Collection, Evaluation, and New Cultivar Breeding of Actinidia chinensis var. chinensis in Wudang Mountains, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Wild A. chinensis var. chinensis
2.2. Propagation of Excellent Selections
2.3. Investigation of Growth Habits and Phenological Aspects
2.4. Fruit Size and Quality
2.5. Data Processing and Analysis
3. Results
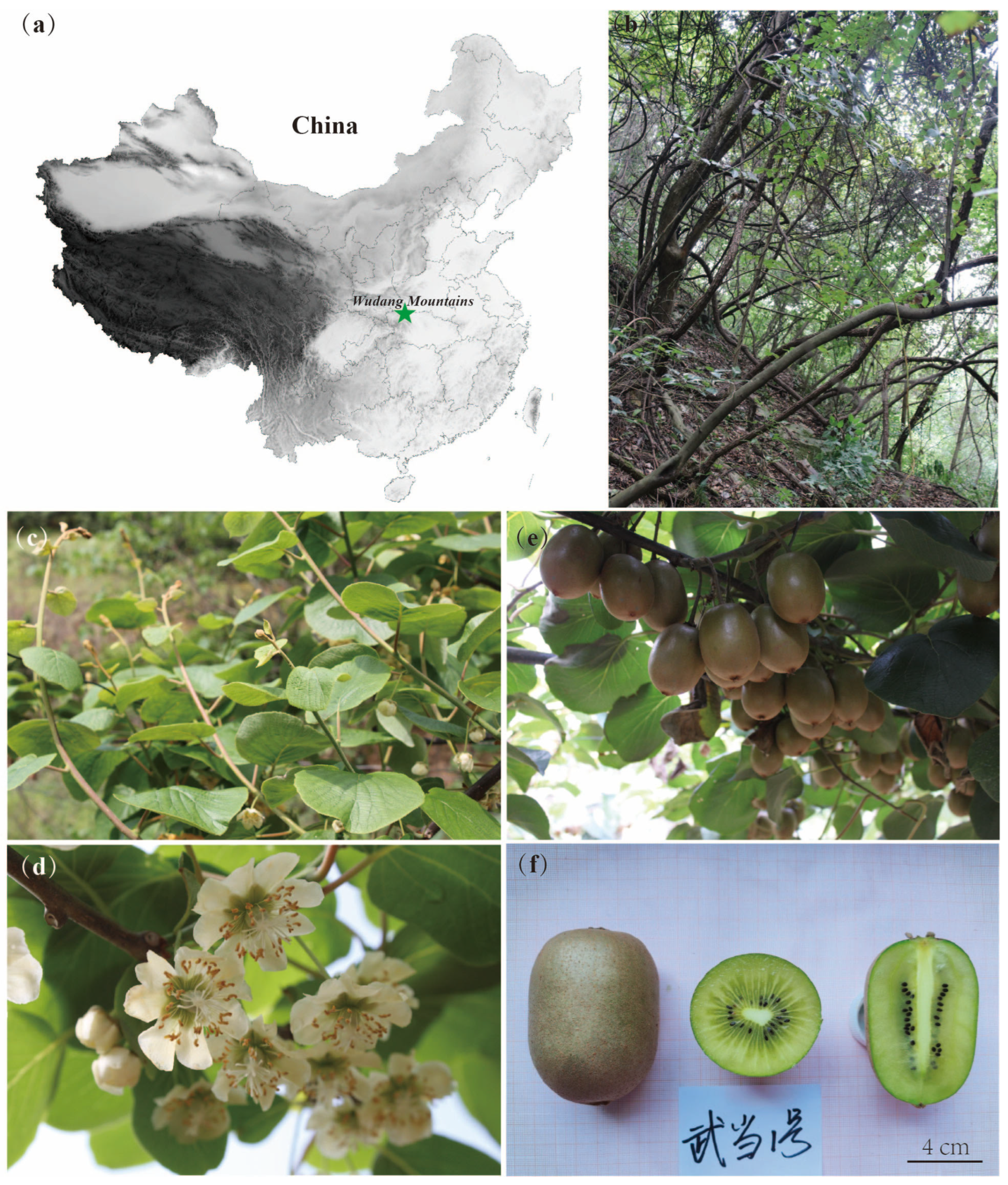
3.1. Collection and Selection of Wild Resources
3.2. The Growth Habits and Phenological Aspect of ‘Wudang 1’ Kiwifruit
3.3. Pomological and Quality Aspects of ‘Wudang 1’ Kiwifruit
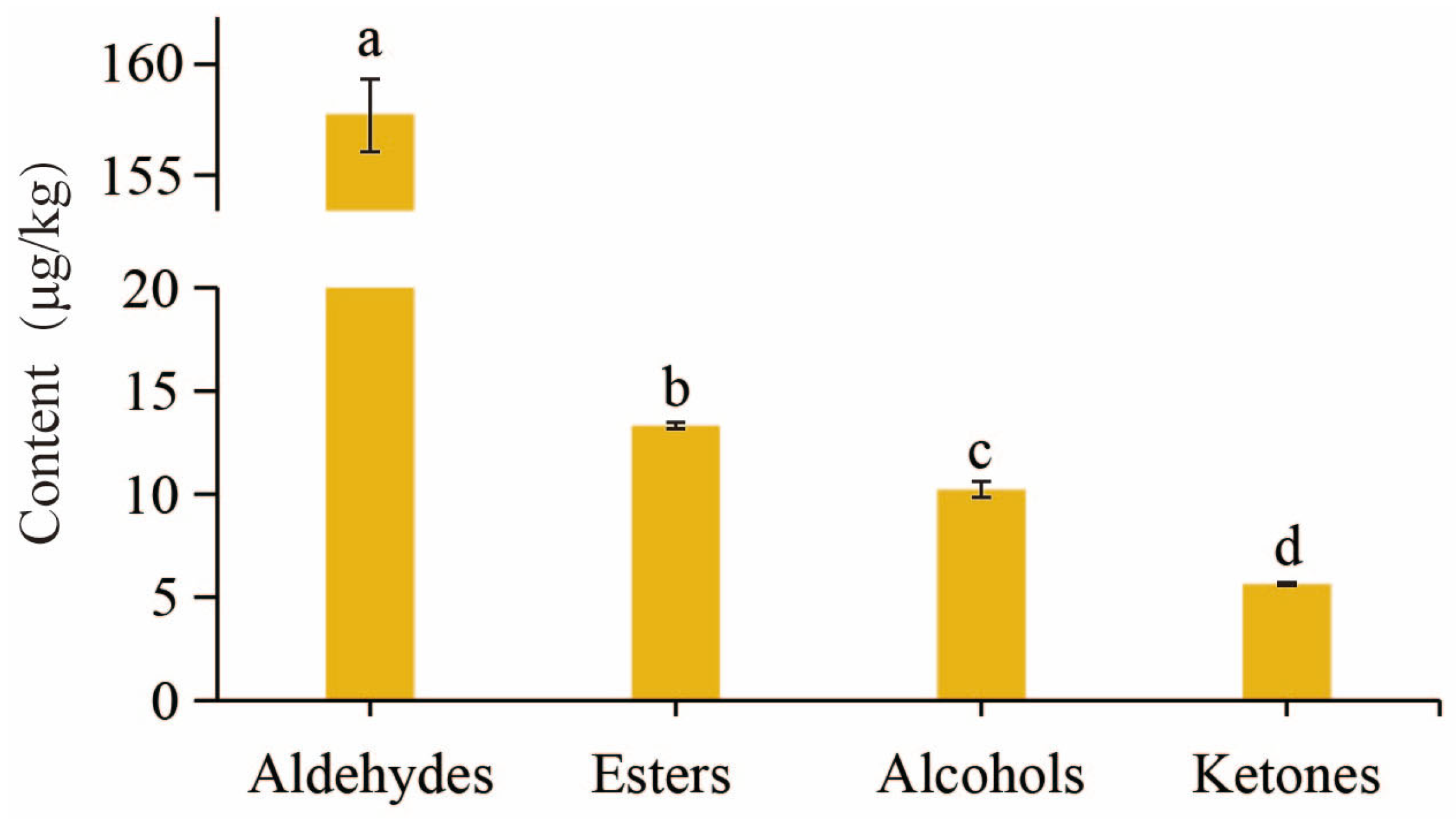
3.4. The Aroma Substances of ‘Wudang 1’ Kiwifruit
3.5. Brief Introduction to Cultivation Techniques of ‘Wudang 1’ Kiwfruit
3.5.1. Soil Selection
3.5.2. Vine Trellis and Pruning
3.5.3. Thinning of Flowers and Fruits
3.5.4. Management of Fertilization and Irrigation
3.5.5. Harvesting and Yield
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H. Genetic resources. In The Kiwifruit Genome; Testolin, R., Huang, H., Ferguson, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 15–36. [Google Scholar]
- Huang, H. KIWIFRUIT: The Genus Actinidia; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2016; pp. 45–167. [Google Scholar]
- Sun, L.; Fang, J. Conservation, Research and utilization of kiwifruit germplasm resources in China. J. Plant Genet. Resour. 2020, 21, 1483–1493. (In Chinese) [Google Scholar] [CrossRef]
- Hu, G.; Li, C.; Yang, B.; Wang, Z.; Shen, S.; Li, Z.; Zhong, C. Analysis and comprehensive evaluation of fruit trait diversity of 72 Actinidia chinensis accessions in Yichang. J. Fruit Sci. 2022, 39, 1540–1552. (In Chinese) [Google Scholar] [CrossRef]
- Deng, X.; Wang, L.; Li, S.; Zhang, S.; Zhang, Z.; Cong, P.; Yi, G.; Chen, X.; Chen, H.; Zhong, C. Retrospection and prospect of fruit breeding for last four decades in China. J. Fruit Sci. 2019, 36, 514–520. (In Chinese) [Google Scholar] [CrossRef]
- Zhong, C.; Huang, W.; Wang, Z.; Li, L.; Li, D.; Zhang, Q.; Zhao, T.; Zhang, P. The breeding progress and development status of the kiwifruit industry in China. Acta Hortic. 2022, 1332, 445–454. [Google Scholar] [CrossRef]
- Richardson, A.; Burdon, J.; Ferguson, R. Kiwifruit Botany, Production and Uses; CAB International: Wallingford, CT, USA, 2023; pp. 2–7. [Google Scholar]
- Huang, H. Actinidia Germplasm Resources in China; China Forestry Publishing House: Beijing, China, 2013; pp. 77–112. [Google Scholar]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef]
- Labra, A.; Zoffoli, J. Response of chlorophyllase and magnesium dechelatase enzymes in yellow- and green-fleshed kiwifruit to degreening at different temperatures. Agronomy 2024, 14, 2481. [Google Scholar] [CrossRef]
- Karp, D.; Gasic, K. Register of new fruit and nut cultivars list 51. HortScience 2022, 57, 1174–1233. [Google Scholar] [CrossRef]
- Zhuang, Q.; Manning, M.; Li, M.; Cheng, C.; He, L.; Wang, L. Survey of wild Actinidia species in the Jinggang mountains of China and identification of associated bacteria. Acta Hortic. 2018, 1218, 287–292. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, H.; Guo, Y.; Jin, P.; Zhou, Q. Geographical distribution and utilization of Actinidia in China: Focusing on archaeological remains. J. Chin. Hist. Geogr. 2025, 40, 28–40+97. (In Chinese) [Google Scholar]
- Jiang, C.; Wu, Y.; Deng, L.; Cai, G.; Bao, C.; Li, K.; Wang, L.; Liu, H. Kiwifruit resources collection in Yunnan Province and phenotypic diversity analysis. J. Southw. For. Univ. (Nat. Sci.) 2021, 41, 38–45. (In Chinese) [Google Scholar]
- Chen, L.; Liu, Y.; Gao, H.; Cao, J.; Qian, J.; Zheng, K.; Jia, D.; Gao, Z.; Xu, X. A model for selecting kiwifruit (Actinidia eriantha) germplasm resources with excellent fruit quality. Foods 2024, 13, 4014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Z.; Liao, G.; Zhong, M.; Huang, C.; Qu, X.; Xu, X. Genetic diversity of wild Actinidia eriantha resources based on SSR markers in Magu Mountain Nature Reserve of China. Eur. J. Hortic. Sci. 2023, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, N.; Wang, X.; Zhang, J.; Wang, X. Comprehensive evaluation of wild Actinidia chinensis resources in Funiu Mountain based on quantitative traits. Acta Agric. Zhejiang 2023, 35, 2354–2363. (In Chinese) [Google Scholar]
- Li, Z.; Man, Y.; Lan, X.; Lan, X.; Wang, Y. Ploidy and phenotype variation of a natural Actnidia arguta population in the east of Daba Mountain located in a region of Shaanxi. Sci. Hortic. 2013, 161, 259–265. [Google Scholar] [CrossRef]
- Hu, G.; Jiang, Q.; Wang, Z.; Li, Z.; Liao, W.; Shen, D.; Zhong, C. Genetic diversity analysis and core collection construction of the Actinidia chinensis complex (kiwifruit) based on SSR markers. Agronomy 2022, 12, 3078. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, C.; Liu, Y.; Zhang, Q.; Sun, X.; Li, D. Agronomic trait variations and ploidy differentiation of kiwiberries in Northwest China: Implication for breeding. Front. Plant Sci. 2017, 8, 711. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, L.; Li, Z. Genetic differentiation of Actinidia chinensis and analysis of gene flow barriers in the Qinling Mountains, the species’ northern distribution boundary. Genet. Resour. Crop Evol. 2018, 65, 881–895. [Google Scholar] [CrossRef]
- Yang, G.; Chen, H.; Zhao, Z.; Yi, T.; Chen, J. Resources and utilization of Taoist Medicinal Plants distributed in Wudang Mountain. J. Chin. Pharm. Sci. 2014, 23, 412–420. [Google Scholar] [CrossRef]
- Wudang Mountains Administrative Committee: Wudang Mountains Overview. Available online: http://www.wudangshan.gov.cn/yxwd_0/wdsgk/202312/t20231201_4358469.shtml (accessed on 4 June 2025).
- Burdon, J.; Pidakala, P.; Martin, P.; Boldingh, H.; Hall, A.; Schaffer, R. Postharvest performance of the yellow-fleshed ’Hort16A’ kiwifruit in relation to fruit maturation. Postharvest Biol. Technol. 2014, 92, 98–106. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, W.; Zhao, Y. Experiment Guidance of Postharvest Physiology and Biochemistry of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007; p. 60. [Google Scholar]
- Ordóñez-santos, L.; Martínez-girón, J.; Arias-jaramillo, M. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017, 233, 96–100. [Google Scholar] [CrossRef]
- GB 5009.124-2016; Determination of Amino Acids in Food Safety National Standards. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- Mota, L.; Aguiar, A.; Ferreira, I.; Pinho, P. Volatile profiling of kiwifruits (Actinidia deliciosa ‘Hayward’) evaluated by HS-SPME and GC-IT/MS: Influence of ripening, training system and storage. Food Bioprocess Technol. 2012, 5, 3115–3128. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, C.; Li, D.; Yan, C.; Yao, X.; Li, Z. Cytotype distribution and chloroplast phylogeography of the Actinidia chinensis complex. BMC Plant Biol. 2021, 21, 325. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, Y. Natural hybridization, introgression breeding, and cultivar improvement in the genus Actinidia. Tree Genet. Genomes 2014, 10, 1113–1122. [Google Scholar] [CrossRef]
- Wu, W.; Peng, J.; Pan, L.; Zhu, X.; Liu, T.; Xiao, L.; Wang, H.; Cheng, J.; Xiao, T. A new kiwifruit cultivar Hanmei. J. Fruit Sci. 2021, 38, 1211–1213. (In Chinese) [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Meng, A. Selection of a new red-fleshed kiwifruit cultivar ‘Hongyang’. Acta Hortic. 2003, 610, 115–117. [Google Scholar] [CrossRef]
- Hu, G.; Xia, W.; Zheng, L.; Rao, H.; Lei, M.; Wang, J.; Zhao, T.; Li, Z.; Zhong, C. Investigation and fruit genetic diversity analysis of wild Actinidia germplasm resources in Tongshan County, Hubei Province. Plant Sci. J. 2021, 39, 620–631. (In Chinese) [Google Scholar] [CrossRef]
- Huang, H.; Wang, S.; Huang, R.; Jiang, Z.; Zhang, Z. ‘Jintao’, a novel, hairless, yellow-fleshed kiwifruit. HortScience 2002, 37, 1135–1136. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, S.; Huang, R.; Zhang, Z.; Huang, H. ‘Wuzhi No 3’ kiwifruit. HortScience 2005, 40, 1923–1924. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Z.; Jiang, Z.; Cheng, Z.; Chen, X.; Wang, Y.; Wang, S. A new male cultivar of Actinidia chinensis ‘Moshan 4’. Acta Hortic. Sin. 2006, 33, 1163. (In Chinese) [Google Scholar] [CrossRef]
- Han, F.; Liu, X.; Li, D.; Zhang, P.; Zhong, C. A new mid-flowering pollenizer cultivar of kiwifruit ‘Moshan Xiong 5’. J. Fruit Sci. 2017, 34, 386–389. (In Chinese) [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Liu, C.; Liao, L.; Liu, Y.; Zhang, J.; Zhong, C.; Li, Z. Full-scale genetic pattern and environmental association of Actinidia chinensis populations across ten mountain systems in China, and its significance for conservation. Tree Genet. Genomes 2023, 19, 52. [Google Scholar] [CrossRef]
- Ma, X.; Han, Z.; Li, T.; Yang, Y.; Su, W.; Li, W. Genetic diversity and population structure analysis of wild kiwifruit (Actinidia spp.) using SRAP markers. Biotechnol. Biotechnol. Equip. 2024, 38, 2373845. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Zhang, C.; Lyu, L.; Li, W. Breeding and growth performance of ‘Ningzhi 4’, a new blackberry cultivar with high yield potential and good quality in China. Plants 2023, 12, 1661. [Google Scholar] [CrossRef]
- Xu, W.; Wei, L.; Cheng, W.; Yi, X.; Lin, Y. Non-destructive assessment of soluble solids content in kiwifruit using hyperspectral imaging coupled with feature engineering. Front. Plant Sci. 2024, 15, 1292365. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Tang, W. Volatile components and their corresponding synthetic gene expression profile in the fruits of Actinidia chinensis. Chin. J. Appl. Environ. 2018, 24, 307–314. (In Chinese) [Google Scholar] [CrossRef]
- Cozzolino, R.; De Giulio, B.; Petriccione, M.; Martignetti, A.; Malorni, L.; Zampella, L.; Laurino, C.; Pellicano, M. Comparative analysis of volatile metabolites, quality and sensory attributes of Actinidia chinensis fruit. Food Chem. 2020, 316, 126340. [Google Scholar] [CrossRef]
- Lan, T.; Gao, C.; Yuan, Q.; Wang, J.; Zhang, H.; Sun, X.; Lei, Y.; Ma, T. Analysis of the aroma chemical composition of commonly planted kiwifruit cultivars in China. Foods 2021, 10, 1645. [Google Scholar] [CrossRef]
- Zhu, X.; Pan, L.; Peng, J.; Wu, W.; Xiao, T.; Ren, X. Study on aroma components of postharvests ‘Wudang 1’kiwifruit. North. Horticult. 2015, 39, 16–21. (In Chinese) [Google Scholar]
- Zheng, H.; Chen, S.; Yuan, X.; Wang, Y.; Dong, J.; Zhang, Q.; Zhong, C. Analysis of mineral elements content and aroma composition differences of 6 kiwifruit varieties from different regions. China Fruits 2024, 68–73+84. (In Chinese) [Google Scholar] [CrossRef]
- Xu, S.; Zhan, P.; Tian, H.; Wang, P. The presence of kiwifruit columella affects the aroma profiles of fresh and thermally treated kiwifruit juice. LWT-Food Sci. Technol. 2022, 165, 113756. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, P.; Liu, R.; He, W.; Gao, G.; Tian, H. Characterization of the formation of key flavor volatiles in kiwifruit (Actinidia deliciosa) during storage by integrating. Eur. Food Res. Technol. 2024, 250, 1017–1029. [Google Scholar] [CrossRef]
- Shikha, T.; Adinath, K.; Debabandya, M.; Kumar, T.; Hena, R.; Amitava, A.; Alokesh, G.; Bharat, M. Volatile organic compounds (VOCs): Biomarkers for quality management of horticultural commodities during storage through e-sensing. Trends Food Sci. Technol. 2020, 106, 417–433. [Google Scholar] [CrossRef]
- Chai, J.; Liao, B.; Li, J.; Liu, H.; Liu, Z. Pollen donor affects the taste and aroma compounds in ‘Cuixiang’ and ‘Xuxiang’ kiwifruit. Sci. Hortic. 2023, 314, 111945. [Google Scholar] [CrossRef]
- Yao, X.; Liu, L.; Yan, M.; Li, D.; Zhong, C.; Huang, H. Exon primed intron-crossing (EPIC) markers reveal natural hybridization and introgression in Actinidia (Actinidiaceae) with sympatric distribution. Biochem. Syst. Ecol. 2015, 59, 246–255. [Google Scholar] [CrossRef]
- Wang, F.; Mo, Q.; Ye, K.; Gong, H.; Qi, B.; Liu, P.; Jiang, Q.; Li, J. Evaluation of the wild Actinidia germplasm for resistance to Pseudomonas syringae pv. actinidiae. Plant Pathol. 2020, 69, 979–989. [Google Scholar] [CrossRef]
- Qi, B.; Wang, F.; Ye, K.; Mo, Q.; Gong, H.; Liu, P.; Jiang, Q.; Li, J. Genetic diversity of 52 species of kiwifruit (Actinidia chinensis Planch.). Horticulturae 2023, 9, 753. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, G.; Li, Z.; Zhong, C.; Yao, X. Characterizing tetraploid populations of Actinidia chinensis for kiwifruit genetic improvement. Plants 2022, 11, 1154. [Google Scholar] [CrossRef]
| Code | Geographic Origins | Key Indicators for Screening | |||
|---|---|---|---|---|---|
| Altitude/m | Longitude/°E | Latitude/°N | Single Fruit Weight/g | Soluble Solids Content/% | |
| WD-03-1 | 730 | 111.04 | 32.44 | 74.52 ± 1.76 a | 15.89 ± 0.57 ab |
| WD-03-2 | 766 | 111.06 | 32.45 | 66.50 ± 1.74 b | 15.03 ± 0.51 ab |
| WD-03-3 | 620 | 111.09 | 32.45 | 75.06 ± 1.65 a | 14.36 ± 0.28 b |
| WD-03-4 | 422 | 111.10 | 32.42 | 50.46 ± 1.18 d | 15.51 ± 0.32 ab |
| WD-03-5 | 331 | 111.08 | 32.38 | 72.00 ± 2.70 a | 15.60 ± 0.88 ab |
| WD-03-6 | 338 | 111.04 | 32.35 | 57.36 ± 1.17 c | 16.32 ± 0.75 a |
| WD-03-7 | 355 | 111.11 | 32.37 | 63.71 ± 1.72 b | 14.77 ± 0.19 ab |
| Cultivars | Bud Burst Period | Leaf Expansion Period | Flowering Period | Expansion Period | Maturity Period | Deciduous Period |
|---|---|---|---|---|---|---|
| Wudang 1 | 11/03–19/03 | 18/03–24/03 | 14/04–26/04 | Early May to early June | Early September | Early to mid December |
| Jinnong | 12/03–20/03 | 19/3–26/03 | 16/04–28/04 | Early May to early June | Late September | Mid to late December |
| Cuiyu | 26/03–6/04 | 5/04–12/04 | 25/04–7/05 | Mid May to mid June | Late October | Mid to late December |
| Cultivars | Bud Burst Rate/% | Shoot Rate/% | Fruit Shoot Rate/% | Fruit Set Rate/% |
|---|---|---|---|---|
| Wudang 1 | 70.00 ± 1.53 b | 93.00 ± 1.73 a | 94.67 ± 0.88 a | 79.67 ± 1.76 a |
| Jinnong | 72.00 ± 1.53 b | 90.00 ± 0.58 ab | 90.00 ± 1.00 b | 77.67 ± 1.33 a |
| Cuiyu | 80.33 ± 3.28 a | 89.00 ± 0.58 b | 94.00 ± 0.58 a | 75.67 ± 0.33 a |
| Indexes | Cultivars | ||
|---|---|---|---|
| Wudang 1 | Jinnong | Cuiyu | |
| Appearance quality | |||
| Single fruit mass/g | 83.22 ± 2.84 ab | 77.19 ± 1.56 b | 89.74 ± 1.82 a |
| Vertical diameter/cm | 62.65 ± 0.66 b | 58.77 ± 1.08 c | 66.40 ± 0.72 a |
| Horizontal diameter/cm | 46.77 ± 0.68 b | 49.06 ± 1.07 b | 53.87 ± 0.87 a |
| Lateral diameter/cm | 42.26 ± 0.64 b | 45.31 ± 0.45 a | 47.04 ± 0.73 a |
| Fruit shape index | 1.34 ± 0.03 a | 1.20 ± 0.02 b | 1.23 ± 0.03 b |
| Internal quality | |||
| Soluble solid content/% | 16.33 ± 0.38 a | 15.70 ± 0.31 a | 15.27 ± 0.37 a |
| Dry matter content/% | 15.28 ± 0.24 a | 14.14 ± 0.14 b | 14.82 ± 0.37 ab |
| Soluble sugar content/% | 12.10 ± 0.42 a | 11.27 ± 0.35 a | 12.50 ± 0.29 a |
| Titratable acidity/% | 1.24 ± 0.03 a | 1.36 ± 0.03 a | 1.26 ± 0.04 a |
| Vitamin C content/(mg/100 g) | 132.10 ± 3.45 a | 93.19 ± 1.43 b | 127.81 ± 1.39 a |
| Amino acid content/(mg/g) | 7.77 ± 0.43 a | 7.01 ± 0.17 a | 7.30 ± 0.38 a |
| Number | Aroma Components | Contents (μg/kg) | CAS Registry Number |
|---|---|---|---|
| 1 | Methyl benzoate | 2.45 ± 0.07 e | 93-58-3 |
| 2 | Hexyl 2-methylbutyrate | 1.49 ± 0.13 efghi | 10032-15-2 |
| 3 | Hexyl acetate | 1.41 ± 0.04 efghi | 142-92-7 |
| 4 | Ethyl acetate | 1.39 ± 0.06 efghi | 141-78-6 |
| 5 | Butyl Caprylate | 1.22 ± 0.06 efghi | 589-75-3 |
| 6 | (E)-11-Tetradecen-1-ol acetate | 0.89 ± 0.07 efghi | 33189-72-9 |
| 7 | Ethyl 4-trans-decenoate | 0.76 ± 0.03 fghi | 76649-16-6 |
| 8 | Ethyl butyrate | 0.52 ± 0.05 hi | 105-54-4 |
| 9 | 2-(2-butoxyethoxy)ethyl benzoate | 0.51 ± 0.06 hi | 5451-84-3 |
| 10 | Propyl octanoate | 0.46 ± 0.04 i | 624-13-5 |
| 11 | 2-Ethylhexyl acetate | 0.45 ± 0.02 i | 103-09-3 |
| 12 | Ethyl hexanoate | 0.44 ± 0.06 i | 123-66-0 |
| 13 | 2-Methylbutyl acetate | 0.35 ± 0.02 i | 624-41-9 |
| 14 | Hexyl hexanoate | 0.32 ± 0.11 i | 6378-65-0 |
| 15 | Ethyl 2-methylbutyrate | 0.28 ± 0.06 i | 7452-79-1 |
| 16 | Butyl acetate | 0.24 ± 0.04 i | 123-86-4 |
| 17 | Hexyl butyrate | 0.14 ± 0.05 i | 2639-63-6 |
| 18 | trans-2-Hexenal | 98.58 ± 2.35 a | 6728-26-3 |
| 19 | Hexanal | 37.80 ± 1.77 b | 1219803-74-3 |
| 20 | 1-Nonanal | 5.75 ± 0.23 d | 124-19-6 |
| 21 | (E)-2-Octenal | 2.37 ± 0.04 ef | 2548-87-0 |
| 22 | trans-2-Nonenal | 2.25 ± 0.09 efg | 18829-56-6 |
| 23 | (R)-(+)-2,2-Dimethyl-1,3-dioxolane-4-carboxaldehyde | 2.18 ± 0.04 efg | 15186-48-8 |
| 24 | Decanal | 2.10 ± 0.09 efgh | 112-31-2 |
| 25 | trans-2-Decenal | 1.37 ± 0.07 efghi | 3913-81-3 |
| 26 | Octanal | 1.11 ± 0.01 efghi | 124-13-0 |
| 27 | trans,cis-2,6-Nonadienal | 0.99 ± 0.04 efghi | 557-48-2 |
| 28 | 2-Heptenal, (Z)- | 0.95 ± 0.02 efghi | 57266-86-1 |
| 29 | 2-Undecenal | 0.54 ± 0.05 hi | 2463-77-6 |
| 30 | 2-Fluoro-4-(1-methylethyl)benzaldehyde | 0.50 ± 0.06 hi | 1289014-70-5 |
| 31 | Benzaldehyde | 0.46 ± 0.04 i | 100-52-7 |
| 32 | 3-Hexenal | 0.39 ± 0.10 i | 4440-65-7 |
| 33 | Citral | 0.31 ± 0.01 i | 5392-40-5 |
| 34 | Geranylacetone | 1.46 ± 0.02 efghi | 3796-70-1 |
| 35 | 6-Methyl-5-hepten-2-one | 1.05 ± 0.04 efghi | 110-93-0 |
| 36 | 1-Penten-3-one | 0.83 ± 0.02 fghi | 1629-58-9 |
| 37 | 1-Octen-3-one | 0.77 ± 0.03 fghi | 4312-99-6 |
| 38 | alpha-Ionone | 0.68 ± 0.03 fghi | 127-41-3 |
| 39 | 2-Undecanone | 0.52 ± 0.05 hi | 112-12-9 |
| 40 | 3-Undecanone | 0.34 ± 0.02 i | 2216-87-7 |
| 41 | trans-2-Hexen-1-ol | 10.23 ± 0.16 c | 928-95-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, T.; Jia, T.; Wu, W.; Peng, J.; Pan, L.; Zhu, X.; Liu, T.; Cheng, J.; Wang, H.; Xiao, L.; et al. Collection, Evaluation, and New Cultivar Breeding of Actinidia chinensis var. chinensis in Wudang Mountains, China. Horticulturae 2025, 11, 739. https://doi.org/10.3390/horticulturae11070739
Xiao T, Jia T, Wu W, Peng J, Pan L, Zhu X, Liu T, Cheng J, Wang H, Xiao L, et al. Collection, Evaluation, and New Cultivar Breeding of Actinidia chinensis var. chinensis in Wudang Mountains, China. Horticulturae. 2025; 11(7):739. https://doi.org/10.3390/horticulturae11070739
Chicago/Turabian StyleXiao, Tao, Tianjiao Jia, Wei Wu, Jiaqing Peng, Liang Pan, Xianbo Zhu, Tao Liu, Junhuan Cheng, Hualing Wang, Lili Xiao, and et al. 2025. "Collection, Evaluation, and New Cultivar Breeding of Actinidia chinensis var. chinensis in Wudang Mountains, China" Horticulturae 11, no. 7: 739. https://doi.org/10.3390/horticulturae11070739
APA StyleXiao, T., Jia, T., Wu, W., Peng, J., Pan, L., Zhu, X., Liu, T., Cheng, J., Wang, H., Xiao, L., Huang, H., Hu, G., & Zou, S. (2025). Collection, Evaluation, and New Cultivar Breeding of Actinidia chinensis var. chinensis in Wudang Mountains, China. Horticulturae, 11(7), 739. https://doi.org/10.3390/horticulturae11070739






