Differential Effects of Arbuscular Mycorrhizal Fungi on Rooting and Physiology of ‘Summer Black’ Grape Cuttings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Substrate
2.2. Arbuscular Mycorrhizal Fungal Inocula
2.3. Experimental Design and Cultivation
2.4. Physiological Parameter Measurements
2.5. Growth and Root Morphological Measurements
2.6. Mycorrhizal Infection and Soil Hyphal Length
2.7. Chemical and Biochemical Analyses
2.8. Statistical Analysis
3. Results
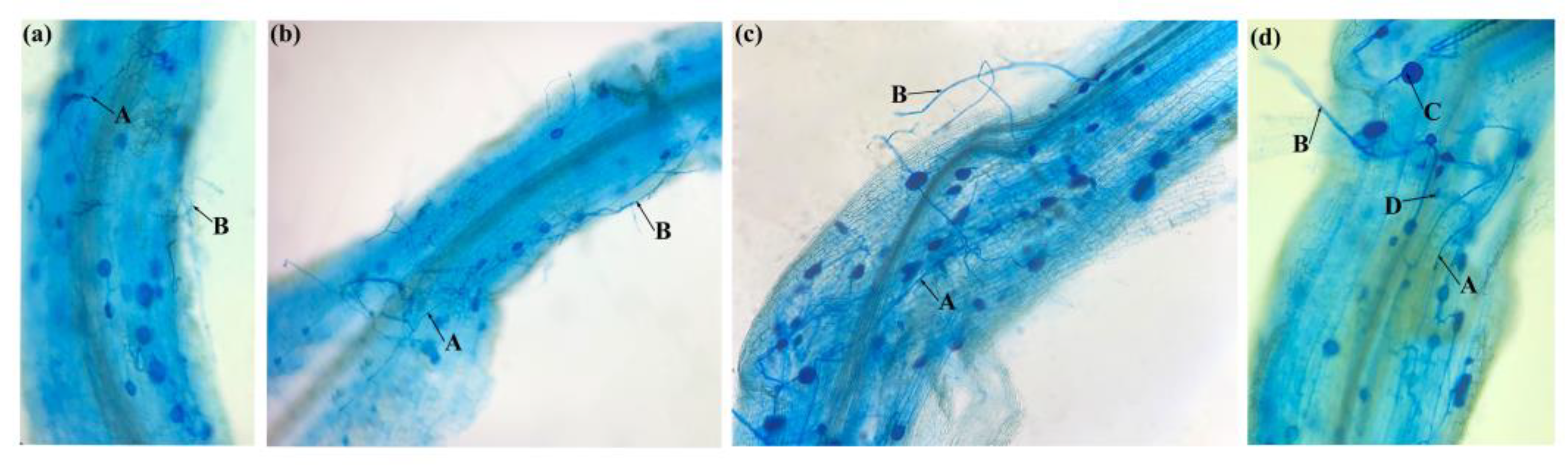
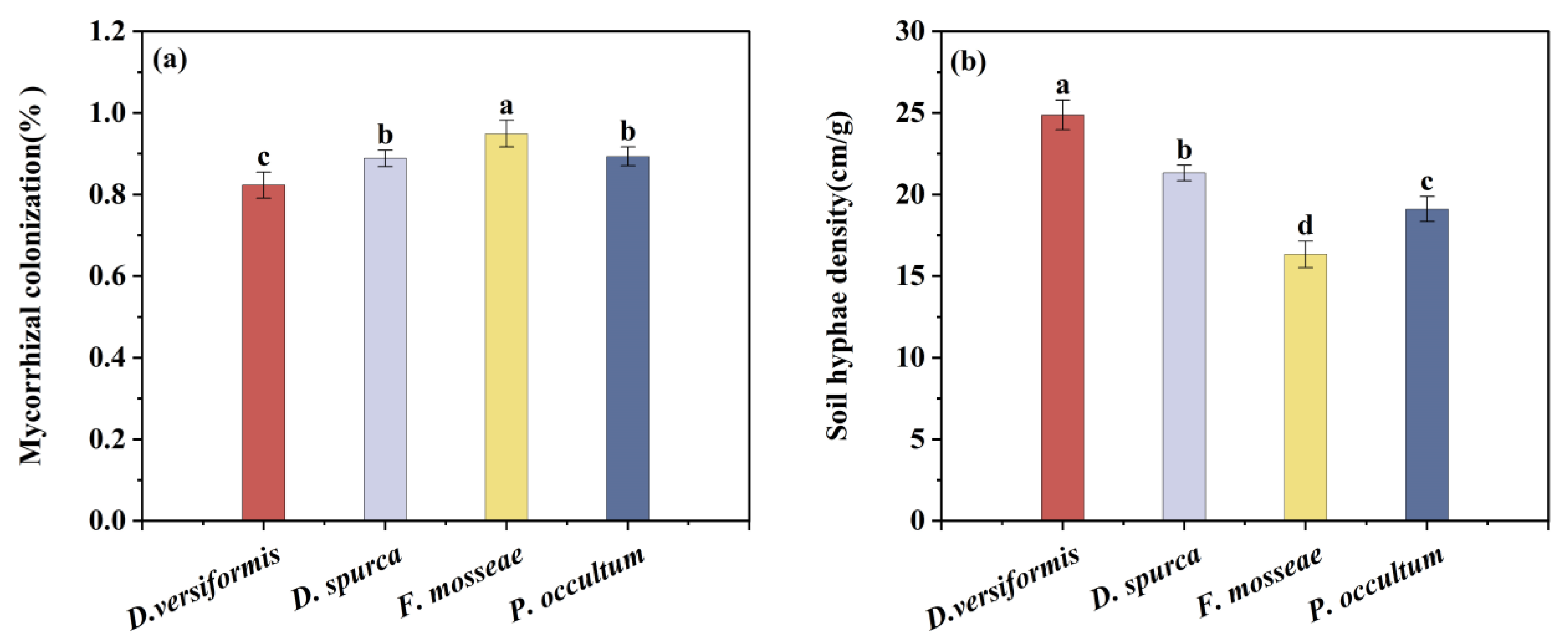
3.1. Mycorrhizal Establishment and Colonization Rate
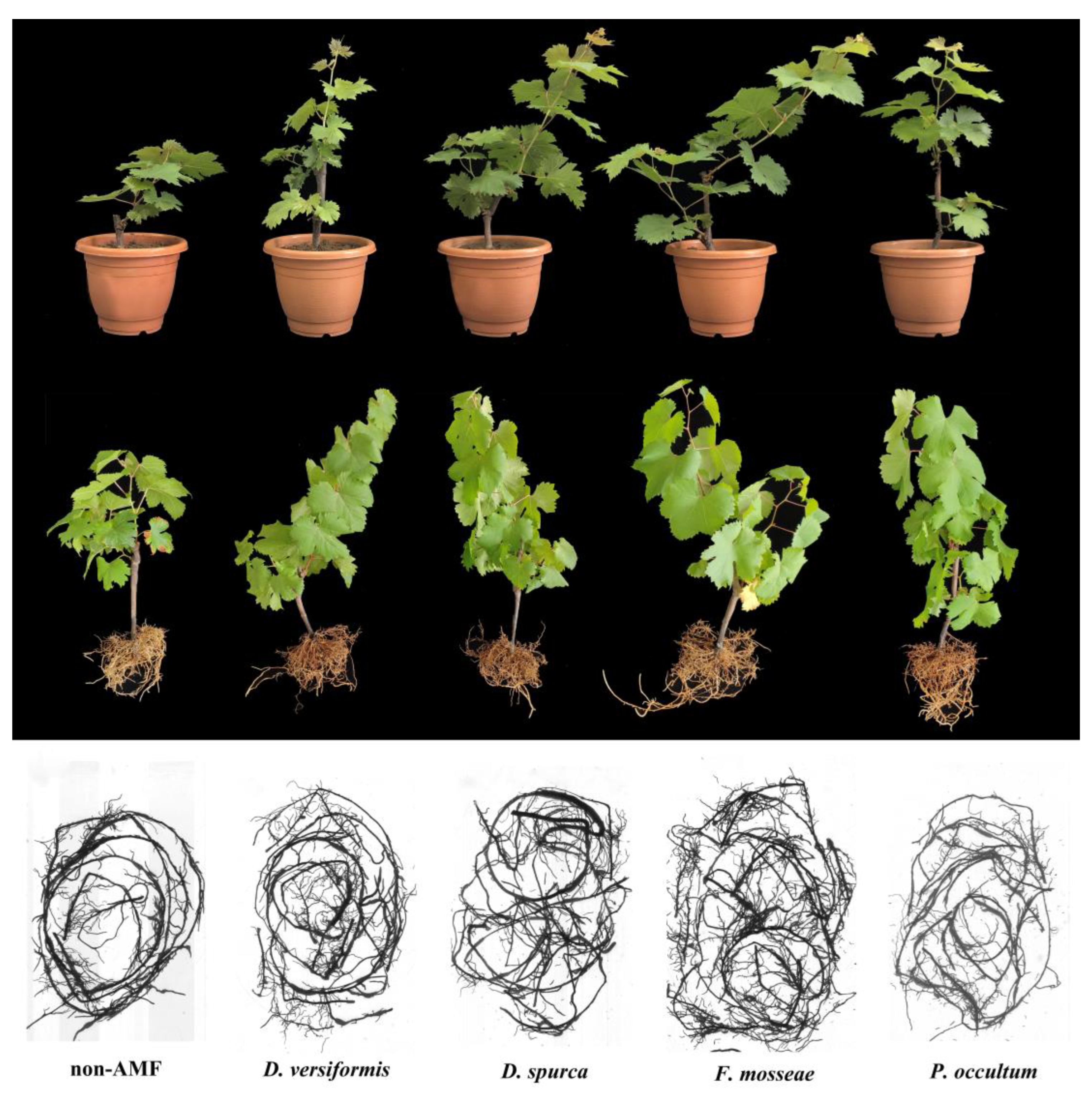
3.2. Effects of AMF Inoculation on Grape Cuttings Growth Parameters
3.3. Effects of AMF Inoculation on Grape Root Architecture
3.4. Effects of AMF Inoculation on Mineral Element Contents in Grapes
3.5. Effects of AMF Inoculation on Antioxidant Enzyme Activities in Grapes
3.6. Effects of AMF Inoculation on Soluble Protein and Soluble Sugar Contents in Grape Tissues
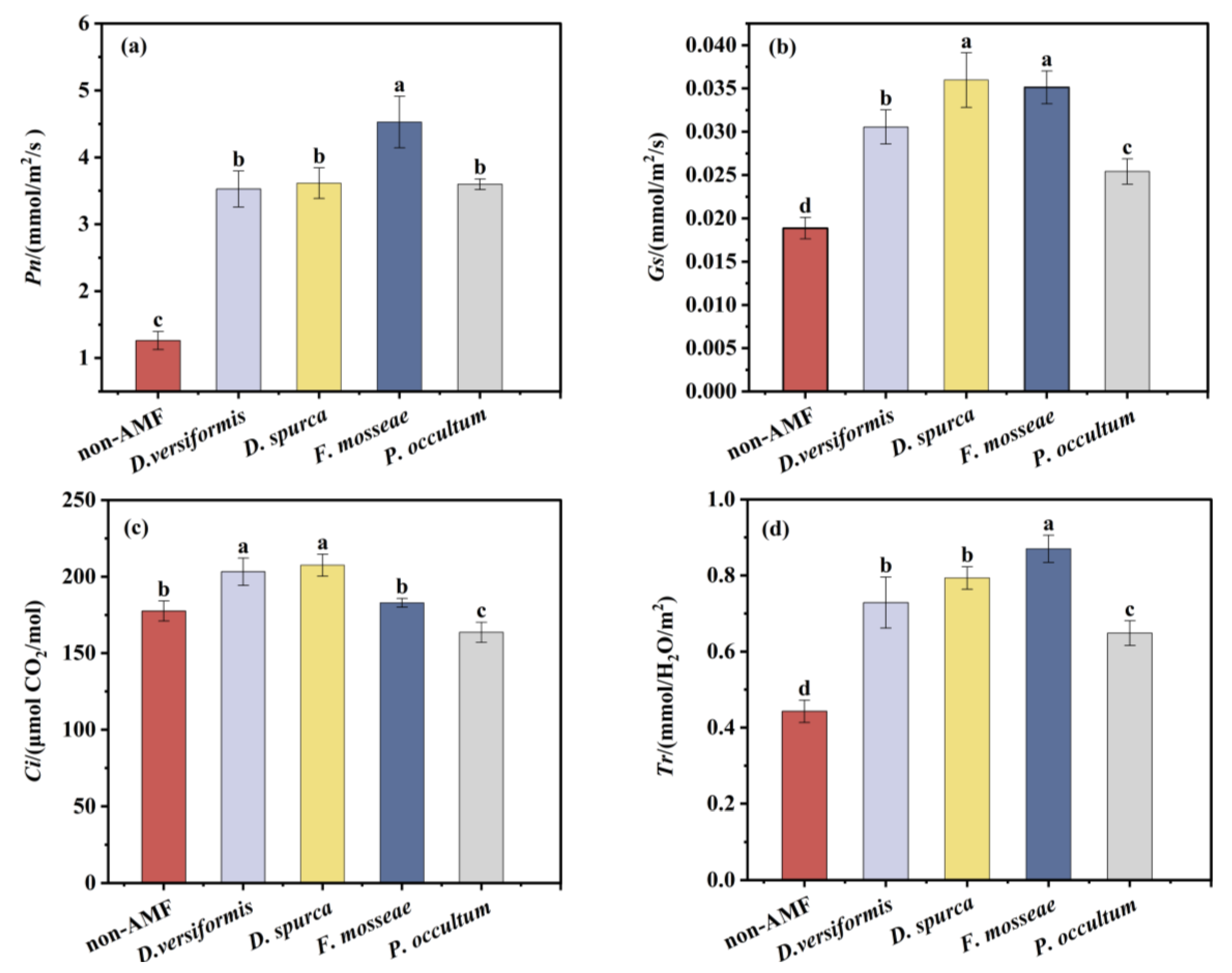
3.7. Effects of AMF Inoculation on Grape Photosynthesis
3.8. Pearson Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bendaali, Y.; Vaquero, C.; González, C.; Morata, A. Contribution of Grape Juice to Develop New Isotonic Drinks with Antioxidant Capacity and Interesting Sensory Properties. Front. Nutr. 2022, 9, 890640. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.-Y. Grape Phytochemicals and Associated Health Benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef] [PubMed]
- Fia, G.; Bucalossi, G.; Proserpio, C.; Vincenzi, S. Unripe Grapes: An Overview of the Composition, Traditional and Innovative Applications, and Extraction Methods of a Promising Waste of Viticulture. Aust. J. Grape Wine Res. 2022, 28, 8–26. [Google Scholar] [CrossRef]
- Chen, Y.P.; Fei, Y.N.; Howell, K.; Chen, D.; Clingeleffer, P.; Zhang, P.Z. Rootstocks for Grapevines Now and into the Future: Selection of Rootstocks Based on Drought Tolerance, Soil Nutrient Availability, and Soil PH. Aust. J. Grape Wine Res. 2024, 2024, 6704238. [Google Scholar] [CrossRef]
- Kohli, P.S.; Maurya, K.; Thakur, J.K.; Bhosale, R.; Giri, J. Significance of Root Hairs in Developing Stress-resilient Plants for Sustainable Crop Production. Plant Cell Environ. 2022, 45, 677–694. [Google Scholar] [CrossRef]
- Mitra, D.; Sierra, B.E.G.; Khoshru, B.; Villalobos, S.D.L.S.; Belz, C.; Chaudhary, P.; Shahri, F.N.; Djebaili, R.; Adeyemi, N.O.; El-Ballat, M.A.; et al. Impacts of Arbuscular Mycorrhizal Fungi on Rice Growth, Development, and Stress Management with a Particular Emphasis on Strigolactone Effects on Root Development. Commun. Soil Sci. Plant Anal. 2021, 52, 1591–1621. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Xie, X.-Y.; Weng, B.; Cai, B.-P.; Dong, Y.-R.; Yan, C.-L. Effects of Arbuscular Mycorrhizal Inoculation and Phosphorus Supply on the Growth and Nutrient Uptake of Kandelia obovata (Sheue, Liu & Yong) Seedlings in Autoclaved Soil. Appl. Soil Ecol. 2014, 75, 162–171. [Google Scholar] [CrossRef]
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Zia Ul Haq, M.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular Mycorrhizal Fungi Symbiosis to Enhance Plant–soil Interaction. Sustainability 2022, 14, 7840. [Google Scholar] [CrossRef]
- Bhantana, P.; Malla, R.; Vista, S.P.; Rana, M.S.; Moussa, M.G.; Joshi, B.D.; Shah, S.; Khadka, D.; Timsina, G.P.; Poude, K. Use of Arbuscular Mycorrhizal Fungi (AMF) and Zinc Fertilizers in an Adaptation of Plant from Drought and Heat Stress. Biomed. J. Sci. Tech. Res. 2021, 38, 30357–30373. [Google Scholar] [CrossRef]
- Asadi, M.; Rasouli, F.; Amini, T.; Hassanpouraghdam, M.B.; Souri, S.; Skrovankova, S.; Mlcek, J.; Ercisli, S. Improvement of Photosynthetic Pigment Characteristics, Mineral Content, and Antioxidant Activity of Lettuce (Lactuca sativa L.) by Arbuscular Mycorrhizal Fungus and Seaweed Extract Foliar Application. Agronomy 2022, 12, 1943. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, L.-J.; Xu, Y.-J.; Hashem, A.; Abd Allah, E.F.; Wu, Q.-S. Growth Performance and Osmolyte Regulation of Drought-stressed Walnut Plants are Improved by Mycorrhiza. Agriculture 2024, 14, 367. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline Accumulation Influenced by Osmotic Stress in Arbuscular Mycorrhizal Symbiotic Plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.H. Arbuscular Mycorrhizal Fungal Responses to Abiotic Stresses: A Review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Fang, Y.-Z.; Wang, Y.-J.; Cao, H.-Y.; Yang, G.-L.; Deng, L.-L.; Zhou, Y.-Y.; Anastopoulos, I.; Wang, X.-R. Arbuscular Mycorrhizal Fungi-induced Mitigation of Heavy Metal Phytotoxicity in Metal Contaminated Soils: A Critical Review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef] [PubMed]
- Mbodj, D.; Effa-Effa, B.; Kane, A.; Manneh, B.; Gantet, P.; Laplaze, L.; Diedhiou, A.G.; Grondin, A. Arbuscular Mycorrhizal Symbiosis in Rice: Establishment, Environmental Control and Impact on Plant Growth and Resistance to Abiotic Stresses. Rhizosphere 2018, 8, 12–26. [Google Scholar] [CrossRef]
- Gujre, N.; Soni, A.; Rangan, L.; Tsang, D.C.W.; Mitra, S. Sustainable Improvement of Soil Health Utilizing Biochar and Arbuscular Mycorrhizal Fungi: A Review. Environ. Pollut. 2021, 268, 115549. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling Mycorrhiza-induced Resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef]
- Aguilera, P.; Ortiz, N.; Becerra, N.; Turrini, A.; Gaínza-Cortés, F.; Silva-Flores, P.; Aguilar-Paredes, A.; Romero, J.K.; Jorquera-Fontena, E.; Borie, F.; et al. Application of Arbuscular Mycorrhizal Fungi in Vineyards: Water and Biotic Stress Under a Climate Change Scenario: New Challenge for Chilean Grapevine Crop. Front. Microbiol. 2022, 13, 826571. [Google Scholar] [CrossRef]
- Meng, L.-L.; Xu, F.-Q.; Zhang, Z.-Z.; Alqahtani, M.D.; Tashkandi, M.A.; Wu, Q.-S. Arbuscular Mycorrhizal Fungi, Especially Rhizophagus intraradices as a Biostimulant, Improve Plant Growth and Root Columbin Levels in Tinospora sagittata. Horticulturae 2023, 9, 1350. [Google Scholar] [CrossRef]
- Luciani, E.; Frioni, T.; Tombesi, S.; Farinelli, D.; Gardi, T.; Ricci, A.M.G.; Sabbatini, P.; Palliotti, A. Effects of A New Arbuscular Mycorrhizal Fungus (Glomus iranicum) on Grapevine Development. BIO Web Conf. EDP Sci. 2019, 13, 04018. [Google Scholar] [CrossRef]
- Cangahuala-Inocente, G.C.; Da Silva, M.F.; Johnson, J.M.; Manga, A.; van Tuinen, D.; Henry, C.; Lovato, P.E.; Dumas-Gaudot, E. Arbuscular Mycorrhizal Symbiosis Eicits Proteome Responses Opposite of P-starvation in SO4 Grapevine Rootstock upon Root Colonisation with Two Glomus Species. Mycorrhiza 2011, 21, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.; Nasrollahpourmoghadam, S.; Mohammadkhani, A. Comparison of Effectiveness of Arbuscular Mycorrhiza Fungi (AMF) on Vitis vinifera under Low Irrigation Conditions. Agricul. Sci. Digest 2021, 41, 119–128. [Google Scholar] [CrossRef]
- Ma, S.-L.; Zhu, L.-J.; Wang, J.-P.; Liu, X.; Jia, Z.-H.; Li, C.; Liu, J.; Zhang, J.-Y.; Zhang, J.-C. Arbuscular Mycorrhizal Fungi Promote Gleditsia sinensis Lam. Root Growth Under Salt Stress by Regulating Nutrient Uptake and Physiology. Forests 2022, 13, 688. [Google Scholar] [CrossRef]
- Liu, C.Y.; Guo, X.N.; Alqahtani, M.D.; Wu, Q.S. Funneliformis Mosseae Exhibits Greater Improved Effects on Root Hair Development and Phosphorus Uptake in Trifoliate Orange than Claroideoglomus etunicatum. Rhizosphere 2025, 34, 101081. [Google Scholar] [CrossRef]
- Wan, Y.-X.; Liang, S.-M.; Wu, Q.-S.; Hashem, A.; Abd_Allah, E.F.; Zou, Y.-N. Serendipita Indica Accelerates Chlorophyll Synthesis Pathway and Photosynthetic Efficiency in Trifoliate Orange Subjected to Water Deficit. Sci. Horti. 2024, 338, 113667. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Ames, R.N. Comparison of Two Methods for Quantifying Extraradical Mycelium of Vesicular-arbuscular Mycorrhizal Fungi. Soil Sci. Soc. Am. J. 1987, 51, 834–837. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. N. Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Poobathy, R.; Sinniah, U.R.; Xavier, R.; Subramaniam, S. Catalase and Superoxide Dismutase Activities and the Total Protein Content of Protocorm-like Bodies of Dendrobium Sonia-28 Subjected to Vitrification. Appl. Biochem. Biotechnol. 2013, 170, 1066–1079. [Google Scholar] [CrossRef]
- Köktepe, T.; Altin, S.; Tohma, H.; Gülçin, İ.; Köksal, E. Purification, Characterization and Selected Inhibition Properties of Peroxidase from Haricot Bean (Phaseolus vulgaris L.). Int. J. Food Prop. 2017, 20 (Suppl. S2), 1944–1953. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.-J.; Bai, J.-P.; Yu, H.; Han, G.-J. Physiological Adaptations of Vigna radiata to Heavy Metal Stress: Soluble Sugar Accumulation and Biomass Enhancement. Plants 2025, 14, 1191. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Liu, C.-Y.; Zhang, D.-J.; Zou, Y.-N.; He, X.-H.; Wu, Q.-H. Mycorrhiza Alters the Profile of Root Hairs in Trifoliate Orange. Mycorrhiza 2016, 26, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Facelli, E.; Smith, S.E.; Smith, F.A. Mycorrhizal Symbiosis—Overview and New Insights into Roles of Arbuscular Mycorrhizas in Agro-and Natural Ecosystem. Australas. Plant Pathol. 2009, 38, 338–344. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y. Mycorrhizal Symbiosis: Evolution, Opportunities, Challenges, and Prospects. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Inoculum Production and Application; Parihar, M., Rakshit, A., Adholeya, A., Chen, Y., Eds.; Springer: Berlin, Germany, 2024; pp. 1–35. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L.X. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of Mycorrhizal Associations Along the Mutualism–parasitism Continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Liu, R.-C.; Xiao, Z.-Y.; Hashem, A.; Abd-Allah, E.F.; Xu, Y.-J.; Wu, Q.-S. Unraveling the Interaction between Arbuscular Mycorrhizal Fungi and Camellia plants. Horticulturae 2021, 7, 322. [Google Scholar] [CrossRef]
- Chen, W.; Koide, R.T.; Eissenstat, D.M. Nutrient Foraging by Mycorrhizas: From Species Functional Traits to Ecosystem Processes. Funct. Ecol. 2018, 32, 858–869. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular Mycorrhiza and Nitrogen: Implications for Individual Plants Through to Ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Sas-Paszt, L.; Głuszek, S.; Derkowska, E.; Sumorok, B.; Lisek, J.; Trzciński, P.; Lisek, A.; Frąc, M.; Sitarek, M.; Przybył, M. Occurrence of Arbuscular Mycorrhizal Fungi in the Roots of Two Grapevine Cultivars in Response to Bioproducts. S. Afr. J. Enol. Vitic. 2019, 40, 1–4. [Google Scholar] [CrossRef]
- Sas-Paszt, L.; Głuszek, S.; Derkowska, E.; Sumorok, B.; Lisek, J.; Trzciński, P.; Lisek, A.; Frąc, M.; Sitarek, M.; Przybył, M.; et al. Diversity of Arbuscular Mycorrhizal Fungi in the Rhizosphere of Solaris and Regent Grapevine Plants Treated with Bioproducts. S. Afr. J. Enol. Vitic. 2020, 41, 83–89. [Google Scholar] [CrossRef]
- Li, Q.-S.; Srivastava, A.-K.; Zou, Y.-N.; Wu, Q.-S. Field Inoculation Responses of Arbuscular Mycorrhizal Fungi Versus Endophytic Fungi on Sugar Metabolism Associated Changes in Fruit Quality of Lane Late Navel Orange. Sci. Horti. 2023, 308, 111587. [Google Scholar] [CrossRef]
- Wu, W.-J.; Xiao, Z.-Y.; Wang, F.-L.; Cheng, J.-Y.; Zou, Y.-N.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Serendipita indica: A Promising Bio-stimulant for Improving Growth, Nutrient Uptake, and Sugar Accumulation in Camellia oleifera. Horticulturae 2024, 10, 936. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- He, Z.-Q.; He, C.-X.; Zhang, Z.-B.; Zou, Z.-R.; Wang, H.-S. Changes of Antioxidative Enzymes and Cell Membrane Osmosis in Tomato Colonized by Arbuscular Mycorrhizae under NaCl Stress. Colloids Surf. B Biointerfaces 2007, 59, 128–133. [Google Scholar] [CrossRef]


| Treatment | Plant Growth Status | Biomass (g FW/plant) | |||||
|---|---|---|---|---|---|---|---|
| Number of Shoots (#/plant) | Shoot Length (cm) | Shoot Diameter (cm) | Leaf Number (#/plant) | Leaf | Shoot | Root | |
| non-AMF | 2.6 ± 0.89 a | 25.3 ± 2.1 c | 4.41 ± 0.37 a | 25 ± 2 a | 21.29 ± 1.35 b | 21.03 ± 1.52 b | 5.74 ± 0.40 a |
| D. versiformis | 1.8 ± 0.00 c | 31.1 ± 2.8 a | 4.18 ± 0.31 a | 23 ± 2 ab | 22.34 ± 0.99 a | 23.35 ± 1.01 a | 5.90 ± 0.43 a |
| D. spurca | 2.4 ± 0.89 a | 22.5 ± 2.5 c | 3.78 ± 0.35 b | 25 ± 2 a | 21.73 ± 1.43 ab | 23.77 ± 1.00 a | 5.03 ± 0.31 ab |
| F. mosseae | 2.0 ± 0.00 b | 28.8 ± 2.7 b | 4.07 ± 0.32 a | 21 ± 2 b | 23.10 ± 1.24 a | 23.10 ± 0.76 a | 4.70 ± 0.30 b |
| P. occultum | 2.4 ± 0.55 a | 16.8 ± 1.7 d | 3.66 ± 0.33 b | 19 ± 1 b | 18.24 ± 1.11 c | 21.12 ± 0.72 b | 3.51 ± 0.14 c |
| Treatment | Total Root Length/cm | Surface Area/cm2 | Volume/cm3 | Primary Adventitious Root | Adventitious Lateral Root (#/Plant) | ||
|---|---|---|---|---|---|---|---|
| 1st-Order | 2nd-Order | 3rd-Order | |||||
| non-AMF | 283.55 ± 25.33 b | 13.81 ± 0.45 b | 4.18 ± 1.75 b | 35 ± 2 c | 1030 ± 72 b | 839 ± 20 b | 77 ± 3 b |
| D. versiformis | 298.40 ± 25.85 ab | 14.07 ± 0.43 ab | 4.76 ± 1.90 ab | 24 ± 2 d | 724 ± 23 d | 806 ± 39 b | 158 ± 13 a |
| D. spurca | 301.40 ± 21.00 ab | 14.17 ± 0.33 ab | 6.03 ± 1.95 ab | 61 ± 4 b | 940 ± 63 c | 1072 ± 73 a | 36 ± 2 c |
| F. mosseae | 308.61 ± 12.78 a | 14.27 ± 0.23 a | 6.21 ± 1.15 a | 74 ± 6 a | 1390 ± 34 a | 684 ± 19 c | 78 ± 6 b |
| P. occultum | 301.80 ± 15.36 ab | 13.94 ± 0.43 ab | 5.19 ± 1.39 ab | 31 ± 2 c | 726 ± 20 d | 556 ± 25 d | 14 ± 1 d |
| Organ | Mineral Element | Non-AMF | D. versiformis | D. spurca | F. mosseae | P. occultum |
|---|---|---|---|---|---|---|
| Leaf | Macronutrients | |||||
| N (g/kg) | 10.62 ± 0.40 b | 10.39 ± 0.14 b | 13.62 ± 0.16 a | 9.45 ± 0.21 c | 8.37 ± 0.15 d | |
| P (g/kg) | 0.61 ± 0.07 c | 1.00 ± 0.03 b | 1.25 ± 0.04 a | 1.24 ± 0.01 a | 0.98 ± 0.03 b | |
| K (g/kg) | 8.26 ± 0.17 a | 5.14 ± 0.02 c | 7.36 ± 0.02 b | 8.40 ± 0.03 a | 7.25 ± 0.04 b | |
| Ca (g/kg) | 15.28 ± 0.33 a | 9.72 ± 0.15 d | 11.25 ± 0.06 c | 13.30 ± 0.36 b | 13.10 ± 0.31 b | |
| Mg (mg/kg) | 2.23 ± 0.03 a | 1.51 ± 0.02 d | 1.72 ± 0.05 c | 2.10 ± 0.03 b | 1.99 ± 0.03 b | |
| Micronutrients | ||||||
| B (mg/kg) | 24.40 ± 0.50 c | 18.87 ± 0.37 d | 25.40 ± 0.54 c | 46.96 ± 0.71 a | 42.00 ± 1.68 b | |
| Cu (mg/kg) | 161.04 ± 3.37 d | 82.58 ± 1.77 e | 290.79 ± 3.38 c | 321.39 ± 4.89 b | 349.16 ± 4.06 a | |
| Fe (mg/kg) | 353.00 ± 9.43 a | 166.38 ± 1.93 e | 307.50 ± 3.50 c | 194.85 ± 3.34 d | 338.23 ± 3.93 b | |
| Mn (mg/kg) | 47.44 ± 0.47 a | 26.15 ± 0.61 e | 30.09 ± 0.47 d | 33.63 ± 0.59 c | 37.75 ± 0.79 b | |
| Zn (mg/kg) | 40.27 ± 1.02 d | 31.96 ± 0.50 e | 49.61 ± 0.75 c | 86.61 ± 3.03 a | 73.44 ± 1.44 b | |
| Root | Macronutrients | |||||
| N (g/kg) | 5.37 ± 0.04 a | 4.50 ± 0.13 b | 4.12 ± 0.02 d | 4.21 ± 0.03 c | 4.25 ± 0.04 c | |
| P (g/kg) | 0.85 ± 0.04 d | 1.86 ± 0.01 b | 1.70 ± 0.04 c | 1.97 ± 0.03 a | 1.66 ± 0.02 c | |
| K (g/kg) | 8.36 ± 0.19 b | 9.97 ± 0.19 a | 7.51 ± 0.26 c | 9.41 ± 0.08 a | 8.44 ± 0.13 b | |
| Ca (g/kg) | 10.23 ± 0.31 a | 9.38 ± 0.11 b | 8.55 ± 0.17 c | 7.84 ± 0.16 d | 7.71 ± 0.16 d | |
| Mg (mg/kg) | 1.51 ± 0.04 b | 1.72 ± 0.01 a | 1.60 ± 0.06 b | 1.45 ± 0.04 bc | 1.34 ± 0.02 c | |
| Micronutrients | ||||||
| B (mg/kg) | 18.38 ± 0.64 a | 12.21 ± 0.29 c | 16.05 ± 0.18 b | 12.95 ± 0.27 c | 15.35 ± 0.31 b | |
| Cu (mg/kg) | 75.27 ± 0.86 c | 198.88 ± 7.01 a | 39.99 ± 0.85 d | 72.16 ± 1.62 c | 94.86 ± 3.34 b | |
| Fe (mg/kg) | 442.66 ± 4.43 d | 582.81 ± 9.05 c | 890.79 ± 18.99 a | 767.25 ± 19.37 b | 347.81 ± 3.48 e | |
| Mn (mg/kg) | 14.00 ± 0.24 b | 14.14 ± 0.28 b | 18.41 ± 0.21 a | 13.59 ± 0.28 bc | 13.37 ± 0.21 c | |
| Zn (mg/kg) | 39.38 ± 1.20 a | 37.37 ± 0.73 b | 26.55 ± 0.31 c | 38.49 ± 0.65 a | 37.14 ± 0.88 b |
| Treatment | Solube Protein Content (mg/g) | Soluble Sugar Content (mg/g) | ||
|---|---|---|---|---|
| Root | Leaf | Root | Leaf | |
| non-AMF | 0.0889 ± 0.0009 c | 0.1114 ± 0.0079 c | 0.0725 ± 0.0051 c | 0.1450 ± 0.0091 d |
| D. versiformis | 0.1198 ± 0.0093 a | 0.1580 ± 0.0139 a | 0.0823 ± 0.0081 a | 0.1622 ± 0.0054 c |
| D. spurca | 0.0902 ± 0.0070 bc | 0.1142 ± 0.0104 bc | 0.0863 ± 0.0069 a | 0.1766 ± 0.0085 b |
| F. mosseae | 0.1013 ± 0.0048 b | 0.1313 ± 0.0122 b | 0.0767 ± 0.0052 b | 0.1960 ± 0.0073 a |
| P. occultum | 0.1183 ± 0.0036 a | 0.1231 ± 0.0085 b | 0.0740 ± 0.0024 bc | 0.2061 ± 0.0096 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.-Y.; Liu, C.-Y.; Hao, Y. Differential Effects of Arbuscular Mycorrhizal Fungi on Rooting and Physiology of ‘Summer Black’ Grape Cuttings. Horticulturae 2025, 11, 825. https://doi.org/10.3390/horticulturae11070825
Peng Y-Y, Liu C-Y, Hao Y. Differential Effects of Arbuscular Mycorrhizal Fungi on Rooting and Physiology of ‘Summer Black’ Grape Cuttings. Horticulturae. 2025; 11(7):825. https://doi.org/10.3390/horticulturae11070825
Chicago/Turabian StylePeng, Yi-Yuan, Chun-Yan Liu, and Yong Hao. 2025. "Differential Effects of Arbuscular Mycorrhizal Fungi on Rooting and Physiology of ‘Summer Black’ Grape Cuttings" Horticulturae 11, no. 7: 825. https://doi.org/10.3390/horticulturae11070825
APA StylePeng, Y.-Y., Liu, C.-Y., & Hao, Y. (2025). Differential Effects of Arbuscular Mycorrhizal Fungi on Rooting and Physiology of ‘Summer Black’ Grape Cuttings. Horticulturae, 11(7), 825. https://doi.org/10.3390/horticulturae11070825






