Fine Mapping of BrTCP1 as a Key Regulator of Branching in Flowering Chinese Cabbage (Brassica rapa subsp. chinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Mapping Populations
2.2. DNA Isolation and Pool Construction
2.3. Genotyping and Linkage Map Construction
2.4. Cloning and Analysis of Gene Sequences
2.5. Determination of Expression Level by RT-qPCR
2.6. Bioinformatics Analysis
2.7. Identification of Homologous Mutants in Arabidopsis
3. Results
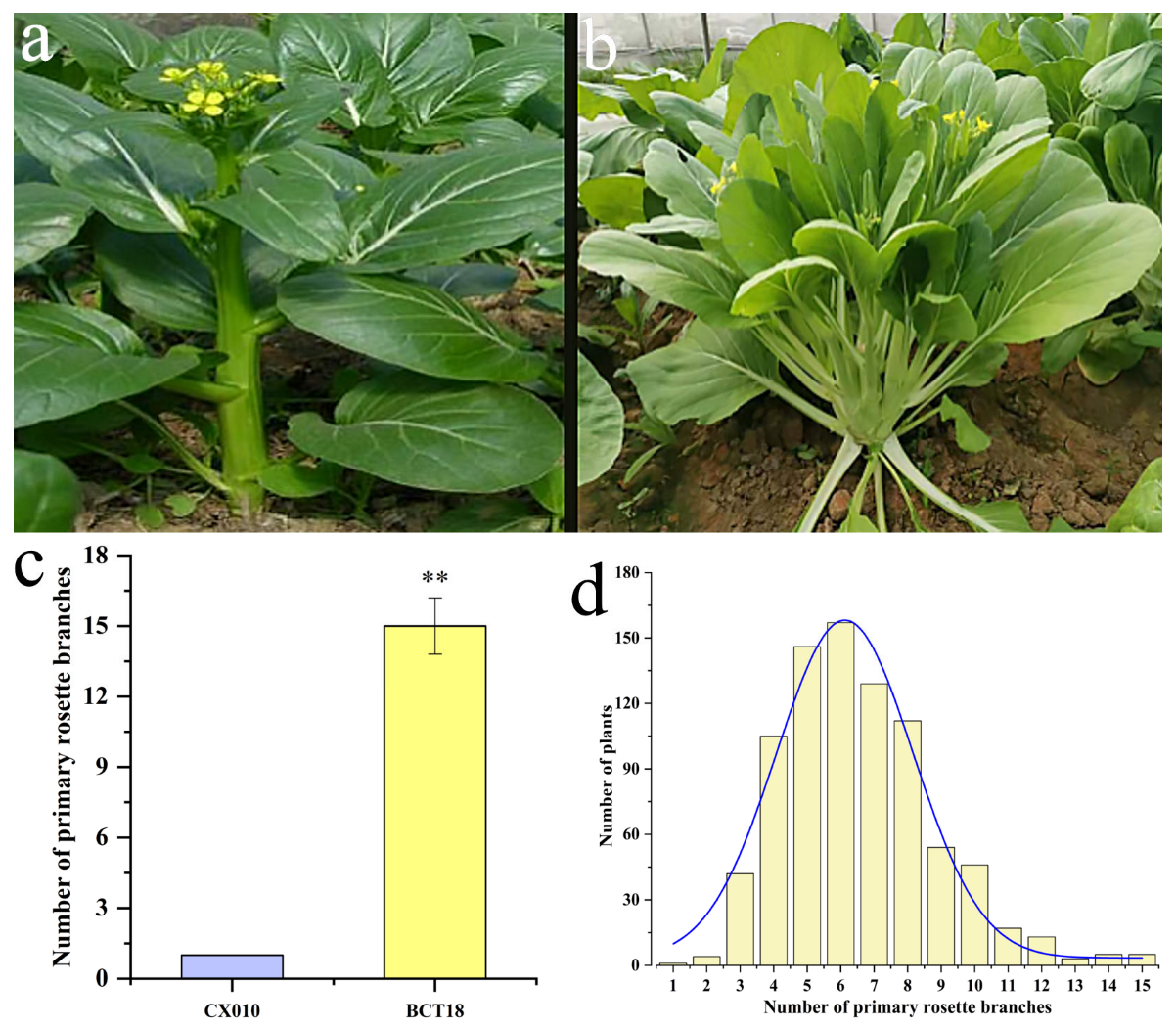
3.1. Phenotypic Observation and Inherent Characteristics of Branching in Flowering Chinese Cabbage
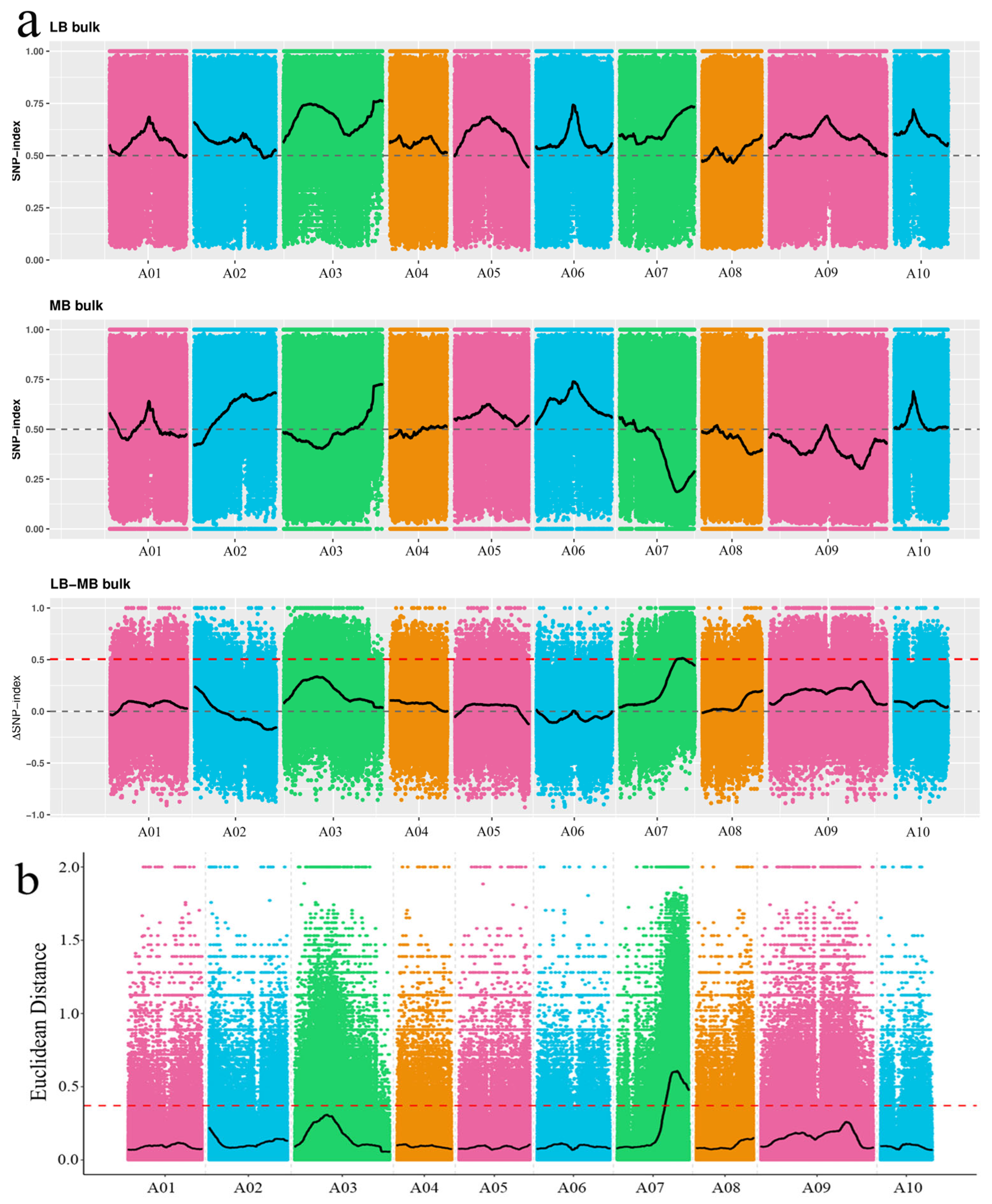
3.2. Preliminary Localization of Branching Candidate Intervals by BSA-Seq
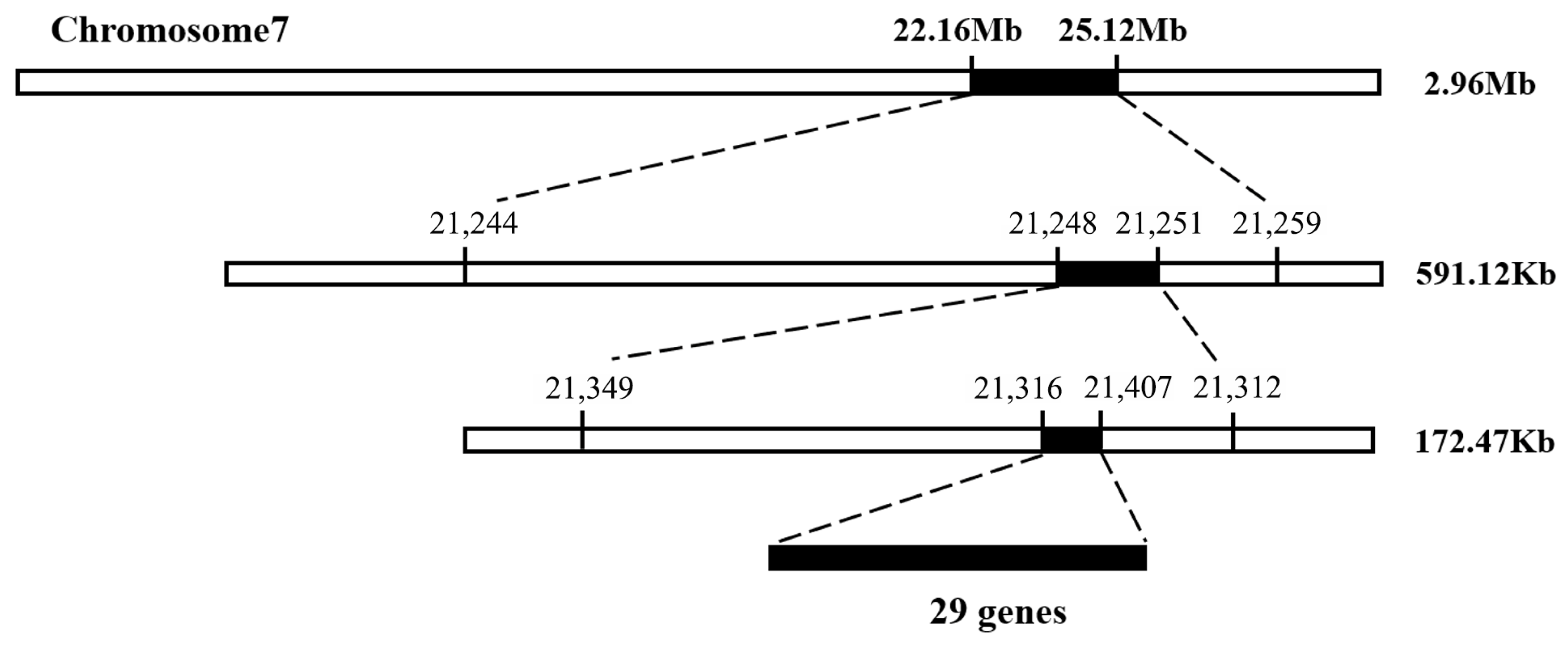
3.3. Fine Mapping Candidate Gene
3.4. Identification of Candidate Gene
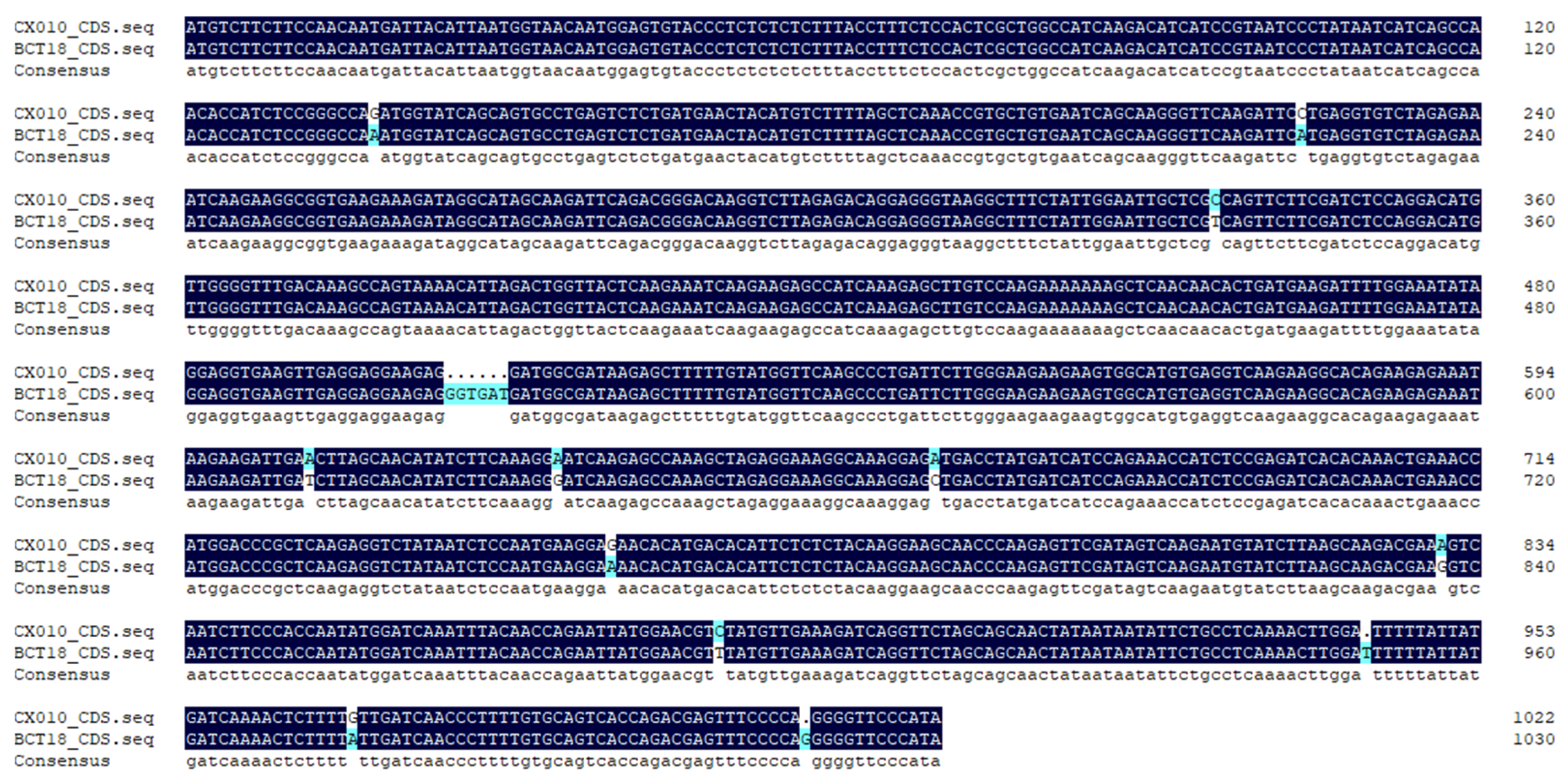
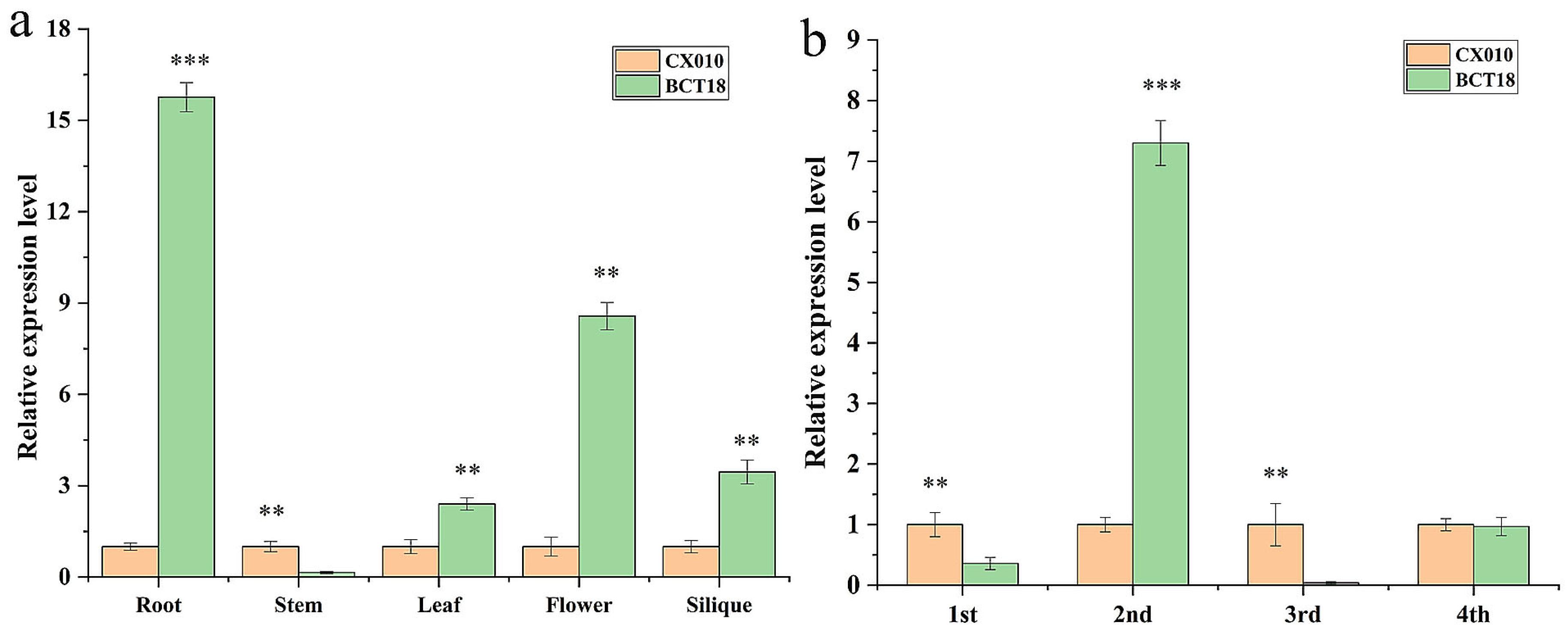
3.5. Cloning and Expression Pattern Analysis of BrTCP1 Gene
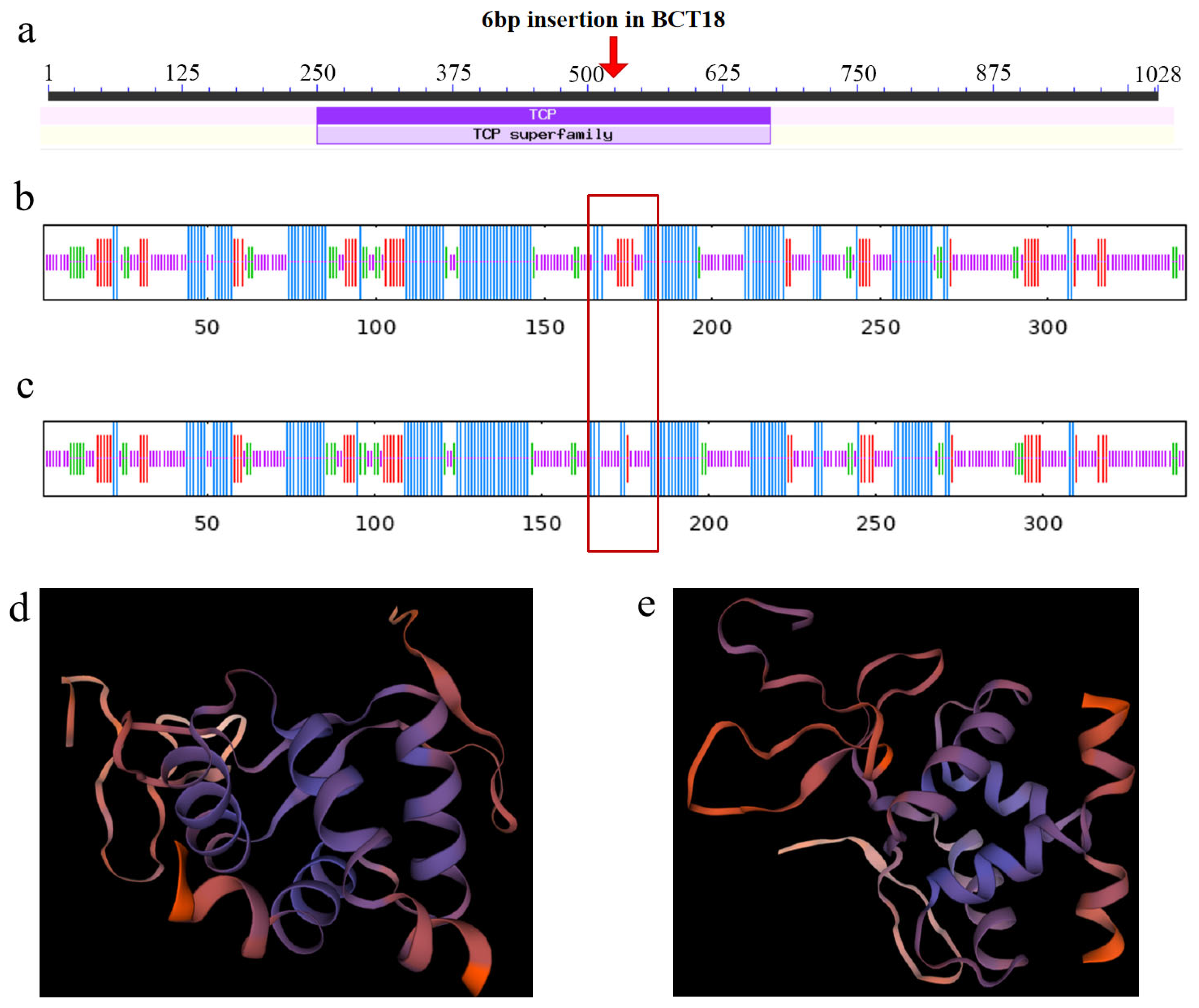
3.6. Structural Analysis of BrTCP1 Protein
3.7. The Arabidopsis Homologous TCP1 Mutant Exhibited a Multi-Branching Phenotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, R. Economic/academic importance of Brassica rapa. In The Brassica Rapa Genome; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–15. [Google Scholar] [CrossRef]
- Guan, J.; Li, J.; Yao, Q.; Liu, Z.; Feng, H.; Zhang, Y. Identification of two tandem genes associated with primary rosette branching in flowering Chinese cabbage. Front. Plant Sci. 2022, 13, 1083528. [Google Scholar] [CrossRef]
- Wang, B.; Smith, S.M.; Li, J. Genetic Regulation of Shoot Architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, H.; Liang, Y.; Li, J.; Wang, Y. Molecular basis underlying rice tiller angle: Current progress and future perspectives. Mol. Plant 2022, 15, 125–137. [Google Scholar] [CrossRef]
- Qi, X.; Zhao, Y.; Cai, N.; Guan, J.; Liu, Z.; Liu, Z.; Feng, H.; Zhang, Y. Characterization and Transcriptome Analysis Reveal Exogenous GA3 Inhibited Rosette Branching via Altering Auxin Approach in Flowering Chinese Cabbage. Agronomy 2024, 14, 762. [Google Scholar] [CrossRef]
- Long, J.; Barton, M.K. Initiation of axillary and floral meristems in Arabidopsis. Dev. Biol. 2000, 218, 341–353. [Google Scholar] [CrossRef]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Wang, X.; Liu, X.; Teng, S.; Hiroshi, F. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Mjomba, F.M.; Zheng, Y.; Liu, H.; Tang, W.; Hong, Z.; Wang, F.; Wu, W. Homeobox is pivotal for OsWUS controlling tiller development and female fertility in rice. G3 Genes Genomes Genet. 2016, 6, 2013–2021. [Google Scholar] [CrossRef]
- Kosugi, S.; Ohashi, Y. DNA binding and dimerization specificity and potential targets for the TCP protein family. Plant J. 2002, 30, 337–348. [Google Scholar] [CrossRef]
- Seale, M.; Bennett, T.; Leyser, O. BRC1 expression regulates bud activation potential but is not necessary or sufficient for bud growth inhibition in Arabidopsis. Development 2017, 144, 1661–1673. [Google Scholar]
- Martínez-Bello, L.; Moritz, T.; López-Díaz, I. Silencing C19-GA 2-oxidases induces parthenocarpic development and inhibits lateral branching in tomato plants. J. Exp. Bot. 2015, 66, 5897–5910. [Google Scholar] [CrossRef]
- Li, G.; Tan, M.; Ma, J.; Cheng, F.; Li, K.; Liu, X.; Zhao, C.; Zhang, D.; Xing, L.; Ren, X. Molecular mechanism of MdWUS2–MdTCP12 interaction in mediating cytokinin signaling to control axillary bud outgrowth. J. Exp. Bot. 2021, 72, 4822–4838. [Google Scholar] [CrossRef] [PubMed]
- Domagalska, M.A.; Leyser, O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef]
- Rameau, C.; Bertheloot, J.; Leduc, N.; Andrieu, B.; Foucher, F.; Sakr, S. Multiple pathways regulate shoot branching. Front. Plant Sci. 2015, 5, 741. [Google Scholar] [CrossRef]
- Li, X.; Xie, Z.; Qin, T.; Zhan, C.; Jin, L.; Huang, J. The SLR1-OsMADS23-D14 module mediates the crosstalk between strigolactone and gibberellin signaling to control rice tillering. New Phytol. 2025, 246, 2137–2154. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Abe, A.; Yoshida, K.; Kosugi, S.; Natsume, S.; Mitsuoka, C.; Uemura, A.; Utsushi, H.; Tamiru, M.; Takuno, S. QTL-seq: Rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, F.; Hu, G.; Yang, Y.; Zhang, L.; Meng, J.; Han, Z.; Zhou, X.; Liu, H.; Ayaad, M. BSA-seq-based identification of a major additive plant height QTL with an effect equivalent to that of Semi-dwarf 1 in a large rice F2 population. Crop J. 2021, 9, 1428–1437. [Google Scholar] [CrossRef]
- Li, B.; Gao, J.; Chen, J.; Wang, Z.; Shen, W.; Yi, B.; Wen, J.; Ma, C.; Shen, J.; Fu, T. Identification and fine mapping of a major locus controlling branching in Brassica napus. Theor. Appl. Genet. 2020, 133, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, H.; Yang, Y.; Wang, S.; Zhang, X.; Zeng, J.; Zhang, F.; Ding, L.; Jiang, J.; Fang, W. BSA-seq identified candidate genes and diagnostic KASP markers for anemone type flower in chrysanthemum. Sci. Hortic. 2024, 327, 112790. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Abe, A.; Kosugi, S.; Yoshida, K.; Natsume, S.; Takagi, H.; Kanzaki, H.; Matsumura, H.; Yoshida, K.; Mitsuoka, C.; Tamiru, M. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 2012, 30, 174–178. [Google Scholar] [CrossRef]
- Liu, C.; Chai, Y.; Tan, C.; Shi, F.; Zhang, Y.; Liu, Z. Brchli1 mutation induces bright yellow leaves by disrupting magnesium chelatase I subunit function in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Front. Plant Sci. 2024, 15, 1450242. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, L.; Tan, C.; Zhao, D.; Liu, Z. Brems1 mutation induced tapetum deficiency leading to male sterility in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Theor. Appl. Genet. 2025, 138, 50. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Suwa, Y.; Suzuki, M.; Kitano, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M.; Ueguchi, C. The OsTB1 gene negatively regulates lateral branching in rice. Plant J. 2003, 33, 513–520. [Google Scholar] [CrossRef]
- Fichtner, F.; Humphreys, J.L.; Barbier, F.F.; Feil, R.; Westhoff, P.; Moseler, A.; Lunn, J.E.; Smith, S.M.; Beveridge, C.A. Strigolactone signalling inhibits trehalose 6-phosphate signalling independently of BRC1 to suppress shoot branching. New Phytol. 2024, 244, 900–913. [Google Scholar] [CrossRef]
- Teichmann, T.; Muhr, M. Shaping plant architecture. Front. Plant Sci. 2015, 6, 233. [Google Scholar] [CrossRef]
- Muntha, S.T.; Zhang, L.; Zhou, Y.; Zhao, X.; Hu, Z.; Yang, J.; Zhang, M. Phytochrome A signal transduction 1 and CONSTANS-LIKE 13 coordinately orchestrate shoot branching and flowering in leafy Brassica juncea. Plant Biotechnol. J. 2019, 17, 1333–1343. [Google Scholar] [CrossRef]
- Li, P.; Su, T.; Zhang, B.; Li, P.; Xin, X.; Yue, X.; Cao, Y.; Wang, W.; Zhao, X.; Yu, Y. Identification and fine mapping of qSB. A09, a major QTL that controls shoot branching in Brassica rapa ssp. chinensis Makino. Theor. Appl. Genet. 2020, 133, 1055–1068. [Google Scholar] [CrossRef]
- Zhou, W.; Tan, C.; Qi, X.; Li, H.; Zhao, Z.; Li, X.; Li, X.; Zhang, X.; Zhang, Y.; Liu, Z. Identification of an IAA conjugate resistant gene BraA07g034950. 3C regulating primary rosette branching in flowering Chinese cabbage. Sci. Hortic. 2024, 338, 113717. [Google Scholar] [CrossRef]
- Cubas, P.; Lauter, N.; Doebley, J.; Coen, E. The TCP domain: A motif found in proteins regulating plant growth and development. Plant J. 1999, 18, 215–222. [Google Scholar] [CrossRef]
- Martín-Trillo, M.; Cubas, P. TCP genes: A family snapshot ten years later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Aguilar-Martínez, J.A.; Poza-Carrion, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Rodríguez-Buey, M.L.; Franco-Zorrilla, J.M.; Cubas, P. A recently evolved alternative splice site in the BRANCHED1a gene controls potato plant architecture. Curr. Biol. 2015, 25, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.T.; Demarest, B.L.; Bisgrove, B.W.; Gorsi, B.; Su, Y.-C.; Yost, H.J. MMAPPR: Mutation mapping analysis pipeline for pooled RNA-seq. Genome Res. 2013, 23, 687–697. [Google Scholar] [CrossRef]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2014, 33, 1–14. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Qi, X.; Fu, S.; Zheng, C.; Wu, C.; Li, X.; Zhang, Y.; Ye, X. Fine Mapping of BrTCP1 as a Key Regulator of Branching in Flowering Chinese Cabbage (Brassica rapa subsp. chinensis). Horticulturae 2025, 11, 824. https://doi.org/10.3390/horticulturae11070824
Liu C, Qi X, Fu S, Zheng C, Wu C, Li X, Zhang Y, Ye X. Fine Mapping of BrTCP1 as a Key Regulator of Branching in Flowering Chinese Cabbage (Brassica rapa subsp. chinensis). Horticulturae. 2025; 11(7):824. https://doi.org/10.3390/horticulturae11070824
Chicago/Turabian StyleLiu, Chuanhong, Xinghua Qi, Shuo Fu, Chao Zheng, Chao Wu, Xiaoyu Li, Yun Zhang, and Xueling Ye. 2025. "Fine Mapping of BrTCP1 as a Key Regulator of Branching in Flowering Chinese Cabbage (Brassica rapa subsp. chinensis)" Horticulturae 11, no. 7: 824. https://doi.org/10.3390/horticulturae11070824
APA StyleLiu, C., Qi, X., Fu, S., Zheng, C., Wu, C., Li, X., Zhang, Y., & Ye, X. (2025). Fine Mapping of BrTCP1 as a Key Regulator of Branching in Flowering Chinese Cabbage (Brassica rapa subsp. chinensis). Horticulturae, 11(7), 824. https://doi.org/10.3390/horticulturae11070824





