Abstract
Active packaging using an edible coating could be an essential and sustainable alternative solution to preserve the properties of fruits and to prevent food loss and food waste. Fruits generate significant food wastes and losses. Reducing food waste is a global priority. For this research, nature-based solutions (NBSs) were applied, using micro-sized chitosan (CsMPs) and selenium microparticles (SeMPs), which are green-synthesized from black tea leaf extracts, and thyme essential oil. In this study, the effects of the new generation active food preservative coating agents formed from combinations of micro-sized chitosan (CsMPs) and selenium (SeMPs), and thyme essential oil (Oil) on the quality of “0900 Ziraat” sweet cherry fruits after harvest were investigated. After the fruits were coated with edible colloid solution, they were stored at 4 °C and 21 °C for 20 days, and quality parameter analyses were performed on days 0, 5, 10, 15, and 20. As a result of this study, it was determined that the application of CsMPs + SeMPs and the subsequent application of CsMPs + SeMPs + Oil from colloid solution coatings reduced weight loss, respiration, and decay rates. Also, it was determined that these applications were the most effective in preserving color values (L*, chroma, and hue), fruit firmness, total soluble solid (TSS) amount, acidity content and total phenolics, anthocyanin, and antioxidant capacity. These results show that CsMPs + SeMPs and CsMPs + SeMPs + Oil applications can be used as edible coatings to preserve the quality of sweet cherry fruits and extend their shelf life after harvest. This study’s results will contribute to obtaining micro-sized composite coating agents/agents produced with new technology to extend the shelf life.
1. Introduction
New sustainable solutions for food packaging are a necessity to prevent food waste and to meet environmental requirements. Food packaging trends have been changing, mainly due to consumer concerns about environmental protection and the prevention of food waste. The development of edible coatings can be much more than packaging, with additional functions such as antioxidant, antimicrobial, and nutritional properties, among others [1].
Türkiye has an excellent potential for sweet cherry production when its ecological and other characteristics are considered. In 2022, sweet cherry production in Türkiye was approximately 660 thousand tons, constituting about 24% of global production [2]. Sweet cherries are mostly consumed as a table fruit due to their superior sensory properties, such as color and flavor, and are also used in the chocolate and pastry industry. Sweet cherry is a significant fruit type for human health. It is one of the best sources of antioxidants, anthocyanins, and phenolic substances. Antioxidants play an essential role in protecting cells from damage caused by free radicals in the body. They are also substances that prevent diseases such as cancer, cardiovascular disease, and Alzheimer’s [3,4,5].
Sweet cherries are highly perishable due to their moderate rates of respiratory activity. Therefore, their shelf life is short, and they are susceptible to rapid microbiological decay during storage [6]. Physical damage and microbial decay cause fruit firmness, quality, discoloration, and post-harvest losses in sweet cherries. Therefore, it is crucial to determine appropriate methods to protect cherries’ post-harvest quality and nutritional value [7].
New advances are needed to minimize post-harvest losses and extend the shelf life of fresh fruits and vegetables. Environmentally friendly technologies are essential for extending their shelf life. Extending fruits’ storage and shelf life by coating them with edible materials has become increasingly popular in recent years. A thin layer of edible material in liquid form on the surface of products is called an edible film or coating. They consist of proteins, polysaccharides, and lipid-based compounds. Edible coatings are biodegradable materials containing essential oils, extracts, polyphenols, and antioxidant agents, individually or in combination [8].
Different biopolymers are used for edible coatings. Chitosan is a polysaccharide frequently used in edible coatings [9]. This polymer can provide endless structural possibilities for chemical and mechanical modifications. Also, it can facilitate innovative properties, applications, and functions. Chitosan is used in bioactive packaging to preserve fresh fruits and is an environmentally friendly application. Chitosan coating can limit respiration and transpiration by forming a selectively permeable film on fruits [10]. The primary source of selenium (Se), an essential trace element for human life, is nutrients in the diet [11]. Inorganic salt forms, sodium selenite and sodium-selenate, are widely used in food fortification and dietary supplementation [12]. Within permissible bounds, micro-sized selenium particles exhibit decreased cytotoxicity to higher organisms (humans, animals, fish, and crop plants), but these particles are also highly bioactive, suppressing bacteria, fungi, and eventually cancerous cells, which increases the usefulness of particles in the fields of biomedicine, pharmacy, and dietary intake [13]. Green-produced micro-sized selenium particles—especially when combined with plant extracts—were added to prescription drugs that contained antioxidant and anticancer agents, as well as ECs, for meat products and agricultural crops [14]. The mixture of chitosan with elements such as selenium, plant essential oils, or their components has significant potential to control the post-harvest decay of fresh fruits [15]. Including plant essential oils in edible coatings increases the antimicrobial effect, ensuring food quality protection against microorganisms [16]. The acceptance of plant volatiles as safe (GRAS) makes it possible to use essential oils in edible coating technology to increase the safety and shelf life of fruits and vegetables. Essential oils, which have antimicrobial effects, are used extensively to prevent microbial spoilage. The antimicrobial effect of essential oils is due to the ability of many oils and aroma compounds they contain to reduce or stop the production of mycotoxins by molds [17].
Colloids are mixtures of microscopically dispersed and insoluble materials in a liquid. They are low-cost due to the need for a small amount of active substance [18]. Colloids, particles with sizes ranging from 1 to 1000 nm, effectively extend the shelf life and freshness of products from production to consumption [19]. Colloids are a general category that includes both nanoparticles and microparticle materials. Although particles smaller than 100 nm are considered nano-sized materials, there are published studies in which larger-sized particles are also accepted as nano-sized. The concentrations of the base materials to be converted into nanoparticles and solvents and the ambient pH level affect the size of the particles to be obtained. It was reported that the average size of chitosan-based nanoparticles obtained in one study varied between 140 and 237 nm Feyzioglu and Tonuk [20], and in another study, it varied between 532 and 716 nm [21].
In this study, micro-sized selenium particles (SeMPs), which are synthesized through green synthesis using black tea leaf extracts, and micro-sized chitosan particles (CsMPs) were combined, and these microparticles were combined with thyme oil to obtain a water-soluble coating material enriched with essential oil for the food industry. This study aimed to investigate the effectiveness of this edible colloid solution in preserving the shelf life of fresh sweet cherry fruits to reduce food loss.
2. Materials and Methods
Low-molecular-weight (150–250 kDa) chitosan (Sigma-Aldrich, St. Louis, MO, USA) and 100% ultrapure acetic acid were used to prepare the chitosan solutions. In addition, sodium tripolyphosphate (TPP; Sigma, St. Louis, MO, USA) was used as a crosslinker, sodium hydroxide (NaOH; Fluka, Switzerland) was used to adjust the pH of the solution, and solid selenium (Na2O3Se; Sodium Selenite) was used for to prepare selenium solutions. The chemicals used were not subjected to any additional purification process, and all these chemicals were preferred over natural products that do not endanger food safety. Thyme essential oil (TEO) was supplied from the oil extraction unit, Erciyes University.
2.1. Synthesizing Microparticles
In this study, micro-sized particles were obtained from both chitosan and selenium. Chitosan microparticles were prepared using the modified ionic gelation method [22]. For synthesis, chitosan polymer (4 mg/mL) was dissolved in distilled water containing acetic acid (0.1 mL/mL) for 24 h with stirring. The pH of the solution was then adjusted to 4.6 with 10 N NaOH solution. TPP (2 mg/mL), a surface surfactant, was added to the chitosan medium at a ratio of 1:5 (TPP:chitosan) to prevent aggregation of particles. The TPP solution was added dropwise to the chitosan solution on a magnetic stirrer at 60 °C and the solution was stirred for 3 h. The resulting products were centrifuged at 12,000 rpm for 30 min. The centrifugation process was repeated 3 times by washing with distilled water to remove unbound structures in the medium.
Selenium was converted into micro-sized particles by green synthesis using black tea leaf extract [23]. To prepare the tea leaf extract, 50 g of dried tea leaves was boiled with 250 mL of distilled water at 85 °C for 120 min. After cooling at room temperature, it was filtered through Whatman No. 1 filter paper and stored at 4 °C for use in the synthesis of selenium microparticles. For the green synthesis, a 5 mM aqueous solution was prepared with solid selenium (Na2O3Se; Sodium Selenite). The extract and aqueous metal solution were mixed in glass beakers at a ratio of 5:3 (extract/metal solution) and allowed to react for about 30 min at room temperature. The formation of selenium particles was determined by measuring the maximum absorbance value in UV-vis spectroscopy (Systonic 119 UV-VIS, Haryana, India) at different time intervals within 30 min depending on the color change. After the reaction, the dark solution was centrifuged at 10,000 rpm for 5 min, the settled solid was washed several times with distilled water, and the final product was dried in an oven at 60 °C for 72 h. The dry fraction was then pulverized using a mortar and pestle and stored for further processing.
2.2. Characterization of Particles
A Zeta Sizer (Malvern, Nano ZS90, Software version 7.11, UK) was used to determine the size and surface charge distribution of the particles. The “dynamic light scattering” (DLS) technique was used to calculate the average size of the particles. An amount of 500 μL of microparticle solution was drawn directly from the medium, loaded into a disposable cuvette model ZEN0040 (PN ZEN0040), and placed in the device. The average Zavg sizes of the particles were 477.3 ± 1.4 nm for chitosan and 583.3 ± 2.6 nm for selenium (Figure 1 and Figure 2).
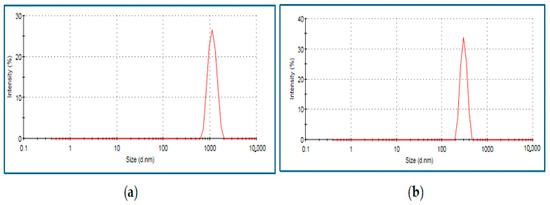
Figure 1.
Chitosan (a) and chitosan microparticle (b) size as density plots.
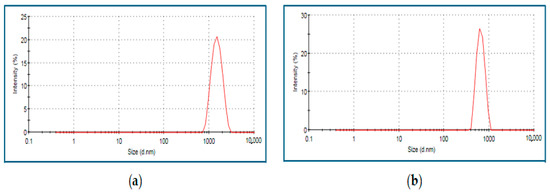
Figure 2.
Selenium (a) and selenium microparticle (b) size as density plots.
The chemical bonding structures of the particles were analyzed by FT-IR spectrophotometry (Fourier transform infrared spectroscopy). Analyses were performed in the wavelength range of 4000–400 cm−1 with a resolution of 4 cm−1. According to the results, a different spectrum due to TPP was observed in both chitosan and selenium particles (Figure 3 and Figure 4).
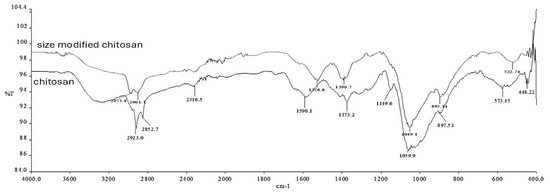
Figure 3.
FTIR plots of chitosan particles.
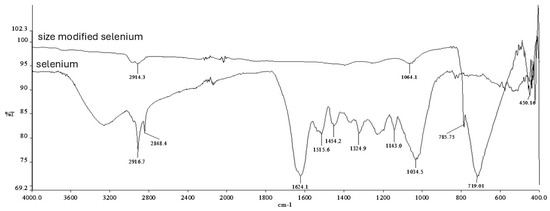
Figure 4.
FTIR plots of selenium particles.
The surface properties of the synthesized particles were comparatively investigated by SEM (scanning electron microscopy ZEISS Gemini SEM 500, Jena, Germany). The results showed that the microparticles exhibited an irregular distribution. Spherical structure was generally observed in particles. It is seen that the particle sizes in the images agree with the results analyzed with the Zeta sizer (Figure 5 and Figure 6).

Figure 5.
30,000× SEM images of sample (chitosan on the left, size modified chitosan on the right).
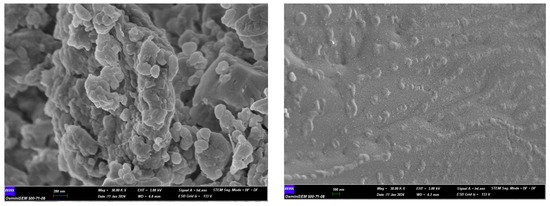
Figure 6.
30,000× SEM images of sample (selenium on the left, size modified selenium on the right).
2.3. Preparation of Colloidal Solutions
The four coating solutions prepared in this study are as follows: 1. CsMPs (Chitosan colloidal solution); 2. CsMPs + Oil (Chitosan colloidal solution + thyme essential oil); 3. CsMPs + SeMPs (Chitosan colloidal solution + Selenium colloidal solution); 4. CsMPs + SeMPs + Oil (Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil). To prepare spraying solutions, chitosan colloidal solution (500 mg) was dissolved in 20 mL of 1% aqueous acetic acid solution overnight and the mixture was stirred continuously to form a clear solution. Additionally, 10 mg of selenium colloidal solution was dissolved in 10 mL of distilled water and kept in a vibrating water bath for 20 min. Selenium colloidal solution was then added to the chitosan colloidal solution and homogenized with continuous stirring. For formulations containing essential oil, a 2.5 mL solution of chitosan or selenium was added to 1000 ppm concentrations of thyme essential oil previously dissolved in methanol (40 mL) using a peristaltic pump for 3 min at 20,000 rpm to obtain stable dispersions. The resulting gel-like viscous solution was poured onto a dust-free ceramic plate and dried at room temperature for 48 h and then kept in a vacuum oven.
The coating solutions were applied to sweet cherry (0900 Ziraat cv.) fruits grown in the research field of Erciyes University Faculty of Agriculture. The fruits were collected early in the morning and at the ideal harvest time and delivered to laboratory conditions within one hour. Cherry fruits were dark-red in color and long-stemmed, and the average fruit weight was 7 g. Some fruits were separated for initial analysis (day 0), while others were used for coating by spraying. The samples were spray-coated to be completely wetted. Coating solutions were prepared to contain 1% of the active ingredient, and the essential oil content was set to 1000 ppm. Control (uncoated) and coated fruits were kept under two different conditions: room conditions (at 21 °C) and cold conditions (at 4 °C). The conditions under which the fruits were kept were approximately 50–60% humidity. The study was planned using a randomized plot experimental design with three replications. In each replicate, 150 g (about 21–22 numbers) of sweet cherry fruits was used. Various quality parameters were analyzed on the 5th, 10th, 15th, and 20th days during the shelf life of the fruits. To determine parameters other than weight loss, samples containing 150 g of fruit with 3 replicates were used separately at each analysis time. The same samples were used at each analysis time throughout the experiment to determine the weight loss.
2.4. Determination of Quality Parameters
In this study, weight loss (%), decay rate (%), respiration rate (mL CO2 kg−1 h−1), fruit color, fruit firmness (N), total soluble solid (TSS) amount (%), titratable acidity (%), total phenolic content (mg/100 g gallic acid), total antioxidant capacity (% inhibition), and total anthocyanin content (µg cy-3-glu/g) were determined during the shelf life of the fruits. The weight loss was determined by finding the difference between the weights at the beginning of shelf life and in each analysis period, and the decay rate was determined by observing the mycelial growth symptoms on the fruit surface. The respiration rate was determined by measuring the amount of CO2 the fruits gave to the external environment. A digital carbon dioxide analyzer was used for this aim [24]. A digital colorimeter measured the fruit color (Minolta, model CR-400, Tokyo, Japan). According to the prepared scale, the a* value is expressed as redness–greenness, and the b* value as yellowness–blueness. Chroma value = (a*2 + b*2)1/2 and hue angle value were determined by the formula hº = tan−1 × b*/a* [25]. Fruit firmness was determined using a 4 mm tip hand penetrometer (Effegi model FT 327), and the TSS amount was determined from the fruit juice sample using a digital refractometer (PAL-1, McCormick Fruit Tech. Yakima, WA, USA). To calculate titratable acidity, a 10 mL sample of fruit juices was diluted with 10 mL of distilled water and titrated with 0.1 mol L−1 sodium hydroxide (NaOH) until pH 8.1 was reached. Based on the amount of NaOH consumed in the titration, it was expressed in terms of malic acid. Among the biochemical properties, the total phenolic content was determined using Folin–Ciocalteu’s chemical, as described in Singleton et al. [26]; the total antioxidant capacity was determined using the DPPH method specified by Brand-Williams et al. [27], and the total anthocyanin content was determined using the pH difference method [28].
This research was conducted using a random plot experimental design with three replicates. The data were analyzed using the SAS 9.1 statistical package program. An analysis of variance was performed, and statistically significant parameter values based on the analysis of variance (p > 0.05) results were compared with the LSD test. Graphs were drawn in Microsoft Office Excel 2016.
3. Results and Discussion
In this study, the weight loss of control and coated sweet cherry fruits during shelf life on different temperature conditions are given in Figure 7. The effect on weight loss of coating applications at 21 °C was statistically significant, but not significant at 4 °C (p < 0.05). The weight loss of control and coated fruits stored at both temperatures increased over 20 days, and this loss was more significant at high temperatures. In cherries kept at 21 °C, lower weight loss was determined with the CsMPs + SeMPs application than other applications on the 20th day of shelf life, followed by the CsMPs + SeMPs + Oil application. The highest weight loss was determined in control fruits. These results obtained from the study are consistent with previous studies showing that colloid solution coatings form a semi-permeable barrier that minimizes respiration and moisture loss, thus effectively reducing weight loss [29,30]. This study exhibited good performance, especially when the micro-sized chitosan and selenium were combined. This situation was explained by Dorazilová et al. [31] as the high synergistic effect of these compounds.
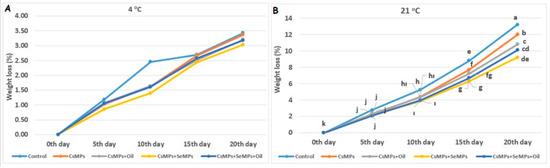
Figure 7.
Weight loss evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; and CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B). Different lowercase letters within each graph indicate significant differences between treatments and storage days, according to the LSD test (p > 0.05). If there is no statistical difference between storage days, no lettering is done.
The changes in the respiration rate of the control and coated sweet cherry fruits during shelf life on different temperature conditions are shown in Figure 8. The effect of coating applications on the respiratory rate of cherry fruits during shelf life was found to be statistically significant (p < 0.05). While the coated fruits had lower respiratory rates than the control fruits on day 0, the lowest values were obtained from the CsMPs + SeMPs and CsMPs + SeMPs + Oil applications. In the cherries kept at 4 °C, the respiratory rate was lower with the CsMPs + SeMPs application than the other applications on the 20th day of the shelf life. On the other hand, all coating applications had the same effect statistically at 21 °C, while the control fruits had a higher respiratory rate than the coated fruits. The respiratory rate of control and coated fruits increased during shelf life, which was more significant at higher temperatures. In addition, lower respiratory rates were determined in coated fruits than in control fruits during shelf life. Respiratory rate, as an indicator of the metabolic activities of fruits, is an essential factor affecting the freshness and post-harvest life of cherries. Generally, the higher the respiratory rate of fruits, the shorter the post-harvest life [32]. It has been demonstrated that coating with micro-sized particles changes the gas exchange properties of fresh products and effectively reduces respiratory rates [33].
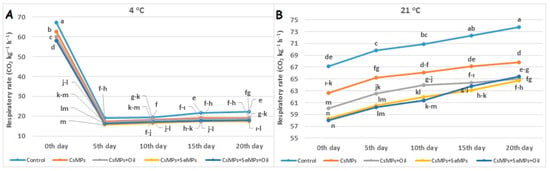
Figure 8.
Respiratory rate evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; and CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B). Different lowercase letters within each graph indicate significant differences between treatments and storage days, according to the LSD test (p > 0.05).
The effect of coating applications on the decay rate measured in cherry fruits used in the experiment was found to be statistically significant at both temperature conditions (p < 0.05, Figure 9). The effect of the temperature factor on the decay rate was much more substantial, and higher rates were observed at elevated temperatures compared with low temperatures. CsMPs + SeMPs and CsMPs + SeMPs + Oil applications showed lower decay rates than the other treatments at both temperature conditions. The highest decay rate was determined in control fruits from the 10th day of shelf life at 4 °C. The decay rate of control fruits was higher than that of coated fruits during shelf life at 21 °C. The lower decay rates of coated fruits are probably related to the antimicrobial properties of micro-sized particle coatings that prevent microbial contamination and delay spoilage in fruits and vegetables. Comparable results to our study were obtained in studies conducted with micro-sized particle coatings [34,35,36].
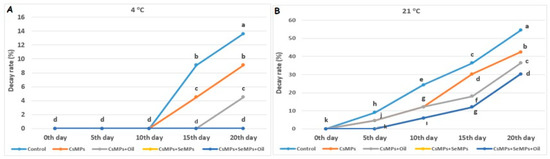
Figure 9.
Decay rate evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B). Different lowercase letters within each graph indicate significant differences between treatments and storage days, according to the LSD test (p > 0.05).
Figure 10 presents the changes in the color characteristics of control and coated sweet cherry fruits during shelf life. The coating applications had a statistically significant effect on L* and hue values at both temperature conditions, while the impact on chroma value was insignificant (p < 0.05). Under both temperature conditions, the color values decreased on the 20th day of the shelf life. On the other hand, the value of cherries kept at low temperatures was generally higher than that of those kept at high temperatures. Also, the L* and hue values were found to be the lowest in the control group, and the highest values occurred in CsMPs + SeMPs and CsMPs + SeMPs + Oil applications. The color values of control and coated fruits decreased during shelf life, and this decrease was more significant at higher temperatures. Also, more color values were determined in coated fruits than in control fruits during shelf life. Fruit color is a critical quality affecting consumer acceptance. This study observed that fruit brightness (L value) decreased during shelf life due to water loss, while coating applications slowed the decrease in the L value compared with the control. Recently, studies have focused on nano/micro particle coatings and some anti-browning agents to reduce enzymatic darkening. Studies have generally reported that nano/micro particle coatings prevent enzymatic darkening and maintain color quality by lowering chlorophyll degradation [37,38,39,40]. The results show that nano/micro particles preserve color brightness in fresh products by reducing oxidative stress.
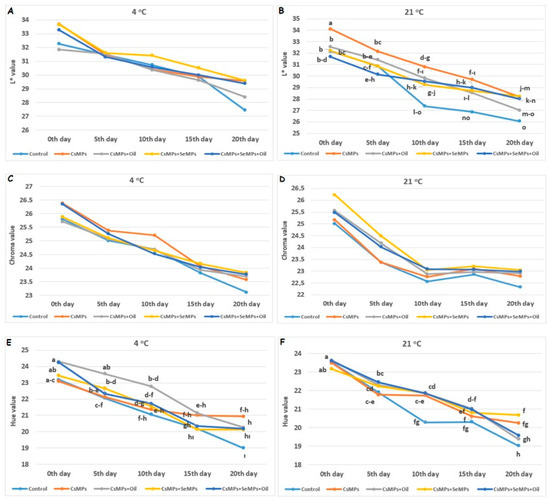
Figure 10.
Color characteristics evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: L* value at 4 °C (A), L* value at 21 °C (B), Chroma value at 4 °C (C), Chroma value at 21 °C (D), Hue value at 4 °C (E), and Hue value at 21 °C (F). Different lowercase letters within each graph indicate significant differences between treatments and storage days, according to the LSD test (p > 0.05). If there is no statistical difference between storage days, no letters are included.
The fruit firmness values of the control and coated sweet cherry fruits during shelf life are shown in Figure 11. The effect on fruit firmness of coating applications at 4 °C was statistically significant, but not significant at 21 °C (p < 0.05). The effect of the temperature factor on fruit firmness was much more substantial, and lower values were observed at elevated temperatures compared with low temperatures. CsMPs + SeMPs and CsMPs + SeMPs + Oil applications showed higher fruit firmness than the other treatments under both temperature conditions. The fruit firmness of the control and coated fruits stored at both temperatures decreased over 20 days, and this decrease was more significant at elevated temperatures. Additionally, a lower decrease was determined in coated fruits than in control fruits during their shelf life. Fruit firmness is a critical quality parameter in preserving cherries’ freshness and commercial value. Slowing down the loss of firmness in cherry fruits allows consumers to be offered fresh and durable fruit for a more extended period. Edible coatings reduce the oxygen uptake of fruits and reduce metabolic activity. As a result, ripening/softening slows down. Results consistent with our study findings have also been reported by various researchers [41,42]. On the other hand, Metin [43] reported that chitosan coating applications delay the decrease in fruit firmness in many fruit species by slowing down enzyme activity.
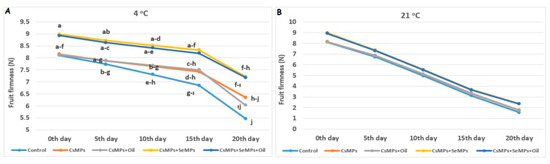
Figure 11.
Fruit firmness evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B) Different lowercase letters within each graph indicate significant differences between treatments and storage days, according to the LSD test (p > 0.05). If there is no statistical difference between storage days, no letters are included.
The amounts of total soluble solids (TSSs) in the control and coated sweet cherry fruits during shelf life are given in Figure 12. The effect of coating applications on the TSS amount measured in cherry fruits used in the experiment was found to be statistically significant at both temperature conditions (p < 0.05). In the cherries kept at 21 °C, a lower amount of TSS was observed with CsMPs + Oil application than other applications on the 20th day of shelf life. The highest TSS amount was determined in control fruits. The TSS amount of the control and coated fruits stored at both temperatures increased over 20 days, which was more significant at high temperatures. The TSS amount represents the percentage of dissolved solids [44]. There may be decreases in the TSS amount due to the respiration of sugars during shelf life in fresh fruits. However, increases in this parameter are more likely to occur due to the concentration effect of water losses [45,46]. On the other hand, the applied coating agents decreased the respiration rate in fruits, delaying the synthesis and utilization of metabolites and thus causing a decrease in the TSS amount [47]. The fact that the control group showed a higher amount of TSS than the treatments in our findings confirms this.
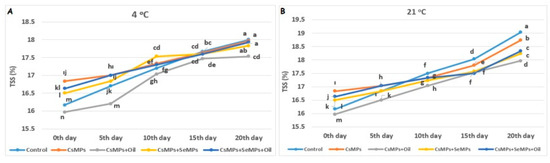
Figure 12.
Evolution of TSS amount in control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B) Different lowercase letters within each graph indicate significant differences between treatments and storage days, according to the LSD test (p > 0.05).
The effect of coating applications on titratable acidity content under both temperature conditions was insignificant (p < 0.05, Figure 13). Under both temperature conditions, the titratable acidity content decreased during shelf life. It is stated that organic acids are consumed by respiration during the shelf life of fruits; therefore, a decrease is observed [48]. It is known that coating application slows down metabolic activity by inhibiting enzyme activity in fruits, which delays synthesis and degradation mechanisms. Therefore, coating applications decelerate the rate of deterioration of organic acids in fruits during their shelf life [49].
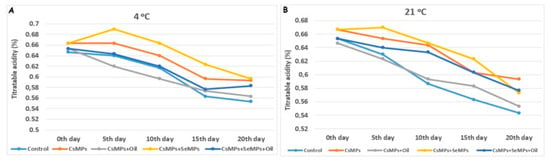
Figure 13.
Titratable acidity content evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B).
In this study, the changes in the total phenolic content of control and coated sweet cherry fruits during shelf life under different temperature conditions are shown in Figure 14. The effect of coating applications on the total phenolic content of cherry fruits stored at both temperatures during shelf life was insignificant (p < 0.05). The total phenolic content of the control and coated fruits increased during their shelf life. In addition, a lower total phenolic content was defined in coated fruits than in control fruits during their shelf life. While the control fruits had a lower total phenolic content than the coated fruits at both temperatures, the lowest values were obtained from the CsMPs + SeMPs application at 4 °C, and CsMPs + SeMPs + Oil application at 21 °C. Phenolic compounds are secondary metabolites that have an essential effect on color and taste formation in fruits. Changes occur in the phenolic content of fresh fruits after harvest due to many factors, such as species, variety, harvest time, cultural practices, and storage time. It was determined that the phenolic compound contents increased by 40–60% with the prolongation of storage time [50]. Our findings indicated a lower total phenolic content in coating solution-treated products than in the control products during the shelf life period. Micro-sized particle applications effectively delayed this increase during storage under shelf-life conditions. Consistent with our findings, the total phenolic content was lower in many cold-stored products with micro or nanocomposite applications compared with control products [42,51,52,53]. The most important effect of micro-sized composite applications in slowing down the increase in phenolic substances can be considered as reducing respiration and metabolic activity accordingly.
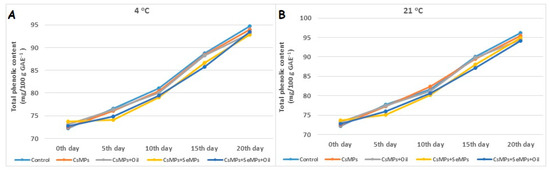
Figure 14.
Total phenolic content evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B).
The effect on the total anthocyanin content of coating applications at both temperatures was statistically significant (p < 0.05, Figure 15). The total anthocyanin content of control and coated fruits stored at both temperatures increased over 20 days, and this increase was slightly more significant at high temperatures. In the cherries stored at both temperatures, lower total anthocyanin content was determined with CsMPs + Oil application than with other applications on the 20th day of shelf life. The highest total anthocyanin content was identified in control fruits. Additionally, a lower total anthocyanin content was determined in coated fruits than in control fruits during shelf life (Figure 15). Anthocyanins are a group of phenolic compounds that are responsible for the red–blue color of fruits [54] and have beneficial effects on human health [55]. In addition, anthocyanins have strong antioxidant properties. Preserving the number of anthocyanins can ensure that the nutritional value of colored fruits such as cherries remains high throughout their shelf life.
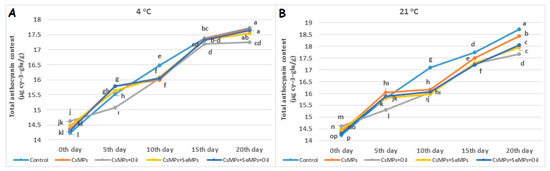
Figure 15.
Total anthocyanin content evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B) Different lowercase letters within each graph indicate significant differences between treatments and storage days, according to the LSD test (p > 0.05).
The effect of coating applications on the total antioxidant capacity measured in cherry fruits used in the experiment was found to be statistically significant under both temperature conditions (p < 0.05, Figure 16). The antioxidant capacity of the control and coated fruits stored at both temperatures decreased over 20 days, and this decrease was more significant at high temperatures. Additionally, a lower decrease was determined in coated fruits than in control fruits during their shelf life. The effect of the temperature factor on the antioxidant capacity was much more significant, and lower values were observed at elevated temperatures compared with low temperatures. CsMPs + SeMPs + Oil application showed higher antioxidant capacity than the other treatments under both temperature conditions. It is known that post-harvest conditions are essential in preserving the nutritional content of fresh fruits. It was determined that modified-size selenium and chitosan coating effectively slowed down the decrease in the antioxidant capacity value in cherry fruits. The total antioxidant capacity in many fresh fruits preserved in cold storage by applying nano/micro-composite was higher than that in control fruits [56,57,58,59]. The researchers stated that the higher antioxidant activity because of the coating process was due to the reduced loss of phenolic compounds.
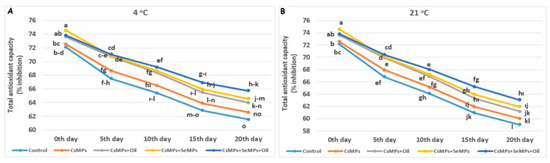
Figure 16.
Total antioxidant capacity evolution of control and coated (CsMPs: Chitosan colloidal solution; CsMPs + Oil: Chitosan colloidal solution + thyme essential oil; CsMPs + SeMPs: Chitosan colloidal solution + Selenium colloidal solution; CsMPs + SeMPs + Oil: Chitosan colloidal solution + Selenium colloidal solution + thyme essential oil) cherry fruits over 20 days (0, 5, 10, 15, and 20 days) under two temperature conditions: 4 °C (A) and 21 °C (B) Different lowercase letters within each graph indicate significant differences between treatments and storage days, according to the LSD test (p > 0.05).
4. Conclusions
This study investigated the effect of some selenium and chitosan-based coating materials and thyme oil additives on the shelf life of sweet cherry fruits. Post-harvest applications aim to extend the shelf life of fruits with minimum losses by preserving their quality characteristics. Coating treatments showed favorable results compared with the control in delaying the weight loss increase due to the progression of storage time. As a result of this study, edible coatings developed from micro-sized selenium and chitosan compositions were reported to have positive effects on reducing decay and preventing the decrease in fruit color, fruit firmness, and total phenolic content values under shelf-life conditions. Considering the decay rate in products kept at 21 °C, edible coatings enabled the shelf life of cherries to be extended by at least 5 days. According to the findings of this study, the CsMPs + SeMPs and CsMPs + SeMPs + Oil treatments not only resulted in a decreased decay rate and weight loss, but also in the preservation of freshness by lowering respiration. The antioxidant capacity in the fruits was preserved owing to the CsMPs + SeMPs + Oil coating. It has been demonstrated that micro-sized chitosan, in combination with micro-sized selenium, may be employed in homogeneous edible coatings. It was determined that post-harvest new active food preservative coating agents were effective in slowing down the changes in physical and biochemical properties of sweet cherry and improving shelf-life conditions. The findings obtained in this study provide data for the design of new micro/nanocomposite coating agents that can be used to extend the post-harvest life of fresh fruits. Edible coating can be an efficient packaging solution to preserve the properties of fruits and to prevent food loss and food waste.
Author Contributions
All authors contributed to the study’s conception and design. E.Y., F.H., M.Y. and A.S. conducted the experiments and collected data. G.C.P. and M.P. analyzed the data and wrote and edited the manuscript. All authors approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Erciyes University Scientific Research Projects Department, grant number FBA-2023-12370.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gaspar, M.C.; Braga, M.E. Edible films and coatings based on agrifood residues: A new trend in the food packaging research. Curr. Opin. Food Sci. 2023, 50, 101006. [Google Scholar] [CrossRef]
- FAOSTAT: FAOSTAT Statistical Database. Available online: http://www.fao.org/faostat/en/#data/ (accessed on 10 December 2024).
- Coşkun, Ö.F.; Toprak, S.; Mavi, K. Some seed properties and molecular analysis with inter-primary binding site (iPBS) retrotranposons markers of edible-seeded watermelon genotypes. Genet. Resour. Crop Evol. 2024, 71, 3151–3162. [Google Scholar] [CrossRef]
- Coskun, O.F.; Gulsen, O. Determination of markers associated with important agronomic traits of watermelon (Citrullus lanatus L.). J. Agric. Sci. Technol. 2024, 26, 1359–1371. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic compounds classification and their distribution in winemaking by products. Eur. Food Res. Technol. 2023, 249, 207–239. [Google Scholar] [CrossRef]
- Alonso, J.; Alique, R. Sweet cherries. In Handbook of Fruits and Fruit Processing; Hui, Y.H., Barta, J., Cano, M.P., Gusek, T.W., Sidhu, J.S., Sinha, N.K., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 359–367. [Google Scholar]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, post-harvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of edible coating in extension of fruit shelf life. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Melo, N.F.C.B.; de MendonçaSoares, B.L.; Diniz, K.M.; Leal, C.F.; Canto, D.; Flores, M.A.; Stamford, T.C.M. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of post-harvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Mueller, A.S.; Mueller, K.; Wolf, N.M.; Pallauf, J. Selenium and diabetes: An enigma? Free Radic. Res. 2009, 43, 1029–1059. [Google Scholar] [CrossRef]
- Nayak, V.; Singh, K.R.; Singh, A.K.; Singh, R.P. Potentialities of selenium nanoparticles in biomedical science. New J. Chem. 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Diab, A.M.; Elzahy, A.F.; Mazrou, K.E.; Tayel, A.A.; Moussa, S.H. Green biosynthesized selenium nanoparticles by cinnamon extract and their antimicrobial activity and application as edible coatings with nano-chitosan. J. Food Qual. 2021, 2021, 6670709. [Google Scholar] [CrossRef]
- Perdones, Á.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, S.; Jia, W.; Guo, T.; Wang, F.; Li, J.; Yao, Z. Natural antimicrobials from plants: Recent advances and future prospects. Food Chem. 2024, 432, 137231. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Črešnar, K.P.; Bikiaris, D.N.; Zemljič, L.F. Colloidal solutions as advanced coatings for active packaging development: Focus on PLA systems. Polymers 2023, 15, 273. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Holster, A.; Kottaki, N.; Tsochantaris, E.; Topsøe, F.; Poulsen, J.; Bache, M.; Liang, X.; Blom, N.S.; Kronholm, J. Water structure, properties and some applications—A Review. Chem. Thermodyn. Therm. Anal. 2022, 6, 100053. [Google Scholar] [CrossRef]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in Nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT-Food Sci. Technol. 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Ilk, S.; Sağlam, N.; Özgen, M.; Korkusuz, F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol. 2017, 94, 653–662. [Google Scholar] [CrossRef]
- Mareedu, T.; Poiba, V.; Vangalapati, M. Green synthesis of iron nanoparticles by green tea and black tea leaves extract. Mater. Today Proc. 2021, 42, 1498–1501. [Google Scholar] [CrossRef]
- Ozturk, B. Effects of modified atmosphere packaging and aloe vera treatments on quality traits of cherry laurel fruit (Prunus laurocerasus L.) during shelf life. Int. J. Agric. Wildl. Sci. 2020, 6, 399–406. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Giusti, M.M.; Rodriguez-Saona, L.E.; Wrolstad, R.E. Spectral characteristics, molar absorptivity and color of pelargonidin derivatives. J. Agric. Food Chem. 1999, 47, 4631–4637. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S. Physico-mechanical and microstructural properties of semolina flour films as influenced by different sorbitol/glycerol concentrations. Int. J. Food Prop. 2018, 21, 983–995. [Google Scholar] [CrossRef]
- Dorazilová, J.; Muchová, J.; Šmerková, K.; Diviš, P.; Kopel, P.; Kociová, S.; Veselý, R.; Pavlináková, V.; Adam, V.; Vojtová, L. Synergistic effect of chitosan and selenium nanoparticles on biodegradation and antibacterial properties of collagenous scaffolds designed for infected burn wounds. Nanomaterials 2020, 10, 1971. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-Abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef]
- Abdulraheem, M.I.; Moshood, A.Y. Effects of nanoparticles on improvement in quality and shelf life of fruits and vegetables. Plant Biol. Crop Res. 2021, 4, 1042. [Google Scholar]
- Indumathi, M.P.; Sarojini, K.S.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Z.; Alsammarraie, F.K.; Kong, F.; Lin, M.; Mustapha, A. Properties and antimicrobial activity of polyvinyl alcohol-modified bacterial nanocellulose packaging films incorporated with silver nanoparticles. Food Hydrocoll. 2020, 100, 105411. [Google Scholar] [CrossRef]
- Aldhanhani, A.R.H.; Kaur, N.; Ahmed, Z.F.R. Antioxidant phytochemicals and antibacterial activities of sidr (Ziziphus spp.) leaf extracts. Acta Hortic. 2022, 1353, 323–332. [Google Scholar] [CrossRef]
- Lichanporn, I.; Techavuthiporn, C.; Wongs-Aree, C. Effect of silver particle-longkong peel extract coating on post-harvest decay and browning in longkong fruit. Hortic. J. 2020, 89, 328–336. [Google Scholar] [CrossRef]
- Ojeda, G.A.; Sgroppo, S.C.; Martín-Belloso, O.; Soliva-Fortuny, R. Chitosan/tripolyphosphate nanoaggregates enhance the anti-browning effect of ascorbic acid on mushroom slices. Postharvest Biol. Technol. 2019, 156, 110934. [Google Scholar] [CrossRef]
- Perez, G.M.J.; Guerrero, Q.D.; Silva, M.E.; Sandoval, R.S.A.; Zaragoza, Z.M.L. The effects of tocopherol nanocapsules/xanthan gum coatings on the preservation of fresh-cut apples: Evaluation of phenol metabolism. Food Bioprocess Technol. 2015, 8, 1791–1799. [Google Scholar] [CrossRef]
- Barikloo, H.; Ahmadi, E. Shelf life extension of strawberry by temperatures conditioning, chitosan coating, modified atmosphere, and clay and silica nanocomposite packaging. Sci. Hortic. 2018, 240, 496–508. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A.; Hallaj, R. Influence of nano-ZnO on microbial growth, bioactive content and post-harvest quality of strawberries during storage. Innov. Food Sci. Emerg. Technol. 2016, 35, 168–176. [Google Scholar] [CrossRef]
- Metin, D. Effects of Postharvest Thyme Oil and Chitosan Applications on Fruit Quality in ‘0900 Ziraat’ Cherry Variety During Storage. Master’s Thesis, Selcuk University, Konya, Turkey, 2022. [Google Scholar]
- Beckles, D.M. Factors affecting the post-harvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Hernandez-Munoz, P.; Almenar, E.; Del Valle, V.; Velez, D.; Gavara, R. Effect of chitosan coating combined with post-harvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT-Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, H.; Guo, X.; Bi, X.; Liu, X.; Xu, Q.; Zheng, Y.I. Effect of chitosan/Nano-TiO2 composite coatings on the post-harvest quality and physicochemical characteristics of mango fruits. Sci. Hortic. 2020, 263, 109135. [Google Scholar] [CrossRef]
- Rivera-Pastrana, D.M.; Béjar, A.A.G.; Martínez-Téllez, M.A.; Rivera-Domínguez, M.; González-Aguilar, G.A. Post-harvest biochemical effects of UV-C irradiation on fruit and vegetables. Rev. Fitotec. Mex. 2007, 30, 361–372. [Google Scholar]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef]
- Valero, D.; Diaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillen, F.; Martinez-Romero, D.; Serrano, M. Post-harvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J. Agric. Food. Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef]
- Kou, X.; He, Y.; Li, Y.; Chen, X.; Feng, Y.; Xue, Z. Effect of abscisic acid (ABA) and chitosan/nano-silica/sodium alginate composite film on the color development and quality of post-harvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao). Food Chem. 2019, 270, 385–394. [Google Scholar] [CrossRef]
- Valizadeh, M.; Behnamian, M.; Dezhsetan, S.; Karimirad, R. Controlled release of turmeric oil from chitosan nanoparticles extends shelf life of Agaricus bisporus and preserves its post-harvest quality. Food Biosci. 2021, 44, 101401. [Google Scholar] [CrossRef]
- Wang, L.; Shao, S.; Madebo, M.P.; Hou, Y.; Zheng, Y.; Jin, P. Effect of nano-SiO2 packing on post-harvest quality and antioxidant capacity of loquat fruit under ambient temperature storage. Food Chem. 2020, 315, 126295. [Google Scholar] [CrossRef]
- Mullen, W.; McGinn, J.; Lean, M.E.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, M.; Rimbach, G.; Rivas-Gonzalo, J.C.; de Pascual-Teresa, S. Antioxidant and cellular activities of anthocyanins and their corresponding vitisins a studies in platelets, monocytes, and human endothelial cells. J. Agric. Food Chem. 2004, 52, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Arabpoor, B.; Yousefi, S.; Weisany, W.; Ghasemlou, M. Multifunctional coating composed of Eryngium campestre L. essential oil encapsulated in nano-chitosan to prolong the shelf-life of fresh cherry fruits. Food Hydrocoll. 2021, 111, 106394. [Google Scholar] [CrossRef]
- Dulta, K.; Koşarsoy Ağçeli, G.; Thakur, A.; Singh, S.; Chauhan, P.; Chauhan, P.K. Development of alginate-chitosan based coating enriched with ZnO nanoparticles for increasing the shelf life of orange fruits (Citrus sinensis L.). J. Polym. Environ. 2022, 30, 3293–3306. [Google Scholar] [CrossRef]
- Emamifar, A.; Mohammadizadeh, M. Preparation and application of LDPE/ZnO nanocomposites for extending shelf life of fresh strawberries. Food Technol. Biotechnol. 2015, 53, 488–495. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, D.H.; Nguyen, H.V. Combination effects of calcium chloride and nano-chitosan on the post-harvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).