Assessment of Genetic Diversity in Walnut (Juglans regia L.) Genotypes from Southern and Southeastern Kazakhstan Using Microsatellite Markers
Abstract
1. Introduction
2. Materials and Methods
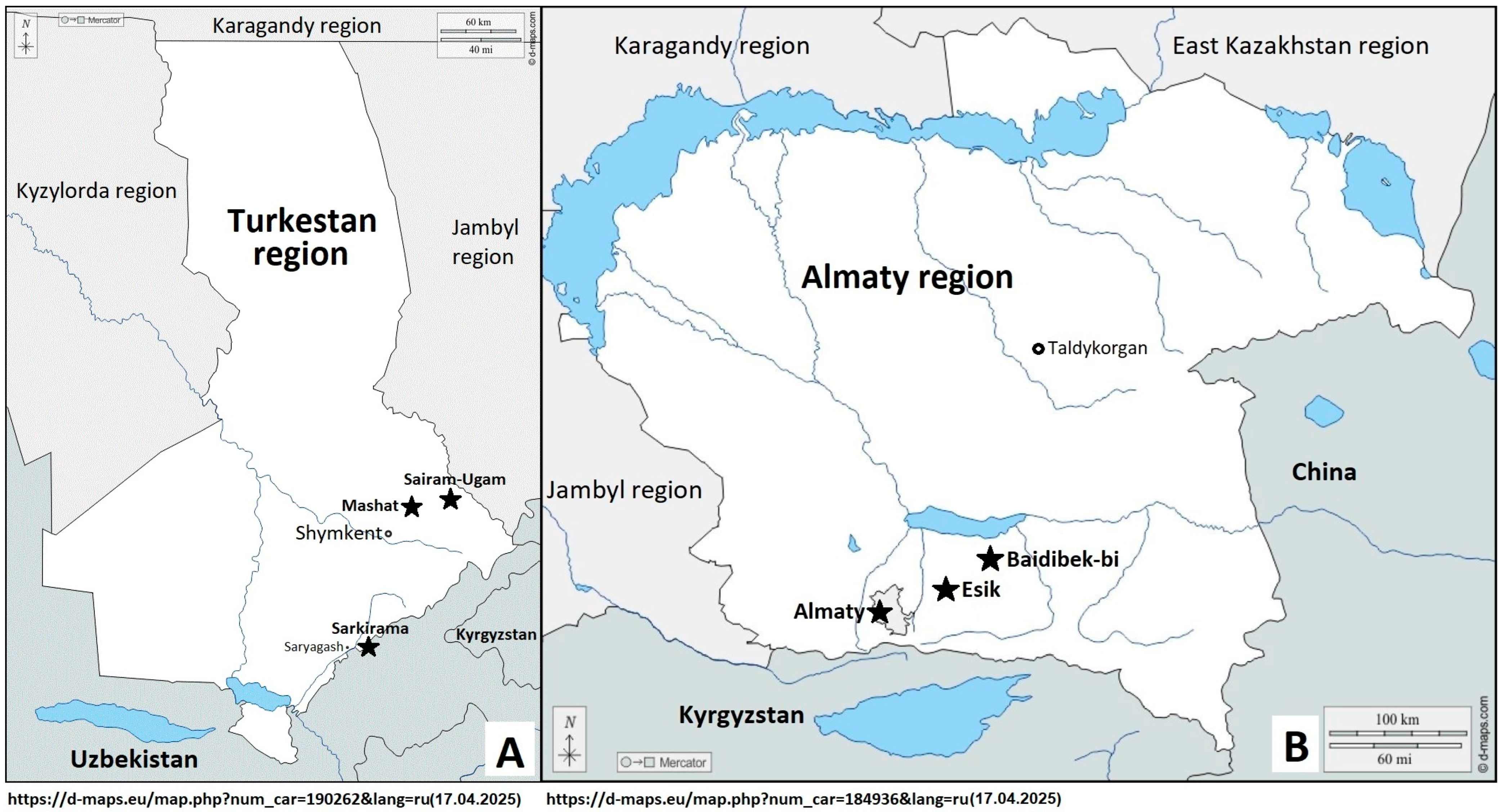
2.1. Plant Materials
2.2. DNA Extraction and Molecular Analysis Using SSR Markers
2.3. Data Analysis
3. Results
3.1. Analysis of the Genetic Diversity of Walnut (Juglans regia)
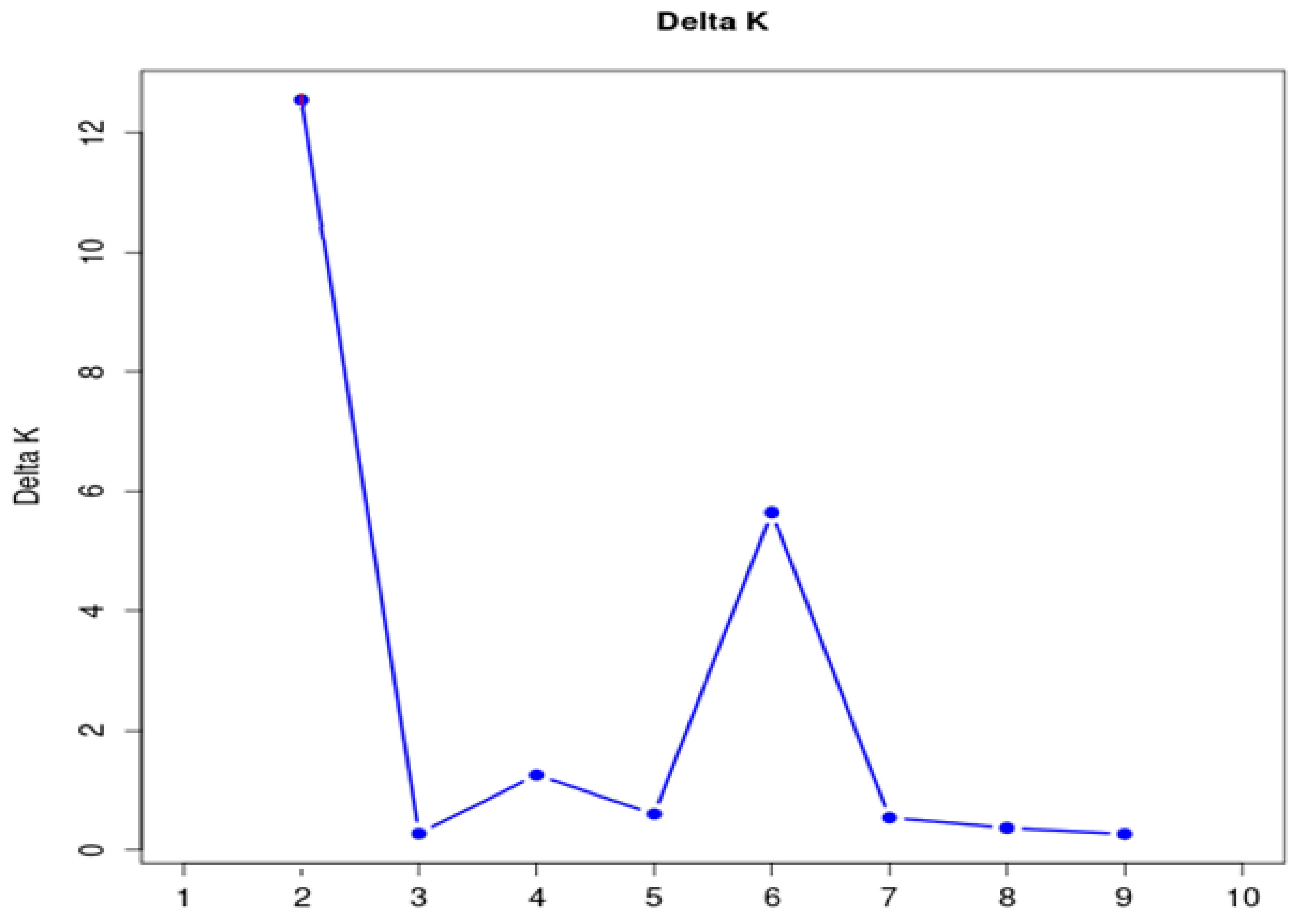
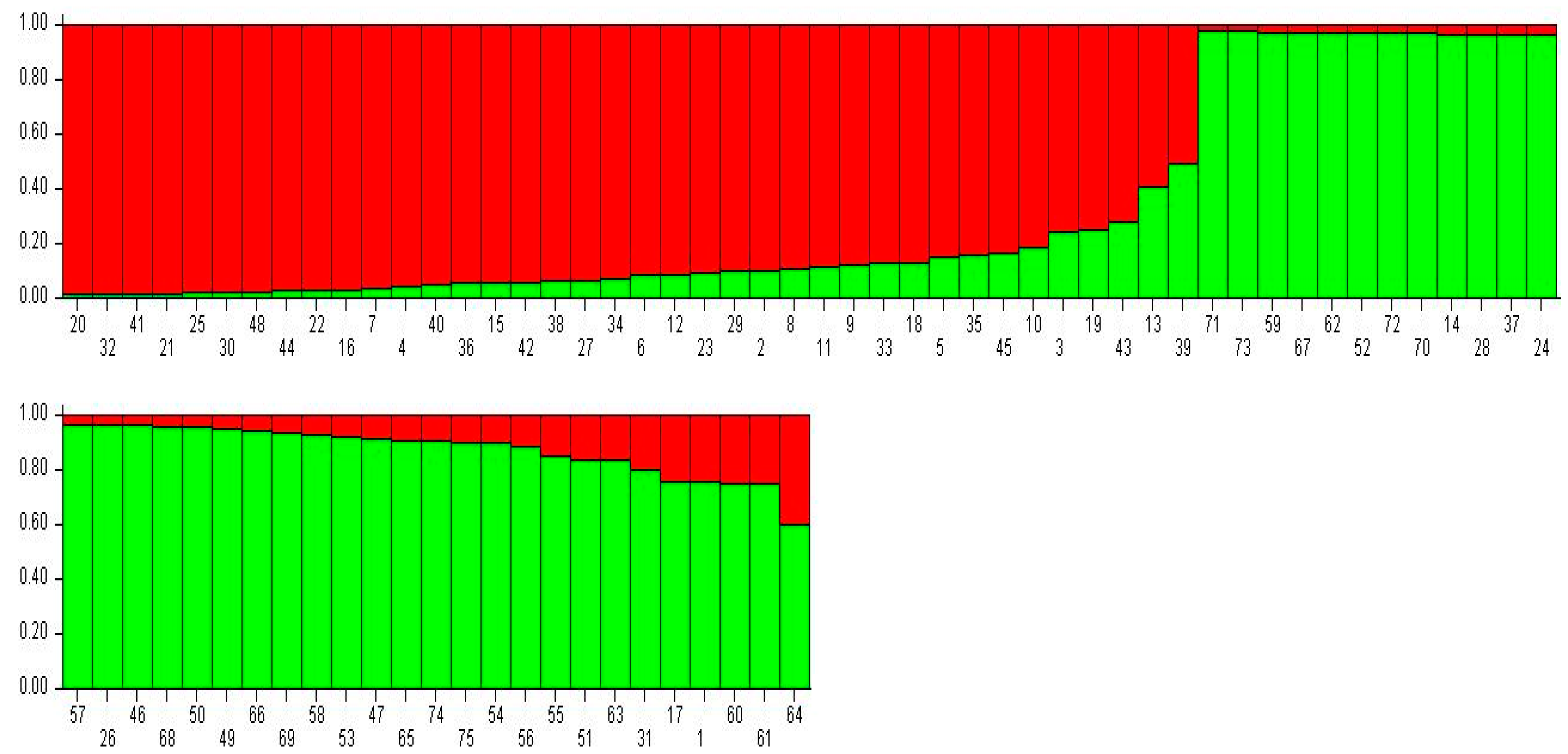
3.2. Population Structure Analysis
3.3. Cluster Analysis Using UPGMA and Principal Coordinate Analysis (PCoA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) chemical composition and research in human health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Ning, D.; Ma, T.; Chen, H.; Zhang, Y.; Sun, X. Comprehensive analysis of the components of walnut kernel (Juglans regia L.) in China. J. Food Qual. 2021, 2021, 9302181. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Ciudad, C.J.; Noé, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- Gandev, S. Budding and grafting of the walnut (Juglans regia L.) and their effectiveness in Bulgaria (Review). Bulgar. J. Agri. Sci. 2007, 13, 683–689. [Google Scholar]
- Pollegioni, P.; Woeste, K.E.; Chiocchini, F.; Del Lungo, S.; Olimpieri, I.; Tortolano, V.; Clark, J.; Hemery, G.E.; Mapelli, S.; Malvolti, M.E. Ancient humans influenced the current spatial genetic structure of common walnut populations in Asia. PLoS ONE 2015, 10, e0135980. [Google Scholar] [CrossRef]
- Vahdati, K. Traditions and folks for walnut growing around the Silk Road. Acta Hortic. 2014, 1032, 19–24. [Google Scholar] [CrossRef]
- McGranahan, G.; Leslie, C. Walnut. In Fruit Breeding; Badenes, M., Byrne, D., Eds.; Handbook of Plant Breeding; Springer: Boston, MA, USA, 2012; pp. 827–846. [Google Scholar]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin, 4th ed.; Oxford University Press: Oxford, UK, 2012; p. 149. [Google Scholar]
- Martinez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. FAO Statistics Division. 2018. Available online: http://faostat.fao.org/site/567/default.aspx#ancor (accessed on 7 December 2018).
- Vahdati, K.; Arab, M.M.; Sarikhani, S.; Sadat-Hosseini, M.; Leslie, C.A.; Brown, P.J. Advances in Persian walnut (Juglans regia L.) breeding strategies. In Advances in Plant Breeding Strategies: Nut and Beverage Crops; Springer: Cham, Switzerland, 2019; pp. 401–472. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Akça, Y.; Yusupov, B.Y.; Erdenov, M.; Vahdati, K. Exploring of walnut genetic resources in Kazakhstan and evaluation of promising selections. Int. J. Hortic. Sci. Technol. 2020, 7, 93–102. [Google Scholar] [CrossRef]
- Kairova, G.; Taskuzhina, A.; Yanin, K.; Ismagulova, E.; Oleichenko, S.; Sarshayeva, M.; Sapakhova, Z.; Gritsenko, D. First evaluation of genetic diversity and population structure of wild and cultivated Juglans regia in Kazakhstan. Genet. Resour. Crop Evol. 2025, 72, 1755–1771. [Google Scholar] [CrossRef]
- Oleichenko, S.N.; Yegizbayeva, T.K.; Apushev, A.K.; Nusipzhanov, N.S. Assessment of promising local walnut forms for the South and South-East of Kazakhstan. Rep. Natl. Acad. Sci. Repub. Kazakhstan 2020, 333, 27–34. [Google Scholar] [CrossRef]
- Bakhytuly, K.; Kokhmetova, A.M.; Umirzakova, A.T.; Kuliev, A.S.; Kumbarbayeva, M.T.; Keishilov, Z.S. Monitoring of the distribution of Juglans regia L. in the southern and south-eastern regions of Kazakhstan and morphological study of walnut fruits. Izd. Natigeler 2024, 2, 238–248. [Google Scholar]
- Terletskaya, N.V.; Shadenova, E.A.; Litvinenko, Y.A.; Ashimuly, K.; Erbay, M.; Mamirova, A.; Nazarova, I.; Meduntseva, N.D.; Kudrina, N.O.; Korbozova, N.K.; et al. Influence of cold stress on physiological and phytochemical characteristics and secondary metabolite accumulation in microclones of Juglans regia L. Int. J. Mol. Sci. 2024, 25, 4991. [Google Scholar] [CrossRef] [PubMed]
- Kushnarenko, S.V.; Rymkhanova, N.K.; Aralbayeva, M.M.; Romadanova, N.V. In vitro cold acclimation is required for successful cryopreservation of Juglans regia L. shoot tips. CryoLetters 2023, 44, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kushnarenko, S.; Aralbayeva, M.; Rymkhanova, N.; Reed, B.M. Initiation pretreatment with Plant Preservative Mixture™ increases the percentage of aseptic walnut shoots. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 964–971. [Google Scholar] [CrossRef]
- Kairov, G.; Ismagulova, E.; Oleichenko, S.; Suleimanova, G.; Basim, H.; Sarshabayeva, M. Resistance of walnut varieties to bacteriosis caused by Pantoea agglomerans in the southern fruit-growing zone of Kazakhstan. Izdenister Natigeler 2024, 2, 238–248. [Google Scholar] [CrossRef]
- Çarpar, H.; Sertkaya, G. First report of Crepis phyllody disease associated with phytoplasma in Crepis foetida in a walnut orchard in Turkey. J. Plant Dis. Prot. 2023, 130, 177–180. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, G.-F.; Zhang, S.-X.; Zhou, H.-J.; Hu, Y.-H.; Woeste, K.E. RAPD derived markers for separating Manchurian walnut (Juglans mandshurica) and Japanese walnut (J. ailantifolia) from close congeners. J. Syst. Evol. 2014, 52, 101–111. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, J.; Pei, D. Genetic analysis of walnut cultivars in China using fluorescent amplified fragment length polymorphism. J. Am. Soc. Hortic. Sci. 2011, 136, 422–428. [Google Scholar] [CrossRef]
- Kafkas, S.; Ozkan, H.; Sutyemez, M. DNA polymorphism and assessment of genetic relationships in walnut genotypes based on AFLP and SAMPL markers. J. Am. Soc. Hortic. Sci. 2005, 130, 585–590. [Google Scholar] [CrossRef]
- Itoo, H.; Shah, R.A.; Qurat, S.; Jeelani, A.; Khursheed, S.; Bhat, Z.A.; Mir, M.A.; Rather, G.H.; Zargar, S.M.; Shah, M.D.; et al. Genome-wide characterization and development of SSR markers for genetic diversity analysis in Northwestern Himalayas walnut (Juglans regia L.). 3 Biotech 2023, 13, 136. [Google Scholar] [CrossRef]
- Arab, M.M.; Brown, P.J.; Abdollahi-Arpanahi, R.; Sohrabi, S.S.; Askari, H.; Aliniaeifard, S.; Mokhtassi-Bidgoli, A.; Mesgaran, M.B.; Leslie, C.A.; Marrano, A.; et al. Genome-wide association analysis and pathway enrichment provide insights into the genetic basis of photosynthetic responses to drought stress in Persian walnut. Hortic. Res. 2022, 9, uhac124. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Aishwarya, V.; Sharma, P.C. Biased distribution of microsatellite motifs in the rice genome. Mol. Genet. Genom. 2007, 277, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Lee Abdullah, T.; Yusop, M.R.; Hanafi, M.M.; Sahebi, M.; Azizi, P.; Shamshiri, R.R. Mining and development of novel SSR markers using next generation sequencing (NGS) data in plants. Molecules 2018, 23, 399. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Z.; Liu, N.; Song, L.; Yan, B.; Xing, Y.; Zhang, Q.; Fang, K.; Zhao, Y.; Chen, X.; et al. Fingerprinting 146 Chinese chestnut (Castanea mollissima Blume) accessions and selecting a core collection using SSR markers. J. Integr. Agric. 2021, 20, 1277–1286. [Google Scholar] [CrossRef]
- Kalia, R.K.; Rai, M.K.; Kalia, S.; Singh, R.; Dhawan, A.K. Microsatellite markers: An overview of the recent progress in plants. Euphytica 2011, 177, 309–334. [Google Scholar] [CrossRef]
- Ali Khan, M.; Shahid Ul, I.; Mohammad, F. Extraction of natural dye from walnut bark and its dyeing properties on wool yarn. J. Nat. Fibers 2016, 13, 458–469. [Google Scholar] [CrossRef]
- Bernard, A.; Barreneche, T.; Donkpegan, A.; Lheureux, F.; Dirlewanger, E. Comparison of structure analyses and core collections for the management of walnut genetic resources. Tree Genet. Genomes 2020, 16, 76. [Google Scholar] [CrossRef]
- Bernard, A.; Marrano, A.; Donkpegan, A.; Brown, P.J.; Leslie, C.A.; Neale, D.B.; Lheureux, F.; Dirlewanger, E. Association and linkage mapping to unravel genetic architecture of phenology-related traits and lateral bearing in Persian walnut (Juglans regia L.). BMC Genom. 2020, 23, 203. [Google Scholar]
- Shah, U.N.; Mir, J.I.; Ahmed, N.; Fazili, K.M. Assessment of germplasm diversity and genetic relationships among walnut (Juglans regia L.) genotypes through microsatellite markers. J. Saudi Soc. Agric. Sci. 2018, 17, 339–350. [Google Scholar] [CrossRef]
- Shah, R.A.; Baksi, P.; Jasrotia, A.; Bhat, D.J.I.; Gupta, R.; Bakshi, M. Genetic diversity of walnut (Juglans regia L.) seedlings through SSR markers in north-western Himalayan region of Jammu. Bangladesh J. Bot. 2020, 49, 1003–1012. [Google Scholar] [CrossRef]
- Pollegioni, P.; Woeste, K.; Chiocchini, F.; Del Lungo, S.; Ciolfi, M.; Olimpieri, I.; Tortolano, V.; Clark, J.; Hemery, G.E.; Mapelli, S.; et al. Rethinking the history of common walnut (Juglans regia L.) in Europe: Its origins and human interactions. PLoS ONE 2017, 12, e0172541. [Google Scholar] [CrossRef]
- Doğan, Y.; Kafkas, S.; Sütyemez, M.; Akça, Y.; Türemiş, N. Assessment and characterization of genetic relationships of walnut (Juglans regia L.) genotypes by three types of molecular markers. Sci. Hortic. 2014, 168, 81–87. [Google Scholar] [CrossRef]
- Nickravesh, M.H.; Vahdati, K.; Amini, F.; Di Pierro, E.A.; Amiri, R.; Woeste, K.; Arab, M.M. Reliable propagation of Persian walnut varieties using SSR marker-based true-to-type validation. HortScience 2023, 58, 64–66. [Google Scholar] [CrossRef]
- Wambulwa, M.C.; Fan, P.Z.; Milne, R.; Wu, Z.Y.; Luo, Y.H.; Wang, Y.H.; Wang, H.; Gao, L.M.; Ye, L.J.; Jin, Y.C.; et al. Genetic analysis of walnut cultivars from Southwest China: Implications for germplasm improvement. Plant Divers. 2022, 44, 530–541. [Google Scholar] [CrossRef]
- Shamlu, F. Genetic diversity of walnut (Juglans regia L.) populations in Iran. based on SSR markers. J. Nuts 2018, 9, 1–12. [Google Scholar]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef]
- Dangl, G.S.; Woeste, K.; Aradhya, M.K.; Koehmstedt, A.; Simon, C.; Potter, D.; Leslie, C.A.; McGranahan, G. Characterization of 14 microsatellite markers for genetic analysis and cultivar identification of walnut. J. Am. Soc. Hortic. Sci. 2005, 130, 348–354. [Google Scholar] [CrossRef]
- Woeste, K.; Burns, R.; Rhodes, O.; Michler, C. Thirty polymorphic nuclear microsatellite loci from black walnut. J. Hered. 2002, 93, 58–60. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Cui, Z.; Shi, W.; Huang, J.; Wang, J. Development of Polymorphic Microsatellite Markers and Identification of Applications for Wild Walnut (Juglans regia L.) in Middle Asia. Diversity 2023, 15, 1073. [Google Scholar] [CrossRef]
- Ye, L.; Shahi Shavvon, R.; Qi, H.; Wu, H.; Fan, P.; Shalizi, M.N.; Khurram, S.; Davletbek, M.; Turuspekov, Y.; Liu, J. Population Genetic Insights into the Conservation of Common Walnut (Juglans regia) in Central Asia. Plant Divers. 2024, 46, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Shahi Shavvon, R.; Qi, H.L.; Mafakheri, M.; Fan, P.Z.; Wu, H.Y.; Bazdid Vahdati, F.; Al-Shmgani, H.S.; Wang, Y.H.; Liu, J. Unravelling the Genetic Diversity and Population Structure of Common Walnut in the Iranian Plateau. BMC Plant Biol. 2023, 23, 201. [Google Scholar] [CrossRef]
- Eser, E.; Topçu, H.; Kefayati, S.; Sütyemez, M.; Islam, M.R.; Kafkas, S. Development of Polymorphic Simple Sequence Repeat Markers in Juglans regia L. Acta Hortic. 2021, 1318, 45–50. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Fatahi, R.; Zamani, Z. Analysis of genetic diversity among some Persian walnut genotypes (Juglans regia L.) using SSR markers. Sci. Hortic. 2011, 130, 146–151. [Google Scholar] [CrossRef]
- Orhan, E.; Eyduran, S.P.; Poljuha, D.; Akin, M.; Weber, T.; Ercisli, S. Genetic diversity detection of seed-propagated walnut (Juglans regia L.) germplasm from Eastern Anatolia using SSR markers. Horticulturae 2020, 32, 67–76. [Google Scholar] [CrossRef]
- Bernard, A.; Barreneche, T.; Lheureux, F.; Dirlewanger, E. Analysis of genetic diversity and structure in a worldwide walnut (Juglans regia L.) germplasm using SSR markers. PLoS ONE 2018, 13, e0208021. [Google Scholar] [CrossRef]
- Plugatar, Y.V.; Suprun, I.I.; Khokhlov, S.Y.; Stepanov, I.V.; Al-Nakib, E.A. Comprehensive Agrobiological Assessment and Analysis of Genetic Relationships of Promising Walnut Varieties of the Nikitsky Botanical Gardens. Vavilov J. Genet. Breed. 2023, 27, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Aradhya, M.K.; Woeste, K.; Velasco, D. Genetic Diversity, Structure and Differentiation in Cultivated Walnut (Juglans regia L.). Acta Hortic. 2010, 861, 127–132. [Google Scholar] [CrossRef]
- Manthos, I.; Sotiropoulos, T.; Karapetsi, L.; Ganopoulos, I.; Pratsinakis, E.D.; Maloupa, E.; Madesis, P. Molecular Characterization of Local Walnut (Juglans regia) Genotypes in the North-East Parnon Mountain Region of Greece. Int. J. Mol. Sci. 2023, 24, 17230. [Google Scholar] [CrossRef]
- Suprun, I.; Stepanov, I.; Anatov, D. Analysis of Genetic Diversity and Relationships of Local Walnut Populations in the Western Caspian Region of the North Caucasus. Horticulturae 2025, 11, 65. [Google Scholar] [CrossRef]
- Samlu, F.; Rezaei, M.; Lawson, S.; Ebrahimi, A.; Biabani, A.; Khan-Ahmadi, A. Genetic Diversity of Superior Persian Walnut Genotypes in Azadshahr, Iran. Physiol. Mol. Biol. Plants 2018, 24, 939–949. [Google Scholar] [CrossRef]
- Bernard, A.; Barreneche, T.; Lheureux, F.; Dirlewanger, E. SSR Genetic Diversity Assessment of the INRAE’s Walnut (Juglans spp.) Germplasm Collection. Acta Hortic. 2020, 1297, 387–394. [Google Scholar] [CrossRef]



| Primer | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| WGA001 | ATTGGAAGGGAAGGGAAATG | CGCGCACATACGTAAATCAC | 56 | Dangl et al., 2005 [42] |
| WGA027 | AACCCTACAACGCCTTGATG | TGCTCAGGCTCCACTTCC | 57 | Woeste et al., 2002 [43] |
| WGA042 | GTGGGTTCGACCGTGAAC | AACTTTGCACCACATCCACA | 55 | Shah et al., 2020 [35] |
| WGA118 | TGTGCTCTGATCTGCCTCC | GGGTGGGTGAAAAGTAGCAA | 60 | Dangl et al., 2005 [42] |
| WGA009 | CATCAAAGCAAGCAATGGG | CCATTGCTCTGTGATTGGG | 56 | Dangl et al., 2005 [42] |
| WGA202 | CCCATCTACCGTTGCACTTT | GCTGGTGGTTCTATCATGGG | 62 | Dangl et al., 2005 [42] |
| WGA276 | CTCACTTTCTCGGCTCTTCC | GGTCTTATGTGGGCAGTCGT | 60 | Dangl et al., 2005 [42] |
| WGA376 | GCCCTCAAAGTGATGAACGT | TCATCCATATTTACCCCTTTCG | 56 | Dangl et al., 2005 [42] |
| Locus | Na | Ne | I | Ho | He |
|---|---|---|---|---|---|
| WGA001 | 6.000 | 3.882 | 1.507 | 0.612 | 0.742 |
| WGA027 | 2.000 | 1.930 | 0.675 | 0.238 | 0.482 |
| WGA042 | 2.000 | 1.943 | 0.679 | 0.341 | 0.485 |
| WGA118 | 3.000 | 2.665 | 1.039 | 0.593 | 0.625 |
| WGA009 | 6.000 | 4.592 | 1.650 | 0.625 | 0.782 |
| WGA202 | 9.000 | 5.619 | 1.916 | 0.571 | 0.822 |
| WGA276 | 10.000 | 6.664 | 2.095 | 0.717 | 0.850 |
| WGA376 | 9.000 | 6.458 | 1.995 | 0.676 | 0.845 |
| Mean | 5.875 | 4.219 | 1.444 | 0.547 | 0.704 |
| Pop | Location | Na | Ne | I | Ho | He |
|---|---|---|---|---|---|---|
| pop1 | S-U | 4.625 | 3.658 | 1.307 | 0.697 | 0.683 |
| pop2 | MA | 2.375 | 1.851 | 0.670 | 0.367 | 0.388 |
| pop3 | SA | 2.500 | 1.877 | 0.642 | 0.290 | 0.363 |
| pop4 | AL | 3.500 | 2.612 | 1.000 | 0.437 | 0.555 |
| pop5 | BA | 1.125 | 1.075 | 0.157 | 0.063 | 0.109 |
| pop6 | ES | 3.875 | 3.420 | 1.187 | 0.634 | 0.639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurzhuma, M.; Kokhmetova, A.; Kumarbayeva, M.; Keishilov, Z.; Bakhytuly, K.; Bolatbekova, A.; Kokhmetova, A.; Mukhametzhanov, K.; Akan, K. Assessment of Genetic Diversity in Walnut (Juglans regia L.) Genotypes from Southern and Southeastern Kazakhstan Using Microsatellite Markers. Horticulturae 2025, 11, 810. https://doi.org/10.3390/horticulturae11070810
Nurzhuma M, Kokhmetova A, Kumarbayeva M, Keishilov Z, Bakhytuly K, Bolatbekova A, Kokhmetova A, Mukhametzhanov K, Akan K. Assessment of Genetic Diversity in Walnut (Juglans regia L.) Genotypes from Southern and Southeastern Kazakhstan Using Microsatellite Markers. Horticulturae. 2025; 11(7):810. https://doi.org/10.3390/horticulturae11070810
Chicago/Turabian StyleNurzhuma, Makpal, Alma Kokhmetova, Madina Kumarbayeva, Zhenis Keishilov, Kanat Bakhytuly, Ardak Bolatbekova, Assiya Kokhmetova, Kanat Mukhametzhanov, and Kadir Akan. 2025. "Assessment of Genetic Diversity in Walnut (Juglans regia L.) Genotypes from Southern and Southeastern Kazakhstan Using Microsatellite Markers" Horticulturae 11, no. 7: 810. https://doi.org/10.3390/horticulturae11070810
APA StyleNurzhuma, M., Kokhmetova, A., Kumarbayeva, M., Keishilov, Z., Bakhytuly, K., Bolatbekova, A., Kokhmetova, A., Mukhametzhanov, K., & Akan, K. (2025). Assessment of Genetic Diversity in Walnut (Juglans regia L.) Genotypes from Southern and Southeastern Kazakhstan Using Microsatellite Markers. Horticulturae, 11(7), 810. https://doi.org/10.3390/horticulturae11070810





