Expression Analysis of the ABF Gene Family in Actinidia chinensis Under Drought Stress and the Response Mechanism to Abscisic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Data Acquisition
2.3. Identification and Physicochemical Properties of the ABF in A. chinensis
2.4. Multiple Sequence Alignment, Phylogenetic Analysis, and Three-Dimensional Structure Analysis of Proteins
2.5. Gene Structure and Conserved Motif Analysis
2.6. Analysis of Promoter Cis-Acting Elements
2.7. Chromosome Localization and Covariate Partitioning
2.8. Transcriptome Sequencing and ABA Content Determination
2.9. qRT-PCR Analysis
3. Results
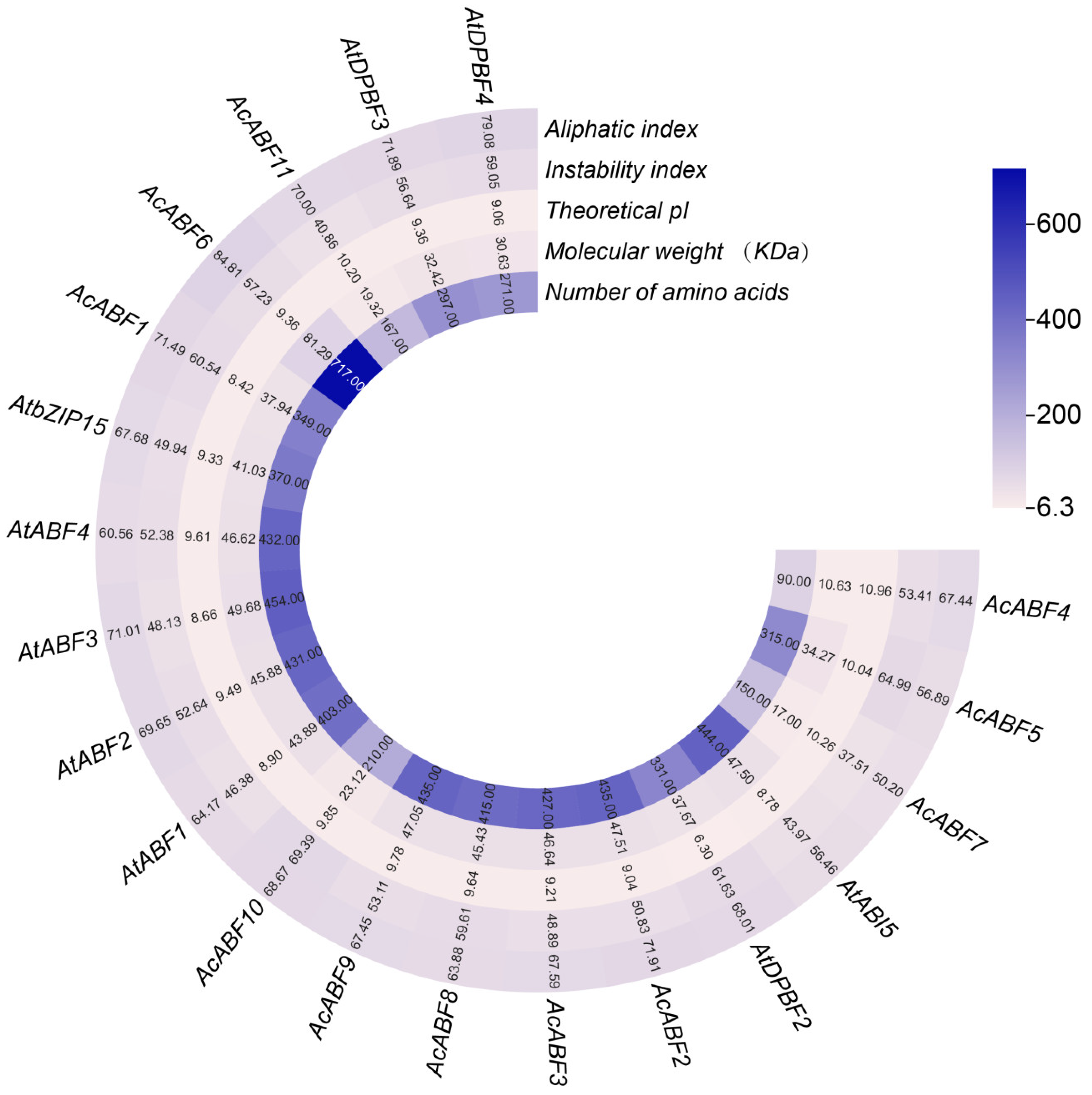
3.1. Identification and Physicochemical Properties of the ABF Gene Family in A. chinensis
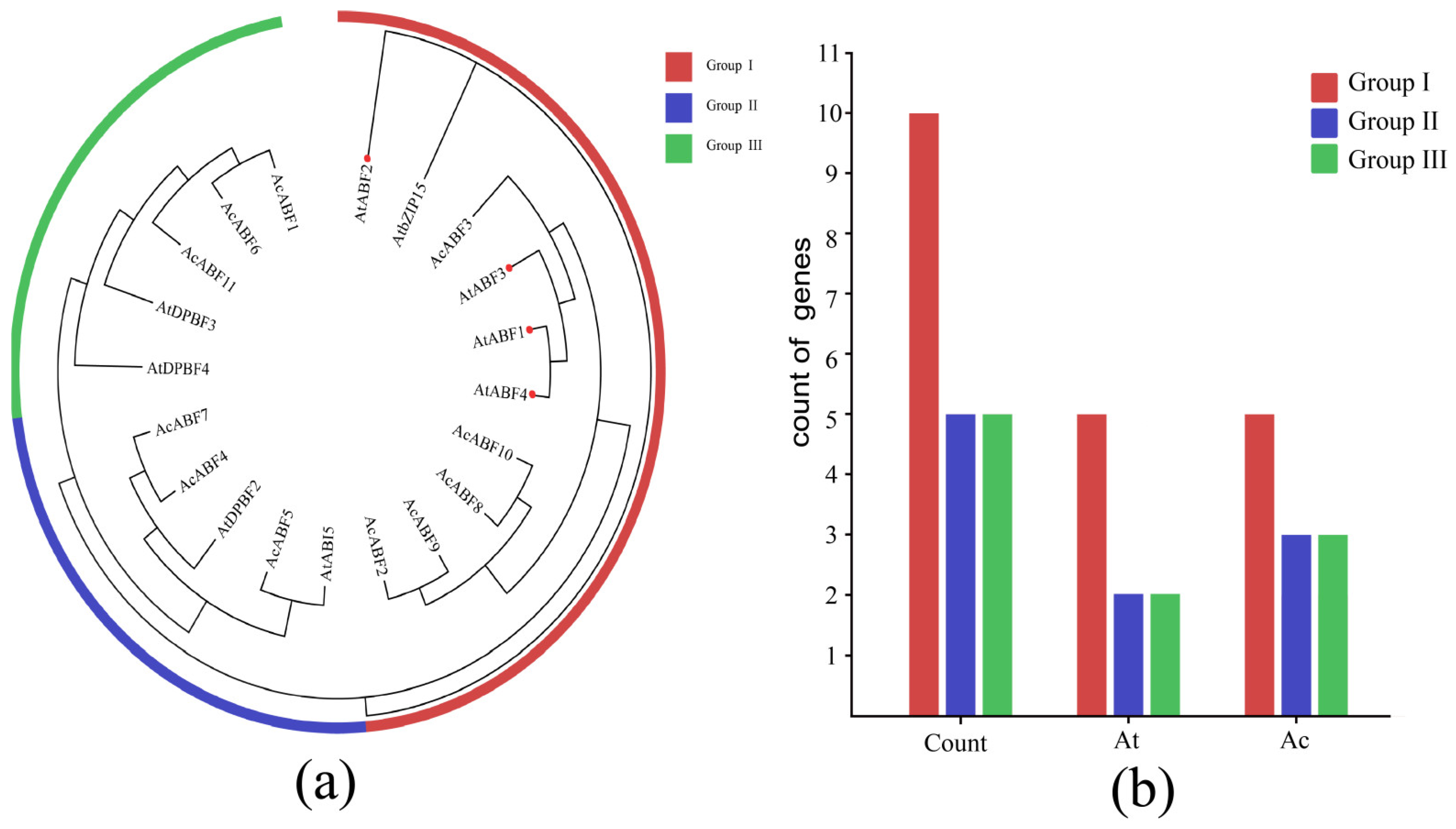
3.2. Phylogenetic Analysis of the ABF Gene Family of A. chinensis
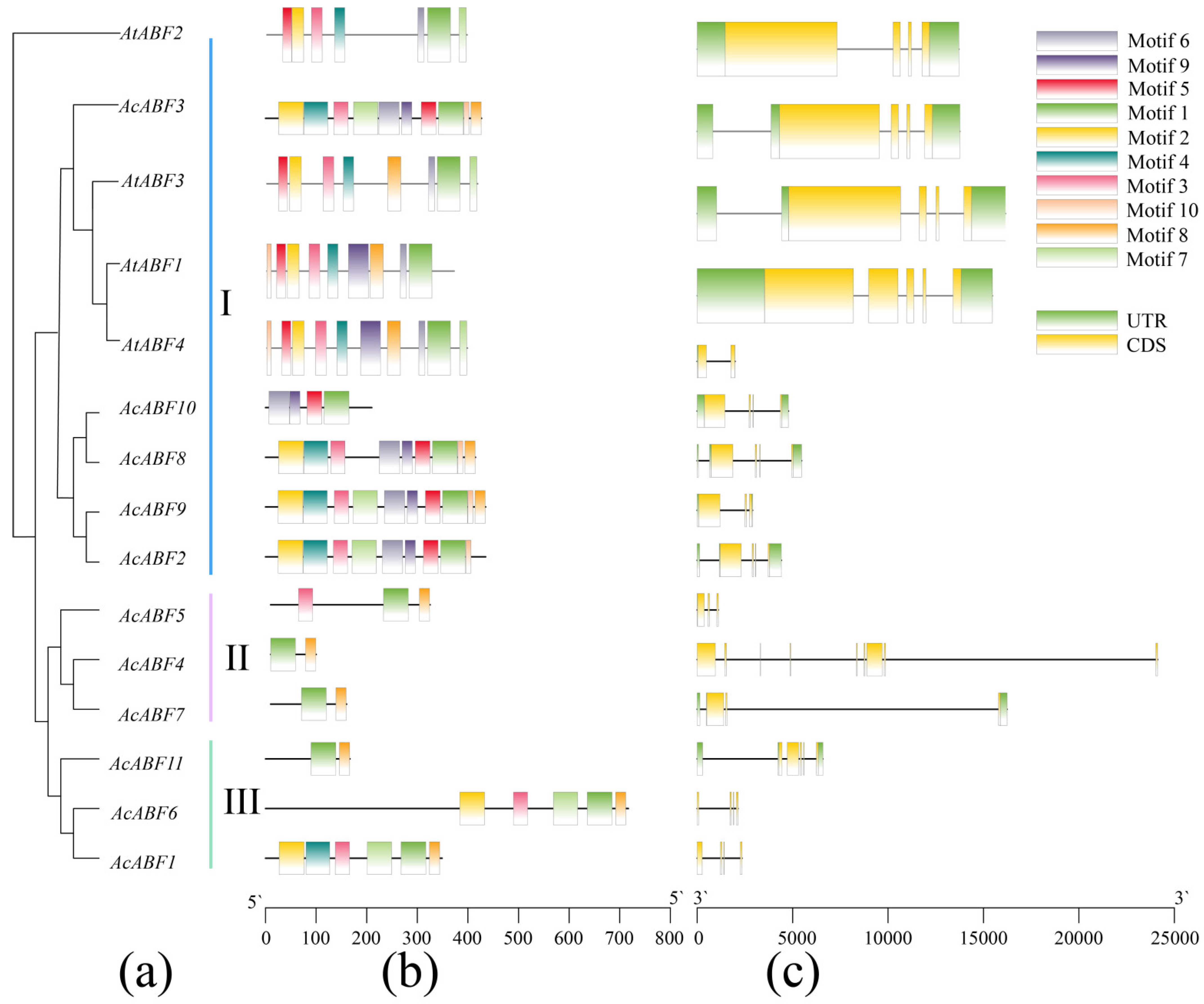
3.3. Analysis of the Gene Structure and Conserved Motifs of the A. chinensis ABF Gene Family
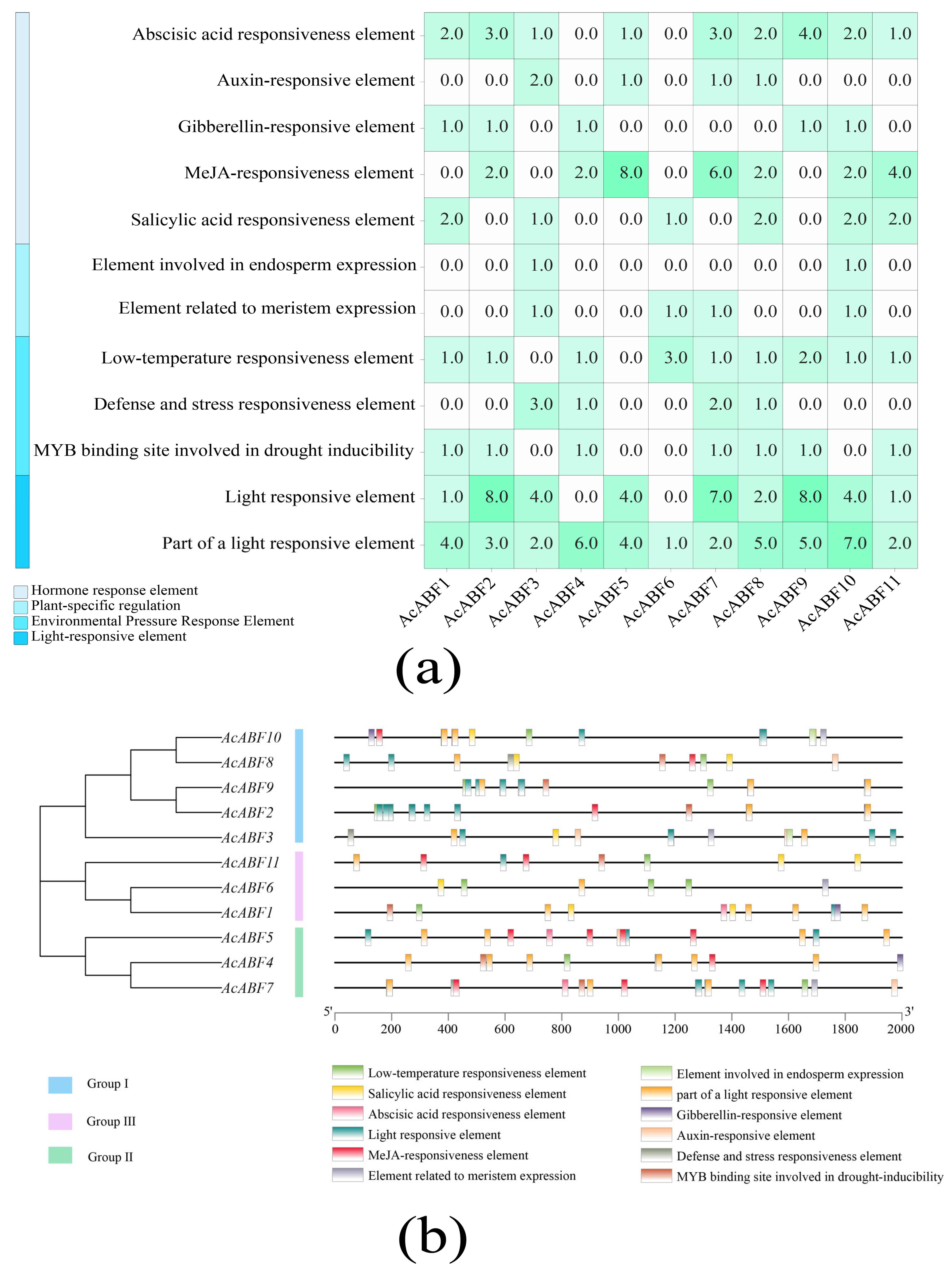
3.4. Analysis of the Promoter Cis-Acting Elements of the A. chinensis ABF Gene Family
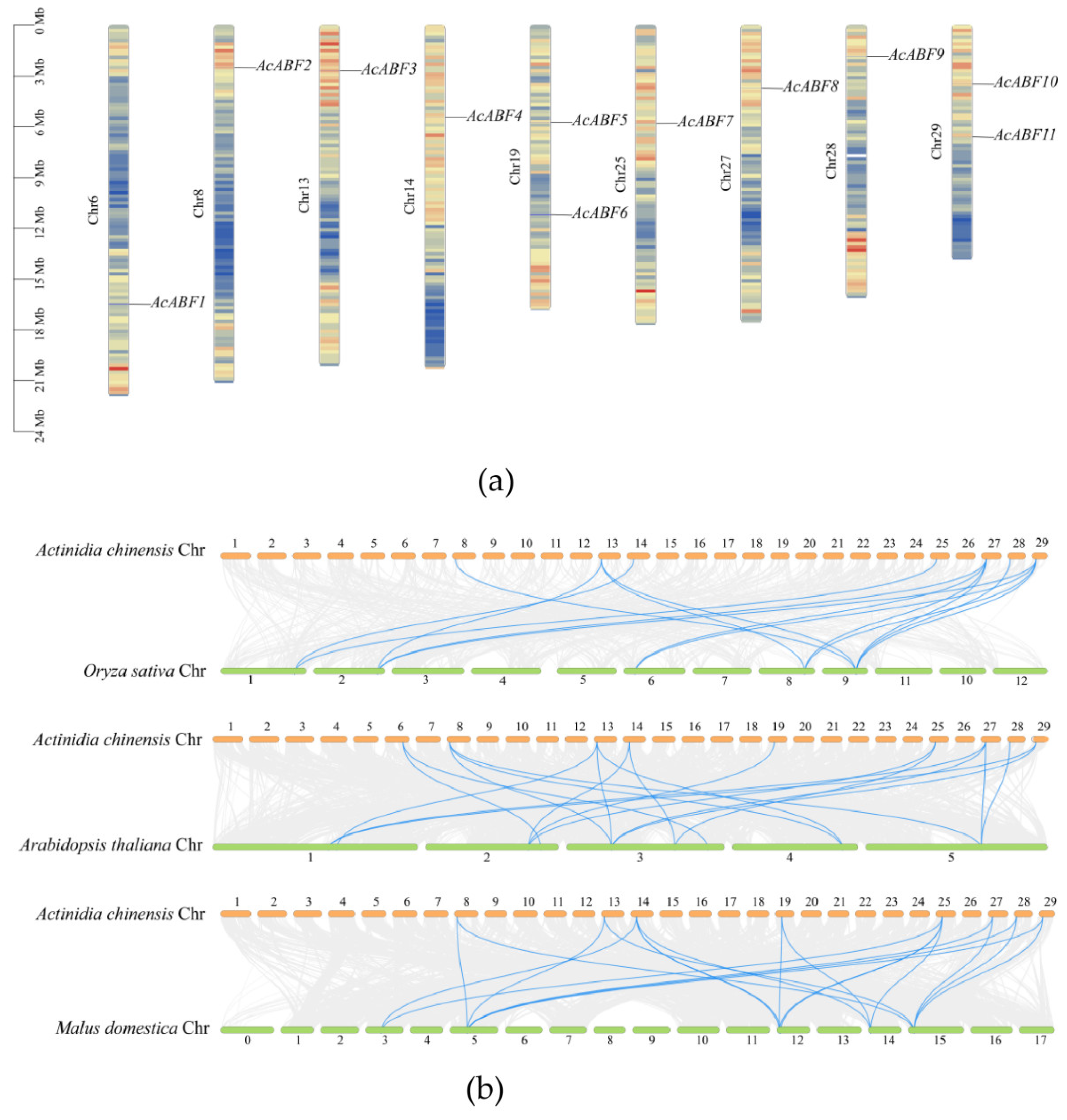
3.5. Chromosomal Localization and Interspecific Covariance of the ABF Gene Family in A. chinensis
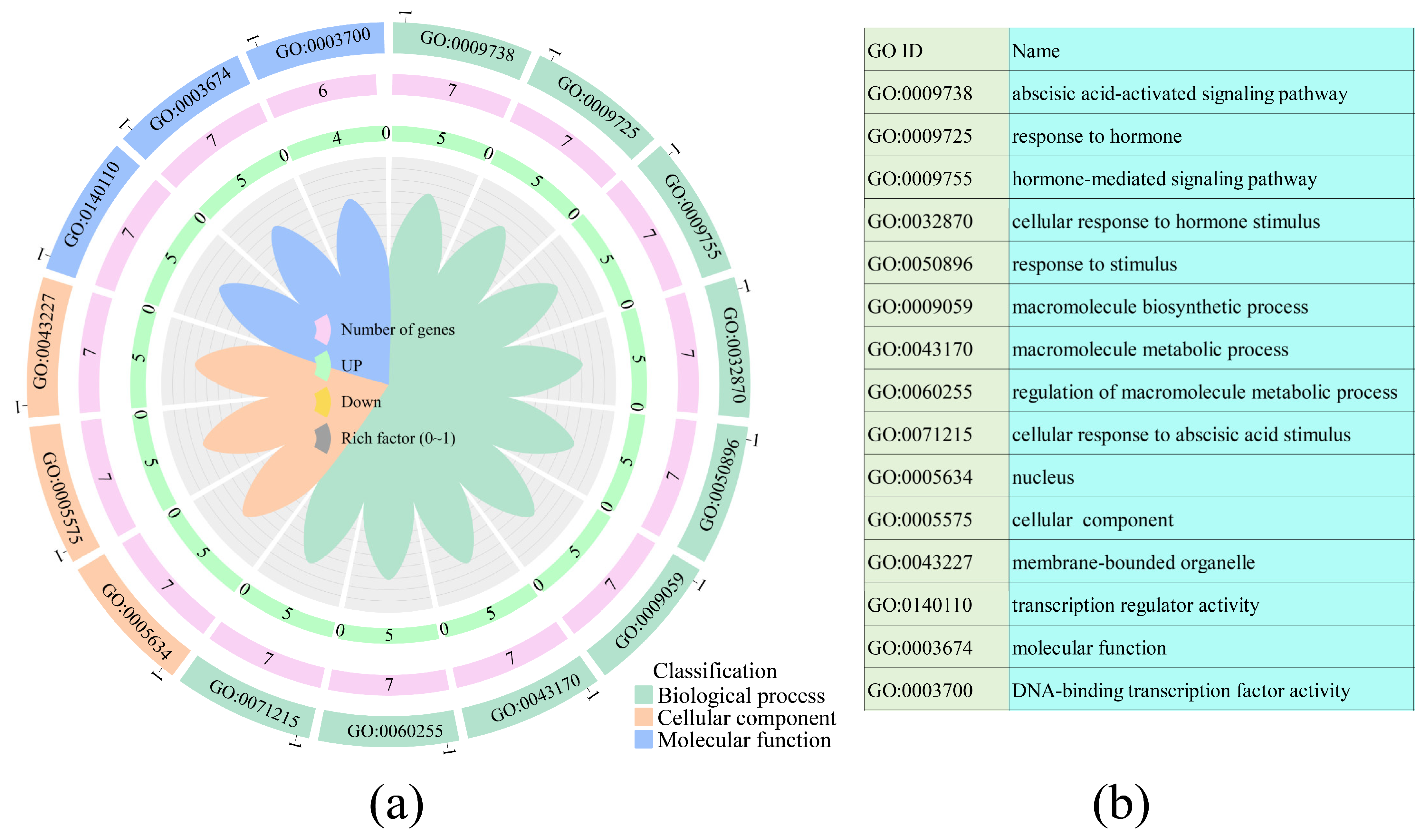
3.6. GO Function Enrichment Analysis
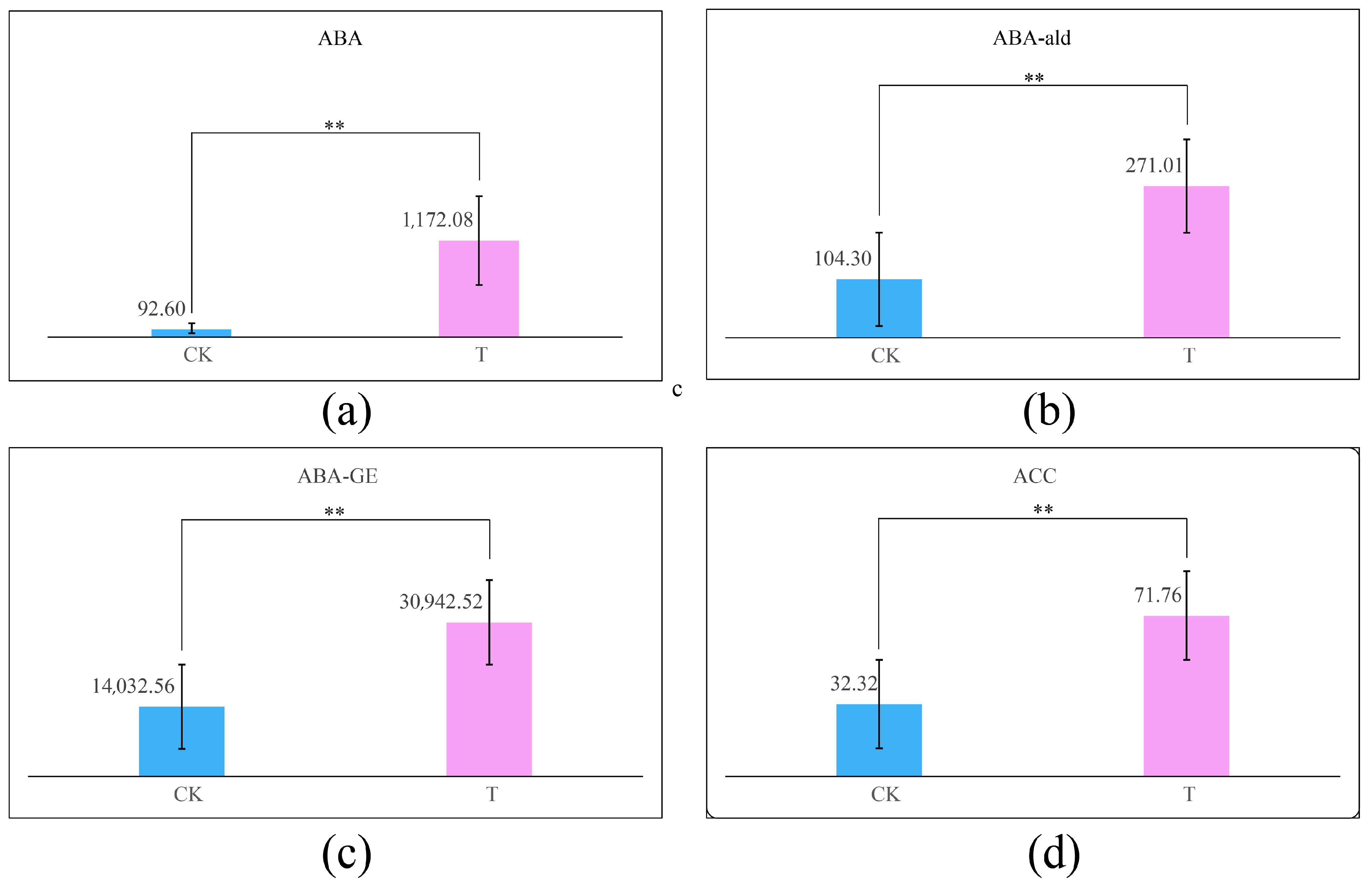
3.7. Changes in the ABA Content in Kiwifruit Leaves Under Drought Stress
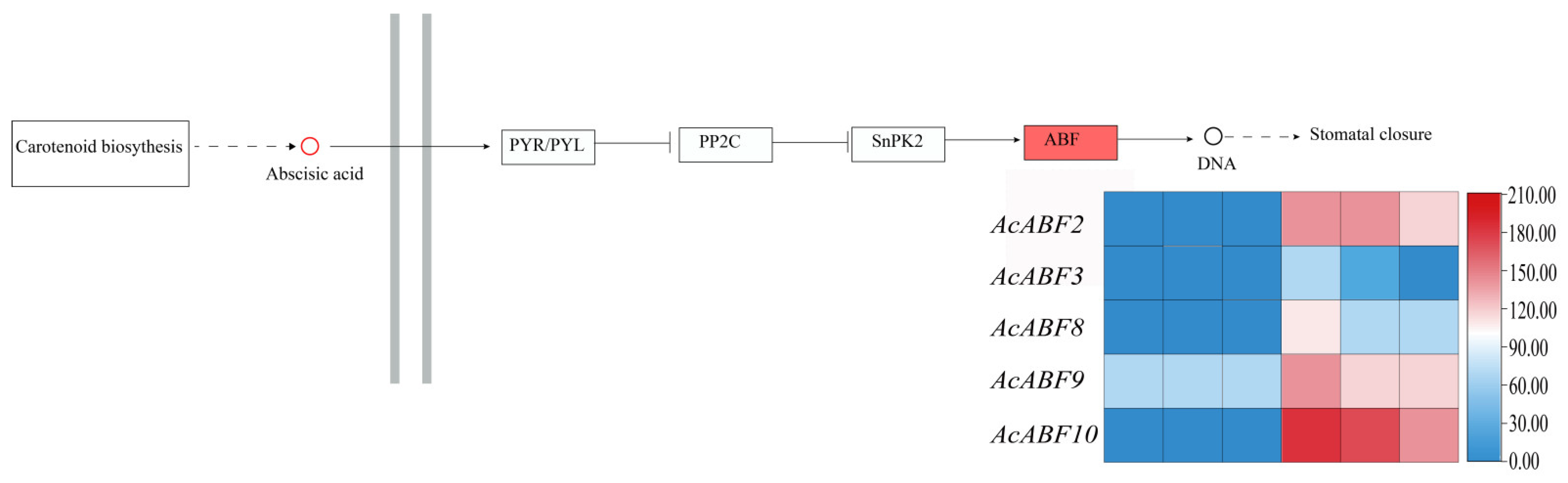
3.8. KEGG Enrichment of the ABF Gene Family in A. chinensis Under Drought Stress
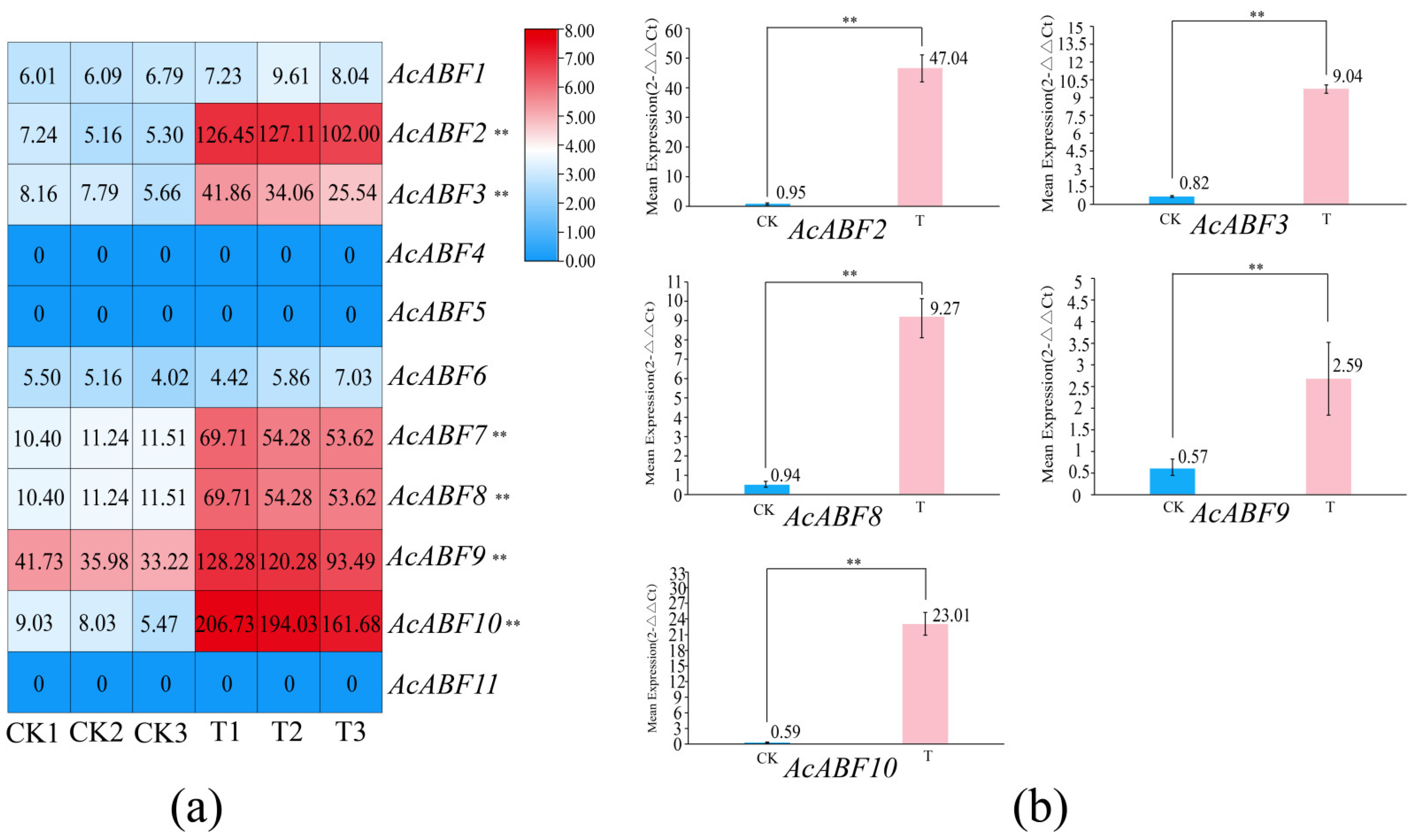
3.9. Expression Pattern and qRT-PCR Analysis of ABF Family Members in A. chinensis Under Drought Stress
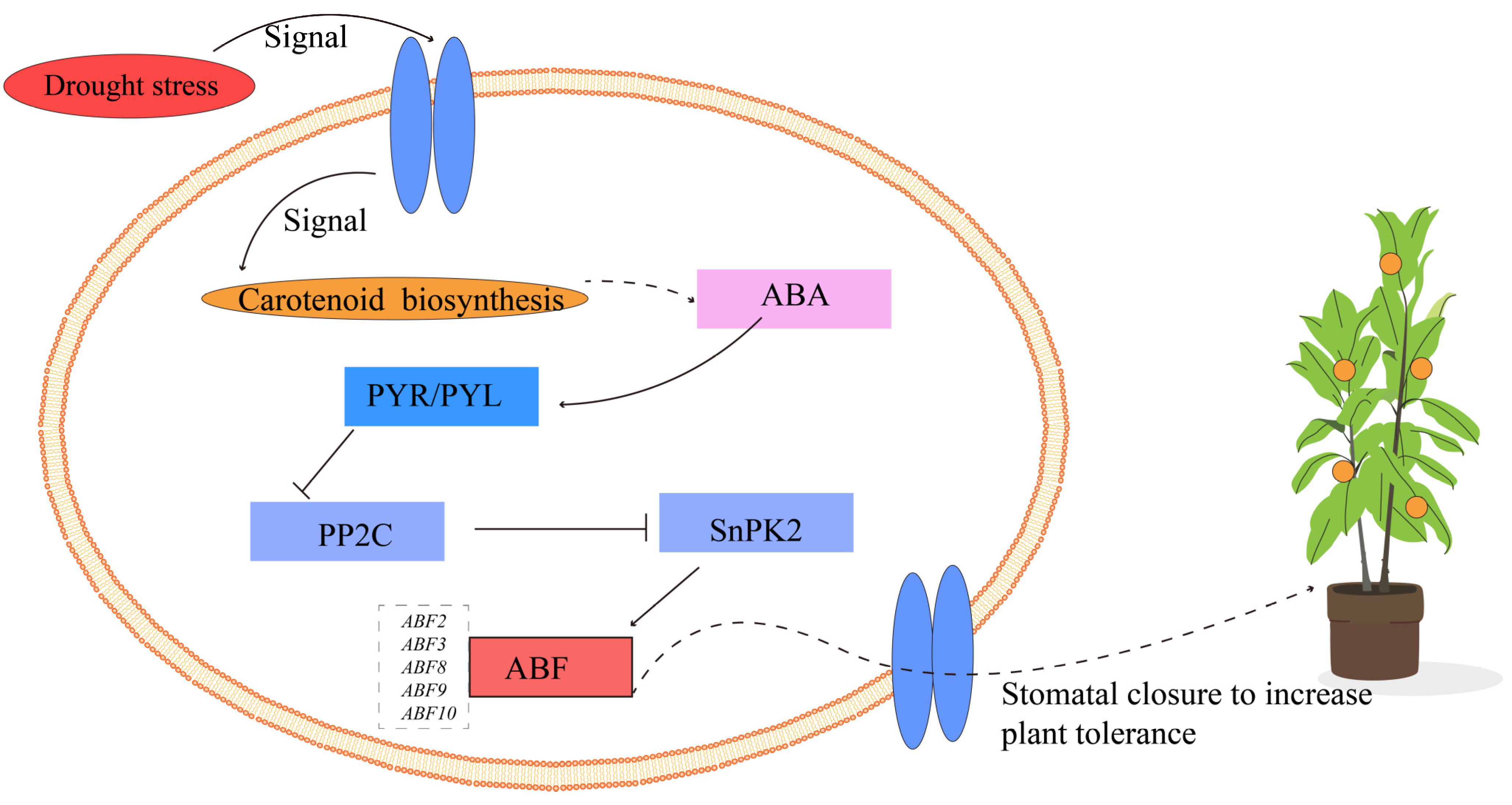
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Ding, J.; Deng, D.; Tang, W.; Sun, H.; Liu, D.; Zhang, L.; Niu, X.; Zhang, X.; Meng, M.; et al. Draft genome of the kiwifruit Actinidia chinensis. Nat. Commun. 2013, 4, 2640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Latocha, P.; Debersaques, F.; Decorte, J. Varietal differences in the mineral composition of kiwiberry Actinidia arguta (Siebold et Zucc.) Planch, ex. Miq. Acta Hortic. 2015, 1096, 479–486. [Google Scholar] [CrossRef]
- Ashraf, I.; Cipriani, G.; De Mori, G. Bridging the gap: Genetic insights into graft compatibility for enhanced kiwifruit production. Int. J. Mol. Sci. 2025, 26, 2925. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, G.; Yang, Y.; Ma, K.; Ren, X.; Li, M.; Liu, Z. Interaction of AcMADS68 with transcription factors regulates anthocyanin biosynthesis in red-fleshed kiwifruit. Hortic. Res. 2022, 10, uhac252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassi, I.; Mian, G.; Troiano, S.; Gori, E.; Iseppi, L. Assessing consumer preferences for new red-pulp kiwifruit: Application of a choice experiment between different countries. Foods 2023, 12, 2865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Zhang, H.Z.; Fu, J.Y.; Du, Y.Y.; Qu, J.; Song, Y.; Wang, P.W. The GmXTH1 gene improves drought stress resistance of soybean seedlings. Mol. Breed. 2021, 42, 3. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2017, 11, e0156362. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, M.A.; Dawood, A.F.M.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Judd, M.J.; McAneney, K.J.; Wilson, K.S. Influence of water stress on kiwifruit growth. Irrig. Sci. 1989, 10, 303–311. [Google Scholar] [CrossRef]
- Chen, J.Y.; Fang, J.B.; Qi, X.J.; Gu, H.; Lin, M.M.; Zhang, W.Y.; Wei, C.G. Research progress on rootstocks of kiwifruit. J. Fruit Sci. 2015, 32, 959–968. [Google Scholar]
- Bao, W.W.; Chen, X.; Li, R.N.; Li, M.; Xie, C.J.; Dou, M.R.; Zhang, K.Z.; Wang, J.; Gao, Z.X.; Liu, Z.D.; et al. Comprehensive assessment of drought resistance and recovery in kiwifruit genotypes using multivariate analysis. Plant J. 2024, 119, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Kumar, A.; Malla, M.A.; Chowdhary, K.; Singh, G.; Ravikanth, G.; Harish; Sharma, S.; Saati-Santamaria, Z.; Menéndez, E.; et al. Approaches for the amelioration of adverse effects of drought stress on crop plants. Front. Biosci. 2021, 26, 928–947. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Sui, N. Transcriptional regulation of bHLH during plant response to stress. Biochem. Biophys. Res. Commun. 2018, 503, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wang, Y.; Wang, J.; Li, Y.; Zhang, Y.; Zhao, X.; Xu, J.; Li, Z.; Zhao, T.; Wang, W.; et al. The basic helix-loop-helix transcription factor gene, OsbHLH38, plays a key role in controlling rice salt tolerance. J. Integr. Plant Biol. 2023, 65, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Geilfus, C.M.; Mithöfer, A.; Ludwig-Müller, J.; Zörb, C.; Muehling, K.H. Chloride-inducible transient apoplastic alkalinizations induce stomatal closure by controlling abscisic acid distribution between leaf apoplast and guard cells in salt-stressed Vicia faba. New Phytol. 2015, 208, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of basal aba in plant growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant. J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, R.; Guo, C.; Hou, H.; Wang, X.; Gao, H. Evolutionary and expression analyses of the apple basic leucine zipper transcription factor Family. Front. Plant Sci. 2016, 7, 376. [Google Scholar] [CrossRef]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F.; bZIP Research Group. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Fu, D. The Roles of BLH Transcription factors in plant development and environmental Response. Int. J. Mol. Sci. 2022, 23, 3731. [Google Scholar] [CrossRef]
- Pan, X.; Wang, C.; Liu, Z.; Gao, R.; Feng, L.; Li, A.; Yao, K.; Liao, W. Identification of ABF/AREB gene family in tomato (Solanum lycopersicum L.) and functional analysis of ABF/AREB in response to ABA and abiotic stresses. PeerJ 2023, 11, e15310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, H.C.; Tsai, M.C.; Wu, S.S.; Chang, I.F. Regulation of ABI5 expression by ABF3 during salt stress responses in Arabidopsis thaliana. Bot. Stud. 2019, 60, 16. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Fiallos-Salguero, M.S.; Li, J.; Li, Y.; Xu, J.; Fang, P.; Wang, Y.; Zhang, L.; Tao, A. Identification of AREB/ABF gene family involved in the response of aba under salt and drought stresses in jute (Corchorus olitorius L.). Plants 2023, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 2012, 147, 15–27. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Hu, Y.F.; Li, J.; Yao, X.; Liu, K. BnaABF2, a bZIP transcription factor from rapeseed (Brassica napus L.), enhances drought and salt tolerance in transgenic Arabidopsis. Bot. Stud. 2016, 57, 12. [Google Scholar] [CrossRef]
- Yang, F.; Sun, X.; Wu, G.; He, X.; Liu, W.; Wang, Y.; Sun, Q.; Zhao, Y.; Xu, D.; Dai, X.; et al. Genome-wide identification and expression profiling of the abf transcription factor family in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2024, 25, 3783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, X.; Lin, M.; Xiao, G.; Wang, Q.; Li, Z. Identification and characterization of the AREB/ABF gene family in three orchid species and functional analysis of DcaABI5 in Arabidopsis. Plants 2024, 13, 774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinkar, R.S.; Rai, M. Plant Tissue Culture:Theory and Practical Approach; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. An Introduction to plant cell, tissue, and organ culture: Current status and perspectives. Methods Mol. Biol. 2024, 2827, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Jia, H.; Wu, M.; Zhong, W.; Jia, D.; Wang, Z. Genome-wide identification and characterization of the TIFY gene family in kiwifruit. BMC Genom. 2022, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, L.; Su, G.; Liu, F.; Zeng, Q.; Li, R. Identification and expression analysis of the lipid phosphate phosphatases gene family reveal their involvement in abiotic stress response in kiwifruit. Front. Plant Sci. 2022, 13, 942937. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server, 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y. TBtools, an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Muñiz García, M.N.; Cortelezzi, J.I.; Fumagalli, M.; Capiati, D.A. Expression of the Arabidopsis ABF4 gene in potato increases tuber yield, improves tuber quality and enhances salt and drought tolerance. Plant Mol. Biol. 2018, 98, 137–152. [Google Scholar] [CrossRef]
- Liu, J.; Shu, D.; Tan, Z.; Ma, M.; Guo, N.; Gao, S.; Duan, G.; Kuai, B.; Hu, Y.; Li, S.; et al. The Arabidopsis IDD14 transcription factor interacts with bZIP-type ABFs/AREBs and cooperatively regulates ABA-mediated drought tolerance. New Phytol. 2022, 236, 929–942. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2014, 38, 35–49. [Google Scholar] [CrossRef]
- Maszkowska, J.; Szymańska, K.P.; Kasztelan, A.; Krzywińska, E.; Sztatelman, O.; Dobrowolska, G. The Multifaceted Regulation of SnRK2 Kinases. Cells 2021, 10, 2180. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Hong, J.; Ha, J.; Kang, J.; Kim, S.Y. ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 2000, 275, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mei, F.; Zhang, Y.; Li, S.; Kang, Z.; Mao, H. Genome-wide analysis of the AREB/ABF gene lineage in land plants and functional analysis of TaABF3 in Arabidopsis. BMC Plant Biol. 2020, 20, 558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Chen, Y.; Hu, Q.; Yang, X.; Zhao, Y.; Lin, Y.; Yuan, J.; Gu, J.; Li, Y.; He, J.; et al. Pseudoresponse regulator 3b and transcription factor ABF3 modulate abscisic acid-dependent drought stress response in soybean. Plant Physiol. 2024, 195, 3053–3071. [Google Scholar] [CrossRef]
- Hossain, M.A.; Cho, J.I.; Han, M.; Ahn, C.H.; Jeon, J.S.; An, G.; Park, P.B. The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice. J. Plant Physiol. 2010, 167, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.S.; Li, M.Y.; Song, Z.; Han, J.P.; Cheng, Z.Q.; Chen, X.J.; Han, D.Z.; Hu, Z.B.; Liu, C.Y.; Yang, M.L.; et al. Characterization of the soybean ABF gene family and the key regulatory function of GmABF1 in salt stress response. Int. J. Biol. Macromol. 2025, 317, 144763. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Du, J.; Tian, L.; Li, M.; Zhang, X.; Zhang, S.; Wan, X.; Chen, Q. Divergent response strategies of CsABF facing abiotic stress in tea plant: Perspectives from drought-tolerance studies. Front. Plant Sci. 2021, 12, 763843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, L.; Wang, J.; Ye, M.; Li, Y.; Guo, B.; Chen, Z.; Li, H.; An, X. Identification and characterization of the Populus AREB/ABF Subfamily. J. Integr. Plant Biol. 2013, 55, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Que, F.; Li, T.; Zhang, R.R.; Khadr, A.; Xu, Z.S.; Tian, Y.S.; Xiong, A.S. DcABF3, an ABF transcription factor from carrot, alters stomatal density and reduces ABA sensitivity in transgenic Arabidopsis. Plant Sci. 2021, 302, 110699. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S.; Liu, J.H.; Chen, X.J. Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol. 2010, 10, 230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, S.; Cai, Y.; Yang, Z.; Joyce, D.C.; Zheng, Y. Effect of MeJA treatment on polyamine, energy status and anthracnose rot of loquat fruit. Food Chem. 2014, 145, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of hormone signaling networks in plant defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef]
- Laura, B.; Silvia, P.; Francesca, F.; Benedetta, S.; Carla, C. Epigenetic control of defense genes following MeJA-induced priming in rice (O. sativa). J. Plant Physiol. 2018, 228, 166–177. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular mechanism for the regulation of ABA homeostasis during plant development and stress Responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014, 202, 35–49. [Google Scholar] [CrossRef]
- Chini, A.; Gimenez-Ibanez, S.; Goossens, A.; Solano, R. Redundancy and specificity in jasmonate signalling. Curr. Opin. Plant Biol. 2016, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef]
- Aleman, F.; Yazaki, J.; Lee, M.; Takahashi, Y.; Kim, A.Y.; Li, Z. An ABA-increased interaction of the PYL6 ABA receptor with MYC2 transcription factor, A putative link of ABA and JA signaling. Scientific Rep. 2016, 6, 28941. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products, biosynthesis and functions. Natural Product Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.Y.; Cho, D.I.; Park, J.H.; Kim, S.Y. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J. 2004, 40, 75–87, Erratum in: Plant J. 2005, 43, 467. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, J.; Gangappa, S.N.; Hettiarachchi, C.; Lin, F.; Andersson, M.X.; Jiang, Y.; Deng, X.W.; Holm, M. Convergence of Light and ABA signaling on the ABI5 promoter. PLoS Genet. 2014, 10, e1004197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muñiz García, M.N.; Giammaria, V.; Grandellis, C.; Téllez-Iñón, M.T.; Ulloa, R.M.; Capiati, D.A. Characterization of StABF1, a stress-responsive bZIP transcription factor from Solanum tuberosum L. that is phosphorylated by StCDPK2 vitro. Planta 2012, 235, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, P. Biomechanics of plant growth. Am. J. Bot. 2006, 93, 1415–1425. [Google Scholar] [CrossRef]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling Pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yang, X.; Lu, J.; Song, F.; Sun, J.; Wang, C.; Lian, J.; Zhao, L.; Zhao, B. OsIAA20, an Aux/IAA protein, mediates abiotic stress tolerance in rice through an ABA pathway. Plant Sci. 2021, 308, 110903. [Google Scholar] [CrossRef] [PubMed]
- Min, M.K.; Kim, R.; Hong, W.J.; Jung, K.H.; Le, J.Y.; Kim, B.G. OsPP2C09 is a bifunctional regulator in both ABA-dependent and independent abiotic stress signaling pathways. Int. J. Mol. Sci. 2021, 22, 393. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ji, C.Y.; Lee, C.J.; Kim, S.E.; Park, S.C.; Kwak, S.S. Orange: A target gene for regulating carotenoid homeostasis and increasing plant tolerance to environmental stress in marginal lands. J. Exp. Bot. 2018, 69, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.; Hagen, G.; Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [CrossRef]
- Chernys, J.T.; Zeevaart, J.A. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol. 2000, 124, 343–354. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2011, 35, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-golec, A.; Szarejko, I. The molecular basis of ABA-mediated plant response to drought. PLoS ONE 2013, 177, 103–134. [Google Scholar]
- Soma, F.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cellular phosphorylation signaling and gene expression in drought stress responses, ABA-dependent and ABA-independent regulatory systems. Plants 2021, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G. Plant abiotic stress response and nutrient use efficiency. Sci. China-Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.K.; Ye, M.; Li, B.; Noel, J.P. Co-evolution of hormone metabolism and signaling networks expands plant adaptive plasticity. Cell 2016, 166, 881–893. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Takahashi, F.; Anderson, J.C.; Ishihama, Y.; Peck, S.C.; Shinozaki, K. Genetics and Phosphoproteomics Reveal a Protein Phosphorylation Network in the Abscisic Acid Signaling Pathway in Arabidopsis thaliana. Sci. Signal. 2013, 6, rs8. [Google Scholar] [CrossRef]
- Kang, J.Y.; Choi, H.I.; Im, M.Y.; Kim, S.Y. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 2002, 14, 343–357. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zi, Y.; Rong, X.; Zhang, Q.; Nie, L.; Wang, J.; Ren, H.; Zhang, H.; Liu, X. Expression Analysis of the ABF Gene Family in Actinidia chinensis Under Drought Stress and the Response Mechanism to Abscisic Acid. Horticulturae 2025, 11, 715. https://doi.org/10.3390/horticulturae11070715
Wang H, Zi Y, Rong X, Zhang Q, Nie L, Wang J, Ren H, Zhang H, Liu X. Expression Analysis of the ABF Gene Family in Actinidia chinensis Under Drought Stress and the Response Mechanism to Abscisic Acid. Horticulturae. 2025; 11(7):715. https://doi.org/10.3390/horticulturae11070715
Chicago/Turabian StyleWang, Haoyu, Yinqiang Zi, Xu Rong, Qian Zhang, Lili Nie, Jie Wang, Hailin Ren, Hanyao Zhang, and Xiaozhen Liu. 2025. "Expression Analysis of the ABF Gene Family in Actinidia chinensis Under Drought Stress and the Response Mechanism to Abscisic Acid" Horticulturae 11, no. 7: 715. https://doi.org/10.3390/horticulturae11070715
APA StyleWang, H., Zi, Y., Rong, X., Zhang, Q., Nie, L., Wang, J., Ren, H., Zhang, H., & Liu, X. (2025). Expression Analysis of the ABF Gene Family in Actinidia chinensis Under Drought Stress and the Response Mechanism to Abscisic Acid. Horticulturae, 11(7), 715. https://doi.org/10.3390/horticulturae11070715





