Critical Levels of Copper, Zinc, and Manganese Toxicity in Soil and Tissues of Plants That Cohabit Vineyards in the Pampa Biome
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the Areas and Sampling of Soil and Tissue
2.2. Dry Mass and Tissue Concentrations of Cu, Zn, and Mn
2.3. Available Cu, Zn, and Mn in the Soil
2.4. Statistical Analysis
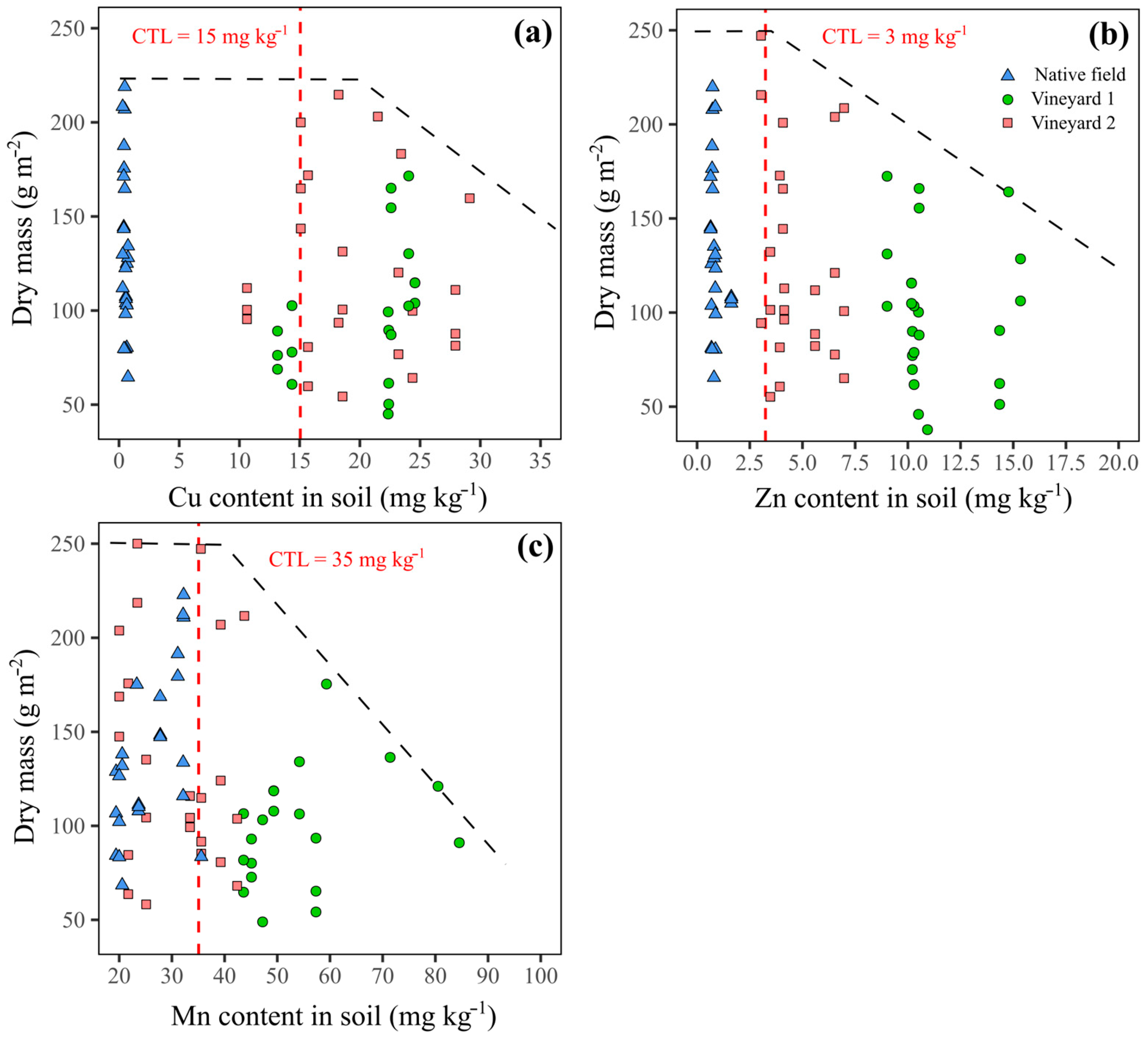
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanfranco, B.; Fernández, E.; Ferraro, B.; de Lima, J.M.S. Historical changes in the Pampas biome, land use, and climate change. In Handbook of Behavioral Economics and Climate Change; Edward Elgar Publishing: Cheltenham, UK, 2022; pp. 162–191. [Google Scholar] [CrossRef]
- Brunetto, G.; Melo, G.W.B.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Fourment, M. Research and innovations in latin american vitiviniculture: A review. Horticulturae 2025, 11, 506. [Google Scholar] [CrossRef]
- Vento, B.; Ginebra, M.; Vallebella, M.; Bonjour, L.; Ontivero, M.; Duplancic, A.; Mezzatesta, D.; Martinez-Carretero, E. Assessing the contribution of spontaneous vegetation to carbon storage and biodiversity in a vineyard of Argentina. Agroecol. Sustain. Food Syst. 2025, 49, 41–62. [Google Scholar] [CrossRef]
- Garavani, A.; Capri, C.; Del Zozzo, F.; Diti, I.; Poni, S.; Gatti, M. Relationship between intra-parcel variability and carbon allocation and sequestration in a mature Barbera (Vitis vinifera L.) vineyard ecosystem. Sci. Hortic. 2023, 309, 111617. [Google Scholar] [CrossRef]
- De Conti, L.; Marques, A.C.R.; Ceretta, C.A.; Tarouco, C.P.; Nicoloso, F.T.; Ferreira, P.A.A.; Brunetto, G. Tolerance and phytoremediation potential of grass species native to South American grasslands to copper-contaminated soils. Int. J. Phytoremediation 2021, 23, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Islam, M.S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Silva, F.B.V.; Nascimento, C.W.A.; Araújo, P.R.M.; Silva, L.H.V.; Silva, R.F. Assessing heavy metal sources in sugarcane Brazilian soils: An approach using multivariate analysis. Environ. Monit. Assess. 2016, 188, 457. [Google Scholar] [CrossRef]
- Poggere, G.; Gasparin, A.; Barbosa, J.Z.; Melo, G.W.; Corrêa, R.S.; Motta, A.C.V. Soil contamination by copper: Sources, ecological risks, and mitigation strategies in Brazil. J. Trace Elem. Min. 2023, 4, 100059. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and molecular mechanisms of plant responses to copper stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Cruz, F.J.R.; da Cruz Ferreira, R.L.; Conceição, S.S.; Lima, E.U.; de Oliveira Neto, C.F.; Galvão, J.R.; Lopes, S.C.; Viegas, I.D.J.M. Copper toxicity in plants: Nutritional, physiological, and biochemical aspects. In Advances in Plant Defense Mechanisms; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Castillo-González, J.; Ojeda-Barrios, D.; Hernández-Rodríguez, A.; González-Franco, A.C.; Robles-Hernández, L.; López-Ochoa, G.R. Zinc metalloenzymes in plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 191–248. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2014, 167, 176–188. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese deficiency in plants: The impact on photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Schwalbert, R.; Milanesi, G.D.; Stefanello, L.; Moura-Bueno, J.M.; Drescher, G.L.; Marques, A.C.R.; Nicoloso, F.T. How do native grasses from South America handle zinc excess in the soil? A physiological approach. Environ. Exp. Bot. 2022, 195, 104779. [Google Scholar] [CrossRef]
- Morsch, L.; Somavilla, L.M.; Trentin, E.; Silva, K.R.; de Oliveira, J.M.S.; Brunetto, G.; Simão, D.G. Root system structure as a criterion for the selection of grapevine genotypes that are tolerant to excess copper and the ability of phosphorus to mitigate toxicity. Plant Physiol. Biochem. 2022, 171, 147–156. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Jia, L.; Chen, H.; Wei, X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol. Environ. Saf. 2013, 89, 150–157. [Google Scholar] [CrossRef]
- Somavilla, L.M.; Simão, D.G.; Hammerschmitt, R.K.; Tiecher, T.L.; Oliveira, J.M.S.; Mayer, N.A.; Pavanello, E.P.; Trentin, E.; Belles, S.W.; Bruneto, G. Structural changes in roots of peach rootstock cultivars grown in soil with high zinc content. Sci. Hortic. 2018, 237, 1–10. [Google Scholar] [CrossRef]
- Girotto, E.; Ceretta, C.A.; Rossato, L.V.; Farias, J.G.; Tiecher, T.L.; De Conti, L.; Nicoloso, F.T. Triggered antioxidant defense mechanism in maize grown in soil with accumulation of Cu and Zn due to intensive application of pig slurry. Ecotoxicol. Environ. Saf. 2013, 93, 145–155. [Google Scholar] [CrossRef]
- Skórka, M.; Sieprawska, A.; Telk, A. The implication of manganese surplus on plant cell homeostasis: A review. J. Plant Growth Regul. 2023, 42, 1327–1341. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Zhou, H.; Wang, R.; Zhang, P.; Wang, H.; Xu, J. Manganese toxicity inhibited root growth by disrupting auxin biosynthesis and transport in Arabidopsis. Front. Plant Sci. 2017, 8, 272. [Google Scholar] [CrossRef]
- Silva, I.C.B.; Marques, A.C.R.; Quadros, F.F.; Sans, G.A.; Soares, V.M.; De Conti, L.; Brunetto, G. Spatial variation of herbaceous cover species community in Cu-contaminated vineyards in Pampa biome. Environ. Sci. Pollut. Res. Int. 2020, 27, 13348–13359. [Google Scholar] [CrossRef]
- Woch, M.W.; Kapusta, P.; Stefanowicz, A.M. Variation in dry grassland communities along a heavy metals gradient. Ecotoxicology 2016, 25, 80–90. [Google Scholar] [CrossRef]
- Vidic, T.; Jogan, N.; Drobne, D.; Vilhar, B. Natural revegetation in the vicinity of the former lead smelter in Žerjav, Slovenia. Environ. Sci. Technol. 2006, 40, 4119–4125. [Google Scholar] [CrossRef]
- Rola, K.; Osyczka, P.; Nobis, M.; Drozd, P. How do soil factors determine vegetation structure and species richness in post-smelting dumps? Ecol. Eng. 2015, 75, 332–342. [Google Scholar] [CrossRef]
- Wong, M. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Dresseno, A.L.; Guido, A.; Balogianni, V.; Overbeck, G.E. Negative effects of an invasive grass, but not of native grasses, on plant species richness along a cover gradient. Austral Ecol. 2018, 43, 949–954. [Google Scholar] [CrossRef]
- Santos, A.P.; Matias, C.A.; Cantoni, F.; Miquelluti, D.J.; Campos, M.L. Critical limits for zinc to forage species. Rev. Ibero-Am. Ciênc. Ambient. 2021, 12, 97–107. [Google Scholar] [CrossRef]
- Webb, R.A. Use of the boundary line in the analysis of biological data. J. Hortic. Sci. 1972, 47, 309–319. [Google Scholar] [CrossRef]
- Makowski, D.; Doré, T.; Monod, H. A new method to analyze relationships between yield components with boundary lines. Agron. Sustain. Dev. 2007, 27, 119–128. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- INMET—Instituto Nacional de Meteorologia. Climatological Normals. Available online: https://portal.inmet.gov.br/normais (accessed on 5 June 2023).
- Streck, E.V.; Kämpf, N.; Dalmolin, R.S.D.; Klamt, E.; Nascimento, P.C.; Schneider, P.; Giasson, E.; Pinto, L.F.S. Solos do Rio Grande do Sul, 3rd ed.; UFRGS: Porto Alegre, Brazil, 2018; 251p. (In Portuguese) [Google Scholar]
- USDA—Soil Survey Staff. Keys to Soil Taxonomy; United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Embrapa—Empresa Brasileira de Pesquisa Agropecuária. Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed. rev. ampl.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; 627p. (In Portuguese) [Google Scholar]
- Tedesco, M.; Gianello, C.; Bissani, C. Análises de Solo, Plantas e Outros Materiais; UFRGS: Porto Alegre, Brazil, 1995; p. 174. (In Portuguese) [Google Scholar]
- Liang, Z.; Qian, S.S.; Wu, S.; Chen, H.; Liu, Y.; Yu, Y.; Yi, X. Using Bayesian change point model to enhance understanding of the shifting nutrients-phytoplankton relationship. Ecol. Modell. 2019, 393, 120–126. [Google Scholar] [CrossRef]
- Kruschke, J.K.; Liddell, T.M. Bayesian data analysis for newcomers. Psychon. Bull. Rev. 2018, 25, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Gelman, A.; Hill, J. Data analysis using regression and multilevel models. In Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: New York, NY, USA, 2007; p. 625. [Google Scholar]
- Plummer, M. rjags: Bayesian Graphical Models Using MCMC; R Package Version 3–13; R Foundation for Statistical Computing: Vienna, Austria, 2016; pp. 1–19. [Google Scholar]
- R Core Team. The R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 15 February 2025).
- Girotto, E.; Ceretta, C.A.; Rossato, L.V.; Farias, J.G.; Brunetto, G.; Miotto, A.; Nicoloso, F.T. Biochemical changes in black oat (Avena strigosa schreb) cultivated in vineyard soils contaminated with copper. Plant Physiol. Biochem. 2016, 103, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.W.; Lourenzi, C.R.; Comin, J.J.; Loss, A.; Girotto, E.; Ludwig, M.P.; Brunetto, G. Effect of organic and mineral fertilizers applications in pasture and no-tillage system on crop yield, fractions and contaminant potential of Cu and Zn. Soil Tillage Res. 2023, 225, 105523. [Google Scholar] [CrossRef]
- Brunetto, G.; Miotto, A.; Ceretta, C.A.; Schmitt, D.E.; Heinzen, J.; de Moraes, M.P.; Girotto, E. Mobility of copper and zinc fractions in fungicide-amended vineyard sandy soils. Arch. Agron. Soil Sci. 2013, 60, 609–624. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Yuan, X.; Browne, P.; Chen, L.; Ji, J. The influences of soil properties on Cu and Zn availability in soil and their transfer to wheat (Triticum aestivum L.) in the Yangtze River delta region, China. Geoderma 2013, 193, 131–139. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Baldi, E.; Miotto, A.; Toselli, M.; Ceretta, C.A.; Brunetto, G. Increasing phosphorus concentration in soil as a possible strategy to overcome Cu excess toxicity symptoms. Acta Hortic. 2018, 1228, 421–426. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Rizzardo, C.; Gottardi, S.; Terzano, R.; Alfeld, M.; Cesco, S. Iron allocation in leaves of Fe-deficient cucumber plants fed with natural Fe complexes. Physiol. Plant. 2015, 154, 82–94. [Google Scholar] [CrossRef]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta 2006, 1763, 595–608. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Chao, Z.F.; Chao, D.Y. Barriers and carriers for transition metal homeostasis in plants. Plant Commun. 2025, 6, 101235. [Google Scholar] [CrossRef] [PubMed]
- Eon, P.; Robert, T.; Goutouly, J.-P.; Aurelle, V.; Cornu, J.-Y. Cover crop response to increased concentrations of copper in vineyard soils: Implications for copper phytoextraction. Chemosphere 2023, 329, 138604. [Google Scholar] [CrossRef]
- Visconti, F.; López, R.; Olego, M.Á. The health of vineyard soils: Towards a sustainable viticulture. Horticulturae 2024, 10, 154. [Google Scholar] [CrossRef]
- Toselli, M.; Baldi, E.; Marcolini, G.; Malaguti, D.; Quartieri, M.; Sorrenti, G.; Marangoni, B. Response of potted grapevines to increasing soil copper concentration. Aust. J. Grape Wine Res. 2009, 15, 85–92. [Google Scholar] [CrossRef]
- De Conti, L.; Ceretta, C.A.; Melo, G.W.B.; Tiecher, T.L.; Silva, L.O.; Garlet, L.P.; Brunetto, G. Intercropping of young grapevines with native grasses for phytoremediation of Cu-contaminated soils. Chemosphere 2019, 216, 147–156. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Marques, D.M.; Veroneze Júnior, V.; Silva, A.B.; Mantovani, J.R.; Magalhães, P.C.; Souza, T.C. Copper toxicity on photosynthetic responses and root morphology of Hymenaea courbaril L. (Caesalpinioideae). Water Air Soil Pollut 2018, 229, 138. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, L.; Qin, J.; Wan, J.; Wang, R.; Li, S.; Xu, J. cGMP is involved in Zn tolerance through the modulation of auxin redistribution in root tips. Environ. Exp. Bot. 2018, 147, 22–30. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef] [PubMed]
- Cambrollé, J.; Mancilla-Leytón, J.M.; Muñoz-Vallés, S.; Figueroa-Luque, E.; Luque, T.; Figueroa, M.E. Evaluation of zinc tolerance and accumulation potential of the coastal shrub Limoniastrum monopetalum (L.) Boiss. Environ. Exp. Bot. 2013, 85, 50–57. [Google Scholar] [CrossRef]
- Fernando, D.R.; Marshall, A.T.; Forster, P.I.; Hoebee, S.E.; Siegele, R. Multiple metal accumulation within a manganese-specific genus. Am. J. Bot. 2013, 100, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Santos, E.F.; Santini, J.M.K.; Paixão, A.P.; Júnior, E.F.; Lavres, J.; Campos, M.; Dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 2017, 113, 6–19. [Google Scholar] [CrossRef]
- Xue, S.; Zhu, F.; Wu, C.; Lei, J.; Hartley, W.; Pan, W. Effects of manganese on the microstructures of Chenopodium ambrosioides L., a manganese tolerant plant. Int. J. Phytoremediation 2016, 18, 710–719. [Google Scholar] [CrossRef]
- Marastoni, L.; Sandri, M.; Pii, Y.; Valentinuzzi, F.; Brunetto, G.; Cesco, S.; Mimmo, T. Synergism and antagonisms between nutrients induced by copper toxicity in grapevine rootstocks: Monocropping vs. intercropping. Chemosphere 2019, 214, 563–578. [Google Scholar] [CrossRef]
- Thiesen, L.A.; Brunetto, G.; Trentin, E.; Silva, A.A.K.; Tabaldi, L.A.; Schwalbert, R.; Nicoloso, F.T. Subcellular distribution and physiological responses of native and exotic grasses from the Pampa biome subjected to excess manganese. Chemosphere 2023, 310, 136801. [Google Scholar] [CrossRef]

| Variable Analyzed | Native Field—NF | Vineyard 1—V1 | Vineyard 2—V2 |
|---|---|---|---|
| Dry mass (g m−2) | 138.03 (±43.83) | 105.20 (±38.87) | 130.73 (±55.19) |
| Cu–shoot (mg kg−1) | 7.53 (±1.77) | 49.11 (±30.25) | 66.81 (±48.49) |
| Zn–shoot (mg kg−1) | 32.11 (±4.11) | 123.87 (±31.33) | 77.19 (±30.24) |
| Mn–shoot (mg kg−1) | 290.22 (±54.41) | 188.49 (±54.80) | 379.13 (±118.22) |
| Cu–soil (mg kg−1) | 0.44 (±0.10) | 23.06 (±4.45) | 18.62 (±4.15) |
| Zn–soil (mg kg−1) | 1.01 (±0.23) | 9.37 (±1.65) | 4.30 (±1.07) |
| Mn–soil (mg kg−1) | 23.15 (±4.88) | 55.33 (±13.17) | 29.31 (±9.48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, F.N.; Morsch, L.; Moura-Bueno, J.M.; Tassinari, A.; Trentin, E.; Marques, A.C.R.; Andreolli, T.; Dias, B.G.; Tabaldi, L.A.; Brunetto, G. Critical Levels of Copper, Zinc, and Manganese Toxicity in Soil and Tissues of Plants That Cohabit Vineyards in the Pampa Biome. Horticulturae 2025, 11, 831. https://doi.org/10.3390/horticulturae11070831
de Oliveira FN, Morsch L, Moura-Bueno JM, Tassinari A, Trentin E, Marques ACR, Andreolli T, Dias BG, Tabaldi LA, Brunetto G. Critical Levels of Copper, Zinc, and Manganese Toxicity in Soil and Tissues of Plants That Cohabit Vineyards in the Pampa Biome. Horticulturae. 2025; 11(7):831. https://doi.org/10.3390/horticulturae11070831
Chicago/Turabian Stylede Oliveira, Filipe Nunes, Letícia Morsch, Jean Michel Moura-Bueno, Adriele Tassinari, Edicarla Trentin, Anderson César Ramos Marques, Talita Andreolli, Bianca Goularte Dias, Luciane Almeri Tabaldi, and Gustavo Brunetto. 2025. "Critical Levels of Copper, Zinc, and Manganese Toxicity in Soil and Tissues of Plants That Cohabit Vineyards in the Pampa Biome" Horticulturae 11, no. 7: 831. https://doi.org/10.3390/horticulturae11070831
APA Stylede Oliveira, F. N., Morsch, L., Moura-Bueno, J. M., Tassinari, A., Trentin, E., Marques, A. C. R., Andreolli, T., Dias, B. G., Tabaldi, L. A., & Brunetto, G. (2025). Critical Levels of Copper, Zinc, and Manganese Toxicity in Soil and Tissues of Plants That Cohabit Vineyards in the Pampa Biome. Horticulturae, 11(7), 831. https://doi.org/10.3390/horticulturae11070831








