Establishment of an Efficient System for Rhizome Proliferation and In Vitro Flowering Induction from Protocorm Explants in Cymbidium goeringii
Abstract
1. Introduction
2. Materials and Methods
2.1. Capsule (Seed) Materials
2.2. Capsule Disinfection
2.3. Seed Pretreatment
2.4. Protocorm Induction
2.5. Rhizome Proliferation
2.6. Rhizome Differentiation
2.7. In Vitro Flowering Induction from Rhizomes
2.8. Data Analysis
3. Results
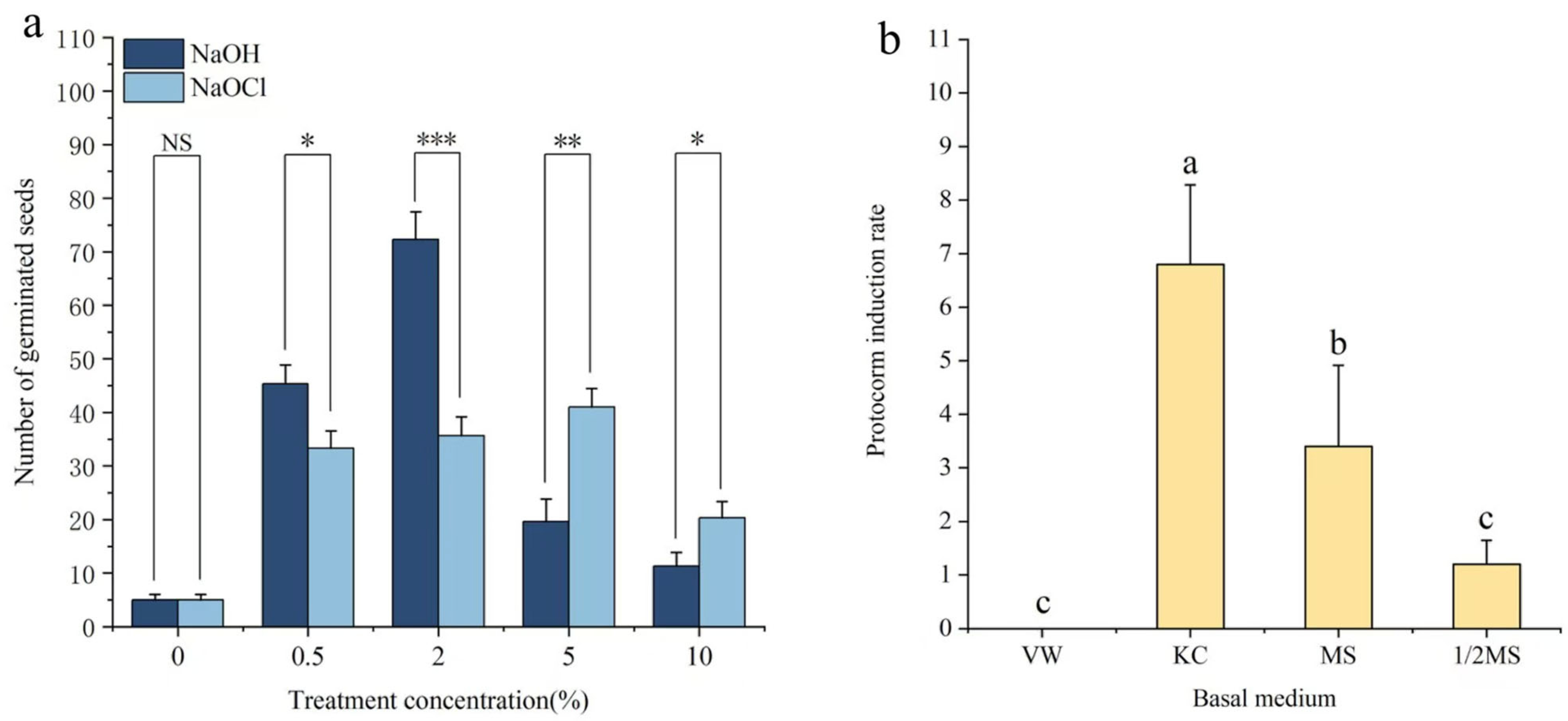
3.1. Effect of Pretreatment on Seed Germination
3.2. Protocorm Induction
3.3. Proliferation of Rhizomes
3.4. Rhizome Differentiation
3.5. In Vitro Flowering from Rhizomes
4. Discussion
4.1. Seed Germination and Protocorm Induction
4.2. Rhizome Proliferation and Differentiation
4.3. In Vitro Flowering Induction from Protocorm-Derived Rhizomes
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.J.; Wang, X.Q. The four gentlemen series: The aesthetic value and selection criteria of Cymbidium goeringii. Zhejiang Landsc. Archit. 2020, 3, 94–96. [Google Scholar]
- Sun, Y.F. In Vitro Germination Using Hybrid Seeds and the Establishment of Its Regeneration System. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2013. [Google Scholar]
- Bu, C.Y.; He, J.Z.; Huang, C.Y.; Ceng, Y.H. A new Cymbidium cultivar ‘sweet princess’. Acta Hortic. Sin. 2019, 46, 2876–2877. [Google Scholar]
- Zhou, H.M.; Mo, Z.L.; Chen, C.M.; Lin, H.F. A new Cymbidium cultivar ‘Shayang Cuidie’. Acta Hortic. Sin. 2022, 49, 2767–2768. [Google Scholar]
- Xu, H.Y.; Yang, M.C.; Zhou, Q.W. An information on orchid industry of Japan. J. Guangxi Agric. Univ. 1996, 15, 180–182. [Google Scholar]
- Zhao, K.K.; Wang, Y.Z.; Du, J.K.; Sun, C.B. A new Cymbidium Cultivar ‘Flying Fairy’. Acta Hortic. Sin. 2024, 51, 173–174. [Google Scholar]
- Zhang, X.Y.; Zhao, F.K.; Mei, H.; Fu, Q.J. A new Cultivar of Cymbidium ‘Xi Zi Niao Niao’. Acta Hortic. Sin. 2024, 51, 167–168. [Google Scholar]
- Zhao, F.K.; Zhang, X.Y.; Mei, H.; Fu, Q.J. A new Cymbidium Cultivar ‘Xian Mei Ren’ by distant hybrid. Acta Hortic. Sin. 2024, 51, 177–178. [Google Scholar]
- Zhang, X.Y.; Zhao, F.K.; Mei, H.; Fu, Q.J. A new cultivar of Cymbidium ‘Cai Hong He’. Acta Hortic. Sin. 2024, 51, 163–164. [Google Scholar]
- He, Y.H.; Hu, F.R.; Sun, C.B.; Chen, Y.; Wang, Y.Z. A new Cymbidium Cultivar ‘Huangyi’. Acta Hortic. Sin. 2022, 49, 125–126. [Google Scholar]
- Teixeira da Silva, J.A.; Zeng, S.; Cardoso, J.C.; Dobránszki, J.; Kerbauy, G.B. In vitro flowering of Dendrobium. Plant Cell Tissue Organ Cult. 2014, 119, 447–456. [Google Scholar] [CrossRef]
- Zeng, W.J. Studies on Tissue Culture of Several Chinese Cymbidiums. Master’s Thesis, Beijing Forestry University, Beijing, China, 2019. [Google Scholar]
- Wang, F.; Xu, B.Q.; Liu, X.J.; Xia, G.H.; Cui, Y.Y. A symbiotic seed germination of Cymbidium goeringii. J. Zhejiang AF Univ. 2013, 30, 136–140. [Google Scholar]
- Huang, L.; He, X.R.; Zheng, L.M.; Cai, J. Seed germination of Cymbidium goeringii in asymbiotic culture. Seed 2003, 6, 40–41. [Google Scholar]
- Zhong, Y.W. Study on seeding sterilizing technology of orchid. Anhui Agric. Sci. Bull. 2007, 13, 87–88. [Google Scholar]
- Shi, F.J.; Mo, Z.Z.; Wei, J.P.; Yang, X.F.; Tian, Y.J.; Tan, G.B. Study on aseptic sowing and rhizome proliferation of Cymbidium sinense. J. Anhui Agric. Sci. 2008, 36, 13968–13969. [Google Scholar]
- Paul, M.; Islam, T.; Sarker, R.H.; Hoque, M.I. In vitro mass propagation of Cymbidium aloifolium (L.) Sw. Plant Tissue Cult. Biotechnol. 2019, 29, 73–79. [Google Scholar] [CrossRef]
- Wang, S.Q. Study on In Vitro Culture and Seed Germination of Cymbidium ensifolium. Master’s Thesis, Shaanxi University of Technology, Hanzhong, China, 2020. [Google Scholar]
- Sun, C.B.; Liu, M.; Shi, J.S.; Guo, F.Q.; Li, X. Aseptic germination of Cymbidium faberi seeds and in vitro plant regeneration. Acta Agric. Zhejiangensis 2008, 20, 231–235. [Google Scholar]
- Zhang, D.X.; Li, C.X.; Wang, Z.X.; Pan, Y.P.; Wang, F.Y.; Zhang, C.Q. Study on in vitro seed germination of Cymbidium faberi seeds and rapid proliferation techniques. Chin. Agric. Sci. Bull. 2009, 25, 159–164. [Google Scholar]
- Mohanty, P.; Paul, S.; Das, M.C.; Kumaria, S.; Tandon, P. A simple and efficient protocol for the mass propagation of Cymbidium mastersii: An ornamental orchid of northeast India. AoB Plants 2012, 2012, pls023. [Google Scholar] [CrossRef]
- Islam, T.; Bhattacharjee, B.; Islam, S.M.S.; Uddain, J.; Subramaniam, S. Axenic seed culture and in vitro mass propagation of malaysian wild orchid Cymbidium finlaysonianum lindl. Pak. J. Bot. 2016, 47, 2361–2367. [Google Scholar]
- Hossain, M.M.; Sharma, M.; Teixeira da Silva, J.A.; Pathak, P. Seed germination and tissue culture of Cymbidium giganteum wall. Ex lindl. Sci. Hortic. 2010, 123, 479–487. [Google Scholar] [CrossRef]
- Nayak, N.R.; Chand, P.K.; Rath, S.P.; Patnaik, S.N. Influence of some plant growth regulators on the growth and organogenesis of Cymbidium aloifolium (L.) Sw. Seed-derived rhizomes in vitro. In Vitro Cell. Dev. Biol. Plant 1998, 34, 185–188. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Liu, Y. In vitro plant regeneration from the immature seeds of Cymbidium faberi. Plant Cell Tissue Organ Cult. 2005, 81, 247–251. [Google Scholar] [CrossRef]
- Chiang, H.F.; Lin, J.R.; Kao, C.Y.; Liu, K.S.; Chu, Y. Rapid mass propagation through multiple shoot induction from rhizome of Chinese Cymbidiums. Acta Hortic. 2010, 878, 213–217. [Google Scholar] [CrossRef]
- Peng, M.; Chen, R.; Wei, Q. Effects of genotype, light, and plant growth regulators on rhizome browning, proliferation, and sprouting in Cymbidium. HortScience 2023, 58, 671–676. [Google Scholar] [CrossRef]
- Shimasaki, K.; Uemoto, S. Micropropagation of a terrestrial Cymbidium species using rhizomes developed from seeds and pseudobulbs. Plant Cell Tissue Organ Cult. 1990, 22, 237–244. [Google Scholar] [CrossRef]
- Islam, S.M.S.; Islam, T.; Bhattacharjee, B.; Mondal, T.K.; Subramaniam, S. In vitro pseudobulb based micropropagation for mass development of Cymbidium finlaysonianum Lindl. Emir. J. Food Agric. 2015, 27, 469–474. [Google Scholar] [CrossRef]
- Duan, W.W.; Jiang, Z.F.; Wu, F.G.; Chu, Y.J.; Chen, Y.J. Study on inhibition of tissue-browning in tissue culture of Phalaenopsis amabilis. Mod. Agric. Sci. Technol. 2017, 137–138, 140. [Google Scholar]
- Liu, X.J.; Xu, W.W.; Cui, Y.H.; Cui, Y.Y. Study on tissue culture and rapid propagation of Cymbidium goeringii. North. Hortic. 2013, 20, 101–104. [Google Scholar]
- Lee, O.R.; Yang, D.C.; Chung, H.J.; Min, B.H. Efficient in vitro plant regeneration from hybrid rhizomes of Cymbidium sinense seeds. Hortic. Environ. Biotechnol. 2011, 52, 303–308. [Google Scholar] [CrossRef]
- Park, H.Y.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In vitro propagation of Cymbidium goeringii Reichenbach fil. through direct adventitious shoot regeneration. Physiol. Mol. Biol. Plants 2018, 24, 307–313. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A. Response of hybrid Cymbidium (Orchidaceae) protocorm-like bodies to 26 plant growth regulators. Bot. Lith. 2014, 20, 3–13. [Google Scholar] [CrossRef]
- Chang, C.; Chang, W.C. Micropropagation of Cymbidium ensifolium var. Misericors through callus-derived rhizomes. In Vitro Cell. Dev. Biol. Plant 2000, 36, 517–520. [Google Scholar] [CrossRef]
- Hasegawa, A.; Ohashi, H.; Goi, M. Effects of BA, rhizome length, mechanical treatment and liquid shaking culture on the shoot formation from rhizome in Cymbidium faberi Rolfe Rolfe. Acta Hortic. 1985, 166, 25–40. [Google Scholar] [CrossRef]
- Qi, Z.Y. Rapad Micro-Propagation and In Vitro Flowering Influence of Cymbidium goeringii. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2017. [Google Scholar]
- Yang, W.Y.; Bai, Y.Y.; Xu, Z.H. Stimulation of shoot regeneration in leaf tissue culture of solanum tuberosum by silver nitrate. Acta Phytophysiol. Sin. 1998, 24, 86–90. [Google Scholar]
- Zhang, X.P.; Zhang, F.F.; Wang, F.; Zhu, G.F. The effect of plant maturity and cytokinin on three varieties of orchid in vitro flowering and proliferation. South. Hortic. 2017, 28, 5–10. [Google Scholar]
- Chen, D.J. Study on Induction of Test-Tube Flower and Its Formation Mechanism in Cymbidium. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2006. [Google Scholar]
- Fu, S.B.; Yang, Y.P.; Ying, Z.; Gao, X.Y.; Zhou, Z. Study on in vitro flowering technology of Cymbidium plant Cymbidium nanulum. Seed 2024, 43, 136–142. [Google Scholar]
- Kostenyuk, I.; Oh, B.; So, I. Induction of early flowering in Cymbidium niveo-marginatum Mak in vitro. Plant Cell Rep. 1999, 19, 1–5. [Google Scholar] [CrossRef]



| Treatment | Orthogonal Factors | Proliferation Rate (%) | Proliferation Coefficient | |||
|---|---|---|---|---|---|---|
| 6-BA (mg/L) | NAA (mg/L) | IBA (mg/L) | AC (g/L) | |||
| 1 | 0.1 | 0.1 | 0.1 | 0.1 | 10.24 | 2.85 |
| 2 | 0.1 | 3.0 | 3.0 | 0.5 | 16.02 | 1.95 |
| 3 | 0.1 | 9.0 | 9.0 | 2.5 | 8.79 | 1.65 |
| 4 | 3.0 | 0.1 | 3.0 | 2.5 | 12.23 | 2.53 |
| 5 | 3.0 | 3.0 | 9.0 | 0.1 | 17.23 | 2.9 |
| 6 | 3.0 | 9.0 | 0.1 | 0.5 | 15.46 | 3.2 |
| 7 | 9.0 | 0.1 | 9.0 | 0.5 | 17.51 | 2.9 |
| 8 | 9.0 | 3.0 | 0.1 | 2.5 | 9.41 | 2.9 |
| 9 | 9.0 | 9.0 | 3.0 | 0.1 | 35.17 | 1.7 |
| Proliferation rate (%) summary | ||||||
| Factors | Level 1 | Level 2 | Level 3 | Range (R) | Best level | |
| 6-BA | 11.683 | 14.973 | 20.697 * | 9.014 | Level 3 (9.0 mg/L) | |
| NAA | 13.327 | 14.22 | 19.807 * | 6.48 | Level 3 (9.0 mg/L) | |
| IBA | 11.703 | 21.14 * | 14.51 | 9.437 | Level 2 (3.0 mg/L) | |
| AC | 20.88* | 16.33 | 10.143 | 10.737 | Level 1 (0.1 g/L) | |
| Proliferation coefficient summary | ||||||
| Factors | Level 1 | Level 2 | Level 3 | Range (R) | Best level | |
| 6-BA | 2.15 | 2.877 * | 2.5 | 0.727 | Level 2 (3.0 mg/L) | |
| NAA | 2.76 * | 2.583 | 2.183 | 0.577 | Level 1 (0.1 mg/L) | |
| IBA | 2.983 * | 2.06 | 2.483 | 0.923 | Level 1 (0.1 mg/L) | |
| AC | 2.483 | 2.683 * | 2.36 | 0.323 | Level 2 (0.5 g/L) | |
| Rhizome Proliferation Rate (%) | |||||
|---|---|---|---|---|---|
| Source of Variance | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value |
| 6-BA | 468.548 | 2 | 234.274 | 6.431 | 0.006 * |
| NAA | 492.136 | 2 | 246.068 | 6.755 | 0.005 * |
| IBA | 266.104 | 2 | 133.052 | 3.653 | 0.041 * |
| AC | 623.413 | 2 | 311.706 | 8.557 | 0.002 * |
| Error | 874.243 | 24 | 36.427 | - | - |
| Rhizome proliferation coefficient | |||||
| Source of variance | Sum of squares | Degree of freedom | Mean square | F value | p value |
| 6-BA | 3.181 | 2 | 1.591 | 1.58 | 0.227 |
| NAA | 2.508 | 2 | 1.254 | 1.246 | 0.306 |
| IBA | 1.91 | 2 | 0.955 | 0.949 | 0.401 |
| AC | 1.619 | 2 | 0.809 | 0.804 | 0.459 |
| Error | 24.157 | 24 | 1.007 | - | - |
| Treatment | Orthogonal Factors | Differentiation Rate (%) | Average Number of Induced Buds | |||
|---|---|---|---|---|---|---|
| Basal Medium | 6-BA (mg/L) | NAA (mg/L) | AgNO3 (mg/L) | |||
| 1 | 1/2MS | 1.0 | 0.1 | 0.02 | 94 | 1.46 |
| 2 | 1/2MS | 5.0 | 0.3 | 0.1 | 84 | 2.04 |
| 3 | 1/2MS | 10 | 0.9 | 0.5 | 91 | 2.53 |
| 4 | MS | 1.0 | 0.3 | 0.5 | 100 | 2.68 |
| 5 | MS | 5.0 | 0.9 | 0.02 | 98 | 2.31 |
| 6 | MS | 10 | 0.1 | 0.1 | 100 | 3.93 |
| 7 | Hyponex 2 | 1.0 | 0.9 | 0.1 | 9 | 0.09 |
| 8 | Hyponex 2 | 5.0 | 0.1 | 0.5 | 15 | 0.3 |
| 9 | Hyponex 2 | 10 | 0.3 | 0.02 | 3 | 0.07 |
| Differentiation rate (%) summary | ||||||
| Factors | Level 1 | Level 2 | Level 3 | Range (R) | Best level | |
| Basal medium | 0.897 | 0.993 * | 0.09 | 0.903 | Level 2 (MS) | |
| 6-BA | 0.677 * | 0.657 | 0.647 | 0.03 | Level 1 (1.0 mg/L) | |
| NAA | 0.697 * | 0.623 | 0.66 | 0.074 | Level 1 (0.1 mg/L) | |
| AgNO3 | 0.65 | 0.643 | 0.687 * | 0.044 | Level 3 (0.5 mg/L) | |
| Summary of average number of induced buds | ||||||
| Factors | Level 1 | Level 2 | Level 3 | Range (R) | Best level | |
| Basal medium | 2.01 | 2.973 * | 0.153 | 2082 | Level 2 (MS) | |
| 6-BA | 1.41 | 1.55 | 2.177 * | 0.767 | Level 3 (10 mg/L) | |
| NAA | 1.897 * | 1.597 | 1.643 | 0.3 | Level 1 (0.1 mg/L) | |
| AgNO3 | 1.28 | 2.02 * | 1.837 | 0.74 | Level 2 (0.1 mg/L) | |
| Rhizome Differentiation Rate | |||||
|---|---|---|---|---|---|
| Source of Variance | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value |
| 6-BA | 0.008 | 2 | 0.004 | 0.174 | 0.841 |
| NAA | 0.043 | 2 | 0.022 | 0.962 | 0.388 |
| AgNO3 | 0.021 | 2 | 0.011 | 0.467 | 0.629 |
| Basal medium | 10.344 | 2 | 5.172 | 228.932 | 0 * |
| Error | 1.333 | 59 | 0.023 | - | - |
| Bud induction rate | |||||
| Source of variance | Sum of squares | Degree of freedom | Mean square | F value | p value |
| 6-BA | 5.617 | 2 | 2.808 | 6.062 | 0.004 * |
| NAA | 0.913 | 2 | 0.456 | 0.985 | 0.38 |
| AgNO3 | 6.228 | 2 | 3.114 | 6.722 | 0.002 * |
| Basal medium | 77.97 | 2 | 38.985 | 84.154 | 0 * |
| Error | 27.332 | 59 | 0.463 | - | - |
| Treatment | Orthogonal Factors | Flower Bud Induction Rate (%) | Normal Flower Bud Formation Rate (%) | |||
|---|---|---|---|---|---|---|
| Basal Medium | 6-BA (mg/L) | NAA (mg/L) | TDZ (mg/L) | |||
| 1 | MS | 1.0 | 0.1 | 0.1 | 10 | 3 |
| 2 | MS | 3.0 | 0.3 | 0.3 | 8 | 4 |
| 3 | MS | 9.0 | 0.9 | 0.9 | 28 | 6.7 |
| 4 | MS (1/3N, 3P) | 1.0 | 0.3 | 0.9 | 8 | 0 |
| 5 | MS (1/3N, 3P) | 3.0 | 0.9 | 0.1 | 15 | 5 |
| 6 | MS (1/3N, 3P) | 9.0 | 0.1 | 0.3 | 36 | 16 |
| 7 | MS (1/5N, 5P) | 1.0 | 0.9 | 0.3 | 4 | 0 |
| 8 | MS (1/5N, 5P) | 3.0 | 0.1 | 0.9 | 8 | 0 |
| 9 | MS (1/5N, 5P) | 9.0 | 0.3 | 0.1 | 24 | 8 |
| Summary ofin vitroflower bud induction rate | ||||||
| Factors | Level 1 | Level 2 | Level 3 | Range (R) | Best level | |
| Basal medium | 0.153 | 0.197 * | 0.12 | 0.077 | 2 MS (1/3N, 3P) | |
| 6-BA | 0.073 | 0.103 | 0.293 * | 0.22 | 3 (9.0 mg/L) | |
| NAA | 0.18 * | 0.133 | 0.157 | 0.047 | 1 (0.1 mg/L) | |
| TDZ | 0.163 * | 0.16 | 0.147 | 0.016 | 1 (0.1 mg/L) | |
| Summary of normal flower bud formation rate | ||||||
| Factors | Level 1 | Level 2 | Level 3 | Range (R) | Best level | |
| Basal medium | 0.047 | 0.07 * | 0.027 | 0.043 | 2 MS (1/3N, 3P) | |
| 6-BA | 0.011 | 0.03 | 0.102 * | 0.091 | 3 (9.0 mg/L) | |
| NAA | 0.064 * | 0.04 | 0.039 | 0.025 | 1 (0.1 mg/L) | |
| TDZ | 0.054 | 0.067 * | 0.022 | 0.045 | 2 (0.3 mg/L) | |
| In Vitro Flower Bud Induction Rate | |||||
|---|---|---|---|---|---|
| Source of Variance | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value |
| Basal medium | 0.046 | 2 | 0.023 | 1.416 | 0.256 |
| 6-BA | 0.382 | 2 | 0.191 | 11.72 | 0 * |
| TDZ | 0.01 | 2 | 0.005 | 0.298 | 0.744 |
| NAA | 0.019 | 2 | 0.01 | 0.595 | 0.557 |
| Error | 0.603 | 37 | 0.016 | - | - |
| Normal flower bud formation rate | |||||
| Source of variance | Sum of squares | Degree of freedom | Mean square | F value | p value |
| Basal medium | 0.014 | 2 | 0.007 | 0.811 | 0.452 |
| 6-BA | 0.073 | 2 | 0.036 | 4.362 | 0.02 * |
| TDZ | 0.016 | 2 | 0.008 | 0.98 | 0.385 |
| NAA | 0.006 | 2 | 0.003 | 0.387 | 0.681 |
| Error | 0.309 | 37 | 0.008 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, Y.; Wang, C.; Yang, Y.; Chen, M.; Ramakrishnan, M.; Fu, B.; Wang, L.; Wei, Q.; Wang, S. Establishment of an Efficient System for Rhizome Proliferation and In Vitro Flowering Induction from Protocorm Explants in Cymbidium goeringii. Horticulturae 2025, 11, 738. https://doi.org/10.3390/horticulturae11070738
Zhi Y, Wang C, Yang Y, Chen M, Ramakrishnan M, Fu B, Wang L, Wei Q, Wang S. Establishment of an Efficient System for Rhizome Proliferation and In Vitro Flowering Induction from Protocorm Explants in Cymbidium goeringii. Horticulturae. 2025; 11(7):738. https://doi.org/10.3390/horticulturae11070738
Chicago/Turabian StyleZhi, Yongqi, Chenhao Wang, Yi Yang, Ming Chen, Muthusamy Ramakrishnan, Bo Fu, Lili Wang, Qiang Wei, and Sen Wang. 2025. "Establishment of an Efficient System for Rhizome Proliferation and In Vitro Flowering Induction from Protocorm Explants in Cymbidium goeringii" Horticulturae 11, no. 7: 738. https://doi.org/10.3390/horticulturae11070738
APA StyleZhi, Y., Wang, C., Yang, Y., Chen, M., Ramakrishnan, M., Fu, B., Wang, L., Wei, Q., & Wang, S. (2025). Establishment of an Efficient System for Rhizome Proliferation and In Vitro Flowering Induction from Protocorm Explants in Cymbidium goeringii. Horticulturae, 11(7), 738. https://doi.org/10.3390/horticulturae11070738








