Characterization of a Gamma Radiation (60Co) Induced Mutant Population of Prickly Pear Cactus (Opuntia velutina F.A.C. Weber) Plants In Vitro Using ISSR Molecular Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Establishment of Apical Shoots
2.2. Radiation Dose and LD50 Determination
2.3. Post-Irradiation In Vitro Shoot Multiplication
2.4. Statistical Analysis
2.5. DNA Extraction and PCR Amplification for ISSR Analysis
2.6. Analysis of Genetic Diversity and Structure
3. Results and Discussion
3.1. Radiosensitivity of In Vitro Meristems
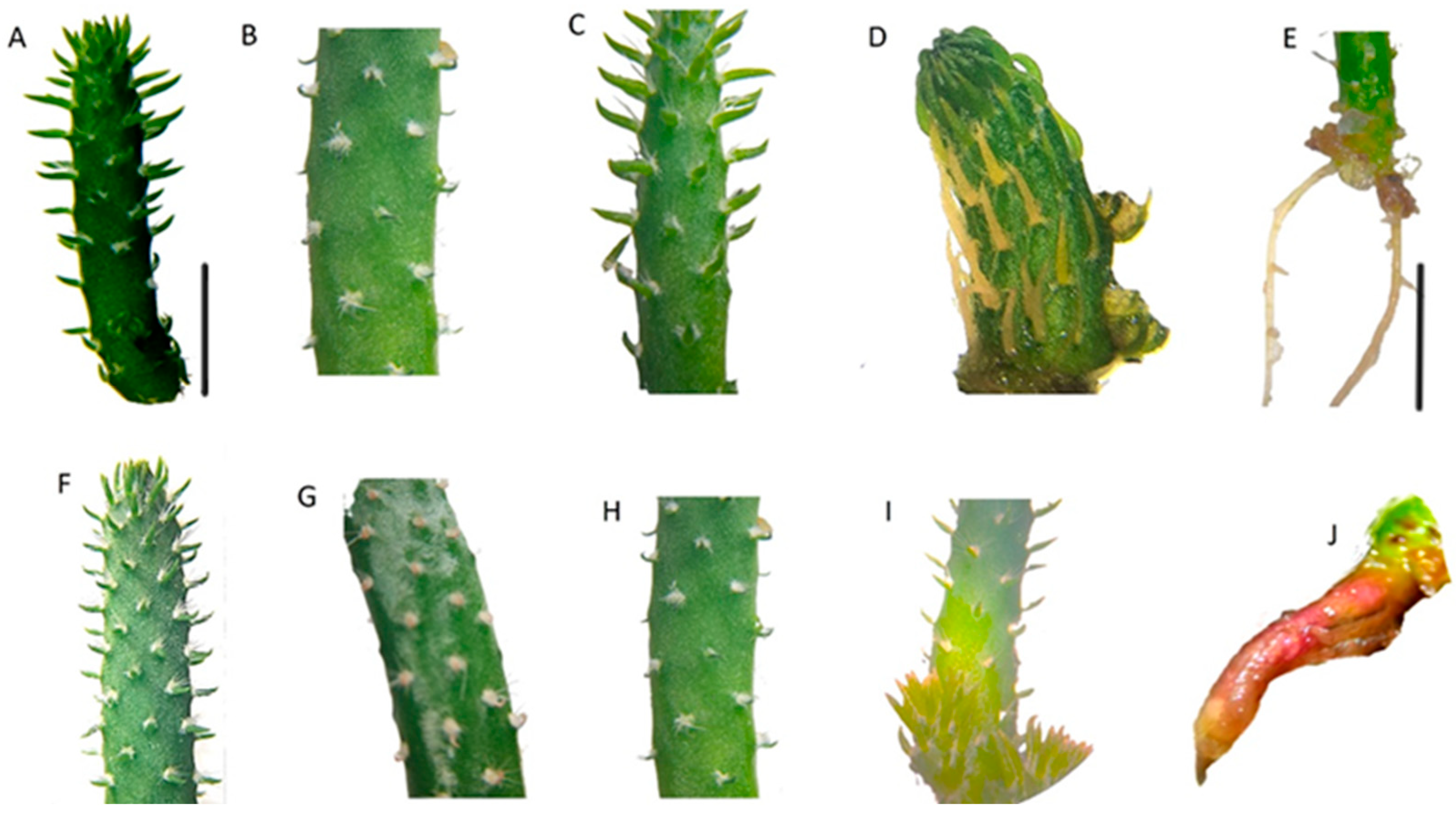
3.2. Morphological Selection of Post-Irradiated In Vitro Seedlings
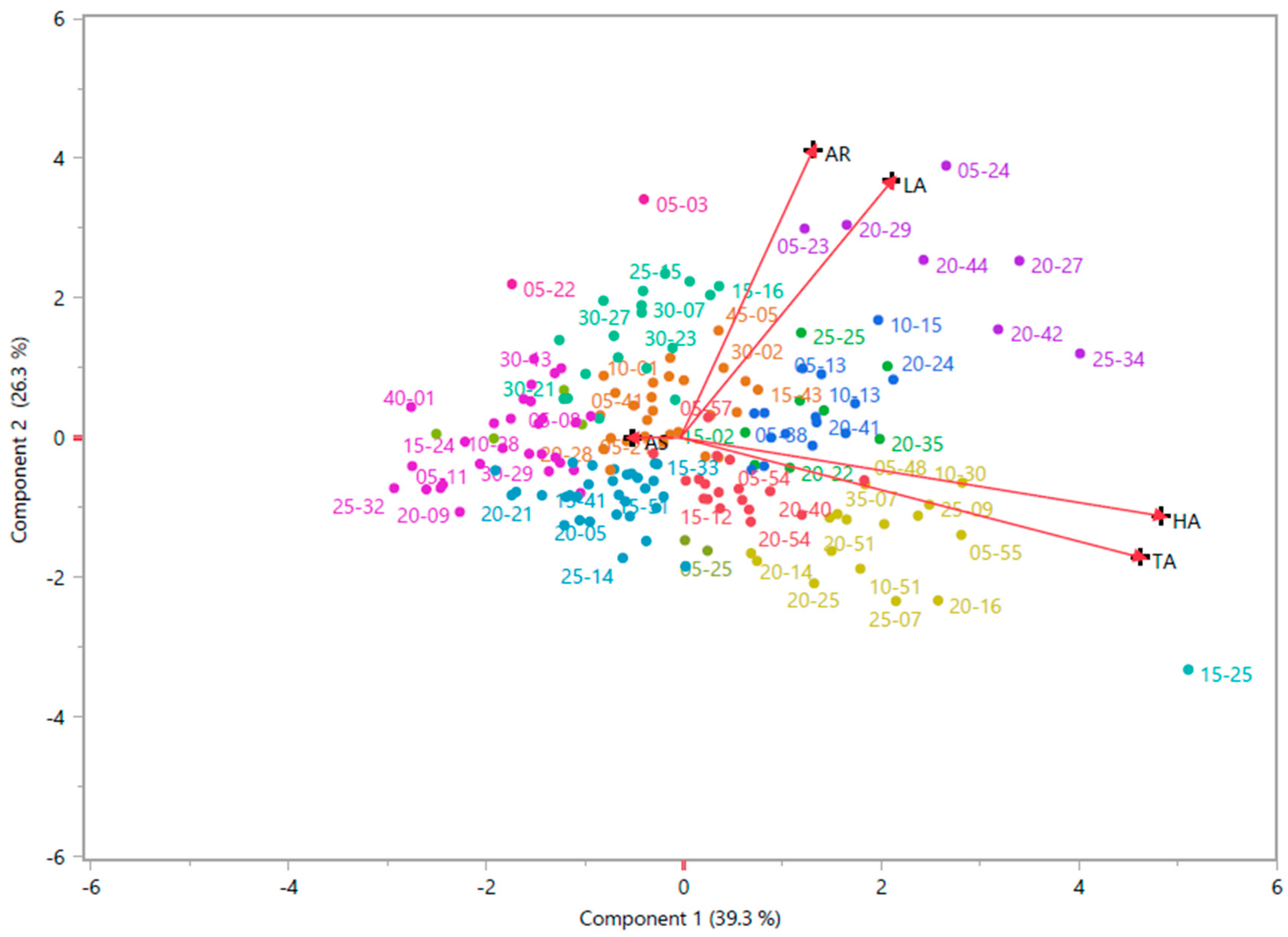
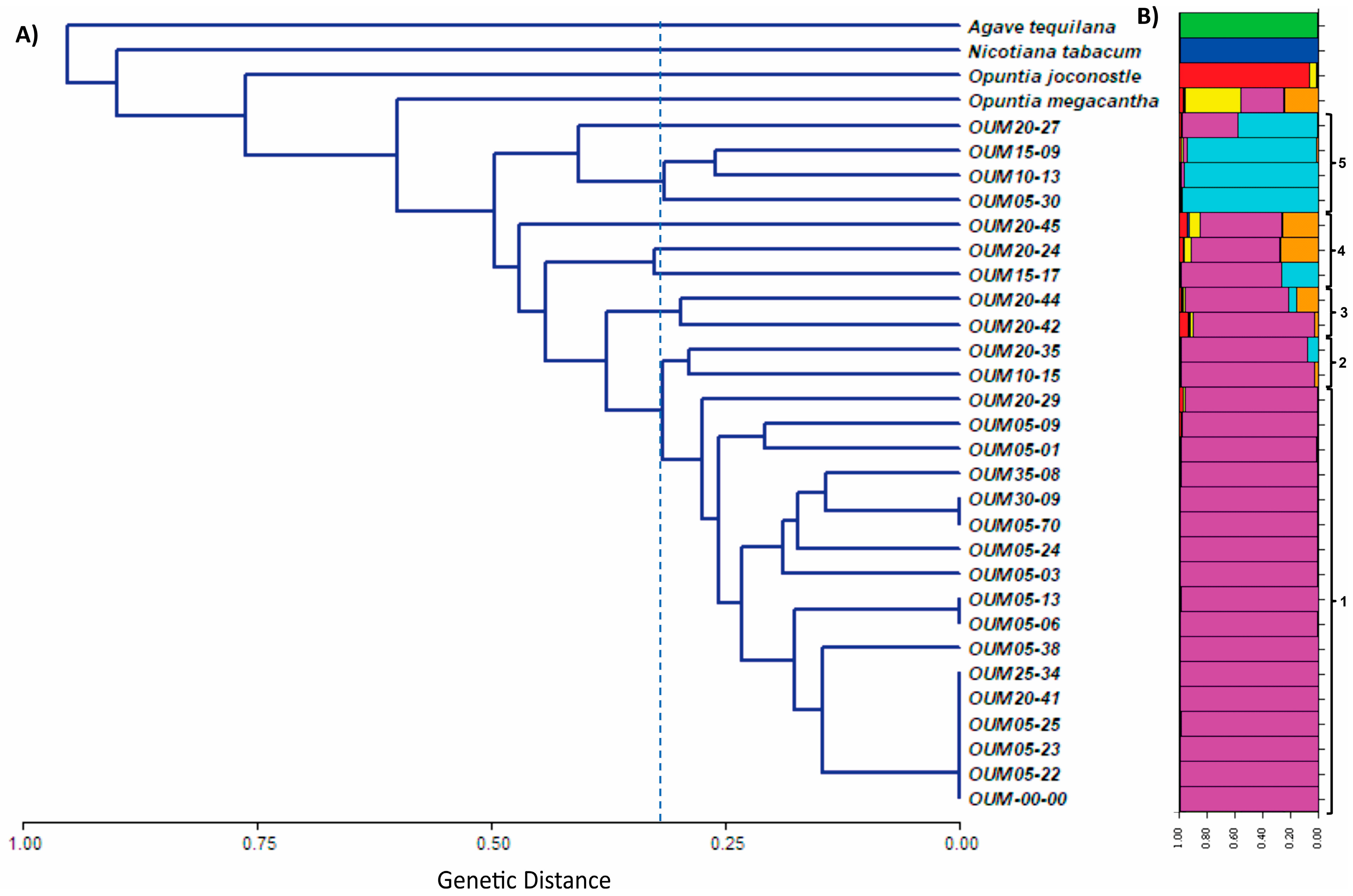
3.3. Cluster Analysis and PCoA
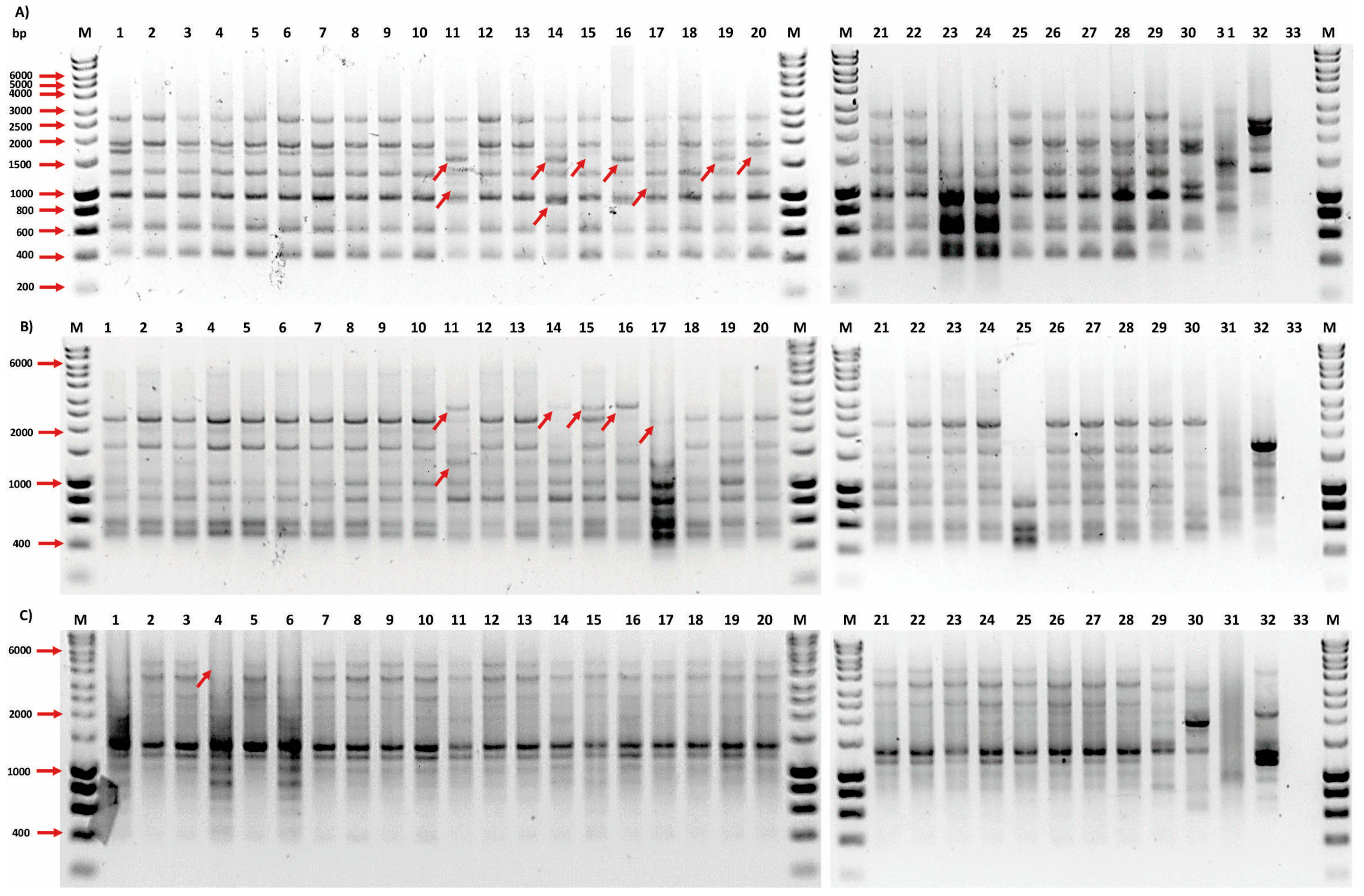
3.4. Analysis of the Mutants by ISSR Molecular Markers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korotkova, N.; Aquino, D.; Arias, S.; Eggli, U.; Franck, A.; Gómez-Hinostrosa, C.; Guerrero, P.; Hernández, H.; Kohlbecker, A.; Köhler, M.; et al. Cactaceae at Caryophyllales.org-A dynamic online species-level taxonomic backbone for the family. Willdenowia 2021, 51, 251–270. [Google Scholar] [CrossRef]
- Reyes-Agüero, J.A.; Aguirre Rivera, J.R.; Flores, J.L. Variación morfológica de Opuntia (Cactaceae) en relación con su domesticación en la altiplanicie meridional de México. Interciencia 2005, 30, 476–484. [Google Scholar]
- Villaseñor, J.L. Checklist of the native vascular plants of Mexico. Rev. Mex. Biodivrs. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Majure, L.C.; Puente, R.; Griffith, M.P.; Judd, W.S.; Soltis, P.S.; Soltis, D.E. Phylogeny of Opuntia s.s. (Cactaceae): Clade delineations, geographic origins, and reticulate evolution. Am. J. Bot. 2012, 99, 847–864. [Google Scholar] [CrossRef]
- Pedraza-Reyes, K.A.; Pérez Olvera, M.A.; Navarro-Garza, H.; Espejel García, A. El sistema familiar de producción de nopal verdura (Opuntia ficus indica L.) en San Pablo Ixquitlán, Méx. Importancia y estrategias de funcionamiento. Rev. Geogr. Agr. 2024, 72, 1–15. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.H.S.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Tilahun, Y.; Welegerima, G. Pharmacological potential of cactus pear (Opuntia ficus indica): A review. J. Pharm. Phytochem. 2018, 7, 1360–1363. [Google Scholar]
- Das, G.; Lim, K.J.; Tantengco, O.A.G.; Carag, H.M.; Gonçalves, S.; Romano, A.; Das, S.K.; Coy Barrera, E.; Shin, H.S.; Gutierrez Grijalva, E.P.; et al. Cactus: Chemical, nutraceutical composition and potential bio-pharmacological properties. Phytother. Res. 2021, 35, 1248–1283. [Google Scholar] [CrossRef]
- Astello-García, M.; Santos-Díaz, M.; Reyes-Agüero, A.; Barba de la Rosa, A. Opuntia spp. as a Source of Bioactive Compounds. In Hispanic Foods: Chemistry and Bioactive Compounds; American Chemical Society: Washington, DC, USA, 2012; pp. 101–111. [Google Scholar] [CrossRef]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A multi-benefit potential to be exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef]
- Mazri, M. Cactus pear (Opuntia spp.) breeding. In Advances in Plant Breeding Strategies: Fruits; Springer: Cham, Switzerland, 2018; pp. 307–341. [Google Scholar] [CrossRef]
- Omar, A.; ElSayed, A.; Mohamed, A. Genetic diversity and ecotypes of Opuntia spp. In Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Springer: Cham, Switzerland, 2021; pp. 181–199. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Mutant Varieties Database. Available online: https://nucleus.iaea.org/sites/mvd/SitePages/Search.aspx (accessed on 16 October 2023).
- Abdelnour-Esquivel, A.; Perez, J.; Rojas, M.; Vargas, W.; Gatica-Arias, A. Use of gamma radiation to induce mutations in rice (Oryza sativa L.) and the selection of lines with tolerance to salinity and drought. In Vitro Cell. Dev. Biol. Plant. 2020, 56, 88–97. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Tallón, C.I.; Pérez-Tornero, O. Inducing mutations in Citrus spp. Sensitivity of different sources of plant material to gamma radiation. App. Radiat. Isot. 2020, 157, 109030. [Google Scholar] [CrossRef] [PubMed]
- Royani, J.I.; Abdullah, L.; Aisyah, S.I. Radio-sensitivity of irradiated seed, plantlets, callus, and in vitro leaves from indigofera zollingeriana Miq by gamma rays. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012061. [Google Scholar] [CrossRef]
- Huerta-Olalde, A.M.; Hernández-García, A.; López-Gómez, R.; Fernández-Pavía, S.P.; Zavala-Páramo, M.G.; Salgado-Garciglia, R. In vitro selection of blackberry (Rubus fruticosus ‘Tupy’) plants resistant to botrytis cinerea using gamma ray-irradiated shoot tips. Plant Biotechnol. 2022, 22, 0312. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Doğan, Y.; Comertpay, G.; Yıldız, M.; Hatipoğlu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N.; et al. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef]
- Bezerra, J.D.C.; de Andrade, A.P.; do Rêgo, M.M.; da Silva, D.S.; do Nascimento Júnior, J.R.S.; Araújo, F.; Valença, R.; do Rêgo, E.R.; Pessoa, A.M.D.; Bruno, R.D.L.A.; et al. Genetic diversity and relationships among Nopalea sp. and Opuntia spp. accessions revealed by RAPD, ISSR and ITS molecular markers. Mol. Biol. Rep. 2022, 49, 6207–6213. [Google Scholar] [CrossRef]
- Bhat, R.S.; Brijesh Patil, M.P.; Tilak, I.S.; Shirasawa, K. Molecular Markers for Mutant Characterization. In Mutation Breeding for Sustainable Food Production and Climate Resilience; Penna, S., Jain, S.M., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Le, K.C.; Ho, T.T.; Paek, K.Y.; Park, S.Y. Low dose gamma radiation increases the biomass and ginsenoside content of callus and adventitious root cultures of wild ginseng (Panax ginseng Mayer). Ind. Crops Prod. 2019, 130, 6–24. [Google Scholar] [CrossRef]
- El-Fiki, A.; Fahmy, E.; Doma, A.A.; Helmy, O.; Adly, M.; El-Metabteb, G. The genetic variation assessment of in vitro irradiated tomato (Lycopersicon esculentum Mill) by SCoT and ISSR markers. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 557–565. [Google Scholar] [CrossRef]
- Sharma, V.; Thakur, M. Gamma irradiations induced morphological and biochemical variations in in vitro regenerated ginger (Zingiber officinale Rosc.)-an invaluable medicinal spice. Int. J. Radiat Biol. 2021, 97, 1696–1704. [Google Scholar] [CrossRef]
- Riviello-Flores, M.D.L.L.; Cadena-Iñiguez, J.; Ruiz-Posadas, L.D.M.; Arévalo-Galarza, M.D.L.; Castillo-Juárez, I.; Soto Hernández, M.; Castillo-Martínez, C.R. Use of gamma radiation for the genetic improvement of underutilized plant varieties. Plants. 2022, 11, 1161. [Google Scholar] [CrossRef]
- Abhang, S.; Sowjanya, P.R.; Singh, N.P.; Kadam, A.R.; Shinde, A.S.; Sangnure, V.R.; Mandave, A. Efficiency of RAPD, ISSR and SSR markers in assessing clonal fidelity of in vitro propagated punica granatum plantlets of cultivar Bhagwa. S. Afr. J. Bot. 2025, 179, 216–223. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; Rabaiolli, S.M.D.S.; Stefanel, C.M. Determining the Polymorphism Information Content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System; Applied Biostatistics, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Pritchardm, J.K.; Stephens, M.; Donelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Princhard, J. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Gourdet, J. Detecting the numer of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Bouzroud, S.; El_Maaiden, E.; Sobeh, M.; Devkota, K.P.; Boukcim, H.; Kouisni, L.; El-Kharrassi, Y. Micropropagation of Opuntia and other cacti species through axillary shoot proliferation: A Comprehensive Review. Front. Plant Sci. 2022, 13, 926653. [Google Scholar] [CrossRef]
- Ángeles-Espino, A.; Valencia-Botín, A.J.; Virgen-Calleros, G.; Ramírez-Serrano, C.; Paredes-Gutiérrez, L.; Hurtado-De la Peña, S. Determinación de la dosis letal (DL50) con Co60 en vitroplántulas de Agave tequilana var. Azul. Rev. Fitotec. Mex. 2013, 36, 381–386. [Google Scholar] [CrossRef]
- Spencer, L.M.M.; Forster, B.P.; Jankuloski, L. Manual de Mejoramiento Por Mutación, 3rd ed.; Food and Agriculture Organization of the United National (FAO): Roma, Italy, 2021. [Google Scholar] [CrossRef]
- Rubio-Ochoa, E.; De la Cruz-Torres, E.; Olalde-Portugal, V.; Pérez-Sánchez, R.E.; Gómez-Leyva, J.F.; García-Saucedo, P.A. Mutagénesis por radiación gamma para mejora genética de plantas de importancia alimentaria. Rev. Mex. Cienc. Agríc. 2024, 15, e-3747. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2015, 30, 1–16. [Google Scholar] [CrossRef]
- Penna, S.; Bhagwat, S.G. Mutagenesis and Selection: Reflections on the In Vivo and In Vitro Approaches for Mutant Development; Springer Nature: Singapore, 2023; pp. 99–127. [Google Scholar]
- Oliveira, N.M.; de Medeiros, A.D.; de Lima Nogueira, M.; Arthur, V.; de Araújo Mastrangelo, T.; da Silva, C.B. Hormetic effects of low-dose gamma rays in soybean seeds and seedlings: A detection technique using optical sensors. Comput. Electron. Agric. 2021, 187, 106251. [Google Scholar] [CrossRef]
- Saloua, K.S. Opuntia ficus indica cladode extract inhibit DNA double-strand breaks and locally multiply damaged sites induced by gamma radiation. J. Genet. Eng. Biotechnol. 2024, 22, 100425. [Google Scholar] [CrossRef]
- Hasim, A.A.; Shamsiah, A.; Hussein, S. Induced mutations using gamma ray and multiplication of plantlet through micro cross section culture of banana (Musa acuminata cv. Berangan). IOP Conf. Ser. Earth Environ. Sci. 2021, 757, 012007. [Google Scholar] [CrossRef]
- Serrano-Fuentes, M.K.; Gómez-Merino, F.C.; Cruz-Izquierdo, S.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Gamma radiation (60Co) induces mutation during in vitro multiplication of vanilla (Vanilla planifolia Jacks. ex Andrews). Horticulturae 2022, 8, 503. [Google Scholar] [CrossRef]
- Volkova, P.Y.; Bondarenko, E.V.; Kazakova, E.A. Radiation hormesis in plants. Curr. Opin. Toxicol. 2022, 30, 100334. [Google Scholar] [CrossRef]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.S.; Reyes-Agüero, A.; Guéraud, F.; de La Rosa, A.P.B. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Adli, B.; Boutekrabt, A.; Touati, M.; Bakria, T.; Touati, A.; Bezini, E. Phenotypic diversity of Opuntia ficus indica (L.) MILL. in the Algerian steppe. S. Afr. J. Bot. 2017, 109, 66–74. [Google Scholar] [CrossRef]
- Amani, E.; Marwa, L.; Hichem, B.S.; Amel, S.H.; Ghada, B. Morphological variability of prickly pear cultivars (Opuntia spp.) established in ex-situ collection in Tunisia. Sci. Hortic. 2019, 248, 163–175. [Google Scholar] [CrossRef]
- Dev, R.; Mangalassery, S.; Dayal, D.; Louhaichi, M.; Hassan, S. Genetic variability, characters association and principal component study for morphological and fodder quality of Opuntia and Nopalea sp. in India. Genet. Resour. Crop Evol. 2024, 71, 2297–2310. [Google Scholar] [CrossRef]
- Pestanana, R.K.N.; Amorim, E.P.; Ferreira, C.F.; de Oliveira Amorim, V.B.; Oliveira, L.S.; da Silva Ledo, C.A.; de Oliveira e Silva, S. Agronomic and molecular characterization of gamma ray induced banana (Musa sp.) mutants using a multivariate statistical algorithm. Euphytica 2011, 178, 151–158. [Google Scholar] [CrossRef]
- Mangi, N.; Baloch, A.W.; Khaskheli, N.K.; Ali, M.; Afzal, W. Multivariate analysis for evaluation of mutant bread wheat lines using metric traits. Integr. Plant Sci. 2021, 1, 29–34. [Google Scholar][Green Version]
- Zulfiqar, S.; Ishfaq, S.; Ikram, M.; Nawaz, M.A.; Rahman, M.U. Characterization of gamma-rays-induced spring wheat mutants for morphological and quality traits through multivariate and GT Bi-plot analysis. Agronomy 2021, 11, 2288. [Google Scholar] [CrossRef]
- Valadez-Moctezuma, E.; Samah, S.; Luna-Paez, A. Genetic diversity of Opuntia spp. varieties assessed by classical marker tools (RAPD and ISSR). Plant Syst. Evol. 2015, 301, 737–747. [Google Scholar] [CrossRef]
- Due, M.S.; Susilowati, A.R.I.; Yunus, A. The effect of gamma rays irradiation on diversity of Musa paradisiaca var sapientum as revealed by ISSR molecular marker. Biodiversitas 2019, 20, 1416–1422. [Google Scholar] [CrossRef]
- Alekseeva, M.; Rusanova, M.; Rusanov, K.; Atanassov, I. Aset of highlypolymorphic microsatellite markers for genetic diversity studies in the genus. Origanum. Plants 2023, 12, 824. [Google Scholar] [CrossRef]
- Porras-Hurtado, L.; Ruiz, Y.; Santos, C.; Phillips, C.; Carracedo, Á.; Lareu, M.V. An overview of STRUCTURE: Applications, parameter settings, and supporting software. Front. Genet. 2013, 4, 98. [Google Scholar] [CrossRef]

| Primer Sequence (5′-3′) | AT * (°C) | TB | PB | BP% | MSR (bp) | PIC | MI |
|---|---|---|---|---|---|---|---|
| (GA)8YC | 46.7 | 13 | 11 | 84.6 | 400–2600 | 0.48 | 4.23 |
| (GA)8YT | 47.4 | 14 | 11 | 78.5 | 500–2250 | 0.47 | 4.07 |
| T(CT)7CC | 48.1 | 10 | 4 | 40.0 | 400–5000 | 0.11 | 0.18 |
| (CT)8RG | 48.1 | 9 | 1 | 11.1 | 300–4000 | 0.01 | 0.00 |
| (GA)8YG | 48.8 | 6 | 4 | 66.6 | 800–3500 | 0.32 | 0.48 |
| (GA)8C | 52.0 | 5 | 2 | 40 | 250–3000 | 0.08 | 0.14 |
| Average | - | 9.6 | 5.5 | 54.5 | - | 0.25 | 1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Ochoa, E.; Cruz-Torres, E.D.l.; Pérez-Sánchez, R.E.; Martínez-Flores, H.E.; Portillo, L.; García-Saucedo, P.A.; Gómez-Leyva, J.F. Characterization of a Gamma Radiation (60Co) Induced Mutant Population of Prickly Pear Cactus (Opuntia velutina F.A.C. Weber) Plants In Vitro Using ISSR Molecular Markers. Horticulturae 2025, 11, 743. https://doi.org/10.3390/horticulturae11070743
Rubio-Ochoa E, Cruz-Torres EDl, Pérez-Sánchez RE, Martínez-Flores HE, Portillo L, García-Saucedo PA, Gómez-Leyva JF. Characterization of a Gamma Radiation (60Co) Induced Mutant Population of Prickly Pear Cactus (Opuntia velutina F.A.C. Weber) Plants In Vitro Using ISSR Molecular Markers. Horticulturae. 2025; 11(7):743. https://doi.org/10.3390/horticulturae11070743
Chicago/Turabian StyleRubio-Ochoa, Eréndira, Eulogio De la Cruz-Torres, Rosa Elena Pérez-Sánchez, Héctor Eduardo Martínez-Flores, Liberato Portillo, Pedro Antonio García-Saucedo, and Juan Florencio Gómez-Leyva. 2025. "Characterization of a Gamma Radiation (60Co) Induced Mutant Population of Prickly Pear Cactus (Opuntia velutina F.A.C. Weber) Plants In Vitro Using ISSR Molecular Markers" Horticulturae 11, no. 7: 743. https://doi.org/10.3390/horticulturae11070743
APA StyleRubio-Ochoa, E., Cruz-Torres, E. D. l., Pérez-Sánchez, R. E., Martínez-Flores, H. E., Portillo, L., García-Saucedo, P. A., & Gómez-Leyva, J. F. (2025). Characterization of a Gamma Radiation (60Co) Induced Mutant Population of Prickly Pear Cactus (Opuntia velutina F.A.C. Weber) Plants In Vitro Using ISSR Molecular Markers. Horticulturae, 11(7), 743. https://doi.org/10.3390/horticulturae11070743






