Soil and Root Responses in Hazelnut Rhizosphere to Inoculate Rhizobacteria Immobilized via JetCutter Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Selection of Bacterial Strains
2.2. Immobilization Process of Gram-Negative Bacteria and Bacillus Safensis
2.3. Preparation and Quantification of Initial Bacterial Inoculum
2.4. Extraction and HPLC-Based Quantification of Auxins from JetCutter-Immobilized PGPR Strains
2.5. Plant Test Conditions
2.6. Determination of Dry Biomass of Plants
2.7. Leaf Gas Exchange and Selected Plant Physiological Parameters
2.8. Soil Microbiological Analyses
2.9. Scanning Electron Microscopy (SEM) Analysis
2.10. Statistical Analysis
3. Results
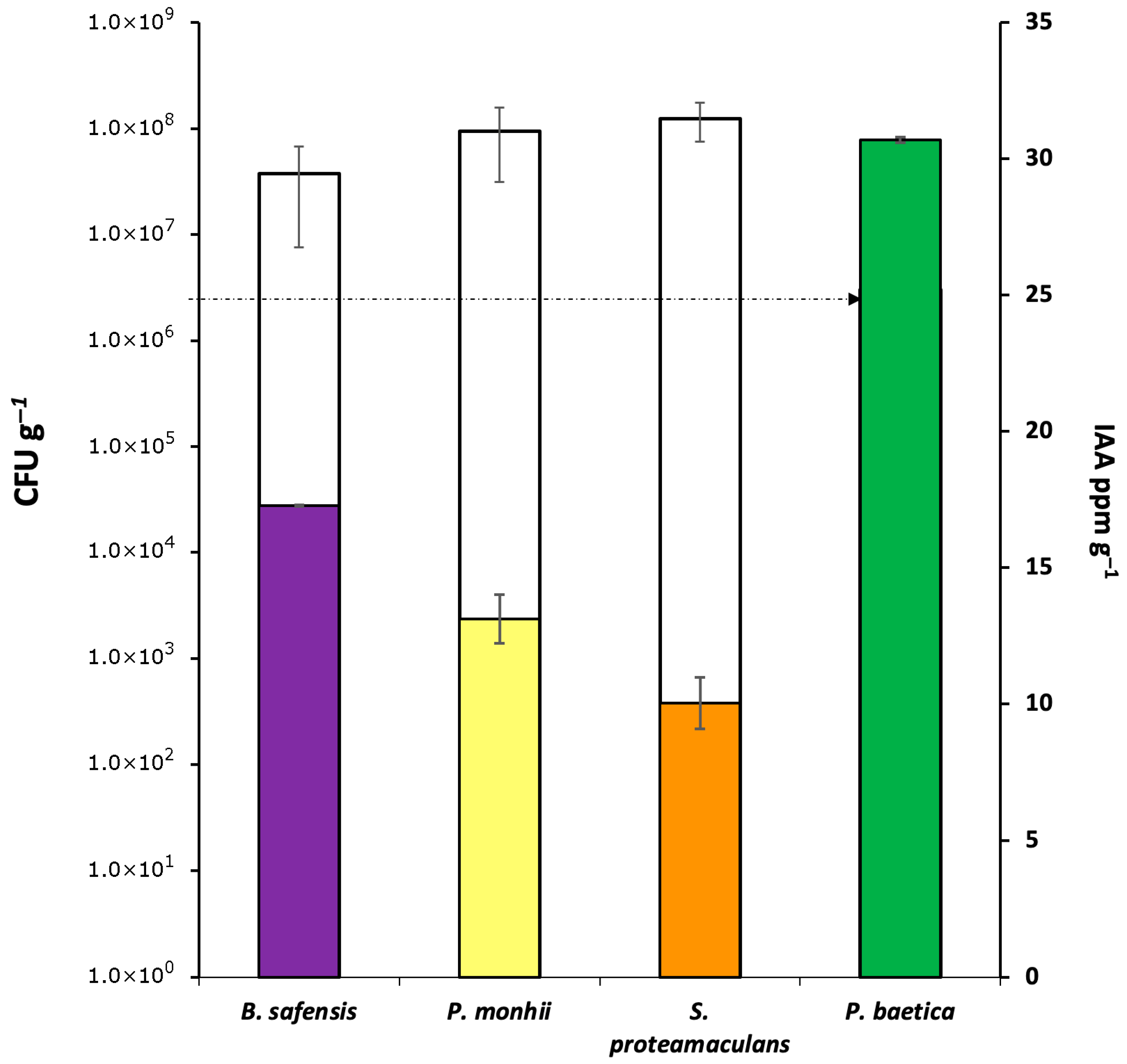
3.1. Microbial Viability and IAA Biosynthesis Efficiency After Immobilization
3.2. Effect of Immobilized Bacterial Strains on Root Biomass and Volume in Hazelnut Plantlets
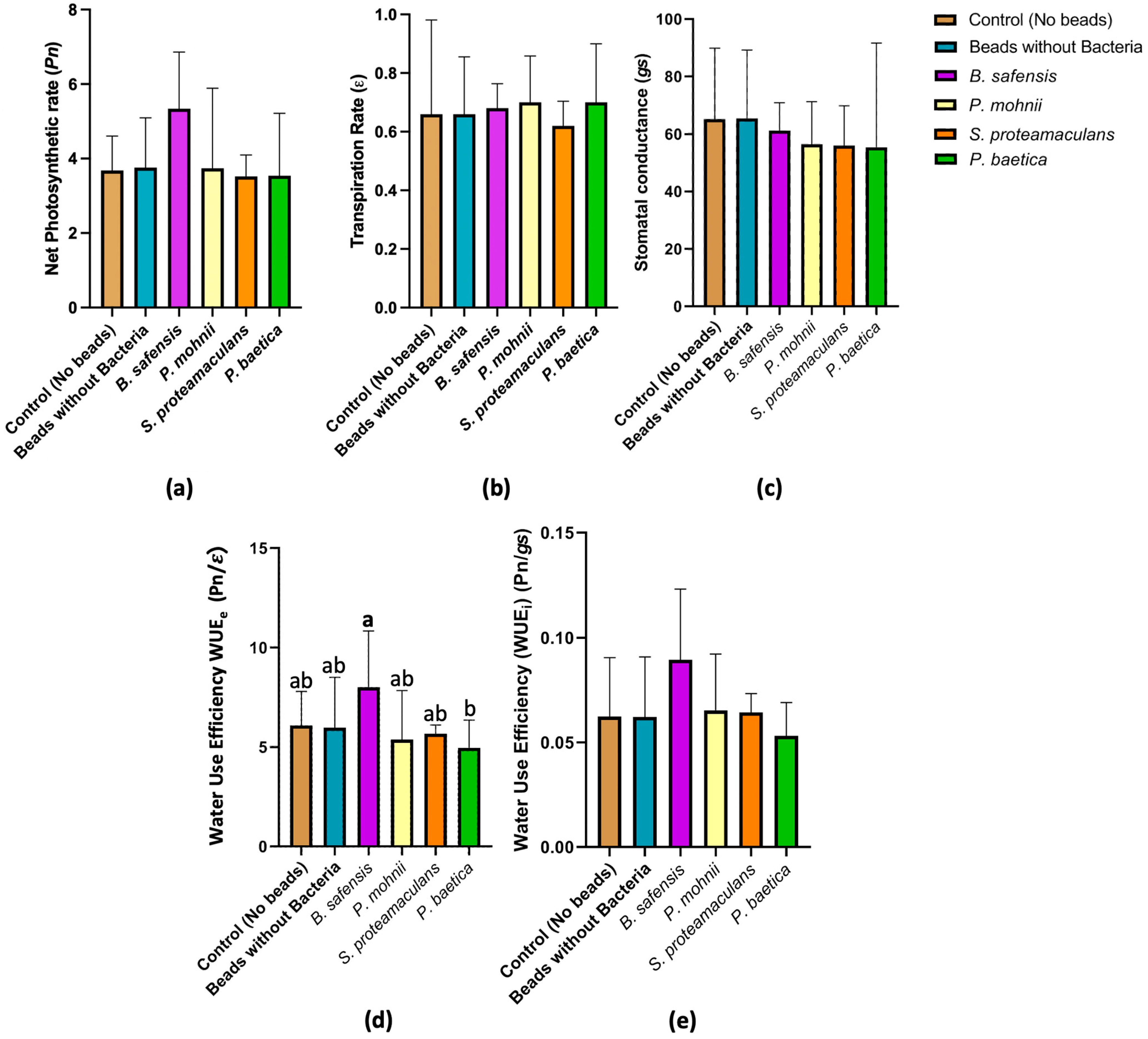
3.3. Trends in Photosynthesis, Stomatal Conductance, and Transpiration in Hazelnut Plantlets
3.4. Trends in Soil Biochemical Activity
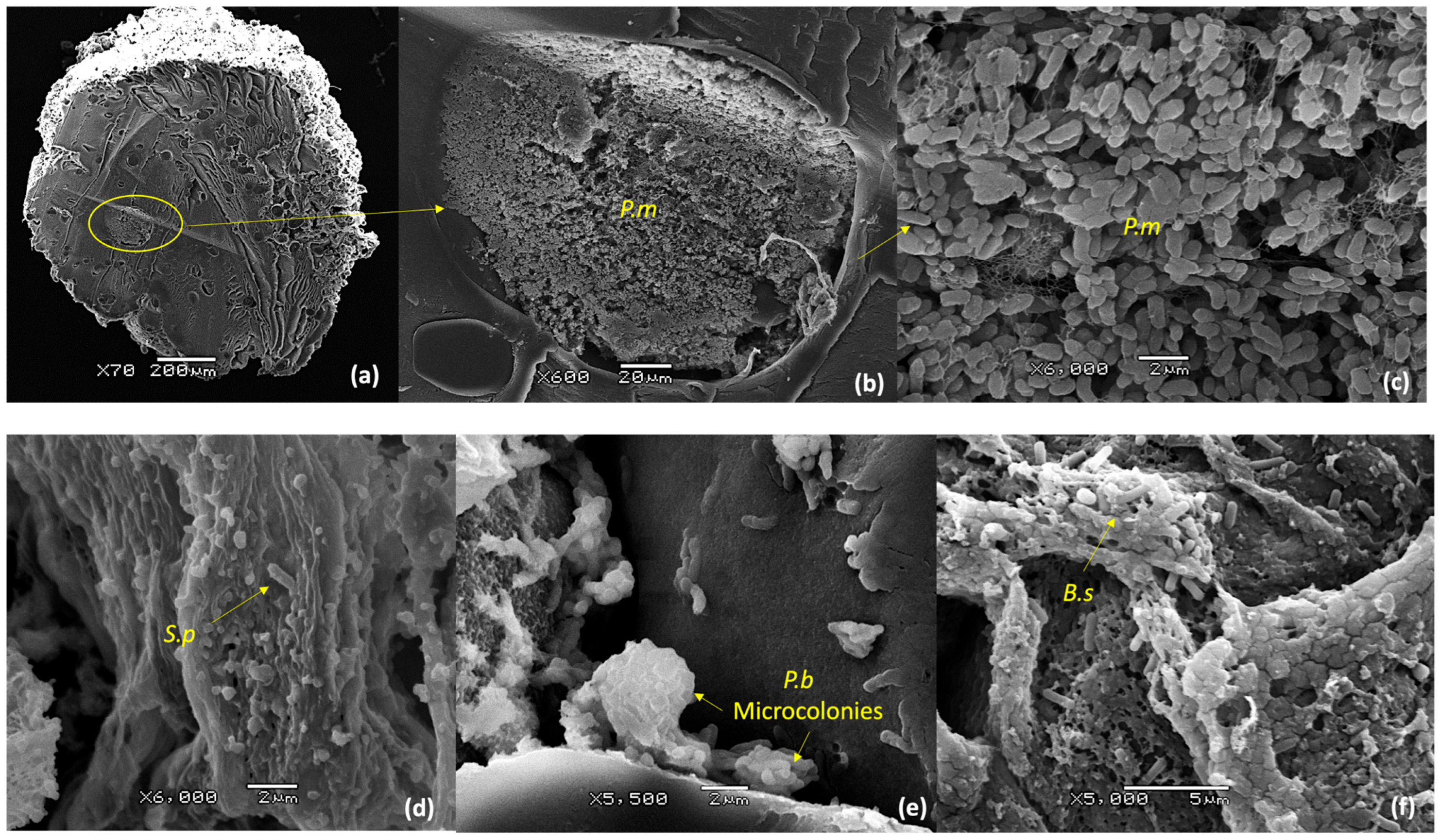
3.5. Scanning Electron Microscopy Analysis
3.6. Multivariate Analysis of Plant Performance and Soil Responses
4. Discussion
4.1. Microbial Viability and IAA Production Efficiency
4.2. Root Development and Morphological Responses to Immobilized PGPR
4.3. Physiological Modulation Through PGPR Inoculation: Gas Exchange Parameters
4.4. Soil Biochemical Responses to PGPR Immobilization
4.5. Multivariate Integration of Plant Growth, Gas Exchange, and Soil Biochemical Activity
4.6. Functional Potential of Immobilized PGPR Strains in the Rhizosphere
4.7. Advantages of JetCutter Technology for PGPR Immobilization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| ANOVA | Analysis of variance |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| CFU | Colony-forming units |
| CIRAS | Compact infrared gas analyzer system |
| CSIC | Consejo Superior de Investigaciones Científicas |
| DNA | Deoxyribonucleic acid |
| EPS | Exopolysaccharides |
| FONDEF | Fondo de Fomento al Desarrollo Científico y Tecnológico |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
| HPLC | High-performance liquid chromatography |
| IAA | Indole-3-acetic acid |
| IU | International Units |
| PGPR | Plant growth-promoting rhizobacteria |
| PCA | Principal component analysis |
| RH | Relative humidity |
| SOD | Superoxide dismutase |
| DOC | Dissolved organic carbon |
References
- Rovira, M. Advances in Hazelnut (Corylus avellana L.) Rootstocks Worldwide. Horticulturae 2021, 7, 267. [Google Scholar] [CrossRef]
- James, S. Plant Growth-Promoting Rhizobacteria: Mechanisms and Agricultural Applications. J. Plant Pathol. Microbiol. 2024, 15, 729. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The Chemistry of Plant–Microbe Interactions in the Rhizosphere and the Potential for Metabolomics to Reveal Signaling Related to Defense Priming and Induced Systemic Resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial Volatiles Promote Growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant Growth Promotion by Volatile Organic Compounds Produced by Bacillus Subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- Win, K.T.; Fukuyo, T.; Keiki, O.; Ohwaki, Y. The ACC Deaminase Expressing Endophyte Pseudomonas spp. Enhances NaCl Stress Tolerance by Reducing Stress-Related Ethylene Production, Resulting in Improved Growth, Photosynthetic Performance, and Ionic Balance in Tomato Plants. Plant Physiol. Biochem. 2018, 127, 599–607. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Iqbal, R.; Manevski, K. Investigating the Effect of Azospirillum brasilense and Rhizobium pisi on Agronomic Traits of Wheat (Triticum aestivum L.). Arch. Agron. Soil. Sci. 2019, 65, 1554–1564. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, S.; Prasad, S.R. PGPR: Renewable Tool for Sustainable Agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 525–530. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The Role of Mycorrhizae and Plant Growth Promoting Rhizobacteria (PGPR) in Improving Crop Productivity under Stressful Environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Kenneth, O.C.; Nwadibe, E.C.; Kalu, A.U.; Unah, U.V. Plant Growth Promoting Rhizobacteria (PGPR): A Novel Agent for Sustainable Food Production. Am. J. Agric. Biol. Sci. 2019, 14, 35–54. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere Bacteria Help Plants Tolerate Abiotic Stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, A.M. Plant Growth Promoting Rhizobacteria (PGPR): Biofertiliser and Biocontrol Agent-Review Article. J. Balkumari Coll. 2019, 8, 42–45. [Google Scholar] [CrossRef]
- Yeşil, Z.B.; Koç, İ. Effects of Drought and Biochar Treatments on Some Morphological and Physiological Parameters in Turkish Hazelnut Seedlings. Memba Kastamonu Üniversitesi Ürünleri Fakültesi Derg. 2024, 10, 211–222. [Google Scholar] [CrossRef]
- Vinci, A.; Di Lena, B.; Portarena, S.; Farinelli, D. Trend Analysis of Different Climate Parameters and Watering Requirements for Hazelnut in Central Italy Related to Climate Change. Horticulturae 2023, 9, 593. [Google Scholar] [CrossRef]
- Nicoletti, R.; Petriccione, M.; Curci, M.; Scortichini, M. Hazelnut-Associated Bacteria and Their Implications in Crop Management. Horticulturae 2022, 8, 1195. [Google Scholar] [CrossRef]
- Soenens, A.; Imperial, J. Biocontrol Capabilities of the Genus Serratia. Phytochem. Rev. 2020, 19, 577–587. [Google Scholar] [CrossRef]
- Amora-Lazcano, E.; Quiroz-González, H.J.; Osornio-Ortega, C.I.; Cruz-Maya, J.A.; Jan-Roblero, J. Plant Growth-Promoting Bacteria Belonging to the Genera Pseudomonas and Bacillus Improve the Growth of Sorghum Seedings in a Low-Nutrient Soil. Bot. Sci. 2021, 100, 56–66. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Zaplatkin, A.N.; Chizhevskaya, E.P.; Gancheva, M.S.; Voshol, G.P.; Malfanova, N.V.; Baganova, M.E.; Khomyakov, Y.V.; Pishchik, V.N. Phytohormone Production by the Endophyte Bacillus safensis TS3 Increases Plant Yield and Alleviates Salt Stress. Plants 2024, 13, 75. [Google Scholar] [CrossRef]
- Kshetri, L.; Naseem, F.; Pandey, P. Role of Serratia sp. as Biocontrol Agent and Plant Growth Stimulator, with Prospects of Biotic Stress Management in Plant. In Microorganisms for Sustainability; Springer: Berlin/Heidelberg, Germany, 2019; Volume 13, pp. 169–200. [Google Scholar]
- Polyanskaya, L.M.; Ivanov, K.E.; Guzev, V.S.; Zvyagintsev, D.G. Estimation of Abundance Dynamics of Gram-Negative Bacteria in Soil. Microbiology 2008, 77, 760–764. [Google Scholar] [CrossRef]
- Wu, W.; Dijkstra, P.; Hungate, B.A.; Shi, L.; Dippold, M.A. In Situ Diversity of Metabolism and Carbon Use Efficiency among Soil Bacteria. Sci. Adv. 2022, 8, eabq3958. [Google Scholar] [CrossRef]
- Rostamikia, Y.; Kouchaksaraei, M.T.; Asgharzadeh, A.; Rahmani, A. The Effect of Plant Growth-Promoting Rhizobacteria on Growth and Physiological Characteristics of Corylus avellana Seedlings. Ecopersia 2016, 4, 1471–1479. [Google Scholar] [CrossRef]
- Rosa, L.; Sangiorgio, M. Global Water Gaps under Future Warming Levels. Nat. Commun. 2025, 16, 1192. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Nour, M.M.; Aljabi, H.R.; AL-Huqail, A.A.; Horneburg, B.; Mohammed, A.E.; Alotaibi, M.O. Drought Responses and Adaptation in Plants Differing in Life-Form. Front. Ecol. Evol. 2024, 12, 1452427. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrera-Bosquet, L.; Morcuende, R.; Avice, J.C.; Nogués, S.; Araus, J.L.; Martínez-Carrasco, R.; Pérez, P. Does Ear C Sink Strength Contribute to Overcoming Photosynthetic Acclimation of Wheat Plants Exposed to Elevated CO2? J. Exp. Bot. 2011, 62, 3957–3969. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How Can We Make Plants Grow Faster? A Source-Sink Perspective on Growth Rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R.; et al. Improved Plant Resistance to Drought Is Promoted by the Root-Associated Microbiome as a Water Stress-Dependent Trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and Assessment of Cell Viability in Formulation of Non-Sporulating Bacterial Inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef]
- Chen, M.; Alexander, M. Survival of Soil Bacteria during Prolonged Desiccation. Soil. Biol. Biochem. 1973, 5, 213–221. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; Silveira, M.P.; Alvim, I.D.; da Costa, T.B.; da Silva, T.L.; Vieira, M.G.A.; Prata, A.S.; Forte, M.B.S. Jet Cutter Technique as a Tool to Achieve High Lipase Hydrolytic Activity. Food Bioprod. Process. 2023, 137, 189–199. [Google Scholar] [CrossRef]
- Martin-Díaz, F.; Baeza-Aranzaez, J.; Giraldo, J.D.; Schoebitz, M.; Urrutia, H.; López-Belchí, M.D. Innovative JetCutter Technology to Scale the Production of a Solid Bacillus pumilus Biofertilizer to Transit to Sustainable Agriculture. ACS Agric. Sci. Technol. 2025, 5, 188–200. [Google Scholar] [CrossRef]
- Jing, D.; Liu, B.; Ma, H.; Liu, F.; Liu, X.; Ren, L. Effects of Inoculation with Different Plant Growth-Promoting Rhizobacteria on the Eco-Physiological and Stomatal Characteristics of Walnut Seedlings under Drought Stress. Agronomy 2023, 13, 1486. [Google Scholar] [CrossRef]
- Lotfi, N.; Soleimani, A.; Çakmakçı, R.; Vahdati, K.; Mohammadi, P. Characterization of Plant Growth-Promoting Rhizobacteria (PGPR) in Persian Walnut Associated with Drought Stress Tolerance. Sci. Rep. 2022, 12, 12725. [Google Scholar] [CrossRef] [PubMed]
- Benítez, S.V.; Carrasco, R.; Giraldo, J.D.; Schoebitz, M. Microbeads as Carriers for Bacillus Pumilus: A Biofertilizer Focus on Auxin Production. J. Microencapsul. 2024, 41, 170–189. [Google Scholar] [CrossRef]
- Rojas-Padilla, J.; De-Bashan, L.E.; Parra-Cota, F.I.; Rocha-Estrada, J.; de los Santos-Villalobos, S. Microencapsulation of Bacillus Strains for Improving Wheat (Triticum turgidum Subsp. durum) Growth and Development. Plants 2022, 11, 2920. [Google Scholar] [CrossRef]
- Alguacil, M.d.M.; Torrecillas, E.; Torres, P.; García-Orenes, F.; Roldán, A. Long-Term Effects of Irrigation with Waste Water on Soil AM Fungi Diversity and Microbial Activities: The Implications for Agro-Ecosystem Resilience. PLoS ONE 2012, 7, e47680. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995. [Google Scholar]
- Anderson, J.; Domsch, K. A Physiological Method for the Quantitative Measurement of Microbial Biomass in Soils. Soil. Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Shmuel, K. Urgencia y Mecanismo de Biosíntesis de Metabolitos Secundarios Microbianos Marinos. Int. J. Sci. Soc. 2022, 4, 138–147. [Google Scholar] [CrossRef]
- Nev, O.A.; Nev, O.A.; van den Berg, H.A. Optimal Management of Nutrient Reserves in Microorganisms under Time-Varying Environmental Conditions. J. Theor. Biol. 2017, 429, 124–141. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Shi, H. Bacterial Strategies in Prolonged Stationary Phase: Tradeoff between Cell Growth, Maintenance, and Recycling. Phys. Rev. Res. 2023, 5, 013119. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- González-Reguero, D.; Robas-Mora, M.; Probanza, A.; Jiménez, P.A. Evaluation of the Oxidative Stress Alleviation in Lupinus albus Var. Orden Dorado by the Inoculation of Four Plant Growth-Promoting Bacteria and Their Mixtures in Mercury-Polluted Soils. Front. Microbiol. 2022, 13, 907557. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 1, 963401. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Front. Sustain. Food Syst. 2021, 4, 618230. [Google Scholar] [CrossRef]
- Neshat, M.; Chavan, D.D.; Shirmohammadi, E.; Pourbabaee, A.A.; Zamani, F.; Torkaman, Z. Canola Inoculation with Pseudomonas Baetica R27N3 under Salt Stress Condition Improved Antioxidant Defense and Increased Expression of Salt Resistance Elements. Ind. Crops Prod. 2023, 206, 117648. [Google Scholar] [CrossRef]
- López-Hernández, J.; García-Cárdenas, E.; López-Bucio, J.S.; Jiménez-Vázquez, K.R.; de la Cruz, H.R.; Ferrera-Rodríguez, O.; Santos-Rodríguez, D.L.; Ortiz-Castro, R.; López-Bucio, J. Screening of Phosphate Solubilization Identifies Six Pseudomonas Species with Contrasting Phytostimulation Properties in Arabidopsis Seedlings. Microb. Ecol. 2023, 86, 431–445. [Google Scholar] [CrossRef]
- Paul, M.; Nayak, D.P.; Thatoi, H. Optimization of Xylanase from Pseudomonas Mohnii Isolated from Simlipal Biosphere Reserve, Odisha, Using Response Surface Methodology. J. Genet. Eng. Biotechnol. 2020, 18, 81. [Google Scholar] [CrossRef]
- Costa-Santos, M.; Mariz-Ponte, N.; Dias, M.C.; Moura, L.; Marques, G.; Santos, C. Effect of Bacillus spp. and Brevibacillus sp. on the Photosynthesis and Redox Status of Solanum lycopersicum. Horticulturae 2021, 7, 24. [Google Scholar] [CrossRef]
- Mukhtar, T.; Ali, F.; Rafique, M.; Ali, J.; Afridi, M.S.; Smith, D.; Mehmood, S.; Amna; Souleimanov, A.; Jellani, G.; et al. Biochemical Characterization and Potential of Bacillus Safensis Strain SCAL1 to Mitigate Heat Stress in Solanum lycopersicum L. J. Plant Growth Regul. 2023, 42, 523–538. [Google Scholar] [CrossRef]
- Wang, T.; Wang, K.; Wang, N.; Cui, D.; Li, S.; Lu, Q.; Zuo, Y. From Intercropping to Monocropping: The Effects of Pseudomonas Strain to Facilitate Nutrient Efficiency in Peanut and Soil. Plant Physiol. Biochem. 2025, 219, 109378. [Google Scholar] [CrossRef]
- Arkhipova, T.; Sharipova, G.; Akhiyarova, G.; Kuzmina, L.; Galin, I.; Martynenko, E.; Seldimirova, O.; Nuzhnaya, T.; Feoktistova, A.; Timergalin, M.; et al. The Effects of Rhizosphere Inoculation with Pseudomonas Mandelii on Formation of Apoplast Barriers, HvPIP2 Aquaporins and Hydraulic Conductance of Barley. Microorganisms 2022, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A.; et al. Insight into Recent Progress and Perspectives in Improvement of Antioxidant Machinery upon PGPR Augmentation in Plants under Drought Stress: A Review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Jurys, A.; Feizienė, D. The Effect of Specific Soil Microorganisms on Soil Quality Parameters and Organic Matter Content for Cereal Production. Plants 2021, 10, 2000. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lopez, J.B.G.; van Veelen, H.P.J.; Kok, D.J.D.; Postma, R.; Thijssen, D.; Sechi, V.; ter Heijne, A.; Bezemer, T.M.; Buisman, C.J.N. Effects of Different Soil Organic Amendments (OAs) on Extracellular Polymeric Substances (EPS). Eur. J. Soil. Biol. 2024, 121, 103624. [Google Scholar] [CrossRef]
- Ng, C.W.W.; Yan, W.H.; Tsim, K.W.K.; So, P.S.; Xia, Y.T.; To, C.T. Effects of Bacillus subtilis and Pseudomonas fluorescens as the Soil Amendment. Heliyon 2022, 8, e11674. [Google Scholar] [CrossRef]
- Mekonnen, E.; Kebede, A.; Nigussie, A.; Kebede, G.; Tafesse, M. Isolation and Characterization of Urease-Producing Soil Bacteria. Int. J. Microbiol. 2021, 2021, 8888641. [Google Scholar] [CrossRef]
- Cruz, D.; Cisneros, R.; Benítez, Á.; Zúñiga-Sarango, W.; Peña, J.; Fernández, H.; Jaramillo, A. Gram-Negative Bacteria from Organic and Conventional Agriculture in the Hydrographic Basin of Loja: Quality or Pathogen Reservoir? Agronomy 2021, 11, 2362. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Zhang, R.; Huang, Z.; Yang, C. Impacts of Pseudomonas Fluorescent Bacterial Fertilizer Addition on the Soil Environment and Fruit Yield under Water Stress in Greenhouse Grape. Front. Microbiol. 2025, 16, 1540628. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil. Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Zilda, D.S.; Yulianti, Y.; Sholihah, R.F.; Subaryono, S.; Fawzya, Y.N.; Irianto, H.E. A Novel Bacillus sp. Isolated from Rotten Seaweed: Identification and Characterization Alginate Lyase Its Produced. Biodiversitas 2019, 20, 1166–1172. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Guan, C.; Mathiesen, G.; Horn, S.J. Expression and Production of Thermophilic Alginate Lyases in Bacillus and Direct Application of Culture Supernatant for Seaweed Saccharification. Algal Res. 2021, 60, 102512. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Q.; Lu, D.; Han, W.; Li, F. A Novel Bifunctional Endolytic Alginate Lyase with Variable Alginate-Degrading Modes and Versatile Monosaccharide-Producing Properties. Front. Microbiol. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Długosz, A.; Długosz, J.; Frąc, M.; Gryta, A.; Breza-Boruta, B. Enzymatic Activity and Functional Diversity of Soil Microorganisms along the Soil Profile—A Matter of Soil Depth and Soil-Forming Processes. Geoderma 2022, 416, 115779. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, G. Dehydrogenase Activity as a Biological Indicator of Soil Health Chemical Science Review and Letters Dehydrogenase Activity as a Biological Indicator of Soil Health. Chem. Sci. Rev. Lett. 2021, 10, 326–329. [Google Scholar]
- Gupta, G.; Chauhan, P.S.; Jha, P.N.; Verma, R.K.; Singh, S.; Yadav, V.K.; Sahoo, D.K.; Patel, A. Secretory Molecules from Secretion Systems Fine-Tune the Host-Beneficial Bacteria (PGPRs) Interaction. Front. Microbiol. 2024, 15, 1355750. [Google Scholar] [CrossRef]
- Bernal, P.; Llamas, M.A.; Filloux, A. Type VI Secretion Systems in Plant-Associated Bacteria. Environ. Microbiol. 2018, 20, 1–15. [Google Scholar] [CrossRef]
- Lucke, M.; Correa, M.G.; Levy, A. The Role of Secretion Systems, Effectors, and Secondary Metabolites of Beneficial Rhizobacteria in Interactions With Plants and Microbes. Front. Plant Sci. 2020, 11, 589416. [Google Scholar] [CrossRef]
- Benitez Hernández, S.V.; Aragón Rojas, S.; Novoa, S.; Madrigal, S.; Núñez, P.; Gómez, P.; Montenegro, M.; Ordoñez, E.; Lancheros, G.; Sánchez, L. Evaluation of a Fermented Millet Product of Bacillus subtilis ATCC 55033 as Potential in Biocontrol of Bacteriosis (Xanthomonas axonopodis) in Gulupa (Passiflora edulis Sims). J. Agric. Sci. Technol. A 2020, 10, 1473–1483. [Google Scholar] [CrossRef]
- Xu, J.M.; Wang, W.J.; Chen, Z.T.; Zhou, Y.Y.; Pan, J.J.; Cheng, F.; Liu, Z.Q.; Zheng, Y.G. Exploring a High-Urease Activity Bacillus Cereus for Self-Healing Concrete via Induced CaCO3 Precipitation. Appl. Microbiol. Biotechnol. 2023, 107, 6351–6362. [Google Scholar] [CrossRef]
- Fitri, L.; Aulia, T.B.; Fauzi, A.; Kamil, G.A. Characterization and Screening of Urease Activity of Ureolytic Bacteria from Landfills Soil in Banda Aceh, Indonesia. Biodiversitas 2023, 24, 910–915. [Google Scholar] [CrossRef]
- Song, H.; Ding, M.Z.; Jia, X.Q.; Ma, Q.; Yuan, Y.J. Synthetic Microbial Consortia: From Systematic Analysis to Construction and Applications. Chem. Soc. Rev. 2014, 43, 6954–6981. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Sustainable Agrobiology: Design and Development of Microbial Consortia. Springer Nature: Berlin/Heidelberg, Germany, 2023; ISBN 9789811995699. [Google Scholar]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting Plant–Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, R.; Leveau, J.H.J.; Jeoh, T. Comparing Fluidized Bed Spray-Coating and Spray-Drying Encapsulation of Non-Spore-Forming Gram-Negative Bacteria. Ind. Biotechnol. 2021, 17, 283–289. [Google Scholar] [CrossRef]
- de-Bashan, L.; Giraldo, J.D.; Cruz-Barrera, M.; Schoebitz, M. Enhancing the Survival Rate and Effectiveness of Plant Growth-Promoting Bacteria through Bioencapsulation Techniques. Biol. Fertil. Soils 2024, 60, 543–556. [Google Scholar] [CrossRef]



| Dry Leaf Biomass (g) | Dry Stem Biomass (g) | Dry Root Biomass (g) | Dry Total Biomass (g) | |||||
|---|---|---|---|---|---|---|---|---|
| ES | ES | ES | ES | |||||
| Control (No beads) | 7.6 | 2.45 | 32.1 | 16.10 | 9.3 | 1.87 | 49.1 | 18.99 |
| Beads without Bacteria | 11.3 | 3.50 | 43.3 | 11.10 | 10.6 | 3.47 | 65.3 | 14.14 |
| B. safensis | 8.4 | 1.23 | 37.8 | 9.87 | 10.5 | 4.10 | 56.8 | 10.60 |
| P. mohnii | 9.8 | 1.85 | 40.9 | 13.74 | 8.4 | 2.32 | 59.2 | 14.45 |
| S. proteomaculans | 10.0 | 3.78 | 40.0 | 14.10 | 10.4 | 3.47 | 60.5 | 18.73 |
| P. baetica | 7.9 | 2.12 | 40.5 | 10.39 | 11.7 | 4.30 | 60.3 | 12.85 |
| Components | |||||
|---|---|---|---|---|---|
| 1. Morphometric and Biomass Parameters | |||||
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| Root dry weight (g) | 0.690 | - | 0.383 | -0.150 | - |
| Root volume (cm3) | −0.208 | −0.342 | −0.225 | −0.629 | 0.181 |
| Root length (cm) | 0.451 | - | 0.534 | 0.442 | −0.321 |
| Stem dry weight (g) | 0.734 | −0.339 | 0.184 | −0.190 | - |
| Leaf dry weight (g) | 0.351 | - | 0.408 | 0.111 | 0.588 |
| 2. Gas Exchange Parameters | |||||
| Photosynthesis (Pn) | - | 0.602 | - | - | - |
| Transpiration (ε) | −0.156 | 0.754 | 0.433 | - | 0.181 |
| Stomatal conductance (gs) | −0.273 | 0.687 | 0.408 | - | - |
| 3. Soil Microbial Activity and Biochemical Indicators | |||||
| Urease µmol NH4+ g−1 h−1 | 0.656 | 0.324 | -0.278 | 0.241 | - |
| Alkaline phosphatase µmol pNP g−1 h−1 | 0.467 | 0.363 | −0.380 | −0.284 | −0.245 |
| Protease µmol NH4+ g−1 h−1 | 0.170 | - | −0.506 | 0.430 | 0.530 |
| β-glucosidase µmol pNP g−1 h−1 | 0.384 | 0.137 | −0.242 | 0.430 | 0.545 |
| Dehydrogenase µg INTF g−1 soil | −0.285 | −0.330 | 0.110 | 0.693 | −0.399 |
| DOC g C kg−1 soil | 0.576 | 0.476 | −0.355 | −0.283 | −0.206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, S.V.; Carrasco, R.; Roldán, A.; Caravaca, F.; Campoy, M.; Cofré, J.; Ortiz, J.; Giraldo, J.D.; Schoebitz, M. Soil and Root Responses in Hazelnut Rhizosphere to Inoculate Rhizobacteria Immobilized via JetCutter Technology. Horticulturae 2025, 11, 808. https://doi.org/10.3390/horticulturae11070808
Benítez SV, Carrasco R, Roldán A, Caravaca F, Campoy M, Cofré J, Ortiz J, Giraldo JD, Schoebitz M. Soil and Root Responses in Hazelnut Rhizosphere to Inoculate Rhizobacteria Immobilized via JetCutter Technology. Horticulturae. 2025; 11(7):808. https://doi.org/10.3390/horticulturae11070808
Chicago/Turabian StyleBenítez, Solange V., Rocío Carrasco, Antonio Roldán, Fuensanta Caravaca, Manuel Campoy, Joaquín Cofré, José Ortiz, Juan D. Giraldo, and Mauricio Schoebitz. 2025. "Soil and Root Responses in Hazelnut Rhizosphere to Inoculate Rhizobacteria Immobilized via JetCutter Technology" Horticulturae 11, no. 7: 808. https://doi.org/10.3390/horticulturae11070808
APA StyleBenítez, S. V., Carrasco, R., Roldán, A., Caravaca, F., Campoy, M., Cofré, J., Ortiz, J., Giraldo, J. D., & Schoebitz, M. (2025). Soil and Root Responses in Hazelnut Rhizosphere to Inoculate Rhizobacteria Immobilized via JetCutter Technology. Horticulturae, 11(7), 808. https://doi.org/10.3390/horticulturae11070808









