Effects of Climatic Fluctuations on the First Flowering Date and Its Thermal Requirements for 28 Ornamental Plants in Xi’an, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Phenological and Meteorological Data
2.3. Statistical Analyses
2.3.1. Identification of Abnormal Years
2.3.2. Comparison of Thermal Requirements
2.3.3. Phenological Models Based on Thermal Requirements
- (1)
- We caused t0 to vary within the range of January 1 to January 31 (with a 1-day step) and Tb (only applicable for method 1) within the range of 0 °C and 10 °C (with a 1 °C step).
- (2)
- For each combination of t0 and Tb, we calculated the thermal requirements for all FFDs in normal years based on Equations (1), (2), or (4). The mean thermal requirement across all normal years was determined as GDDb for method 1, GDDSb for method 2, and GDDHb for method 3.
- (3)
- We simulated the FFD for each year using different parameter sets of t0 and Tb (accumulating GDD, GDDS, or GDDH on a daily step, and the date on which GDD exceeded GDDb GDDSb, or GDDHb was regarded as the simulated FFD). Furthermore, we calculated the root mean square error (RMSE) between the simulated FFD and observed FFD for each parameter set. The parameters t0 and Tb (only applicable for method 1) yielding the smallest root mean square error (RMSE) were selected as the optimal parameters for the final model.
3. Results
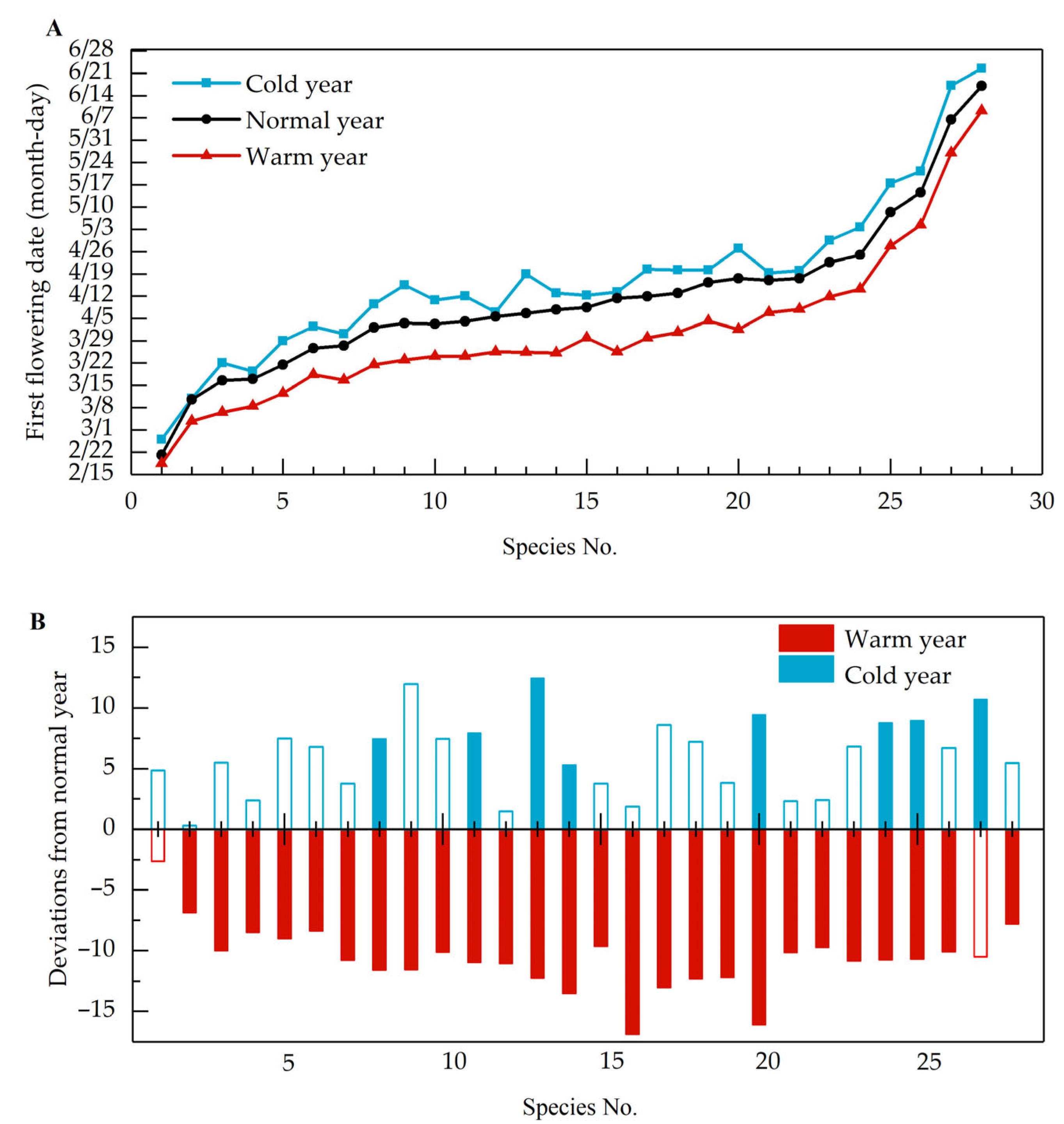
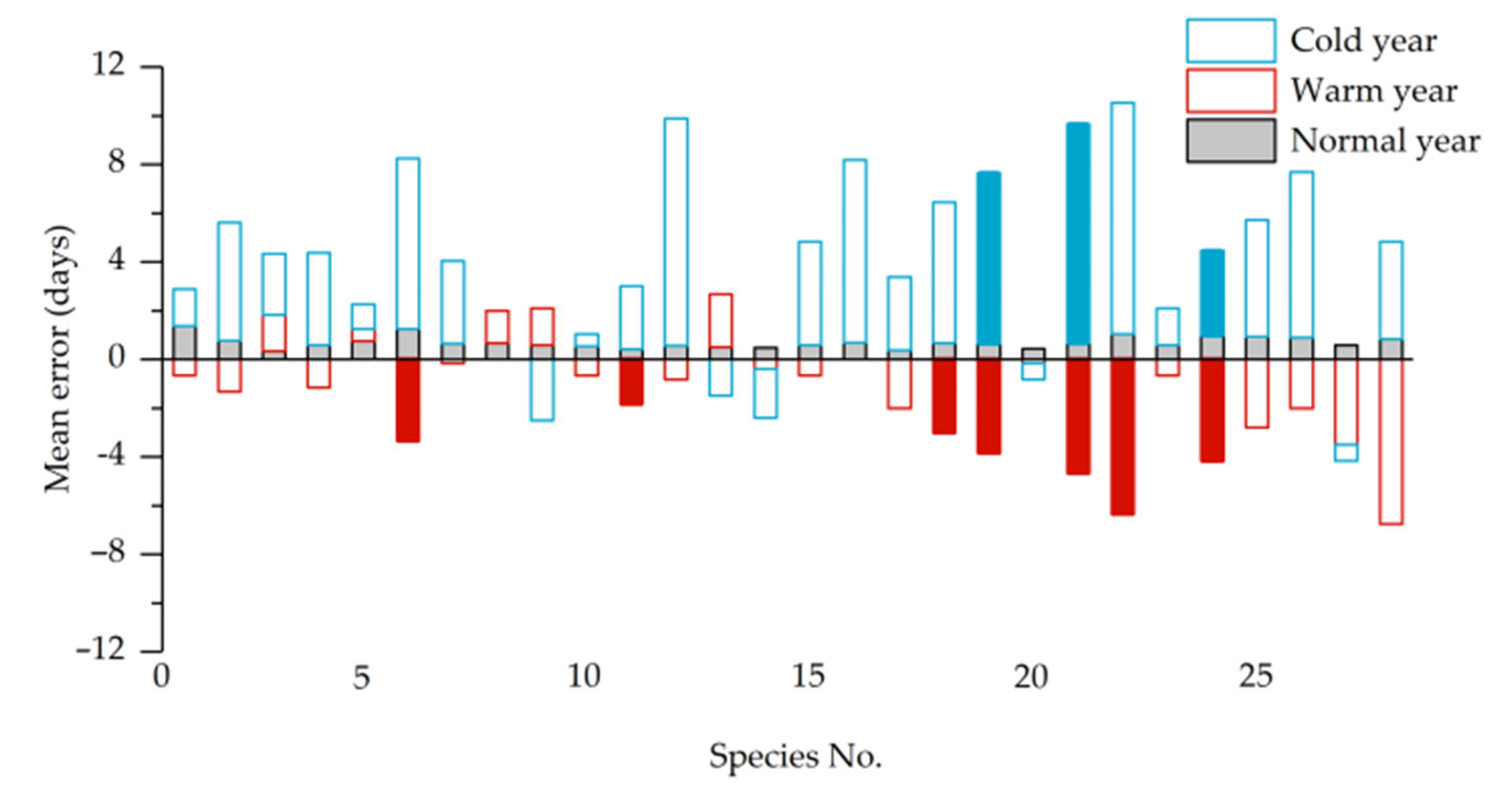
3.1. Phenological Shifts in Years with Abnormal Climate
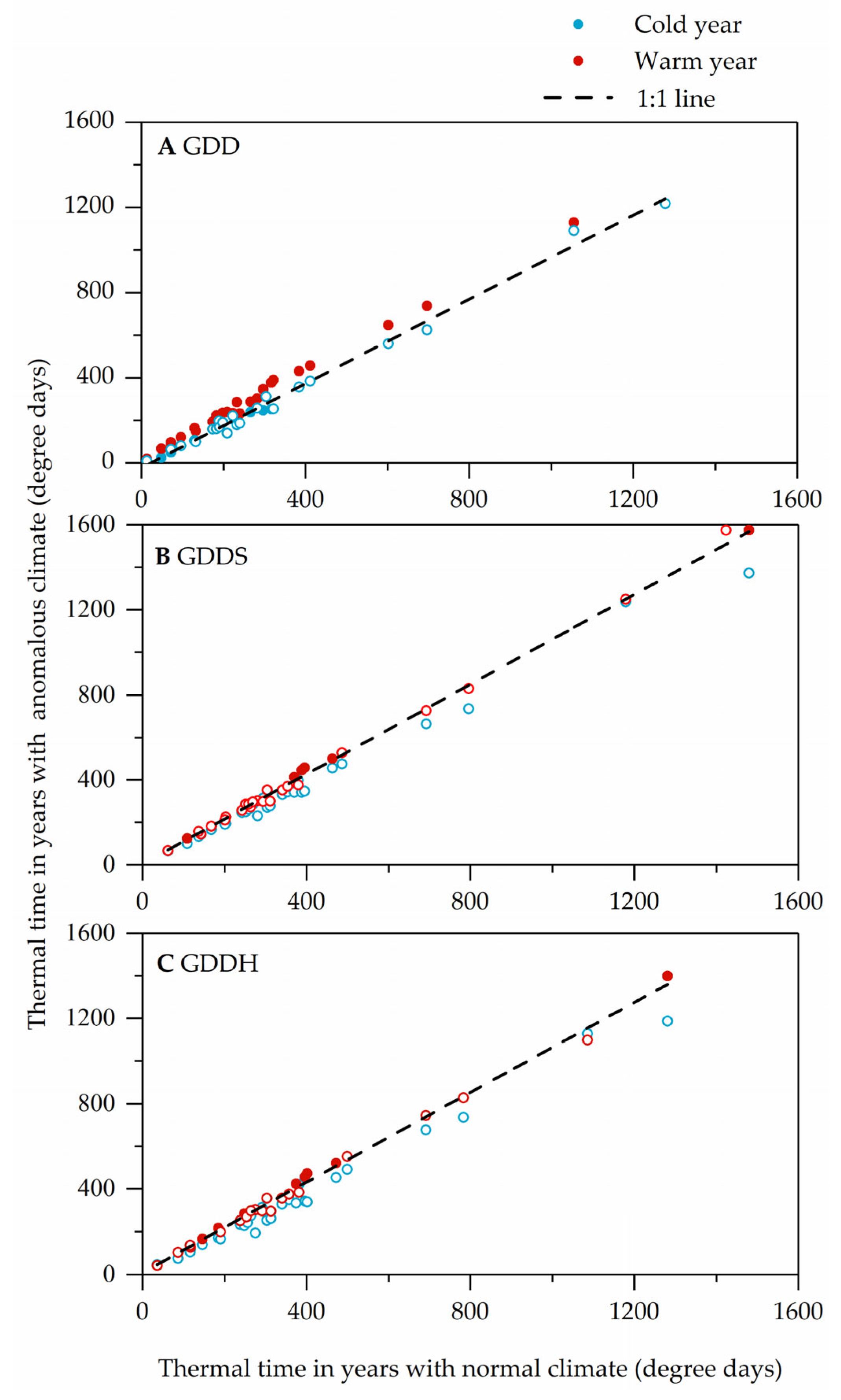
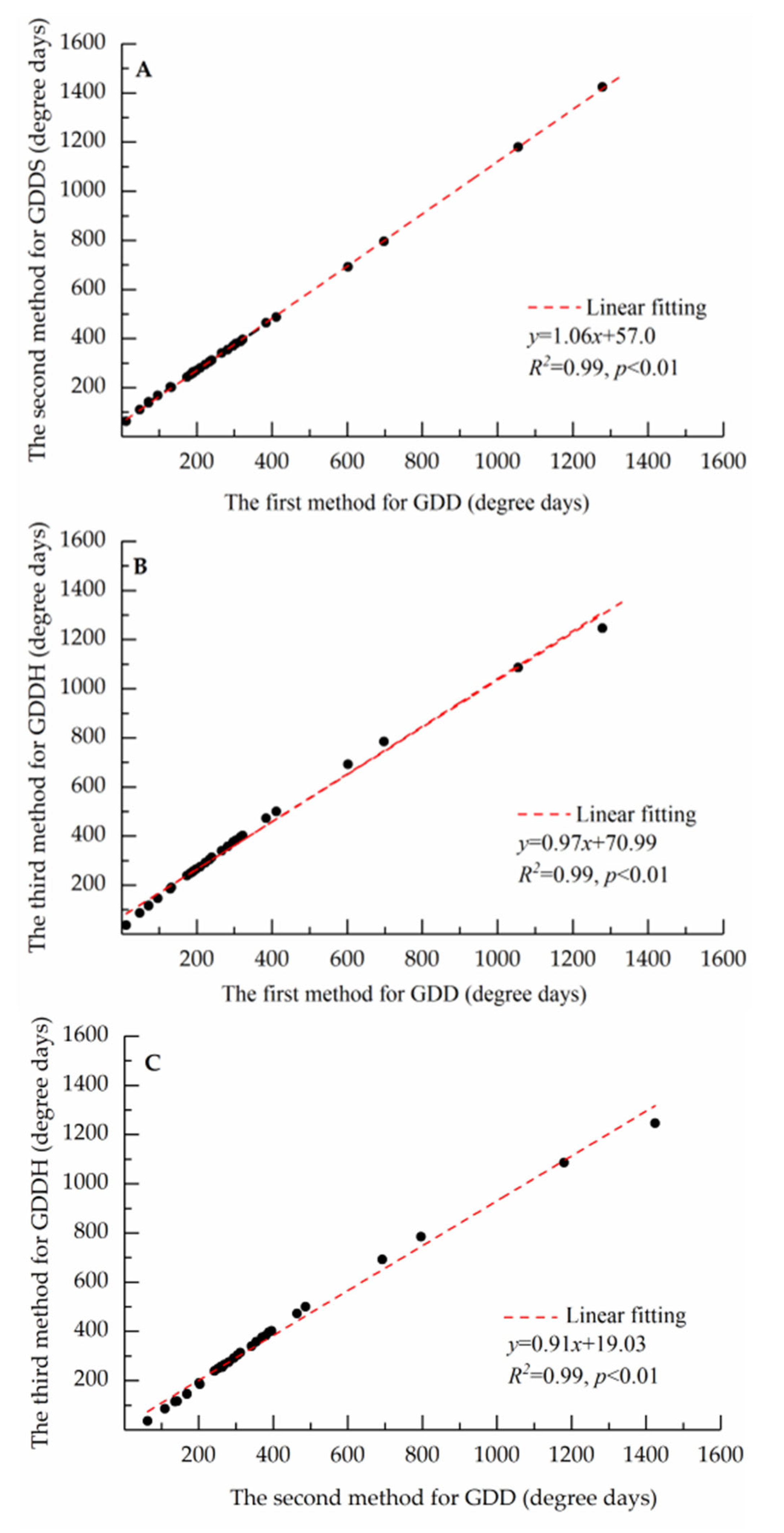
3.2. Changes in Thermal Requirements with Abnormal Climate
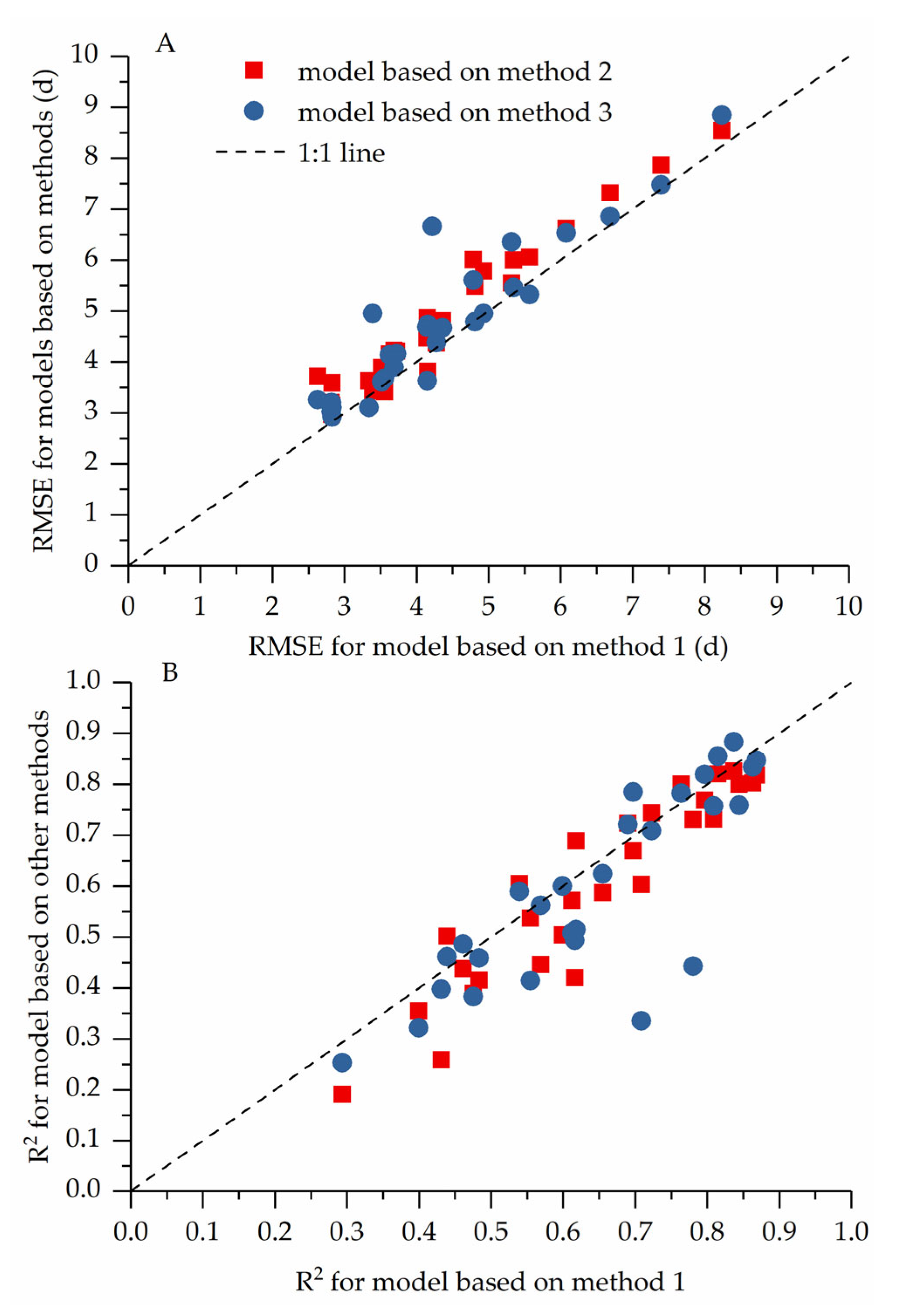
3.3. Model Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Schillerberg, T.A.; Tian, D. Global Assessment of Compound Climate Extremes and Exposures of Population, Agriculture, and Forest Lands Under Two Climate Scenarios. Earth’s Future 2024, 12, e2024EF004845. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, S.; Li, M.; He, Y. Summer heatwaves in China during 1961–2021: The impact of humidity. Atmos. Res. 2024, 304, 107366. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Xia, J.; Guo, Y.; Fu, Y.H. Effects of Vegetation Phenology on Ecosystem Water Use Efficiency in a Semiarid Region of Northern China. Front. Plant Sci. 2022, 13, 945582. [Google Scholar]
- Zhou, S.; Yu, B.; Zhang, Y. Global concurrent climate extremes exacerbated by anthropogenic climate change. Sci. Adv. 2023, 9, eabo1638. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Z.; Zhang, Y. Soil Moisture Dominates the Forest Productivity Decline During the 2022 China Compound Drought-Heatwave Event. Geophys. Res. Lett. 2023, 50, e2023GL104539. [Google Scholar] [CrossRef]
- Lyu, J.; Shi, Y.; Liu, T.; Xu, X.; Liu, S.; Yang, G.; Peng, D.; Qu, Y.; Zhang, S.; Chen, C.; et al. Extreme drought-heatwave events threaten the biodiversity and stability of aquatic plankton communities in the Yangtze River ecosystems. Commun. Earth Environ. 2025, 6, 171. [Google Scholar] [CrossRef]
- Vieira, J.; Matos, P.; Mexia, T.; Silva, P.; Lopes, N.; Freitas, C.; Correia, O.; Santos-Reis, M.; Branquinho, C.; Pinho, P. Green spaces are not all the same for the provision of air purification and climate regulation services: The case of urban parks. Environ. Res. 2018, 160, 306–313. [Google Scholar] [CrossRef]
- Martilli, A.; Krayenhoff, E.S.; Nazarian, N. Is the Urban Heat Island intensity relevant for heat mitigation studies? Urban Clim. 2020, 31, 100541. [Google Scholar] [CrossRef]
- Raciti, S.M.; Hutyra, L.R.; Newell, J.D. Mapping carbon storage in urban trees with multi-source remote sensing data: Relationships between biomass, land use, and demographics in Boston neighborhoods. Sci. Total Environ. 2014, 500–501, 72–83. [Google Scholar] [CrossRef]
- Song, J.; Zhou, S.; Yu, B.; Li, Y.; Liu, Y.; Yao, Y.; Wang, S.; Fu, B. Serious underestimation of reduced carbon uptake due to vegetation compound droughts. NPJ Clim. Atmos. Sci. 2024, 7, 23. [Google Scholar] [CrossRef]
- Ghanbari, M.; Arabi, M.; Georgescu, M.; Broadbent, A.M. The role of climate change and urban development on compound dry-hot extremes across US cities. Nat. Commun. 2023, 14, 3509. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Wang, H.; Rutishauser, T.; Dai, J. Phenological response to climate change in China: A meta-analysis. Glob. Chang. Biol. 2015, 21, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, L.; Zhong, D.; Liu, P.; Chi, Y. Declined benefit of earlier spring greening on summer growth in northern ecosystems under future scenarios. Agric. For. Meteorol. 2024, 351, 110019. [Google Scholar] [CrossRef]
- Meng, L.; Mao, J.; Zhou, Y.; Richardson, A.D.; Lee, X.; Thornton, P.E.; Ricciuto, D.M.; Li, X.; Dai, Y.; Shi, X.; et al. Urban warming advances spring phenology but reduces the response of phenology to temperature in the conterminous United States. Proc. Natl. Acad. Sci. USA 2020, 117, 4228–4233. [Google Scholar] [CrossRef]
- Han, G.; Xu, J. Land Surface Phenology and Land Surface Temperature Changes Along an Urban–Rural Gradient in Yangtze River Delta, China. Environ. Manag. 2013, 52, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Dai, J.; Wang, W.; Li, J.; Feng, C.; Du, J. Phenological changes in herbaceous plants in China’s grasslands and their responses to climate change: A meta-analysis. Int. J. Biometeorol. 2020, 64, 1865–1876. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, S.; Tao, Z.; Dai, J.; Ge, Q. Changes in flowering phenology of woody plants from 1963 to 2014 in North China. Int. J. Biometeorol. 2019, 63, 579–590. [Google Scholar] [CrossRef]
- Guralnick, R.; Crimmins, T.; Grady, E.; Campbell, L. Phenological response to climatic change depends on spring warming velocity. Commun. Earth Environ. 2024, 5, 634. [Google Scholar] [CrossRef]
- Lin, S.; Wang, H.; Ge, Q.; Hu, Z. Effects of chilling on heat requirement of spring phenology vary between years. Agric. For. Meteorol. 2022, 312, 108718. [Google Scholar] [CrossRef]
- Rauschkolb, R.; Herben, T.; Kattge, J.; Knickmann, B.; Linstädter, A.; Menzel, A.; Mora, K.; Nordt, B.; Vitasse, Y.; Weigelt, P.; et al. The performance of growing degree day models to predict spring phenology of herbaceous species depends on the species’ temporal niche. Funct. Ecol. 2025. [Google Scholar] [CrossRef]
- Wheeler, K.I.; Dietze, M.C.; LeBauer, D.; Peters, J.A.; Richardson, A.D.; Ross, A.A.; Thomas, R.Q.; Zhu, K.; Bhat, U.; Munch, S.; et al. Predicting spring phenology in deciduous broadleaf forests: NEON phenology forecasting community challenge. Agric. For. Meteorol. 2024, 345, 109810. [Google Scholar] [CrossRef]
- Clark, J.S.; Salk, C.; Melillo, J.; Mohan, J. Tree phenology responses to winter chilling, spring warming, at north and south range limits. Funct. Ecol. 2014, 28, 1344–1355. [Google Scholar] [CrossRef]
- Carter, J.M.; Orive, M.E.; Gerhart, L.M.; Stern, J.H.; Marchin, R.M.; Nagel, J.; Ward, J.K. Warmest extreme year in U.S. history alters thermal requirements for tree phenology. Oecologia 2017, 183, 1197–1210. [Google Scholar] [CrossRef]
- Hsu, H.; Yun, K.; Kim, S. Variable warming effects on flowering phenology of cherry trees across a latitudinal gradient in Japan. Agric. For. Meteorol. 2023, 339, 109571. [Google Scholar] [CrossRef]
- Yan, J.; Cai, Z.; Chen, Z.; Zhang, B.; Li, J.; Xu, J.; Ma, R.; Yu, M.; Shen, Z. Relationship between Chilling Accumulation and Heat Requirement for Flowering in Peach Varieties of Different Chilling Requirements. Agronomy 2024, 14, 1637. [Google Scholar] [CrossRef]
- Zhang, P.; Ning, P.; Cao, R.; Xu, J. Analysis of Climate Change Characteristics in Xi’an Based on the Visibility Graph. Front. Phys. 2021, 9, 702064. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Ma, Z.; Yang, H.; Jia, W. Urban Morphology Influencing the Urban Heat Island in the High-Density City of Xi’an Based on the Local Climate Zone. Sustainability 2024, 16, 3946. [Google Scholar] [CrossRef]
- Wan, M.; Liu, X. Zhong Guo Wu Hou Guan Ce Fang Fa; Science Press: Beijing, China, 1979. [Google Scholar]
- Chow, D.; Levermore, G.J. New algorithm for generating hourly temperature values using daily maximum, minimum and average values from climate models. Build. Serv. Eng. Res. Technol. 2007, 28, 237–248. [Google Scholar] [CrossRef]
- Shahzad, K.; Zhu, M.; Cao, L.; Hao, Y.; Zhou, Y.; Liu, W.; Dai, J. Phylogenetic conservation in plant phenological traits varies between temperate and subtropical climates in China. Front. Plant Sci. 2024, 15, 1367152. [Google Scholar] [CrossRef]
- Hunter, A.F.; Lechowicz, M.J. Predicting the Timing of Budburst in Temperate Trees. J. Appl. Ecol. 1992, 29, 597–604. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Smith, R.I. Thermal Time, Chill Days and Prediction of Budburst in Picea sitchensis. J. Appl. Ecol. 1983, 20, 951–963. [Google Scholar] [CrossRef]
- Sarvas, R. Investigations on the annual cycle of development of forest trees. Active period. Finl. Metsantutkimuslaitos Julk. 1972, 76, 110. [Google Scholar]
- Hänninen, H. Modelling bud dormancy release in trees from cool and temperate regions. Acta For. Fenn 1990, 213, 7660. [Google Scholar] [CrossRef]
- Anderson, J.; Richardson, E.; Kesner, C. Validation of chill unit and flower bud phenology models for ‘Montmorency’ sour cherry. Acta Hortic. 1986, 184, 71–78. [Google Scholar] [CrossRef]
- Luedeling, E.; Zhang, M.; McGranahan, G.; Leslie, C. Validation of winter chill models using historic records of walnut phenology. Agric. For. Meteorol. 2009, 149, 1854–1864. [Google Scholar] [CrossRef]
- Menzel, A.; Helm, R.; Zang, C. Patterns of late spring frost leaf damage and recovery in a European beech (Fagus sylvatica L.) stand in south-eastern Germany based on repeated digital photographs. Front. Plant Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Li, D.; Stucky, B.J.; Deck, J.; Baiser, B.; Guralnick, R.P. The effect of urbanization on plant phenology depends on regional temperature. Nat. Ecol. Evol. 2019, 3, 1661–1667. [Google Scholar] [CrossRef]
- Fitchett, J.M.; Raik, K. Phenological advance of blossoming over the past century in one of the world’s largest urban forests, Gauteng City-Region, South Africa. Urban For. Urban Green. 2021, 63, 127238. [Google Scholar] [CrossRef]
- Austin, M.W.; Smith, A.B.; Olsen, K.M.; Hoch, P.C.; Krakos, K.N.; Schmocker, S.P.; Miller-Struttmann, N.E. Climate change increases flowering duration, driving phenological reassembly and elevated co-flowering richness. New Phytol. 2024, 243, 2486–2500. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Zhang, T.; Zhao, Y.; Sun, Y.; Ma, M. Warming promotes divergent shift in sequential phenophases of alpine meadow plants. Agric. For. Meteorol. 2025, 368, 110521. [Google Scholar] [CrossRef]
- Cleland, E.E.; Allen, J.M.; Crimmins, T.M.; Dunne, J.A.; Pau, S.; Travers, S.E.; Zavaleta, E.S.; Wolkovich, E.M. Phenological tracking enables positive species responses to climate change. Ecology 2012, 93, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Kamimori, M.; Hosomi, A. Evaluation of Chilling and Heat Requirements for the Budbreak of Delaware Grape in Osaka, Japan. Am. J. Enol. Vitic. 2024, 75, 750011. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, J.; Jiang, H.; Fu, Y.H. A comparative study of 17 phenological models to predict the start of the growing season. Front. For. Glob. Chang. 2023, 5, 1032066. [Google Scholar] [CrossRef]
- Wang, H.; Lin, S.; Dai, J.; Ge, Q. Modeling the effect of adaptation to future climate change on spring phenological trend of European beech (Fagus sylvatica L.). Sci. Total Environ. 2022, 846, 157540. [Google Scholar] [CrossRef]
- Laube, J.; Sparks, T.H.; Estrella, N.; Höfler, J.; Ankerst, D.P.; Menzel, A. Chilling outweighs photoperiod in preventing precocious spring development. Glob. Change Biol. 2014, 20, 170–182. [Google Scholar] [CrossRef]
- Zhu, M.; Dai, J.; Wang, H.; Alatalo, J.M.; Liu, W.; Hao, Y.; Ge, Q. Mapping 24 woody plant species phenology and ground forest phenology over China from 1951 to 2020. Earth Syst. Sci. Data 2024, 16, 277–293. [Google Scholar] [CrossRef]
- Richardson, A.D.; Anderson, R.S.; Arain, M.A.; Barr, A.G.; Bohrer, G.; Chen, G.; Chen, J.M.; Ciais, P.; Davis, K.J.; Desai, A.R.; et al. Terrestrial biosphere models need better representation of vegetation phenology: Results from the North American Carbon Program Site Synthesis. Glob. Chang. Biol. 2012, 18, 566–584. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, F.; Zheng, J.; Chen, L.; Hänninen, H.; Wu, J. Temperature sum models in plant spring phenology studies: Two commonly used methods have different fields of application. J. Exp. Bot. 2024, 75, 6011–6016. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Regnie, P.; Yuan, W. New insights on plant phenological response to temperature revealed from long-term widespread observations in China. Glob. Chang. Biol. 2018, 24, 2066–2078. [Google Scholar] [CrossRef]

| No. | Species | Life Form | Provenance | N | First Flowering Date (Month-Day) |
|---|---|---|---|---|---|
| 1 | Corylus heterophylla | Shrub or small tree | Temperate | 32 | 2-21 |
| 2 | Amygdalus davidiana | Tree | Temperate | 43 | 3-9 |
| 3 | Forsythia suspensa | Shrub | Temperate | 32 | 3-14 |
| 4 | Yulania denudate | Tree | Subtropical | 42 | 3-16 |
| 5 | Cerasus tomentosa | Shrub | Temperate | 32 | 3-20 |
| 6 | Pyrus betulifolia | Tree | Temperate | 32 | 3-25 |
| 7 | Cerasus yedoensis | Tree | Temperate | 41 | 3-26 |
| 8 | Syringa oblata | Shrub or small tree | Temperate | 44 | 4-1 |
| 9 | Pterocarya stenoptera | Tree | Subtropical | 32 | 4-2 |
| 10 | Acer pictum subsp. mono | Tree | Temperate | 42 | 4-2 |
| 11 | Cercis chinensis | Shrub | Temperate | 43 | 4-3 |
| 12 | Chaenomeles sinensis | Shrub or small tree | Subtropical | 34 | 4-3 |
| 13 | Poncirus trifoliata | Small tree | Subtropical | 34 | 4-5 |
| 14 | Fraxinus chinensis | Tree | Temperate | 30 | 4-6 |
| 15 | Juglans regia | Tree | Temperate | 38 | 4-7 |
| 16 | Platanus orientalis | Tree | Temperate | 30 | 4-9 |
| 17 | Xanthoceras sorbifolium | Shrub or small tree | Temperate | 32 | 4-9 |
| 18 | Wisteria sinensis | Liana | Subtropical | 37 | 4-11 |
| 19 | Morus alba | Tree | Temperate | 34 | 4-14 |
| 20 | Broussonetia papyrifera | Tree | Subtropical | 30 | 4-15 |
| 21 | Paeonia suffruticosa | Shrub | Temperate | 42 | 4-15 |
| 22 | Pinus tabuliformis | Tree | Temperate | 36 | 4-16 |
| 23 | Cornus controversa | Tree | Subtropical | 32 | 4-21 |
| 24 | Robinia pseudoacacia | Tree | Temperate | 41 | 4-24 |
| 25 | Diospyros kaki | Tree | Subtropical | 38 | 5-8 |
| 26 | Ailanthus altissima | Tree | Temperate | 36 | 5-14 |
| 27 | Albizia julibrissin | Tree | Temperate | 34 | 6-7 |
| 28 | Ligustrum lucidum | Tree | Subtropical | 32 | 6-16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Dai, J.; Gao, X.; Tao, Z. Effects of Climatic Fluctuations on the First Flowering Date and Its Thermal Requirements for 28 Ornamental Plants in Xi’an, China. Horticulturae 2025, 11, 772. https://doi.org/10.3390/horticulturae11070772
Huang W, Dai J, Gao X, Tao Z. Effects of Climatic Fluctuations on the First Flowering Date and Its Thermal Requirements for 28 Ornamental Plants in Xi’an, China. Horticulturae. 2025; 11(7):772. https://doi.org/10.3390/horticulturae11070772
Chicago/Turabian StyleHuang, Wenjie, Junhu Dai, Xinyue Gao, and Zexing Tao. 2025. "Effects of Climatic Fluctuations on the First Flowering Date and Its Thermal Requirements for 28 Ornamental Plants in Xi’an, China" Horticulturae 11, no. 7: 772. https://doi.org/10.3390/horticulturae11070772
APA StyleHuang, W., Dai, J., Gao, X., & Tao, Z. (2025). Effects of Climatic Fluctuations on the First Flowering Date and Its Thermal Requirements for 28 Ornamental Plants in Xi’an, China. Horticulturae, 11(7), 772. https://doi.org/10.3390/horticulturae11070772







