Genetic Merit of Parents and Heterosis in Cassava (Manihot esculenta Crantz)
Abstract
1. Introduction
2. Materials and Methods
2.1. Genotypes
2.2. Crosses
2.3. Experimental Design and Evaluations
2.4. Data Analysis
2.5. Ranking, Selection Gains and Heterosis
3. Results
3.1. Phenotypic Analysis and Predictions
3.2. Correlations and Rankings
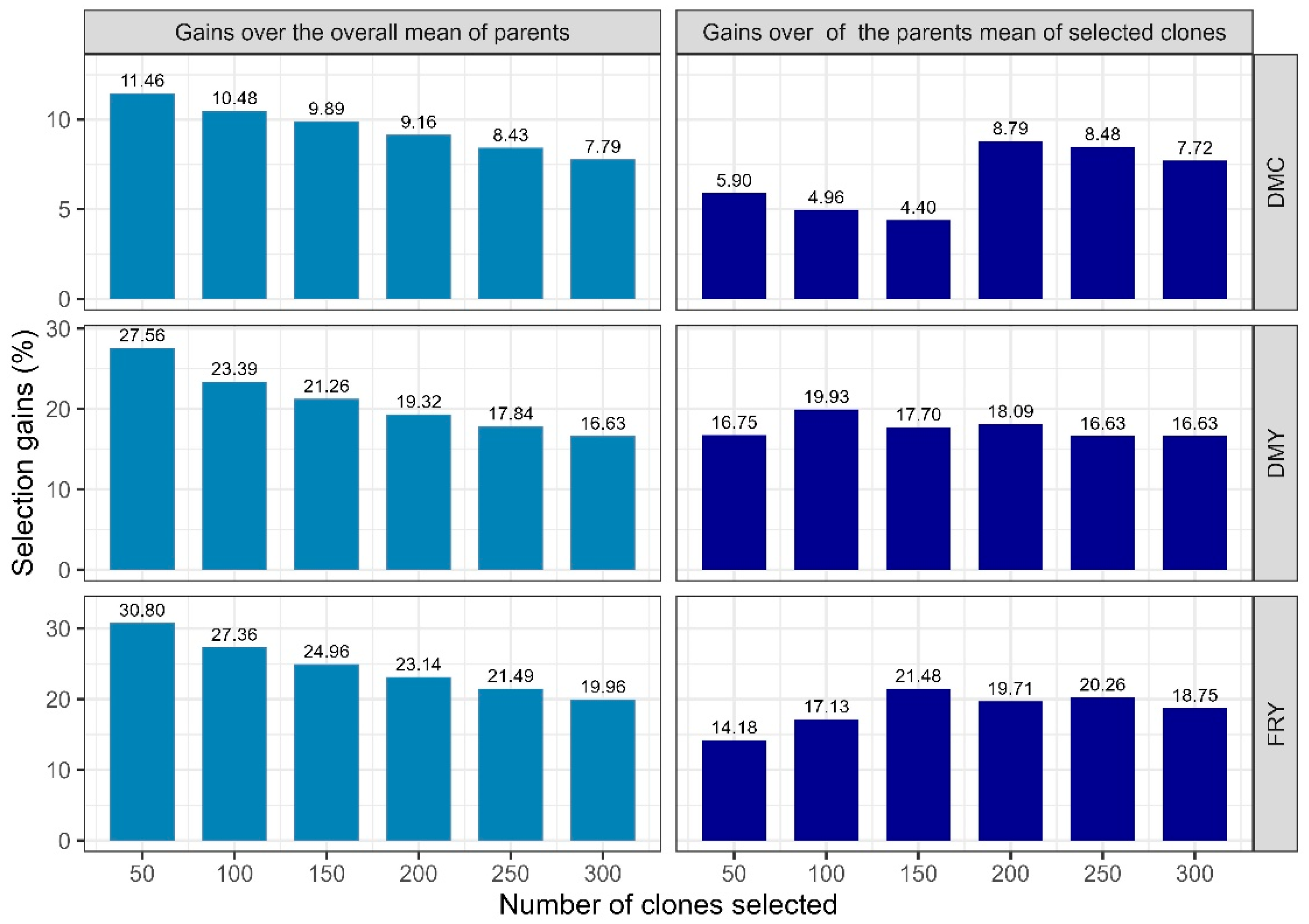
3.3. Selection Gains
3.4. Heterosis
3.4.1. Fresh Root Yield (FRY)
3.4.2. Dry Matter Content (DMC)
3.4.3. Dry Matter Yield (DMY)
4. Discussion
4.1. Phenotypic Analysis and Predictions
4.2. Correlations and Rankings
4.3. Selection Gains
4.4. Heterosis
4.4.1. Fresh Root Yield (FRY)
4.4.2. Dry Matter Content (DMC)
4.4.3. Dry Matter Yield (DMY)
5. Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allem, A.C. The origins and taxonomy of cassava. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CAB International: Walingford, UK, 2002; pp. 1–16. [Google Scholar] [CrossRef]
- Swamy, K.R.M. Origin, distribution, taxonomy, botanical description, genetics and cytogenetics, genetic diversity and breeding of cassava. Int. J. Curr. Res. 2024, 16, 29429–29450. [Google Scholar] [CrossRef]
- Ceballos, H.; Rojanaridpiched, C.; Phumichai, C.; Becerra, L.A.; Kittipadakul, P.; Iglesias, C.; Gracen, V.E. Excellence in cassava breeding: Perspectives for the future. Crop Breed. Genet. Genom. 2020, 2, e200008. [Google Scholar] [CrossRef][Green Version]
- Kistler, L.; Freitas, F.O.; Gutaker, R.M.; Maezumi, S.Y.; Ramos-Madrigal, J.; Simon, M.F.; Flores, J.M.M.; Drovetski, S.V.; Loiselle, H.; Oliveira, E.J.; et al. Historic manioc genomes illuminate traditional maintenance of diversity under long-lived clonal cultivation. Science 2025, 387, eadq0018. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organizations of the United Nations (FAO). FAOSTAT. n.d. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 15 July 2024).
- Ceballos, H.; Kulakow, P.; Hershey, C. Cassava breeding: Current status, bottlenecks and the potential of biotechnology tools. Trop. Plant Biol. 2012, 5, 73–87. [Google Scholar] [CrossRef]
- Malik, A.I.; Kongsil, P.; Nguyễn, V.A.; Ou, W.; Sholihin; Srean, P.; Sheela, M.N.; López-Lavalle, L.A.B.; Utsumi, Y.; Lu, C.; et al. Cassava breeding and agronomy in Asia: 50 years of history and future directions. Breed. Sci. 2020, 70, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Grüneberg, W.; Mwanga, R.; Andrade, M.; Espinoza, J. Selection methods part 5: Breeding clonally propagated crops. In Plant Breeding and Farmer Participation; Cecarelli, S., Guimarães, E.P., Weltzien, E., Eds.; FAO: Rome, Italy, 2009; pp. 275–322. [Google Scholar] [CrossRef]
- Ceballos, H.; Kawuki, R.S.; Gracen, V.E.; Yencho, G.C.; Hershey, C.H. Conventional breeding, marker-assisted selection, genomic selection and inbreeding in clonally propagated crops: A case study for cassava. Theor. Appl. Genet. 2015, 128, 1647–1667. [Google Scholar] [CrossRef]
- Hu, W.; Ji, C.; Liang, Z.; Ye, J.; Ou, W.; Ding, Z.; Zhou, G.; Tie, W.; Yan, Y.; Yang, J.; et al. Resequencing of 388 cassava accessions identifies valuable loci and selection for variation in heterozygosity. Gen. Biol. 2021, 22, 316. [Google Scholar] [CrossRef]
- Zhang, X.; Holley, R.; Egesi, C.N.; Gemenet, D.C.; Moreta, D.; Giomode, W. Towards transforming cassava breeding: Harnessing inbred-parent-based hybrid breeding strategies. Trop. Plants 2024, 3, e025. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F. Introduction to Quantitative Genetics, 4th ed.; Addison Wesley Longman: Harlow, UK, 1996; 464p. [Google Scholar] [CrossRef]
- Viana, J.M.S.; Cruz, C.D.; Cardoso, A.A. Theory and analysis of partial diallel crosses. Genet. Mol. Biol. 1999, 22, 591–599. [Google Scholar] [CrossRef]
- Onofri, A.; Terzaroli, N.; Russi, L. Linear models for diallel crosses: A review with R functions. Theor. Appl. Genet. 2021, 134, 585–601. [Google Scholar] [CrossRef]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and Hybrid Crop Breeding: A Multidisciplinary Review. Front. Genet. 2021, 12, 643761. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, R. Reinventing quantitative genetics for plant breeding: Something old, something new, something borrowed, something BLUE. Heredity 2020, 125, 375–385. [Google Scholar] [CrossRef]
- Weigel, K.A.; VanRaden, P.M.; Norman, H.D.; Grosu, H. A 100-Year Review: Methods and impact of genetic selection in dairy cattle—From daughter–dam comparisons to deep learning algorithms. J. Dairy Sci. 2017, 100, 10234–10250. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, R. Predictive breeding in maize during the last 90 years. Crop Sci. 2021, 61, 2872–2881. [Google Scholar] [CrossRef]
- Ozimati, A.; Kawuki, R.; Esuma, W.; Kayondo, S.I.; Pariyo, A.; Wolfe, M.; Jannink, J.L. Genetic variation and trait correlations in an East African cassava breeding population for genomic selection. Crop Sci. 2019, 59, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Federer, W.T. Augmented (or Hoonuiaku) designs. Hawaii. Plant. Rec. 1956, 55, 191–208. [Google Scholar]
- Kawano, K.; Fukuda, W.M.G.; Cenpukdee, U. Genetic and environmental effects on dry matter content of cassava root. Crop Sci. 1987, 27, 69–74. [Google Scholar] [CrossRef]
- Henderson, C.R. General flexibility of linear model techniques for sire evaluation. J. Dairy Sci. 1974, 57, 963–972. [Google Scholar] [CrossRef]
- Malécot, G. Les mathématiques de l’hérédité; Barnéoud frères: Paris, France, 1948; 63p. [Google Scholar]
- Coster, A. pedigree: Pedigree Functions. R Package Version 1.4. 2013. Available online: https://CRAN.R-project.org/package=pedigree (accessed on 2 February 2025).
- Mrode, R.A. Linear Models for the Prediction of Animal Breeding Values, 3rd ed.; CABI International: Wallingford, UK, 2014; 360p. [Google Scholar]
- Wolfe, M.D.; Del Carpio, D.P.; Alabi, O.; Ezenwaka, L.C.; Ikeogu, U.N.; Kayondo, I.S.; Lozano, R.; Okeke, U.G.; Ozimati, A.A.; Williams, E.; et al. Prospects for genomic selection in cassava breeding. Plant Genome 2017, 10, 1–19. [Google Scholar] [CrossRef]
- Covarrubias-Pazaran, G. Genome-assisted prediction of quantitative traits using the R package sommer. PLoS ONE 2016, 11, e0156744. [Google Scholar] [CrossRef]
- Parkes, E.; Aina, O.; Kingsley, A.; Iluebbey, P.; Bakare, M.; Agbona, A.; Akpotuzor, P.; Labuschagne, M.; Kulakow, P. Combining ability and genetic components of yield characteristics, dry matter content, and total carotenoids in provitamin a cassava F1 cross-progeny. Agronomy 2020, 10, 1850. [Google Scholar] [CrossRef]
- Zacarias, A.M.; Labuschagne, M.T. Diallel analysis of cassava brown streak disease, yield and yield related characteristics in Mozambique. Euphytica 2010, 176, 309–320. [Google Scholar] [CrossRef]
- Athanase, N.; Rob, M. Gene action and heterosis in F1 clonal progenies of cassava for β-Carotene and farmers’ preferred traits. Heliyon 2019, 5, e01807. [Google Scholar] [CrossRef] [PubMed]
- Barandica, O.J.; Pérez, J.C.; Lenis, J.I.; Calle, F.; Morante, N.; Pino, L.; Hershey, C.H.; Ceballos, H. Cassava Breeding II: Phenotypic Correlations through the Different Stages of Selection. Front. Plant Sci. 2016, 7, 1649. [Google Scholar] [CrossRef] [PubMed]
- Resende, M.D.V. Genética Biométrica e Estatística no Melhoramento de Plantas Perenes; Embrapa Florestas: Colombo, Brazil, 2002; 975p. [Google Scholar] [CrossRef]
- Resende, M.D.V.; Duarte, J.B. Precisão e controle de qualidade em experimentos de avaliação de cultivares. Pesqui. Agropecu. Trop. 2007, 37, 182–194. [Google Scholar]
- Torres, L.G.; Vilela de Resende, M.D.; Azevedo, C.F.; Fonseca e Silva, F.; de Oliveira, E.J. Genomic selection for productive traits in biparental cassava breeding populations. PLoS ONE 2019, 14, e0220245. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.R.B.; Sousa, M.B.E.; Oliveira, E.J.; Resende, M.D.V.; Azevedo, C.F. Cassava yield traits predicted by genomic selection methods. PLoS ONE 2019, 14, e0224920. [Google Scholar] [CrossRef]
- Freitas, J.P.X.; Diniz, R.P.; Santos, V.S.; Oliveira, E.J. Genetic parameters and selection gains in early clonal evaluation trials: Implications for cassava breeding. Euphytica 2018, 214, 1–16. [Google Scholar] [CrossRef]
- Andrade, L.R.B.; Sousa, M.B.; Wolfe, M.; Jannink, J.L.; Resende, M.D.V.; Azevedo, C.F.; Oliveira, E.J. Increasing cassava root yield: Additive dominant genetic models for selection of parents and clones. Front. Plant Sci. 2022, 13, 1071156. [Google Scholar] [CrossRef]
- Yuanjit, P.; Vuttipongchaikij, S.; Wonnapinij, P.; Ceballos, H.; Kraichak, E.; Jompuk, C.; Kittipadakul, P. Evaluation of Yield Potential and Combining Ability in Thai Elite Cassava Varieties for Breeding Selection. Agronomy 2023, 13, 1546. [Google Scholar] [CrossRef]
- Cach, N.T.; Lenis, J.I.; Perez, J.C.; Morante, N.; Calle, F.; Ceballos, H. Inheritance of useful traits in cassava grown in subhumid conditions. Plant Breed. 2006, 125, 177–182. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J.B. Quantitative Genetics in Maize Breeding; Springer Science + Business Media: New York, NY, USA, 2010; 663p. [Google Scholar] [CrossRef][Green Version]
- Lynch, M.; Walsh, B. Genetics an Analysis of Quantitative Traits, 1st ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 1998; 980p. [Google Scholar] [CrossRef]
- Garcia, A.A.F.; Frisch, M.; Weng, Y.; Varshney, R.; Sorrells, M.; Fang, D.D. Heterosis and hybrid breeding. Theor. Appl. Genet. 2025, 138, 69. [Google Scholar] [CrossRef] [PubMed]
- Cach, N.T.; Perez, J.C.; Lenis, J.I.; Calle, F.; Morante, N.; Ceballos, H. Epistasis in the Expression of Relevant Traits in Cassava (Manihot esculenta Crantz) for Subhumid Conditions. J. Hered. 2005, 96, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Calle, F.; Perez, J.C.; Gaitán, W.; Morante, N.; Ceballos, H.; Llano, G.; Álvarez, E. Diallel inheritance of relevant traits in cassava (Manihot esculenta Crantz) adapted to acid-soil savannas. Euphytica 2005, 144, 177–186. [Google Scholar] [CrossRef]
- Perez, J.C.; Ceballos, H.; Calle, F.; Morante, N.; Gaitán, W.; Llano, G.; Alvarez, E. Within-family genetic variation and epistasis in cassava (Manihot esculenta Crantz) adapted to the acid-soils environment. Euphytica 2005, 145, 77–85. [Google Scholar] [CrossRef]
- Nonaka, E.; Sirén, J.; Somervuo, P.; Ruokolainen, L.; Ovaskainen, O.; Hanski, I. Scaling up the effects of inbreeding depression from individuals to metapopulations. J. Anim. Ecol. 2019, 88, 1202–1214. [Google Scholar] [CrossRef]
- Rojas, M.C.; Pérez, J.C.; Ceballos, H.; Baena, D.; Morante, N.; Calle, F. Analysis of inbreeding depression in eight S1 cassava families. Crop Sci. 2009, 49, 543–548. [Google Scholar] [CrossRef]
- Kawuki, R.S.; Nuwamanya, E.; Labuschagne, M.T.; Herselman, L.; Ferguson, M.E. Segregation of selected agronomic traits in six S1 cassava families. J. Plant Breed. Crop Sci. 2018, 3, 154–160. [Google Scholar]
- Freitas, J.P.X.; Santos, V.S.; Oliveira, E.J. Inbreeding depression in cassava for productive traits. Euphytica 2016, 209, 137–145. [Google Scholar] [CrossRef]
- Jiwuba, L.; Ogbonna, A.; Ikeogu, U.; Okeakpu, F.; Eluwa, K.; Ekaette, E.; Njoku, D.; Okocha, P.; Egesi, C.; Okogbenin, E. Analysis of inbreeding depression in five S1 Cassava families of elite varieties at the seedling nursery and clonal evaluation trial stages. F1000Research 2024, 13, 966. [Google Scholar] [CrossRef]
- Jansky, S.H.; Charkowski, A.O.; Douches, D.S.; Gusmini, G.; Richael, C.; Bethke, P.C.; Spooner, D.M.; Novy, R.G.; De Jong, H.; De Jong, W.S.; et al. Reinventing Potato as a Diploid Inbred Line–Based Crop. Crop Sci. 2016, 56, 1412–1422. [Google Scholar] [CrossRef]
- Muthoni, J.; Shimelis, H.; Melis, R. Production of hybrid potatoes: Are heterozygosity and ploidy levels important? Aust. J. Crop Sci. 2019, 13, 687–694. [Google Scholar] [CrossRef]
- Grüneberg, W.J.; De Boeck, B.; Diaz, F.; Eyzaguirre, R.; Low, J.W.; Reif, J.C.; Campos, H. Heterosis and Responses to Selection in Orange-Fleshed Sweetpotato (Ipomoea batatas L.) Improved Using Reciprocal Recurrent Selection. Front. Plant Sci. 2022, 13, 793904. [Google Scholar] [CrossRef]
- Lentini, Z.; González, Á.; Tabares, E.; Buitrago, M.E.; Wêdzony, M. Studies on gynogenesis induction in cassava (Manihot esculenta Crantz) unpollinated ovule culture. Front. Plant Sci. 2020, 11, 365. [Google Scholar] [CrossRef]
- Perera, P.I.P.; Ordoñez, C.A.; Lopez-Lavalle, L.A.B.; Dedicova, B. A milestone in the doubled haploid pathway of cassava. Protoplasma 2014, 251, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Hyde, P.T.; Setter, T.L. Long-day photoperiod and cool temperature induce flowering in cassava: Expression of signalling genes. Front. Plant Sci. 2022, 13, 973206. [Google Scholar] [CrossRef]
- Cress, C.E. Heterosis of the hybrid related to gene frequency differences between two populations. Genetics 1966, 53, 269–274. [Google Scholar] [CrossRef]
- Sánchez, T.; Salcedo, E.; Ceballos, H.; Dufour, D.L.; Mafla, G.; Morante, N.; Calle, F.; Pérez, J.C.; Debouck, D.G.; Jaramillo, G.; et al. Screening of starch quality traits in cassava (Manihot esculenta Crantz). Starke 2009, 61, 12–19. [Google Scholar] [CrossRef]
- Bechoff, A.; Tomlins, K.; Fliedel, G.; Lopez-Lavalle, L.A.B.; Westby, A.; Hershey, C.; Dufour, D. Cassava traits and end-user preference: Relating traits to consumer liking, sensory perception, and genetics. Crit. Rev. Food Sci. Nutr. 2018, 58, 547–567. [Google Scholar] [CrossRef]
- Ceballos, H.; Pérez, J.C.; Joaqui Barandica, O.; Lenis, J.I.; Morante, N.; Calle, F.; Pino, L.; Hershey, C.H. Cassava Breeding I: The value of breeding value. Front. Plant Sci. 2016, 7, 1227. [Google Scholar] [CrossRef]


| Parameters | FRY | DMC | DMY |
|---|---|---|---|
| The mean of clones a | 25.23 | 34.35 | 8.69 |
| The mean of parents a | 25.71 | 34.86 | 8.96 |
| Minimum prediction for clones a | 18.89 | 27.60 | 6.84 |
| Maximum prediction for clones a | 36.20 | 40.13 | 12.88 |
| Clones above the mean b | 0.45 | 0.50 | 0.46 |
| Heritability | 0.16 | 0.47 | 0.15 |
| Selection accuracy | 0.67 | 0.75 | 0.64 |
| Prediction ability | 0.19 | 0.46 | 0.20 |
| Bias of prediction | 0.0036 | 0.0024 | −0.0307 |
| Rank. | FRY | DMC | DMY |
|---|---|---|---|
| 1st | BGM 1709 (34.56) | BGM 1116 (39.50) | BGM 0254 (11.57) |
| 2nd | 2003 14-11 (30.79) | BGM 0254 (38.81) | BGM 1709 (11.47) |
| 3rd | BGM 0254 (29.95) | BGM 1632 (37.34) | BGM 2024 (10.57) |
| 4th | BGM 2024 (29.94) | BGM 2038 (36.75) | 2003 14-11 (9.51) |
| 5th | BGM 1660 (27.22) | BRS Gema de Ovo (36.73) | BGM 1660 (9.47) |
| 6th | BGM 0212 (26.36) | BGM 1660 (36.26) | BGM 1632 (9.44) |
| 7th | BGM 1632 (25.78) | BGM 2024 (35.61) | BGM 1116 (9.12) |
| 8th | BRS Jari (25.69) | BGM 1709 (34.90) | BGM 0212 (9.09) |
| 9th | BGM 0131 (23.66) | BGM 0212 (33.92) | BRS Gema de Ovo (8.39) |
| 10th | BGM 2038 (23.27) | BGM 1807 (33.82) | BGM 2038 (8.25) |
| 11th | BRS Gema de Ovo (23.26) | BRS Dourada (33.50) | BRS Dourada (7.73) |
| 12th | BRS Dourada (22.05) | BGM 0131 (32.80) | BGM 1807 (7.65) |
| 13th | BGM 1807 (21.95) | BRS Jari (31.28) | BRS Jari (7.59) |
| 14th | BGM 1116 (21.37) | BGM 1722 (31.12) | BGM 0131 (7.54) |
| 15th | BGM 1722 (19.79) | 2003 14-11 (30.54) | BGM 1722 (7.02) |
| Trait | Number of Clones | Number of Families | Number of Parents Crossed |
|---|---|---|---|
| FRY | 50 | 6 | 6 |
| 100 | 10 | 8 | |
| 150 | 16 | 13 | |
| 200 | 19 | 13 | |
| 250 | 23 | 14 | |
| 300 | 26 | 14 | |
| DMC | 50 | 8 | 7 |
| 100 | 10 | 7 | |
| 150 | 10 | 7 | |
| 200 | 16 | 10 | |
| 250 | 19 | 11 | |
| 300 | 26 | 13 | |
| DMY | 50 | 7 | 7 |
| 100 | 12 | 10 | |
| 150 | 14 | 12 | |
| 200 | 21 | 14 | |
| 250 | 23 | 14 | |
| 300 | 27 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, V.d.S.; Pereira, H.D.; Sampaio Filho, J.S.; Andrade, L.R.B.d. Genetic Merit of Parents and Heterosis in Cassava (Manihot esculenta Crantz). Horticulturae 2025, 11, 714. https://doi.org/10.3390/horticulturae11070714
Santos VdS, Pereira HD, Sampaio Filho JS, Andrade LRBd. Genetic Merit of Parents and Heterosis in Cassava (Manihot esculenta Crantz). Horticulturae. 2025; 11(7):714. https://doi.org/10.3390/horticulturae11070714
Chicago/Turabian StyleSantos, Vanderlei da Silva, Helcio Duarte Pereira, Juraci Souza Sampaio Filho, and Luciano Rogério Braatz de Andrade. 2025. "Genetic Merit of Parents and Heterosis in Cassava (Manihot esculenta Crantz)" Horticulturae 11, no. 7: 714. https://doi.org/10.3390/horticulturae11070714
APA StyleSantos, V. d. S., Pereira, H. D., Sampaio Filho, J. S., & Andrade, L. R. B. d. (2025). Genetic Merit of Parents and Heterosis in Cassava (Manihot esculenta Crantz). Horticulturae, 11(7), 714. https://doi.org/10.3390/horticulturae11070714







