME 2016, 1, 188–204. [Google Scholar]
- Khan, M.K.I.; Mujawar, L.H.; Schutyser, M.A.I.; Schroën, K.; Boom, R. Deposition of Thin Lipid Films Prepared by Electrospraying. Food Bioproc Tech. 2013, 6, 3047–3055. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional Bionanocomposite Films Based on Konjac Glucomannan/Chitosan with Nano-ZnO and Mulberry Anthocyanin Extract for Active Food Packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Malekshahi, G.; ValizadehKaji, B. Effects of Postharvest Edible Coatings to Maintain Qualitative Properties and to Extend Shelf-Life of Pomegranate (Punica Granatum. L). Int. J. Hortic. Sci. Technol. 2021, 8, 67–80. [Google Scholar] [CrossRef]
- Satriaji, K.P.; Garcia, C.V.; Kim, G.H.; Shin, G.H.; Kim, J.T. Antibacterial Bionanocomposite Films Based on CaSO4-Crosslinked Alginate and Zinc Oxide Nanoparticles. Food Packag. Shelf Life 2020, 24, 100510. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Liu, Y.; Rashid, A.; Qayum, A.; Zhou, C.; Han, X.; Ren, X.; Chi, Z.; Chi, R.; et al. Preparation Technology and Preservation Mechanism of Novel Ag NPs-Loaded ZIF-67 Packaging Film. Food Packag. Shelf Life 2024, 45, 101338. [Google Scholar] [CrossRef]
- Chávez-Magdaleno, M.E.; Luque-Alcaraz, A.G.; Gutiérrez-Martínez, P.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M. Effect of Chitosan-Pepper Tree (Schinus Molle) Essential Oil Biocomposites on the Growth Kinetics, Viability and Membrane Integrity of Colletotrichum gloeosporioides. Rev. Mex. Ing. Quim. 2018, 17, 29–45. [Google Scholar] [CrossRef]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P. Synthesis and in Vitro Antifungal Efficacy of Cu–Chitosan Nanoparticles against Pathogenic Fungi of Tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef]
- Ali, E.A.; Eweis, M.; Elkholy, S.; Ismail, M.N.; Elsabee, M. The Antimicrobial Behavior of Polyelectrolyte Chitosan-Styrene Maleic Anhydride Nano Composites. Macromol. Res. 2018, 26, 418–425. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, H.; Guo, X.; Bi, X.; Liu, X.; Xu, Q.; Wang, Q.; Li, W.; Li, X.; Shui, Y.; et al. Effect of Chitosan/Nano-TiO2 Composite Coatings on the Postharvest Quality and Physicochemical Characteristics of Mango Fruits. Sci. Hortic. 2020, 263, 109135. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose Acetate/AgNPs-Organoclay and/or Thymol Nano-Biocomposite Films with Combined Antimicrobial/Antioxidant Properties for Active Food Packaging Use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Barnela, M.; Chopra, M.; Manuja, A.; Chaudhury, A. Synthesis, Characterization and in Vitro Evaluation of Cytotoxicity and Antimicrobial Activity of Chitosan–Metal Nanocomposites. J. Chem. Technol. Biotechnol. 2015, 90, 867–873. [Google Scholar] [CrossRef]
- Teixeira Pigozzi, M.; Mendes Silva, V.; Mendes, F.; Oliveira, I.; Moraes, A.R.; Lopes, E. Post-Harvest Quality of Papaya Coated with Polivinilic Alcohol and Maize Starch. Ciência E Agrotecnologia 2021, 45. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible Films and Coatings: Characteristics and Properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in Edible Coatings: A Novel Strategy for Food Preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef] [PubMed]
- Jampílek, J.; Kráĺová, K. Chapter 15—Nanocomposites: Synergistic Nanotools for Management of Mycotoxigenic Fungi. In Nanomycotoxicology; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press: New York, NY, USA, 2020; pp. 349–383. ISBN 978-0-12-817998-7. [Google Scholar]
- Mallakpour, S.; Sadaty, M.A. Thiamine Hydrochloride (Vitamin B1) as Modifier Agent for TiO2 Nanoparticles and the Optical, Mechanical, and Thermal Properties of Poly(Vinyl Chloride) Composite Films. RSC Adv. 2016, 6, 92596–92604. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Du, Y.; Wang, L.; Tong, C.; Hu, Y.; Pang, J.; Yan, Z. Preparation and Characterization of Konjac Glucomannan-Based Bionanocomposite Film for Active Food Packaging. Food Hydrocoll. 2019, 89, 682–690. [Google Scholar] [CrossRef]
- Md Nor, S.; Ding, P. Trends and Advances in Edible Biopolymer Coating for Tropical Fruit: A Review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of Coating Thickness on Viscosity of Coating Solution Applied to Fruits and Vegetables by Dipping Method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Barciela, P.; Carpena, M.; Prieto, M.A. Edible Coatings as a Natural Packaging System to Improve Fruit and Vegetable Shelf Life and Quality. Foods 2023, 12, 3570. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Wang, M.; Miao, X.; Guo, F.; Deng, Z.; Bian, F.; Xiao, T.; Chen, C. Optimized Hybrid Edible Surface Coating Prepared with Gelatin and Cellulose Nanofiber for Cherry Tomato Preservation. Int. J. Biol. Macromol. 2024, 279, 134822. [Google Scholar] [CrossRef]
- Chettri, S.; Sharma, N.; Mohite, A.M. Formulation of Extracted Soyabean Starch Based Edible Coatings by Different Methods and Their Impact on Shelf Life of Sapota Fruit. J. Saudi Soc. Agric. Sci. 2024, 23, 205–211. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, X.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. Effect of Different Coating Methods on Coating Quality and Mango Preservation. Food Packag. Shelf Life 2023, 39, 101133. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Schmid, M.; Müller, K. Effect of Dipping and Vacuum Impregnation Coating Techniques with Alginate Based Coating on Physical Quality Parameters of Cantaloupe Melon. J. Food Sci. 2018, 83, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; De, J.; Berrios, J.; Sambo, P.; McHugh, T.H. Electrostatic and Conventional Spraying of Alginate-Based Edible Coating with Natural Antimicrobials for Preserving Fresh Strawberry Quality. Food Bioproc Tech. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Rodríguez, G.M.; Sibaja, J.C.; Espitia, P.J.P.; Otoni, C.G. Antioxidant Active Packaging Based on Papaya Edible Films Incorporated with Moringa Oleifera and Ascorbic Acid for Food Preservation. Food Hydrocoll. 2020, 103, 105630. [Google Scholar] [CrossRef]
- Kumar, A.V.; Hasan, M.; Mangaraj, S.; M, P.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and Its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kathuria, D.; Singh, N. Edible Films and Coatings, Its Chemical Crosslinking, Starch-Protein Interaction and Application in Food System: A Systematic Review. Int. J. Biol. Macromol. 2025, 306, 141726. [Google Scholar] [CrossRef]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/Wax Blend Extrusion for Production of Edible Films as Carriers of Potassium Sorbate—A Comparative Study of Waxes and Potassium Sorbate Effect. Food Packag. Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Sapna; Sharma, C.; Pathak, P.; Gautam, S. Chitosan Edible Coatings Loaded with Bioactive Components for Fruits and Vegetables: A Step Toward Sustainable Development Goals. Food Bioproc Tech. 2025, 18, 4975–5009. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a Biopolymer With Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef]
- Khamis, Y.; Hashim, A. Inhibitory Effect of Clay/Chitosan Nanocomposite against Penicillium digitatum on Citrus and Its Possible Mode of Action. Jordan. J. Biol. Sci. 2020, 13, 349–355. [Google Scholar]
- Al-Dhabaan, F.A.; Shoala, T.; Ali, A.A.M.; Alaa, M.; Abd-Elsalam, K.A. Chemically-Produced Copper, Zinc Nanoparticles and Chitosan—Bimetallic Nanocomposites and Their Antifungal Activity against Three Phytopathogenic Fungi. Int. J. Ofagricultural Technol. 2017, 13, 753–769. [Google Scholar]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active Packaging from Chitosan-Titanium Dioxide Nanocomposite Film for Prolonging Storage Life of Tomato Fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.F.; Youssef, K.; Abd-Elsalam, K.A. Ecofriendly Nanomaterials for Controlling Gray Mold of Table Grapes and Maintaining Postharvest Quality. Eur. J. Plant Pathol. 2019, 154, 377–388. [Google Scholar] [CrossRef]
- de Menezes, F.L.G.; de Lima Leite, R.H.; dos Santos, F.K.G.; Aria, A.I.; Mendes Aroucha, E.M. TiO2 Incorporated into a Blend of Biopolymeric Matrices Improves Film Properties and Affects the Postharvest Conservation of Papaya Fruits under UV Light. Food Chem. 2024, 433, 137387. [Google Scholar] [CrossRef]
- Zubair, M.; Rauf, Z.; Nawaz, H.; Shahzad, S.; Ullah, A. A Review of Recent Advances in Starch Derived Bionanocomposites for Food Packaging Applications. Nano-Struct. Nano-Objects 2024, 39, 101204. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Martínez-Ortiz, M.A.; Palma-Rodríguez, H.M.; Montalvo-González, E.; Sáyago-Ayerdi, S.G.; Utrilla-Coello, R.; Vargas-Torres, A. Effect of Using Microencapsulated Ascorbic Acid in Coatings Based on Resistant Starch Chayotextle on the Quality of Guava Fruit. Sci. Hortic. 2019, 256, 108604. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing Shelf Life of Strawberries (Fragaria Ssp) by Using a Banana Starch-Chitosan-Aloe Vera Gel Composite Edible Coating. Int. J. Food Sci. Technol. 2020, 55, 92–98. [Google Scholar] [CrossRef]
- Kaur, M.; Kalia, A.; Thakur, A. Effect of Biodegradable Chitosan–Rice-Starch Nanocomposite Films on Post-Harvest Quality of Stored Peach Fruit. Starch-Stärke 2017, 69, 1600208. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Lv, J.; Zhang, X.; Li, Y.; Han, X.; Zhang, W. Development of Starch-Based Films Reinforced with Curcumin-Loaded Nanocomplexes: Characterization and Application in the Preservation of Blueberries. Int. J. Biol. Macromol. 2024, 264, 130464. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Gómez-Hoyos, C.; Zuluaga-Gallego, R.; López-Córdoba, A. Alginate-Edible Coatings for Application on Wild Andean Blueberries (Vaccinium meridionale Swartz): Effect of the Addition of Nanofibrils Isolated from Cocoa By-Products. Polymers 2020, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Emamifar, A.; Bavaisi, S. Nanocomposite Coating Based on Sodium Alginate and Nano-ZnO for Extending the Storage Life of Fresh Strawberries (Fragaria × ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]
- Saputri, A.E.; Praseptiangga, D.; Rochima, E.; Panatarani, C.; Joni, I.M. Mechanical and Solubility Properties of Bio-Nanocomposite Film of Semi Refined Kappa Carrageenan/ZnO Nanoparticles. AIP Conf. Proc. 2018, 1927, 030040. [Google Scholar] [CrossRef]
- Ni, Y.; Shi, S.; Li, M.; Zhang, L.; Yang, C.; Du, T.; Wang, S.; Nie, H.; Sun, J.; Zhang, W.; et al. Visible Light Responsive, Self-Activated Bionanocomposite Films with Sustained Antimicrobial Activity for Food Packaging. Food Chem. 2021, 362, 130201. [Google Scholar] [CrossRef]
- Mehmood, Z.; Sadiq, M.B.; Khan, M.R. Gelatin Nanocomposite Films Incorporated with Magnetic Iron Oxide Nanoparticles for Shelf Life Extension of Grapes. J. Food Saf. 2020, 40, e12814. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Sun, Y.; Chen, B.; Wu, X.; Le, T. Improvement of Banana Postharvest Quality Using a Novel Soybean Protein Isolate/Cinnamaldehyde/Zinc Oxide Bionanocomposite Coating Strategy. Sci. Hortic. 2019, 258, 108786. [Google Scholar] [CrossRef]
- Motamedi, E.; Nasiri, J.; Malidarreh, T.R.; Kalantari, S.; Naghavi, M.R.; Safari, M. Performance of Carnauba Wax-Nanoclay Emulsion Coatings on Postharvest Quality of ‘Valencia’ Orange Fruit. Sci. Hortic. 2018, 240, 170–178. [Google Scholar] [CrossRef]
- Resende, N.S.; Gonçalves, G.A.S.; Reis, K.C.; Tonoli, G.H.D.; Boas, E.V.B. V Chitosan/Cellulose Nanofibril Nanocomposite and Its Effect on Quality of Coated Strawberries. J. Food Qual. 2018, 2018, 1727426. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.R.M. Antimicrobial Activity, Physical, Mechanical and Barrier Properties of Sugar Palm Based Nanocellulose/Starch Biocomposite Films Incorporated with Cinnamon Essential Oil. J. Mater. Res. Technol. 2021, 11, 144–157. [Google Scholar] [CrossRef]
- Salehudin, M.H.; Salleh, E.; Mamat, S.N.H.; Muhamad, I.I. Starch Based Active Packaging Film Reinforced with Empty Fruit Bunch (EFB) Cellulose Nanofiber. Procedia Chem. 2014, 9, 23–33. [Google Scholar] [CrossRef]
- Hashem, A.H.; El-Naggar, M.E.; Abdelaziz, A.M.; Abdelbary, S.; Hassan, Y.R.; Hasanin, M.S. Bio-Based Antimicrobial Food Packaging Films Based on Hydroxypropyl Starch/Polyvinyl Alcohol Loaded with the Biosynthesized Zinc Oxide Nanoparticles. Int. J. Biol. Macromol. 2023, 249, 126011. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, M.S.; Salama, H.E. Development of Alginate-Based Edible Coatings of Optimized UV-Barrier Properties by Response Surface Methodology for Food Packaging Applications. Int. J. Biol. Macromol. 2022, 212, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Active Bilayer Films Based on Cassava Starch Incorporating ZnO Nanorods and PVA Electrospun Mats Containing Rosemary Extract. Food Hydrocoll. 2020, 108, 106054. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Cao, Y.; Lan, T.; Chen, H.; Qin, Y. Effects of PLA Film Incorporated with ZnO Nanoparticle on the Quality Attributes of Fresh-Cut Apple. Nanomaterials 2017, 7, 207. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Qaisar, M.; Jahangir, M.; Abbasi, K.S.; Khan, S.U.; Ali, N.; Liaquat, M. Influence of CMC- and Guar Gum-Based Silver Nanoparticle Coatings Combined with Low Temperature on Major Aroma Volatile Components and the Sensory Quality of Kinnow (Citrus Reticulata). Int. J. Food Sci. Technol. 2016, 51, 2345–2352. [Google Scholar] [CrossRef]
- Yan, X.; Meng, F.; Van, T.T.; Tanaka, F.; Tanaka, F. Impact of Chitosan, Trans-Cinnamaldehyde, Poly (Vinyl Alcohol) and TiO2 Bio-Nanocomposites on Preservation and Flavor of Postharvest Button Mushroom (Agaricus Bisporus). LWT 2025, 217, 117377. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The Synergistic Effects of Cinnamon Essential Oil and Nano TiO2 on Antimicrobial and Functional Properties of Sago Starch Films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Junqueira-Gonçalves, M.P.; Salinas, G.E.; Bruna, J.E.; Niranjan, K. An Assessment of Lactobiopolymer-Montmorillonite Composites for Dip Coating Applications on Fresh Strawberries. J. Sci. Food Agric. 2017, 97, 1846–1853. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic Starch/Clay Nanocomposites Loaded with Essential Oil Constituents as Packaging for Strawberries − In Vivo Antimicrobial Synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Jha, P. Effect of Plasticizer and Antimicrobial Agents on Functional Properties of Bionanocomposite Films Based on Corn Starch-Chitosan for Food Packaging Applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef]
- Dam, X.T.; Duong, T.M.; Mai, D.H.; Thai, H.; Vu, V.A.; Vu, Q.T.; Ngo, T.C.Q.; Nguyen, T.A.; Nguyen, T.C. Preparation of the Novel Bio-Nanocomposites Based on Chitosan, Piper Betle Leaf Extract and MgO Nanoparticles for Chili Preservation. Polym. Eng. Sci. 2024, 64, 2795–2811. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Liu, Y.; Song, Y.; Wu, T.; Zhang, M. Using Soy Protein SiOx Nanocomposite Film Coating to Extend the Shelf Life of Apple Fruit. Int. J. Food Sci. Technol. 2017, 52, 2018–2030. [Google Scholar] [CrossRef]
- Wu, L.; Lv, S.; Wei, D.; Zhang, S.; Zhang, S.; Li, Z.; Liu, L.; He, T. Structure and Properties of Starch/Chitosan Food Packaging Film Containing Ultra-Low Dosage GO with Barrier and Antibacterial. Food Hydrocoll. 2023, 137, 108329. [Google Scholar] [CrossRef]
- Huang, X.; Wang, F.; Hu, W.; Zou, Z.; Tang, Q.; Li, H.; Xu, L. Smart Packaging Films Based on Corn Starch/Polyvinyl Alcohol Containing Nano SIM-1 for Monitoring Food Freshness. Int. J. Biol. Macromol. 2024, 256, 128373. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccolli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Development of Quaternary Nanocomposites Made up of Cassava Starch, Cocoa Butter, Lemongrass Essential Oil Nanoemulsion, and Brewery Spent Grain Fibers. J. Food Sci. 2021, 86, 1979–1996. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of Antimicrobial Nanoemulsions as Edible Coatings: Impact on Safety and Quality Attributes of Fresh-Cut Fuji Apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Dai, X.; Dong, F.; Dong, Z.; Bai, Z.; Mao, L. Enhanced Antibacterial and Antioxidant Activities of Chlorogenic Acid Loaded Sweet Whey/Starch Active Films for Edible Food Packaging. LWT 2024, 199, 116118. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, S.; Das, D. Rice Husk Fiber-Reinforced Starch Antimicrobial Biocomposite Film for Active Food Packaging. J. Clean. Prod. 2023, 421, 138525. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Cai, J. Robust Design of Starch Composite Nanofibrous Films for Active Food Packaging: Towards Improved Mechanical, Antioxidant, and Antibacterial Properties. Int. J. Biol. Macromol. 2024, 260, 129329. [Google Scholar] [CrossRef]


| Host | Disease | Pathogens | References |
|---|---|---|---|
| Oranges | Green mold, blue mold, sour rot | Penicillium digitatum, Penicillium italicum, Geotrichum citri aurantii. | [53,54,55,56] |
| Papaya | Soft rot, anthracnose | Rhizopus oryzae, Mucor irregularis, Gilbertella persicaria, Colletotrichum plurivorum, Colletotrichum brevisporum, Colletotrichum truncatum, Colletotrichum fructicola. | [57,58] |
| Grapes | Anthracnose, gray mold | Colletotrichum gloeosporioides, Botrytis cinerea. | [59,60,61] |
| Pome fruit | Blue mold, bull’s eye rot, bitter rot | Penicillium expansum, Neofabraea spp, Colletotrichum spp. | [62,63] |
| Mango | Anthracnose, stem-end rot | Colletotrichum gloeosporioides, Colletotrichum asianum, Colletotrichum fructicola, Colletotrichum siamense, Dothiorella spp. | [35,37,64] |
| Loquat | Anthracnose | Colletotrichum acutatum | [36] |
| Peach | Brown rot | Monilinia fructicola | [65] |
| Plum | Brown rot | Monilinia fructicola | [65] |
| Apples | Blue mold, anthracnose | Penicillium expansum, Botrytis cinerea, Penicillium expansum. | [62,66] |
| Kiwi | Soft rot | Phomopsis spp., Botryosphaeria dothidea | [40] |
| Cherry | Brown rot | Monilinia fructicola | [65] |
| Banana | Anthracnose | Colletotrichum musae | [67] |
| Apricot | Brown rot | Monilinia fructicola | [65] |
| Mume fruit | Brown rot | Monilinia fructicola | [65] |
| Litchi | Soft rot | Alternaria alternata | [68] |
| Blueberry | Gray mold | Botrytis cinerea | [34] |
| Tomatoes | Anthracnose, soft rot | Alternaria alternata | [32,33] |
| Nanomaterial | Biopolymer | Complementary Materials | Pathogen | Fruit | References |
|---|---|---|---|---|---|
| Cellulose nanofibrils (3–5%) | Chitosan | Acetic acid | - | Strawberries | [170] |
| Cellulose nanofibrils (0.1–0.3%) | Alginate | Glycerol | - | Blueberries | [163] |
| Nanocrystalline cellulose (0.05 g) | Sugar palm starch | Glycerol, sorbitol, cinnamon EO (0.8–2%), tween-80 | Escherichia coli, Bacillus subtilis, Staphylococcus aureus | - | [171] |
| Cellulose nanofiber (2–10%) | Wheat starch/chitosan | Glycerol | Escherichia coli, Bacillus subtilis | - | [172] |
| Cellulose nanofiber (0.1–0.3%) | Gelatin | - | - | Cherry tomato | [141] |
| ZnO-NPs (0.25–1.25%) | Yam starch | Sodium tripolyphosphate, sorbitol, microcrystalline cellulose, eugenol | Escherichia coli, Staphylococcus aureus | - | [87] |
| ZnO/Halloysite (3–5%) | Sodium alginate | Xanthan gum, CaCl2, licorice root extract | Escherichia coli, Staphylococcus aureus | - | [105] |
| ZnO-NPs (50 mg/mL) | Hydroxypropyl starch | PVA, palmitic acid, glycerol | Escherichia coli, Staphylococcus aureus, Fusarium oxysporum, Aspergillus niger, Penicillium expansum, Aspergillus flavus | - | [173] |
| ZnO-NPs (1–5%) | Alginate | Aloe vera gel, glycerol | Escherichia coli, Syncephalastrum racemosum, Staphylococcus aureus | Tomatoes | [174] |
| ZnO nanorods (1%) | Cassava starch | Plasticizers, glycerol, sorbitol, citric acid, sodium hypophosphite, PVA, rosemary extract | Escherichia coli | - | [175] |
| ZnO-NPs (1–3%) | PLA | Cinnamaldehyde | - | Apple | [176] |
| ZnO-NPs (2 mg/mL) | Soybean protein isolate | Cinnamaldehyde, glycerol, tween-80 | - | Bananas | [168] |
| Nano ZnO (0.25–1.25 g/L) | Alginate | Glycerol | - | Strawberries | [164] |
| ZnO-NPs (10 ppm), Ag-NPs (10 ppm) | Chitosan/rice starch | - | Escherichia coli, Staphylococcus aureus | Peach | [161] |
| Ag-NPs/COFs (1–5%) | Carboxymethyl chitosan | Glycerol | Escherichia coli, Staphylococcus aureus, Bacillus cereus, Cronobacter sakazakii, Listeria monocytogenes, Salmonella | Citrus | [104] |
| Ag-NPs (3–5%) | Cellulose acetate/gelatin | Triethyl citrate, organo-clay, thymol | Escherichia coli, Salmonella, Pseudomonas, Aspergillus niger, Aspergillus flavus, Staphylococcus aureus | - | [129] |
| Ag-NPs (0.03 mg/L) | Gum guar | Carboxymethyl cellulose | - | Kinnow | [177] |
| AgNO3 (40 μL, 1M) | Rice starch | PVA | Salmonella typhimurium, Staphylococcus aureus | - | [88] |
| Ag-NPs (0.5%), TiO2-NPs (0.5–1%) | PLA | - | Escherichia coli, Listeria monocytogenes | - | [86] |
| TiO2 (0.01%) | Chitosan/alginate | Cinnamon EO, acetic acid, glycerol | - | Mango | [143] |
| Nano-TiO2 (0.02%) | Chitosan | PVA, tween-80, trans-cinnamaldehyde, acetic acid | Escherichia coli, Staphylococcus aureus | Mushrooms | [178] |
| Nano TiO2 (0.01–0.03 g) | Chitosan | Glycerin, acetic acid | - | Mango | [128] |
| TiO2 (1–5%) | Sago starch | Glycerol, sorbitol, cinnamon EO | Escherichia coli, Salmonella typhimurium, Staphylococcus aureus | - | [179] |
| TiO2 (1–2%) | Chitosan | Acetic acid, glycerol | - | Papaya | [156] |
| Nano clay (1%) | Chitosan | Acetic acid | Penicillium digitatum | Oranges | [152] |
| Nano clay (5%) | Whey protein | Calcium caseinate, potassium sorbate, glycerol | - | Strawberries | [180] |
| Nano clay (4.8%) | Corn starch | Glycerol, carvacrol, thymol | Botrytis cinerea | Strawberries | [181] |
| Nano clay (0.01 g) | Corn starch/Chitosan | Glycerol, sorbitol, potassium sorbate | Rhodococcus opacus, Aspergillus niger | - | [182] |
| Nano clay (0.5–1%) | Carnauba Wax/ beeswax | Oleic acid, ammonia | - | Oranges | [169] |
| MgO-NPs (3–10%) | Chitosan | Acetic acid, sodium tripolyphosphate, Piper betle leaf extract | Escherichia coli, Staphylococcus aureus | Chili peppers | [183] |
| Nano SiOx (0.1–0.4 g/g) | Soy protein | Glycerol | - | Apples | [184] |
| Graphene oxide (0.03–0.1%) | Corn starch/Chitosan | Carboxymethyl cellulose, citric acid, urea | Escherichia coli, Staphylococcus aureus | Tomatoes | [185] |
| Cu-MOF (0.1–0.9 g) | Sodium alginate | α-Lipoic acid, glycerol | Botrytis cinerea, Escherichia coli, Staphylococcus aureus | Peaches, grapes, blueberries | [51] |
| Substituted imidazolate MOF (5–20 mg) | Corn starch | PVA, glycerol | Escherichia coli, Staphylococcus aureus | - | [186] |
| Lemongrass EO (5–10%) | Cassava starch | Glycerol, tween-80, cocoa butter, brewery spent grain | Escherichia coli, Staphylococcus aureus | - | [187] |
| Nanoemulsion (lemongrass EO) (0.1–1%) | Alginate | Tween-80 | Escherichia coli | Fuji apples | [188] |
| Schinus molle EO (100 μL) | Chitosan | Acetic acid | Colletotrichum gloeosporioides | - | [125] |
| Chlorogenic acid (0.5–3%) | Corn starch/sweet whey | Glycerol | Escherichia coli | Banana | [189] |
| Benzalkonium chloride (2.5 mL) | Corn starch | Glycerol, sorbitol, rice husk fiber | Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae. | Strawberries | [190] |
| Tannic acid (1–8%) | Corn starch | Fe3+ | Escherichia coli, Staphylococcus aureus | - | [191] |
| Curcumin-loaded nanocomplexes (2–11%) | Corn starch | Perilla seed protein isolate, glycerol | - | Blueberries | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Silva, J.M.; López-García, U.M.; Gutierrez-Martinez, P.; Flores-Ramírez, A.Y.; Ramos-Bell, S.; Moreno-Hernández, C.; Rivas-García, T.; González-Estrada, R.R. Bionanocomposite Coating Film Technologies for Disease Management in Fruits and Vegetables. Horticulturae 2025, 11, 832. https://doi.org/10.3390/horticulturae11070832
Sánchez-Silva JM, López-García UM, Gutierrez-Martinez P, Flores-Ramírez AY, Ramos-Bell S, Moreno-Hernández C, Rivas-García T, González-Estrada RR. Bionanocomposite Coating Film Technologies for Disease Management in Fruits and Vegetables. Horticulturae. 2025; 11(7):832. https://doi.org/10.3390/horticulturae11070832
Chicago/Turabian StyleSánchez-Silva, Jonathan M., Ulises M. López-García, Porfirio Gutierrez-Martinez, Ana Yareli Flores-Ramírez, Surelys Ramos-Bell, Cristina Moreno-Hernández, Tomás Rivas-García, and Ramsés Ramón González-Estrada. 2025. "Bionanocomposite Coating Film Technologies for Disease Management in Fruits and Vegetables" Horticulturae 11, no. 7: 832. https://doi.org/10.3390/horticulturae11070832
APA StyleSánchez-Silva, J. M., López-García, U. M., Gutierrez-Martinez, P., Flores-Ramírez, A. Y., Ramos-Bell, S., Moreno-Hernández, C., Rivas-García, T., & González-Estrada, R. R. (2025). Bionanocomposite Coating Film Technologies for Disease Management in Fruits and Vegetables. Horticulturae, 11(7), 832. https://doi.org/10.3390/horticulturae11070832