Abstract
Yellow shoulder disorder (YSD) is characterized by discolored regions beneath the fruit’s epidermis, impacting the ripening process and rendering tomatoes unsuitable for marketing. YSD poses a significant challenge in high-tunnel (HT) tomato production, a system that has gained prominence for its ability to extend growing seasons and enhance crop quality. This review delves into the various factors influencing YSD occurrence, including soil nutritional status, weather, plant variety, and the interactions between these factors, contributing to the occurrence of YSD in HT microclimate. The severity of YSD symptoms, ranging from minor to significant discoloration, highlights the complexity of this disorder. This review highlights research gaps on the effects of temperature, relative humidity, nutrient imbalance, soil water management, clay minerals, and how their interactions influence YSD in HT microclimates, emphasizing the need for comprehensive studies to understand the complex relationships between soil health, nutrient management, and tomato quality in HT microclimates and the need for further research to sustain high-quality tomato production in HTs.
1. Introduction
High tunnels (HTs), also known as “hoop houses”, are plastic-covered greenhouse structures in which crops are typically produced in-ground in the native soil. They differ from greenhouses, where production typically occurs above ground in soilless media and the growing microclimate is more actively controlled through heating and cooling units. HTs are a semi-controlled environment used worldwide for extending growing seasons and improving crop quality [1] by protecting the crop from adverse weather conditions, including frost, temperature fluctuations, wind, and excessive rainfall. The plastic covering allows for the capture of passive solar energy, resulting in soil and air temperatures warming earlier in the spring, extending the frost-free period in the fall [2]. This enables year-round crop production in temperate climates and environments where open-field production may be limited [3]. The extension of the growing season can help farmers access early-season markets at times when open-field production is limited. Crop quality is improved due to control of soil moisture, soil-borne foliar pathogens, and some insect pests [2,4].
The global adoption of HTs has increased in recent decades, concomitant with the increase in protected agriculture systems worldwide. HTs are used to produce a variety of crops such as vegetables, small fruits, tree fruits, and flowers [4]. The total global production area in HTs is difficult to enumerate, as estimates may be grouped with all protected agriculture systems (e.g., greenhouse systems), and HTs are referred to using different terms around the world (e.g., hoop houses, polyethylene greenhouses). However, global production in protected agriculture systems is significant. The global greenhouse production area is estimated to be between 473,000 and 500,000 hectares [5] and is greatest in Europe, followed by Asia, Africa, the Middle East, North–Central America, South America, and Oceania. When low tunnel and HT structures are accounted for, the total production area in protected agriculture systems exceeds 3 million hectares [6].
The use of HTs has also significantly increased in the United States, and the rising adoption of HT is attributed to the advantages in production described above. Additionally, the US Department of Agriculture programs (e.g., Natural Resource Conservation Service High-Tunnel System Initiative) have incentivized HT adoption through cost-sharing programs, which totaled over USD 170 million between 2010 and 2020 [7]. This does not account for structures purchased independently by farmers. In the United States, the use of HT for tomato, Lycopersicon esculentum, cultivation has increased dramatically [8]. Tomatoes produced in HTs may produce fruits with improved marketable yield and quality (achieved by protection from adverse environmental conditions such as low temperatures, overhead moisture, wind, and hail [9]). This thereby resulted in a potentially profitable “out-of-season” harvest, decreased culling, and decreased pesticide usage [10].
Despite various advantages of HT production, achieving yield potential in these systems often requires intensive management practices. This may include higher fertilizer rates, reduced crop rotation, and increased planting density as compared to open-field environments [11]. There is a growing concern that such intensive management may make HT soils prone to degradation [12]. Challenges such as increased soil and air temperatures, lack of crop rotations, frequent tillage, high crop nutrient demand, and irrigation may drive nutrient imbalances, loss of organic matter, and soil salinization in HT systems [13,14,15]. These factors can contribute to a decline in soil quality over time. As soil quality declines, tomato yield and quality may be reduced. Further, issues such as soil compaction and decreased microbial activity can arise due to the unique HT environment. These factors can contribute to declining soil fertility, which may ultimately increase the risk of ripening disorders in HT tomato fruit [13,16].
Fruit ripening disorders affect tomato marketable yield and fruit quality [17]. They are considered complex challenges and are a function of interacting with edaphic and microclimatic factors [18]. One common fruit ripening disorder in tomato production is yellow shoulder disorder (YSD). “Yellow shoulder” is defined as the yellow ring circling the abscission zone in tomatoes [18]. It is also characterized by distinct demarcations between yellow or yellow–orange tissue and red tissue in red tomato cultivars [19]. It was originally described as a concern to the processing tomato industry, as the disorder leads to a persistent white appearance in tomato tissue even after undergoing processing, rendering fruit unmarketable [17,18,20,21]. Although YSD and other fruit ripening disorders are not unique to HT systems, they can occur at increased rates than in the open field, particularly in temperate climates. The severity of this disorder can vary from minor, exhibiting internal spotting, to more serious cases with extensive hardened areas that appear yellow to white [19,22] (Figure 1).

Figure 1.
Example of YSD in tomato in order of increasing severity (Photo credit: Sapana Pandey).
YSD is a commonly cited problem in open field and protected agriculture systems globally, with marketable yield reductions documented in the USA [18,19,23,24,25], India [26], Nepal [27], Southeastern Africa [28], Japan [29], China [30], Switzerland [31], Spain [32], and Mexico [33]. It is a particular problem in HT systems, where the factors influencing YSD occurrence are exacerbated by the HT microclimate and intensive production practices. This review explores the factors driving the presence and severity of YSD that may be exacerbated by known edaphic and microclimatic conditions present in HTs. These driving factors and their interactions in HTs are conceptualized in Figure 2, with a synthesis of the literature, knowledge gaps, and suggestions for future applied research described in this context below.
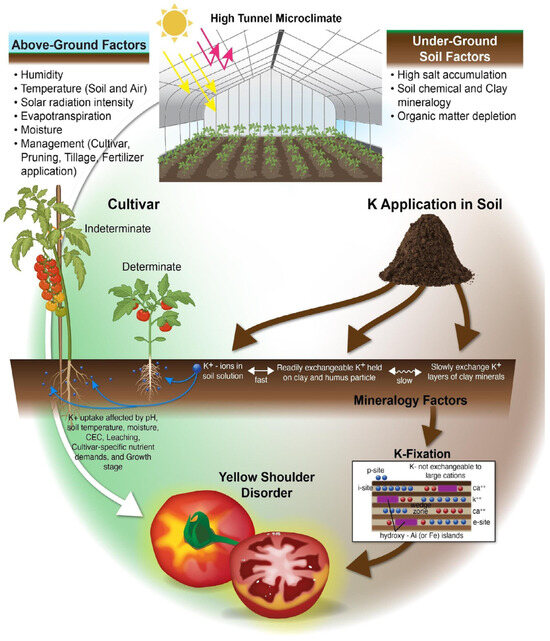
Figure 2.
Potential factors affecting YSD in HT tomato production. (Concept by Sapana Pandey and Krista Jacobsen, illustration by Matthew Hazard, University of Kentucky).
2. Methods
A literature review was conducted in 2023–2024 to identify, synthesize, and document all peer-reviewed primary literature and university extension resources related to tomato fruit quality and ripening disorders in HT tomato production systems. The search terms “high tunnel”, “yellow shoulder” or “ripening disorder”, “potassium AND YSD”, “tomato AND YSD”, and all combinations of these terms were queried on Web of Science, ResearchGate, and Google Scholar search engines. These resulted in a total of 78 peer-reviewed journal articles (including published conference abstracts) and 24 university extension resources reviewed for this work.
3. Results
Literature reviewed indicated that factors such as soil nutritional status, weather conditions, plant genetics, variety, and their interactions are significant contributors to YSD [18,23,24,25,34,35,36]. The sections below synthesize the literature in the context of what is known about the mechanisms influencing YSD in the context of HT production systems. Areas where HTs may provide unique study systems to improve our understanding of the mechanisms affecting YSD and opportunities for improved management to decrease YSD incidence are also discussed.
3.1. High-Tunnel Microclimates Favor the Development of YSD by Affecting Lycopene Synthesis
Foundational work in YSD associated the prevalence of the disorder with high pericarp temperature in the shoulder region of tomatoes [37], as well as the higher relative humidity levels experienced in controlled environments such as HTs [38]. Brust et al. [39] found that temperatures greater than 30 °C cause a reduction in lycopene synthesis. Lycopene, an antioxidant carotenoid pigment, is responsible for the distinct red color observed in ripe tomatoes [40,41,42]. The concentration of lycopene in tomato fruits was completely inhibited, particularly when exposed to temperatures exceeding 32 °C, across various tomato cultivars [23,43]. An increase in direct sunlight exposure led to a corresponding rise in tomato shoulder or surface temperature, subsequently diminishing the lycopene content [44]. However, this foundational mechanistic work was not conducted in the context of tomato growth habit (determinate versus indeterminate) nor utilizing modern tomato plant cultivars. Similarly, lycopene and carotenoid synthesis are highly active and sensitive to temperature during the breaker-to-pink stages of tomato ripening [45,46]. YSD symptoms commonly develop during this period. However, the temporal dynamics of YSD occurrence across different tomato growth stages and the regulation of key metabolic genes for lycopene synthesis are not analyzed under HT microclimatic conditions. This presents a promising area for future research, particularly in understanding how microclimate conditions in HT influence pigment development during critical ripening stages.
Reducing high temperatures in HTs during summer months may be limited due to the lack of active cooling infrastructure. However, management of the canopy and the tomato crop through trellising and pruning practices may decrease the negative effect of high temperatures on lycopene synthesis. The shoulder of the tomato fruit has greater exposure to direct rays of the sun, thus experiencing higher temperatures in this tissue region, leading to this region of tissue being more susceptible to temperature aspects of this ripening disorder. Thus, shading by the foliage may be important for maximizing lycopene content in tomato crops grown in warm regions with high solar radiation.
HT (and greenhouse) tomato crops are typically pruned to increase air circulation, decrease nutrient competition, and improve root architecture, resulting in a crop canopy with reduced susceptibility to foliar disease, improved nutrient uptake, and improved fruit yield and quality [47]. While some sunlight is needed for lycopene synthesis [48], excess pruning exposing tomato fruits to extreme sunlight radiation during periods of high temperatures and/or solar radiation could increase the risk of YSD. The effect of increasing fruit shading through pruning strategies to maintain leaf canopy around ripening fruit, row orientation, and breeding for foliage patterns that accommodate partial shading has not been investigated till this review. Additionally, the use of shade cloth with different percentages of shade levels depending on crop and HT latitude could increase the production of uniformly ripened tomatoes. For example, shade cloth with a moderate shade level (e.g., 30%) has been suggested to enhance tomato yield in the midwestern US [49].
Conversely, in a mild summer, this practice can lead to a decline in overall tomato yield in the later part of the season. Shade cloth may prove more beneficial when cultivating indeterminate tomatoes that are pruned and trellised to a single stem. Due to reduced foliage and elevated temperatures in the upper levels of HT, these tomatoes are more susceptible to YSD damage from excessive heat and are likely to produce superior fruit when provided with shade [49]. However, there is still a research gap that needs to be addressed regarding the appropriate use of shade cloth with shade level percentage for different latitudes having different solar intensities, particularly in tomato shoulder in HTs.
3.2. K Regulation of Ripening May Be Difficult to Achieve in HT Microclimate Conditions
Abiotic stresses, such as salinity, have detrimental effects on plant growth, development, and yield [50]. Salt stress adversely impacts various physiological processes in plants, including but not limited to photosynthesis, protein synthesis, and energy and lipid metabolism [51]. The detrimental effects of salt stress on plants primarily occur through processes like osmotic stress, ion toxicity, nutrient imbalance, and oxidative stress [52]. Such issues can cause physiological stress in tomato plants, which can result in YSD. Potassium plays a role in plant nutrition, growth, enzyme homeostasis, and the regulation of osmotic pressure [53]. Thus, K fertilizer applications can mitigate YSD by addressing salt and heat stress and influencing plant hormones, as observed by higher expression of plant hormone-related genes with exogenous K application [54]. However, the effectiveness of exogenous K application depends heavily on the plant’s root health and nutrient uptake capacity, both of which can be significantly compromised by high soil temperatures [55].
3.3. High Soil Temperatures in HTs Increase the Risk of YSD by Affecting Tomato Root Growth, Nutrient Uptake, and Source-Sink Dynamics
High soil temperatures (above 32–40.5 °C) may indirectly affect YSD in tomatoes through impeding root growth, reducing nutrient uptake, diminishing nutrient-assimilation proteins, and slowing down the rate of nutrient absorption by roots [55]. High temperatures have the potential to disturb the sink-source dynamics between roots and shoots, impacting the carbon balance within tomato plants. High-temperature stress (>38 °C) in HTs (or greenhouses) can reduce the efficiency of leaf photosynthesis, leading to greater carbohydrate demand, which in turn increases dark respiration [56]. This disruption exerts an influence on both the vegetative and reproductive growth of tomato plants, ultimately causing a decline in yield and fruit quality [57,58,59], although such effects differ by genotype [60]. Thus, there is a need for further research on the optimum temperature for root growth and K uptake and reduction of YSD for different tomato cultivars in HT production systems.
Significant interaction exists between temperature and soil K level and K and Mg uptake. The soil temperature of 16 °C during the day/8 °C at night, coupled with high soil K levels, increased the risk of Mg deficiency in tomatoes. Potassium uptake by tomato shoots increased by 26.9% under low temperatures. In contrast, the shoots exhibited a reduction of 11.8% in Mg uptake, while the roots displayed a rise of 48.6%. Furthermore, the K/Mg ratio in shoots and roots was significantly higher at lower temperatures (16 °C during the day/8 °C at night). Such K and Mg imbalance in roots and shoots of tomato plants affected by temperature can play an important role in YSD, and further research is needed in terms of HT tomato production [61].
The effect of high temperatures on K uptake has also been shown to increase the incidence of YSD. Zhang et al. [62] found that YSD increased in severity at an air temperature of 32 °C in comparison to 20 °C, despite similar levels of K application. At a temperature of 32 °C, the YSD index exhibited a strong negative linear correlation with K uptake (R2 = 0.81, p < 0.01). Increasing the concentration of K in the nutrient solution at 32 °C effectively enhanced K uptake. This enhancement led to a subsequent reduction in the YSD disorder. Zhang et al. [62] suggested that the effect of high temperature on YSD could be minimized with sufficient K application. However, the exact amount of K required to minimize YSD and the efficacy of application method (e.g., applied in soil pre-plant versus fertigation) in HT systems during high-temperature conditions remain unknown. Additionally, specific temperature thresholds and key physiological indicators, such as K uptake rate and root activity under HT conditions, could be a promising area for future research. These physiological responses, however, are also closely linked to water management practices, which directly influence nutrient mobility and availability in the soil.
3.4. Common Irrigation Practices in HT Systems May Limit Soil K Availability and Increase the Risk of YSD
Soil moisture plays an important role in soil health and the regulation of soil biological activity [63,64,65]. However, high rates of fertilizer use and lack of rainfall may cause fertilizer salt accumulation, soil compaction, and soil pH fluctuation [2]. Further, compared to open fields, where soil moisture typically increases with depth, well-drained HTs may experience drier subsoil conditions despite the surface soil appearing well-irrigated [1]. Further, surface soil moisture may be variable, as the only water in HT systems is provided through irrigation. Specific to K dynamics, low soil moisture levels can contribute to reduced K availability and absorption through reduced K solubility and by limiting root growth, resulting in K deficiency [66,67,68]. To date, no direct relationship has been found in the literature that associates soil moisture dynamics with risk of YSD.
In addition to K dynamics, little work has been conducted that explores irrigation regimes that optimize nutrient availability, crop production, and sustained soil productivity. To date, irrigation recommendations in HTs have been based on crop growth. There is a need for improved understanding of how irrigation can be managed for crop water demand, improved crop root architecture for nutrient and water use uptake efficiency, and balancing nutrient availability with leaching of excess salts [69].
3.5. Nutrient Imbalances Driven by Intensive Production Practices Increase Risk for YSD
Increased crop production intensity through increased crop biomass and lack of rotation is also accompanied by greater rates of yield and crop debris removal in HTs. Crop debris removal is a primary method to reduce pathogens in systems with limited crop rotation. This increases rates of nutrient removal, which may exacerbate nutrient imbalances.
Specific to YSD, high rates of K removal through removal of tomato crop biomass may decrease soil K levels over time. Multiple studies have shown that YSD can be influenced by the levels of soil-exchangeable K and Mg. Research conducted by Hartz et al. examined the relationship between soil exchangeable levels of K, Ca, and Mg and the occurrence of YSD. The research study observed a negative correlation between soil exchangeable K levels and the occurrence of YSD. In contrast, a positive correlation was observed between soil-exchangeable Mg and the YSD, while exchangeable Ca did not show a significant relationship with them. Currently, the ‘Hartz ratio’ (K/√Mg) is a predictive tool used to assess the risk of ripening disorders based on soil nutrient levels [18].
Although previous studies have demonstrated a direct association between increased K nutrition and a decrease in color disorders [19,70,71], high K levels can increase K uptake while reducing Mg uptake [19,72]. This complex interplay also affects the absorption of other nutrients, such as sodium, calcium, and nitrogen [73]. There is currently a lack of understanding of how cation interactions can be managed to decrease the incidence of YSD. Further, at the time of this writing, we did not find any research incorporating nutrient budgeting approaches or soil organic matter dynamics in HT systems. Such work could inform improved fertilizer recommendations and nutrient management strategies, and K management in particular, to minimize the risk of YSD in HTs.
3.6. Soil Mineralogy Influences K Fixation, Potentially Increasing the Risk of YSD
In addition to nutrient imbalance driven by intensive production practices, soil processes, such as physical (soil texture, structure, and moisture), chemical (type of minerals, competing cations), and biological factors (root exudates and OM), impact K availability [74]. High clay content, combined with specific clay minerals, tends to restrict the movement of K, resulting in reduced availability for plant absorption [75]. For example, K fixation is caused by clay mineral particles like vermiculite and mica, where the site of isomorphic substitution is located in the tetrahedral sheet in clay mineral interlayers [74,75,76,77]. It has been reported that 80% of applied K fertilizers can be fixed in interlayer sites in vermiculite minerals [78,79]. Such fixed K is not readily available for immediate crop uptake but may gradually be released and absorbed by crops during the growing season under optimal temperature and moisture conditions [80], and such an issue could be one of the driving factors responsible for YSD.
Recent studies have shown that K uptake by plants is a function of the amount of K released from clay particles, which was significantly dependent on the mineralogical composition of the clay minerals [81]. Research has shown that smectite-dominated soils have less non-exchangeable K than vermiculite, hydroxy-interlayered vermiculite, and illite-dominated soils [82]. Similarly, beidelitic dominant clays were reported to fix almost 80% of applied K in aquic soils [83]. Furthermore, it has been shown that there is a positive correlation between clay concentration in calcareous soils and their ability to fix K [84]. Thus, it is important to consider both the quantity and nature of the dominant clay minerals present in an HT in order to understand potential K availability and fertilizer response. The evaluation of the inherent ability of soils to supply K to crops, as well as the identification of key soil characteristics related to their K status, are becoming increasingly important in sustainable agriculture [85]. To date, this work is lacking in the context of both open field and HT production, which has different microclimate conditions than the open field.
Further, the presence of other cations, Mg and Ca, has been observed to affect K fixation processes. High concentrations of these cations within the soil solution have the potential to engage in competition with K for the occupation of binding sites on soil colloids [74]. There is a need for work on how cation competition for binding sites affects K availability based on soil mineralogy, physicochemical characteristics, and soil moisture regime, particularly in HT soil [81,82]. This knowledge gap in HT systems could lead to better K fertilizer recommendations by understanding the impact of cation competition on K’s ability to prevent YSD and may need to be tailored to specific soil types, management practices, and tomato cultivars.
3.7. Management to Reduce YSD May Be Cultivar- and Growth-Habit Specific
Plant nutrient absorption is proportional to both the total root length and the rate of inflow per unit of root length [86]. As such, root length and nutrient absorption are important to consider while managing YSD in HTs, which can be managed by in-season nutrient management and matching fertilizer application to the root foraging area. This may require differential management based on growth habits, as determinate cultivars have a more limited root length and architecture [86], although the overall K demand may be lower than an indeterminate cultivar due to smaller growth habits. In addition to facilitating K transport from the soil to the surface of the roots, root characteristics such as root architecture, root exudates, and root membrane transporters also influence K supply to plants [86].
Plants with indeterminate growth habits with extensive root architecture have greater overall nutritional demands due to their continuous fruit production and greater biomass. By curbing vegetative growth, which competes for assimilates, pruning redirects resources toward roots or fruits, leading to a harmonious balance between vegetative and reproductive growth for higher fruit quality [87]. Furthermore, Lhamo et al. [88] found that pruned tomato plants had an early fruit set and maturity, fewer fruits overall but heavier and larger fruits, a greater leaf-to-fruit ratio, less competition of nutrient distribution in plant parts, and a longer reproductive phase of tomatoes, all of which can affect tomato quality by optimizing K use efficiency in fruits and reducing the vulnerability of indeterminate tomatoes to YSD. Different processing tomato varieties suitable for canning, such as ‘Amish Paste’, ‘BoxCar Willie’, ‘Marglobe Improved’, ‘Old Brooks’, and ‘Rutgers’, are among those that have shown less susceptibility to YSD [89]. However, future research is needed to study the YSD resistance and susceptibility of different tomato cultivars in HT conditions along with the study regarding the amount of K removed during crop debris removal, considering cultivar (determinate and indeterminate) and genotype for effective fertilizer recommendation, particularly K, to minimize YSD in HT.
3.8. Interaction Between Genetic and Environmental Factors Could Be Responsible for YSD
Genetics has been identified as an underlying cause of YSD. Specifically, the symptoms have been attributed to a gene associated with nonuniform ripening genotypes or green shoulder genotypes (u+/u+) [19]. Several researchers reported that the uniform green genotypes (u) had a higher level of resistance to YSD than the green shoulder genotypes [19,37,70,90,91]. However, Strobel et al. [92] observed that the green-shouldered cultivar ‘Walter’ exhibited resistance to YSD. In contrast, findings by Mattia et al. [93] indicated that ‘Walter’ had intermediate resistance to YSD, yet 18 F2 generation segregated plants with green shoulders had significantly more YSD than those with uniform shoulder genes in spring but not in the fall experiment and concluded that selecting plants that have uniform ripening will not eliminate the problem of YSD. Such conflicting findings underscore the necessity for further epigenetic research to understand the role of gene and environmental interactions in the development of tomato cultivars that are susceptible to YSD. On the other hand, Scott et al. [90] proposed that green or darker pigments on tomato shoulders increase heat absorption from sunlight, thereby making fruit more susceptible to YSD. However, Mattia et al. [93] found no evidence supporting the notion that the darkness of green pigmentation on tomato shoulders leads to increased incidence of YSD. They proposed that solar radiation might interact with genetic mechanisms related to green shoulders, causing a breakdown in chromoplast formation, which in turn reduces cell size [45], leading to the production of carotenoids and resulting in YSD. However, the exact molecular expression and phenotypic mechanism remain unknown as of this review.
3.9. Interactions Between Main Factors Result in Complex Mechanisms Associated with YSD
The quality of tomatoes cultivated in HTs can be influenced by the interaction between different variables described above, whether directly or indirectly. Physiological stress in plants can arise due to various factors, including high soil temperatures, decline in soil fertility, and increased soil salinity. The aforementioned factors can exert a detrimental influence on the quality of fruits. The nutritional composition of tomatoes can also be influenced by nutrient imbalances resulting from intensive production methods, potentially affecting their overall nutritional quality.
The implementation of HT agriculture may lead to increased soil salinity and alkalinity levels, particularly in regions where the irrigation water has a notable salt content [12,14]. The accumulation of salt in the soil can arise from a combination of limited airflow within HT and evapotranspiration from crops. The occurrence of elevated soil salinity levels can result in the disturbance of osmotic equilibrium in plants, hindering the uptake of water and nutrients. This can adversely affect the quality of tomatoes, causing YSD in HT. Research to study the optimum amounts of salts present in HT soil for enhancing maximum water and nutrient uptake, particularly K, has yet to be studied and may be an important aspect to consider in managing YSD in HT systems.
As noted above, Hartz et al. [18] found a correlation between the soil exchangeable (K/√Mg) and the percentage of YSD to be more pronounced than that of the individual parameters of the soil exchangeable K or K activity ratio alone. Similarly, the application of K or gypsum (calcium sulfate (CaSO4) has been used to increase the K/√Mg ratio in the soil by forming insoluble compounds such as magnesium sulfate (MgSO4) and reducing the availability of Mg in the soil solution, which resulted in a significant decrease in YSD. Working to increase K availability and K transport between roots and shoots through such indirect effects is a promising strategy, but work should be conducted in the context of HT tomato systems to understand the potential efficacy in reducing YSD in HT microclimates.
An increase in the occurrence of YSD was also observed in conjunction with higher relative humidity levels. This correlation was found to be influenced by both short-wave radiation and an amplified effective radiation load [38,94]; also observed was a significant decrease in nutrient uptake, particularly for K and Ca in greenhouse tomatoes that were supplied with high relative humidity. Color disorders in tomatoes have also been associated with high pericarp fruit temperatures and increased environmental humidity levels [19]. High-tunnel microclimates may experience both high temperatures [9,95,96] and high relative humidity [9], warranting investigation into these interactions.
4. Reducing the Risk of YSD
There are a variety of management factors that may be adjusted to minimize the effect of YSD in HT tomatoes, based on the (albeit incomplete) mechanistic understanding we have of this disorder to date. Adjusting planting dates to avoid extremely high temperatures and relative humidity in high tunnels during the fruit initiation phase could reduce the severity of YSD. Growers should maintain continuous operation of fan–jet or horizontal airflow systems in greenhouses during high humidity periods, especially at night and on rainy days. They may also consider using exhaust fans, when available, to circulate air in HTs with closed sides to minimize high air temperature and better air circulation to monitor and maintain air temperatures within the normal growth range to reduce plant stress. Ensuring proper ventilation in HTs to exchange internal air with fresh outdoor air, reducing internal relative humidity by opening sidewalls, end walls, and/or ridge vents, and maintaining adequate plant spacing to prevent the development of high humidity microclimates caused by closely spaced plants and overlapping canopies may help to minimize YSD to some extent. Training and pruning by removing suckers and old leaves up to the first bunch of fruit to improve airflow and reduce humidity may also help better K utilization, ultimately reducing YSD susceptibility. Maintaining adequate pH levels and, more importantly, avoiding excessive use of fertilizer to minimize nutrient imbalance in the soil. Efficient and optimum fertilizer application based on soil test results along with in-season foliar testing should be considered.
5. Conclusions
The complex interactions between management practices, climatic drivers, and the cultural factors that influence YSD in the HT system have not been sufficiently studied. Improved fertilizer recommendations, particularly for K based on soil tests, not limited to only routine tests but also clay mineralogy affecting K fixation, are needed to minimize YSD in HTs. Further research is needed to study the effects of complex interactions between different driving factors, such as the relationship between temperature and YSD index, K application, and fruit color development at different growth stages with cultivar-specific response, and the interaction between temperature/humidity control and irrigation regimes and their joint impact on YSD. Additionally, a significant research gap exists in understanding the role of the Hartz ratio and its implications on nutrient transport between roots and shoots, the effectiveness of different shading intensities across latitudes and cultivars, and how key metabolic genes involved in lycopene synthesis are affected under HT conditions. Finally, an integrated breeding program to develop YSD-resistant varieties for different climatic regions with an optimized nutrient regime should be conducted to support sustained high-quality tomato production in HT growing systems.
Author Contributions
S.P.: writing—original draft. C.J.M.: review and conceptualization. H.P.: review and conceptualization. K.J.: review, conceptualization, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The materials supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
This work was supported by the Department of Horticulture and The Food Connection at the University of Kentucky.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| CEC | cation exchange capacity |
| EQIP | Environmental Quality Incentive Program |
| HT | high tunnel |
| USDA-NRCS | United States Department of Agriculture–Natural Resources Conservation Service |
| YSD | yellow shoulder disorder |
References
- Montri, A.; Biernbaum, J.A. Management of the soil environment in high tunnels. HortTechnology 2009, 19, 34–36. [Google Scholar] [CrossRef]
- Blomgren, T.; Frisch, T.; Moore, S. High Tunnels: Using Low-Cost Technology to Increase Yields, Improve Quality and Extend the Season. Regional Farm and Food Project and Cornell University. Retrived from: Agriculture. 2007. Available online: https://u.osu.edu/vegprolab/crop-environment-publications/high-tunnels-using-low-cost-technology-to-increase-yields-improve-quality-and-extend-the-season/ (accessed on 10 November 2023).
- Wells, O.S.; Loy, J.B. Rowcovers and high tunnels enhance crop production in the Northeastern United States. HortTechnology 1993, 3, 92–95. [Google Scholar] [CrossRef]
- Lamont, W.J., Jr. Overview of the use of high tunnels worldwide. HortTechnology 2009, 19, 25–29. [Google Scholar] [CrossRef]
- Rabobank. World Vegetable Map 2018. RaboResearch Food & Agribusiness. 2018. Available online: https://research.rabobank.com/far/en/sectors/regional-food-agri/world_vegetable_map_2018.html (accessed on 28 September 2023).
- Hadley, D. Controlled Environment Horticulture Industry Potential in NSW. University of New England. 2017. Available online: https://www.une.edu.au/about-une/faculty-of-science-agriculture-business-and-law/unebs/centre-for-agribusiness/documents/controlled-environment-horticulture-industry-potential-hadley.pdf (accessed on 15 December 2023).
- U.S. Department of Agriculture-Natural Resource Conservation Service. EQIP and RCPP-EQIP Contracted High Tunnels 2012–2021. 2021. Available online: https://www.nrcs.usda.gov/programs-initiatives/eqip-high-tunnel-initiative (accessed on 15 February 2024).
- Frey, C.J.; Zhao, X.; Brecht, J.K.; Black, Z.E.; Zhao, X. High tunnel and grafting effects on organic tomato plant disease severity and root-knot nematode infestation in a subtropical climate with sandy soils. HortScience 2020, 55, 46–54. [Google Scholar] [CrossRef]
- O’Connell, S.; Rivard, C.; Peet, M.M.; Harlow, C.; Louws, F. High tunnel and field production of organic heirloom tomatoes: Yield, fruit Quality, disease, and microclimate. HortScience 2012, 47, 1283–1290. [Google Scholar] [CrossRef]
- Kaiser, C.; Ernst, M. High Tunnel Tomatoes. CCD-CP-62; Center for CROP Diversification, University of Kentucky College of Agriculture, Food and Environment: Lexington, KY, USA, 2017; Available online: https://publications.ca.uky.edu/ccd-cp-62 (accessed on 24 December 2023).
- Guan, W. A New Soil Test for High Tunnel Growers; Purdue University Cooperative Extension Service: West Lafayette, IN, USA, 2020; ISSUE: 668; Available online: https://vegcropshotline.org/article/a-new-soil-test-for-high-tunnel-growers/ (accessed on 15 December 2024).
- Rudisill, M.A.; Bordelon, B.P.; Turco, R.F.; Hoagland, L.A. Sustaining soil quality in intensively managed high tunnel vegetable production systems: A role for green manures and chicken litter. HortScience 2015, 50, 461–468. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K. Tomato tolerance to abiotic stress: A review of most often engineered target sequences. Plant Growth Regul. 2017, 83, 175–198. [Google Scholar] [CrossRef]
- Hajime, A.; Hane, S.; Hoshino, Y.; Hirata, T. Cover Crop Use in Tomato Production in Plastic High Tunnel. Hortic. Environ. Biotechnol. 2009, 50, 324–328. [Google Scholar]
- Wildung, D.; Johnson, P. High Tunnel Production Manual for Commercial Growers; University of Minnesota: Minneapolis, MN, USA, 2012; Available online: http://www.plantgrower.org/uploads/6/5/5/4/65545169/high-tunnel-manual-2012.pdf (accessed on 7 December 2023).
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Higgins, G.; Scheufele, S. Tomato, Physiological Ripening Disorders; University of Massachusetts Agriculture and Landscape Program: Amherst, MA, USA, 2019; Available online: https://ag.umass.edu/vegetable/fact-sheets/tomato-physiological-ripening-disorders (accessed on 17 December 2023).
- Hartz, T.K.; Miyao, G.; Mullen, R.J.; Brittan, K.L. Potassium requirements for maximum yield and fruit quality of processing tomato. Am. Soc. Hortic. Sci. 1999, 124, 199. [Google Scholar] [CrossRef]
- Picha, D.H. Physiological factors associated with yellow shoulder expression in tomato fruit. J. Am. Soc. Hortic. Sci. 1987, 112, 798–801. [Google Scholar] [CrossRef]
- Schlimme, D.V.; Corey, K.A.; Frey, B.C. Evaluation of lye and steam peeling using four processing tomato cultivars. J. Food Sci. 1984, 49, 1415–1418. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Agricultural Marketing Service. Fruit and Vegetable Division. Fresh Products Branch. United States Standards for Grades of Fresh Tomatoes: Effective. 1 October 1991 (Reprinted—January 1997). 1997. Available online: https://www.ams.usda.gov/sites/default/files/media/Tomato_Standard%5B1%5D.pdf (accessed on 2 March 2023).
- Bogash, S.; Orzolek, M. Yellow Shoulder on Tomato. In Proceedings of the Greenhouse and High Tunnels Session. 2012; pp. 1–7. Available online: http://www.hort.cornell.edu/expo/proceedings/2012/Greenhouse%20and%20High%20Tunnels/High%20Tunnels%20Bogash.pdf (accessed on 27 May 2024).
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Maynard, E.T.; Calsoyas, I.S.; Malecki, J. Potassium Applications and Yellow Shoulder disorder of Tomatoes in High Tunnels; Purdue University: West Lafayette, IN, USA, 2016; Available online: https://docs.lib.purdue.edu (accessed on 24 January 2024).
- Sacks, E.J.; Francis, D.M. Genetic and environmental variation for tomato flesh color in a population of modern breeding lines. J. Am. Soc. Hortic. Sci. 2001, 126, 221–226. [Google Scholar] [CrossRef]
- Eshu, S.; Rangare, S.B.; Yadav, V.; Rangare, N.R. Physiological disorders in tomato (Solanum lycopersicum Mill.)—An abnormalities. Trends Biosci. 2014, 7, 3779–3785. [Google Scholar]
- Shrestha, S.L.; Sah, R.L. Evaluation of tomato cultivars for central Terai of Nepal. Nepal J. Sci. Technol. 2015, 15, 11–16. [Google Scholar] [CrossRef]
- Masarirambi, M.T.; Mhazo, N.; Oseni, T.O.; Shongwe, V.D. Common physiological disorders of tomato (Lycopersicon esculentum) fruit found in Swaziland. J. Agric. Soc. Sci. 2009, 5, 123–127. Available online: http://www.fspublishers.org (accessed on 27 November 2023).
- Suzuki, K. Physiological Disorders and Their Management in Greenhouse Tomato Cultivation at High Temperatures. In Adaptation to Climate Change in Agriculture; Iizumi, T., Hirata, R., Matsuda, R., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Qin, M.; Xie, Z.; Chen, R.; Zhang, Y. Effects of supplemental lighting on potassium transport and fruit coloring of tomatoes grown in hydroponics. Int. J. Mol. Sci. 2021, 22, 2687. [Google Scholar] [CrossRef]
- Redondo, I.B. Comparison of Six Selections of the Heirloom Tomato. 2013. Available online: https://www.ub.edu/masterae/wp-content/uploads/2014/05/CULTIVO-DE-HORTALIZAS-H33.pdf (accessed on 15 January 2024).
- Romero-Aranda, R.; Fernández-Muñoz, R.; López-Casado, G.; Cuartero, J. Yellow Shoulder Disorder in Tomatoes Under Natural and Controlled Conditions; Department of Plant Breeding, La Mayora Experimental Station, Spanish National Research Council (CSIC): Algarrobo-Costa, Spain, 2004; Volume 53, pp. 34–35. Available online: https://www.researchgate.net/publication/265818400_Avances_en_la_genetica_de_la_fisiopatia_de_la_mancha_solar_en_tomate (accessed on 28 September 2023).
- Hernandez-Perez, O.I.; Valdez-Aguilar, L.A.; Alia-Tejacal, I.; Cartmill, A.D.; Cartmill, D.L. Tomato fruit yield, quality, and nutrient status in response to potassium: Calcium balance and electrical conductivity in the nutrient solution. J. Soil Sci. Plant Nutr. 2020, 20, 484–492. [Google Scholar] [CrossRef]
- Jarquín-Enríquez, L.; Mercado-Silva, E.; Maldonado, J.L.; Lopez-Baltazar, J. Lycopene content and color index of tomatoes are affected by the greenhouse cover. Sci. Hortic. 2013, 155, 43–48. [Google Scholar] [CrossRef]
- Shaheen, M.R.; Ayyub, C.M.; Amjad, M.; Waraich, E.A. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions. J. Sci. Food Agric. 2015, 96, 2698–2704. [Google Scholar] [CrossRef]
- Yui, S.; Ishii, T.; Fujino, M.; Yanokuchi, Y.; Kataoka, S.; Ishiuchi, D.; Uchiumi, T.; Matsunaga, H.; Okimura, M.; Kawazu, Y. Breeding and characteristics of ʻTomato intermediate mother plant No 10’, high lycopene tomato breeding line for staked culture. Breed. Res. 2009, 11, 95–99. [Google Scholar] [CrossRef]
- Venter, F. Investigation on green-back of tomatoes. Acta Hortic. 1966, 4, 99–101. [Google Scholar] [CrossRef]
- Lipton, W.J. Effects of high humidity and solar radiation on temperature and color of tomato fruits. J. Am. Soc. Hortic. Sci. 1970, 95, 680–684. [Google Scholar] [CrossRef]
- Brust, J. Yellow Shoulders in Tomato: A Big Problem This Season. Weekly Crop Update. University of Delaware Cooperative Extension. 2011. Available online: https://sites.udel.edu/weeklycropupdate/?p=3457 (accessed on 25 September 2024).
- Hwang, I.; Kim, Y.; Han, J.; Nou, I.S. Orange color is associated with CYC-B expression in tomato fleshy fruit. Mol. Breed. 2016, 36, 42. [Google Scholar] [CrossRef]
- Preedy, V.R.; Watson, R.R. (Eds.) Tomatoes and Tomato Products: Nutritional, Medicinal and Therapeutic Properties; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Stommel, J.; Abbott, J.A.; Saftner, R.A.; Camp, M. Sensory and objective quality attributes of beta-carotene and lycopene-rich tomato fruit. J. Am. Soc. Hortic. Sci. 2005, 130, 244. [Google Scholar] [CrossRef]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Helyes, L.; Lugasi, A.; Pek, Z. Effect of natural light on surface temperature and lycopene content of vine-ripened tomato fruit. Can. J. Plant Sci. 2007, 87, 927–929. [Google Scholar] [CrossRef]
- Francis, D.M.; Barringer, S.A.; Whitmoyer, R.E. Ultrastructural characterization of Yellow Shoulder Disorder in a uniform ripening tomato genotype. HortScience 2000, 35, 1114–1117. [Google Scholar] [CrossRef]
- UMass Extension. Tomato Physiological Ripening Disorders. In Vegetable Program Fact Sheet; University of Massachusetts Amherst: Amherst, MA, USA, 2022; Available online: https://www.umass.edu/agriculture-food-environment/vegetable/fact-sheets/tomato-physiological-ripening-disorders (accessed on 20 June 2025).
- Ambroszczyk, A.M.; Cebula, S.; Sekara, A. The effect of plant pruning on the light conditions and vegetative development of eggplant (Solanum melongena L.) in greenhouse cultivation. Veg. Crops Res. Bull. 2008, 68, 57–70. [Google Scholar] [CrossRef]
- Cox, S.E.; Stushnoff, C.; Sampson, D.A. Relationship of fruit color and light exposure to lycopene content and antioxidant properties of tomato. Can. J. Plant Sci. 2003, 83, 913–919. [Google Scholar] [CrossRef]
- Guan, W. Whether to Put Shade Cloth on High Tunnel Tomatoes. Vegetable Crops Hotline, ISSUE: 619 2016. Available online: https://vegcropshotline.org/article/temperature-and-light-intensity-in-a-high-tunnel-covered-with-30-black-shade-cloth/ (accessed on 16 December 2023).
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Rajora, N.; Bhardwaj, S.; Sudhakaran, S.S.; Kumar, A.; Raturi, G.; Chakraborty, K.; Gupta, O.P.; Devanna, B.N.; Tripathi, D.K. Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiol. Biochem. 2021, 162, 110–123. [Google Scholar] [CrossRef]
- Zhu, J. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Schroeder, J.I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 1994, 370, 655–658. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Du, S.; Wang, G.; Zhang, J.; Jiang, J. Effects of exogenous (K+) potassium application on plant hormones in the roots of Tamarix ramosissima under NaCl stress. Genes 2022, 13, 1803. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat Stress Decreases Levels of Nutrient-Uptake and -Assimilation Proteins in Tomato Roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Stommel, J.R. Pollen viability and fruit set of tomato genotypes under optimum and high-temperature regimes. HortScience 1995, 30, 115–117. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Zhou, T.; Liu, Y.; Raza, S.; Zhou, J. Effects of high potassium and low temperature on the growth and magnesium nutrition of different tomato cultivars. HortScience 2018, 53, 710–714. [Google Scholar] [CrossRef]
- Zhang, Y.; Suzuki, K.; Liu, H.; Nukaya, A.; Kiriiwa, Y. Fruit yellow-shoulder disorder as related to mineral element uptake of tomatoes grown in high temperature. Sci. Hortic. 2018, 242, 25–29. [Google Scholar] [CrossRef]
- Biernbaum, J. Water, Soil, and Fertility Management in Organic High Tunnels; Michigan State University: East Lansing, MI, USA, 2013; Available online: https://www.canr.msu.edu/hrt/uploads/535/78622/HighTunnelWaterSoilFertility2013-10pgs.pdf (accessed on 21 November 2023).
- Gaskell, M.; Fouche, B.; Smith, S.R.; Koike, S.; Lanini, T.; Mitchell, J.P. Organic Vegetable Production in California—Science and Practice. HortTechnology 2000, 10, 699. [Google Scholar] [CrossRef]
- Lei, L.; McDonald, L.M. Soil moisture and temperature effects on nitrogen mineralization in a high tunnel farming system. Commun. Soil Sci. Plant Anal. 2019, 50, 2140–2150. [Google Scholar] [CrossRef]
- Gluck, B.I.; Hanson, E.J. Effect of drip irrigation and winter precipitation on distribution of soil salts in three season high tunnels. Acta Hortic. 2013, 987, 99–104. [Google Scholar] [CrossRef]
- Camberato, J. Low Soil Moisture and Compaction Promote Potassium Deficiency. In Pest & Crop Newsletter; College of Agriculture, Purdue University: West Lafayette, IN, USA, 2020; Available online: https://extension.entm.purdue.edu/newsletters/pestandcrop/article/low-soil-moisture-and-compaction-promote-potassium-deficiency/ (accessed on 22 May 2024).
- Lawrence, D.; Majumdar, A.; Glover, T.; Boozer, R. High Tunnel Irrigation and Fertigation. Alabama Cooperative Extension System. 23 February 2022. Available online: https://www.aces.edu/blog/topics/crop-production/high-tunnel-irrigation-and-fertigation/ (accessed on 24 August 2023).
- Pierre, J.F.; Jacobsen, K.L.; Wszelaki, A.; Butler, D.; Velandia, M.; Woods, T.; Sideman, R.; Grossman, J.; Coolong, T.; Hoskins, B.; et al. Sustaining soil health in high tunnels: A paradigm shift toward soil-centered management. HortTechnology 2024, 34, 594–603. [Google Scholar] [CrossRef]
- Picha, D.H.; Hall, C.B. Influences of potassium, cultivar, and season on tomato graywall and blotchy ripening. J. Am. Soc. Hortic. Sci. 1981, 106, 704–708. [Google Scholar] [CrossRef]
- Van Lune, P.; Van Goor, B.J. Ripening disorders of tomatoes as affected by the K/Ca ratio in the culture solution. J. Hortic. Sci. 1977, 52, 173–180. [Google Scholar] [CrossRef]
- Trankner, M.; Tavakol, E.; Jakli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhao, X.H.; Jiang, C.J.; Wang, X.G.; Han, Y.; Wang, J.; Yu, H. Effect of potassium deficiency on root growth and nutrient uptake in maize (Zea mays L.). Agric. Sci. 2017, 8, 1263–1277. [Google Scholar] [CrossRef]
- Contii, M.E.; Horra, A.M.; de la Effron, D.; Zourarakis, D. Factors affecting potassium fixation on Argentine agricultural soils. Commun. Soil Sci. Plant Anal. 2001, 32, 2679–2690. [Google Scholar] [CrossRef]
- Portela, E.; Monteiro, F.; Fonseca, M.; Abreu, M.M. Effect of soil mineralogy on potassium fixation in soils developed on different parent material. Geoderma 2019, 343, 226–234. [Google Scholar] [CrossRef]
- Liu, Y.J.; Laird, D.A.; Barak, P. Release and fixation of ammonium & potassium under long-term fertility management. Soil Sci. Soc. Am. J. 1997, 61, 310–313. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, M.; Zhang, W. Factors affecting potassium fixation in seven soils under 15- year long-term fertilization. Chin. Sci. Bull. 2009, 54, 1773–1780. [Google Scholar] [CrossRef]
- Hartz, T.K.; Johnstone, P.R.; Francis, D.M.; Miyao, E.M. Processing Tomato Yield and Fruit Quality Improved with Potassium Fertigation. HortScience 2005, 40, 1862–1867. [Google Scholar] [CrossRef]
- Cassman, K.G.; Bryant, D.C.; Roberts, B.A. Comparison of soil test methods for predicting cotton response to soil and fertilizer potassium on potassium-fixing soils. Commun. Soil Sci. Plant Anal. 1990, 21, 1727–1743. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Potassium in crop production. Adv. Agron. 1980, 33, 59–110. [Google Scholar]
- Binner, I.; Dultz, S.; Schellhorn, M.; Schenk, M.K. Potassium adsorption and release properties of clays in peat-based horticultural substrates for increasing the cultivation safety of plants. Appl. Clay Sci. 2017, 145, 28–36. [Google Scholar] [CrossRef]
- Raheb, A.; Heidari, A. Effects of clay mineralogy and physicochemical properties on potassium availability under soil aquic conditions. J. Soil Sci. Plant Nutr. 2012, 12, 747–761. [Google Scholar] [CrossRef]
- Bajwa, M.I. Soil clay mineralogy in relation to fertility management: Effect of soil clay mineral composition on potassium fixation under conditions of upland rice soils. Fertil. Res. 1981, 2, 193–197. [Google Scholar] [CrossRef]
- Ghiri, M.N.; Abtahi, A. Factors affecting potassium fixation in calcareous soils of southern Iran. Arch. Agron. Soil Sci. 2012, 58, 335–352. [Google Scholar] [CrossRef]
- Oborn, I.; Andrist-Rangel, Y.; Askekaard, M.; Grant, C.A.; Watson, C.A.; Edwards, A.C. Critical aspects of potassium management in agricultural systems. Soil Use Manag. 2005, 21, 102–112. [Google Scholar] [CrossRef]
- Kuchenbuch, R.; Claassen, N.; Jungk, A. Potassium availability in relation to soil moisture. Plant Soil 1986, 95, 221–231. [Google Scholar] [CrossRef]
- Hesami, A.; Sarikhani, S.; Hosseini, S.S. Effect of shoot pruning and flower thinning on quality and quantity of semi-determinate tomato (Lycopersicon esculentum Mill.). Not. Sci. Biol. 2012, 4, 108–111. [Google Scholar] [CrossRef]
- Lhamo, T.; Gyalmo, T.; Pem, T.; Bajgai, Y. Effect of different pruning systems on yield and quality of tomato grown under greenhouse. Bhutanese J. Agric. 2022, 5, 71–82. [Google Scholar] [CrossRef]
- Goodspeed, D. How to Prevent Yellow Shoulder Disorder on Tomatoes. 2021. Available online: https://blog.jungseed.com/how-to-prevent-yellow-shoulder-disorder-on-tomatoes/ (accessed on 24 January 2024).
- Scott, J.W. Yellow shoulder. In Compendium of Tomato Diseases; APS Press: St. Paul, MN, USA, 2014. [Google Scholar]
- Yeager, T. The uniform fruit color gene in the tomato. Proc. Am. Soc. Hortic. Sci. 1935, 33, 512. [Google Scholar]
- Strobel, J.W.; Hayslip, N.C.; Burgis, D.S.; Everett, P.H. Walter: A Determinate Tomato Resistant to Races 1 and 2 of the Fusarium wilt Pathogen. Florida Agricultural Experiment Station Circular. 1969. Available online: https://www.apsnet.org/publications/phytopathology/backissues/Documents/1974Articles/Phyto64n12_1507.PDF (accessed on 11 September 2023).
- Mattia, M.R.; Scott, J.W. Effect of immature green tomato fruit color on yellow shoulder incidence and soluble solids content of ripe fruit. J. Am. Soc. Hortic. Sci. 2017, 142, 444–453. [Google Scholar] [CrossRef]
- Gislerod, H.R.; Selmer-Olsen, A.R.; Mortensen, L.M. The effect of air humidity on nutrient uptake of some greenhouse plants. Plant Soil 1987, 102, 193–196. [Google Scholar] [CrossRef]
- Zhao, X.; Carey, E.E. Summer production of lettuce, and microclimate in high tunnel and open field plots in Kansas. HortTechnology 2009, 19, 113–119. [Google Scholar] [CrossRef]
- Hoskins, B. High Tunnel Soil Management Update. In Proceedings of the New England Vegetable & Fruit Conference and Trade Show, NEVFC, Manchester, UK, 12–14 December 2017; Available online: https://projects.sare.org/wp-content/uploads/2017-NEVFC-Proceedings-FINAL.pdf (accessed on 18 February 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).