Storage Morphological and Biochemical Performance of Highbush Blueberries (Vaccinium corymbosum L.) Grown Under Photoselective Nets
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Setup
2.2. Fruit Harvest and Storage Conditions
2.3. Fruit Maturity Parameters
2.4. Extraction and Analysis of Sugars, Organic Acids and Phenolic Compounds
2.5. Analysis of Volatile Organic Compounds
2.6. Statistical Analyses
3. Results
3.1. Maturity Parameters
3.2. Primary Metabolites
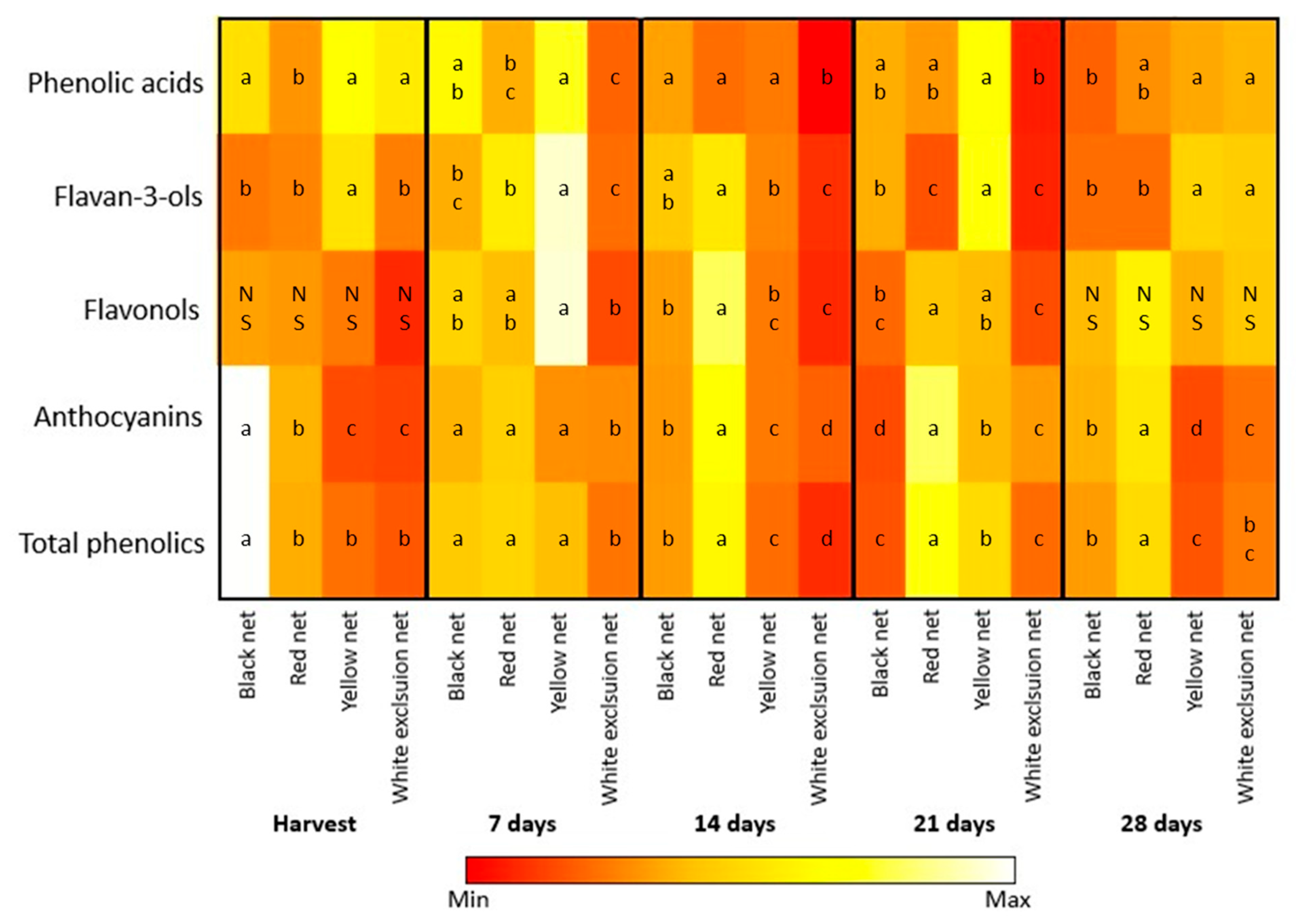
3.3. Phenolic Compounds
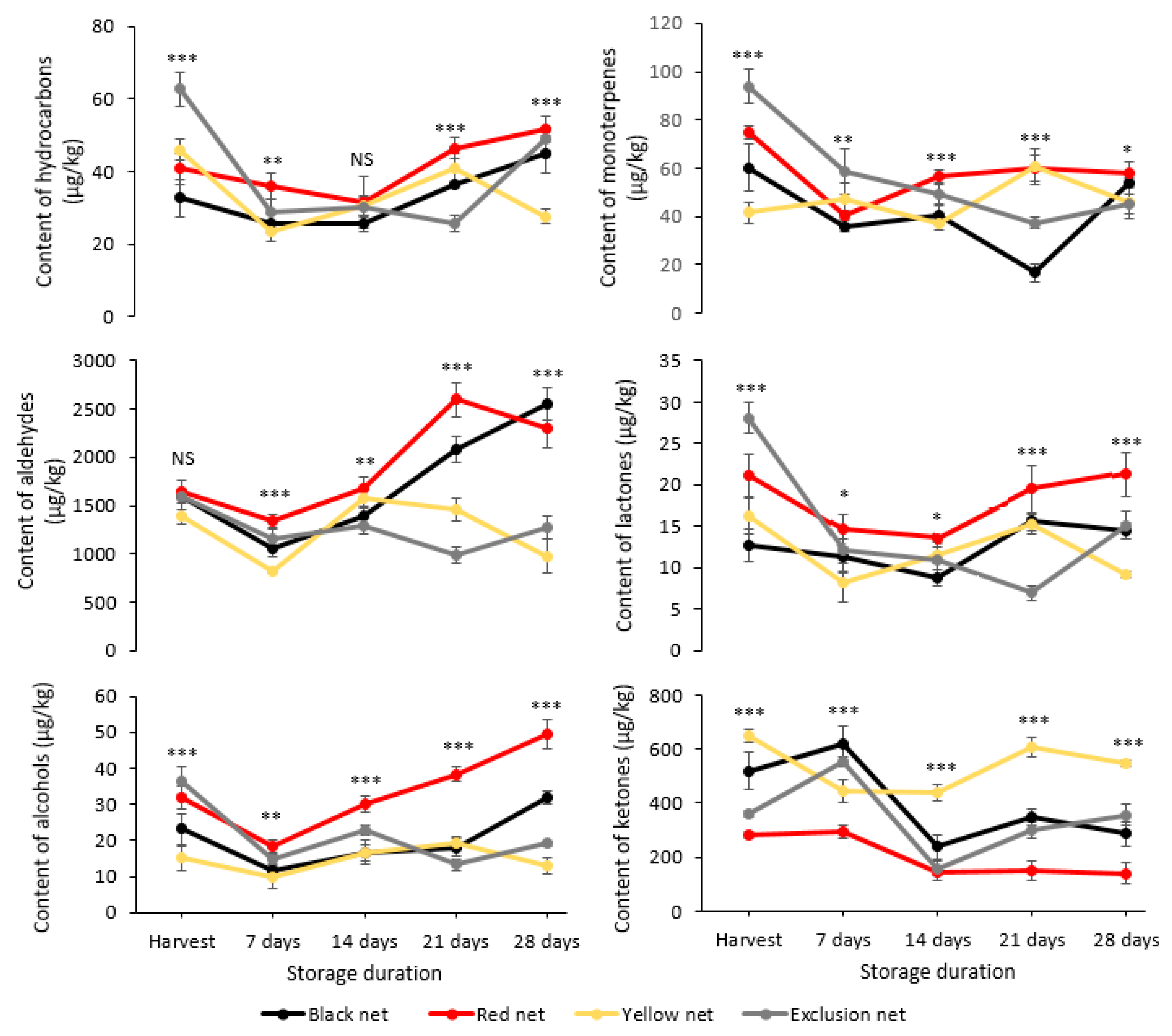
3.4. Volatile Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barcia, M.T.; Jacques, A.C.; Pertuzatti, P.B.; Zambiazi, R.C. Determinação de Ácido Ascórbico e Tocoferóis Em Frutas Por CLAE. Semin. Cienc. Agrar. 2010, 31, 381. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, J.Y.; Li, J.; Zhang, H.; Li, Y.; Farooq, S.; Bacha, S.A.S.; Wang, J. Evaluation of Sugar and Organic Acid Composition and Their Levels in Highbush Blueberries from Two Regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Ikegaya, A.; Toyoizumi, T.; Ohba, S.; Nakajima, T.; Kawata, T.; Ito, S.; Arai, E. Effects of Distribution of Sugars and Organic Acids on the Taste of Strawberries. Food Sci. Nutr. 2019, 7, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Smrke, T.; Veberic, R.; Hudina, M.; Jakopic, J. Pot and Ridge Production of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars under High Tunnels. Agriculture 2022, 12, 438. [Google Scholar] [CrossRef]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Muñoz, P.D. A Review of the Fruit Volatiles Found in Blueberry and Other Vaccinium Species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Grisenti, M.; Ajelli, M.; Betta, E.; Algarra, A.A.; Cappellin, L.; Aprea, E.; Gasperi, F.; Biasioli, F.; et al. Exploring Blueberry Aroma Complexity by Chromatographic and Direct-Injection Spectrometric Techniques. Front. Plant Sci. 2017, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Smrke, T.; Veberic, R.; Hudina, M.; Stamic, D.; Jakopic, J. Comparison of Highbush Blueberry (Vaccinium corymbosum L.) under Ridge and Pot Production. Agriculture 2021, 11, 929. [Google Scholar] [CrossRef]
- Smrke, T.; Grohar, M.C.; Indihar, E.; Veberic, R.; Jakopic, J. Does Photoselective Netting Influence Ripening, Maturity Parameters and Chemical Composition of Highbush Blueberry (Vaccinium corymbosum L.) Fruit? Sci. Hortic. 2024, 337, 113555. [Google Scholar] [CrossRef]
- Smrke, T.; Veberic, R.; Hudina, M.; Zitko, V.; Ferlan, M.; Jakopic, J. Fruit Quality and Yield of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars Grown in Two Planting Systems under Different Protected Environments. Horticulturae 2021, 7, 591. [Google Scholar] [CrossRef]
- Smrke, T.; Vodnik, D.; Veberic, R.; Sircelj, H.; Lenarcic, D.; Jakopic, J. Growing Highbush Blueberries (Vaccinium corymbosum L.) in a Protected Environment—How Much Does a Microclimate Matter? S. Afr. J. Bot. 2023, 160, 260–272. [Google Scholar] [CrossRef]
- Smrke, T.; Stajner, N.; Cesar, T.; Veberic, R.; Hudina, M.; Jakopic, J. Correlation between Destructive and Non-Destructive Measurements of Highbush Blueberry (Vaccinium corymbosum L.) Fruit during Maturation. Horticulturae 2023, 9, 501. [Google Scholar] [CrossRef]
- Milivojević, J.; Radivojević, D.; Ruml, M.; Dimitrijević, M.; Maksimović, J.D. Does Microclimate under Grey Hail Protection Net Affect Biological and Nutritional Properties of “Duke” Highbush Blueberry (Vaccinium corymbosum L.)? Fruits 2016, 71, 161–170. [Google Scholar] [CrossRef]
- McDermott, L.; Nickerson, L. Evaluation of Insect Exclusion and Mass Trapping as Cultural Controls of Spotted Wing Drosophila in Organic Blueberry Production. N. Y. Fruit Q. 2014, 22, 28. [Google Scholar]
- Cormier, D.; Veilleux, J.; Firlej, A. Exclusion Net to Control Spotted Wing Drosophila in Blueberry Fields. IOBC-WPRS Bull. 2015, 109, 181–184. [Google Scholar]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Romero-Bravo, S.; Del Pozo, A. Productivity and Fruit Quality of Vaccinium corymbosum Cv. Elliott under Photo-Selective Shading Nets. Sci. Hortic. 2013, 153, 143–149. [Google Scholar] [CrossRef]
- Shahak, Y.; Gussakovsky, E.E.; Gal, E.; Ganelevin, R. ColorNets: Crop Protection and Light-Quality Manipulation in One Technology. Acta Hortic. 2004, 659, 143–151. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light Quality Manipulation Improves Vegetable Quality at Harvest and Postharvest: A Review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Cobo, N.; del Pozo, A. Spectral Irradiance, Gas Exchange Characteristics and Leaf Traits of Vaccinium corymbosum L. “Elliott” Grown under Photo-Selective Nets. Environ. Exp. Bot. 2012, 75, 142–149. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Stamps, R.H.; Giglia, F.F. Environmental Modification inside Photoselective Shadehouses. HortScience 2013, 48, 975–979. [Google Scholar] [CrossRef]
- Zoratti, L.; Jaakola, L.; Häggman, H.; Giongo, L. Modification of Sunlight Radiation through Colored Photo-Selective Nets Affects Anthocyanin Profile in Vaccinium spp. Berries. PLoS ONE 2015, 10, e0135935. [Google Scholar] [CrossRef]
- Shahak, Y. Photo-Selective Netting for Improved Performance of Horticultural Crops. A Review of Ornamental and Vegetable Studies Carried out in Israel. Acta Hortic. 2008, 770, 161–168. [Google Scholar] [CrossRef]
- Forney, C.F. Postharvest Issues in Blueberry and Cranberry and Methods to Improve Market-Life. Acta Hortic. 2009, 810, 785–798. [Google Scholar] [CrossRef]
- Cantwell, M. Properties and Recommended Conditions for Long-Term Storage of Fresh Fruits and Vegetables; University of California at Davis: Davis, CA, USA, 2001. [Google Scholar]
- Lee, T.C.; Zhong, P.J.; Chang, P.T. The Effects of Preharvest Shading and Postharvest Storage Temperatures on the Quality of “Ponkan” (Citrus Reticulata Blanco) Mandarin Fruits. Sci. Hortic. 2015, 188, 57–65. [Google Scholar] [CrossRef]
- Buthelezi, M.N.D.; Soundy, P.; Jifon, J.; Sivakumar, D. Spectral Quality of Photo-Selective Nets Improves Phytochemicals and Aroma Volatiles in Coriander Leaves (Coriandrum sativum L.) after Postharvest Storage. J. Photochem. Photobiol. B 2016, 161, 328–334. [Google Scholar] [CrossRef]
- Fallik, E.; Alkalai-Tuvia, S.; Parselan, Y.; Aharon, Z.; Elmann, A.; Matan, E.; Yehezkel, H.; Offir, Y.; Ratner, K.; Zur, N.; et al. Can Colored Shade Nets Maintain Sweet Pepper Quality during Storage and Marketing? Acta Hortic. 2009, 830, 37–43. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Manojlović, M. Color Shade Nets Improve Vegetables Quality at Harvest and Maintain Quality During Storage. Contemp. Agric. 2018, 67, 9–19. [Google Scholar] [CrossRef]
- Retamales, J.B.; Montecino, J.M.; Lobos, G.A.; Rojas, L.A. Colored Shading Nets Increase Yields and Profitability of Highbush Blueberries. Acta Hortic. 2008, 770, 193–197. [Google Scholar] [CrossRef]
- Smrke, T.; Weber, N.C.; Veberic, R.; Hudina, M.; Jakopic, J. Modified Atmospheric CO2 Levels for Maintenance of Fruit Weight and Nutritional Quality upon Long-Term Storage in Blueberry (Vaccinium corymbosum L.) ‘Liberty’. Horticulturae 2021, 7, 478. [Google Scholar] [CrossRef]
- Mallik, A.U.; Hamilton, J. Harvest Date and Storage Effect on Fruit Size, Phenolic Content and Antioxidant Capacity of Wild Blueberries of NW Ontario, Canada. J. Food Sci. Technol. 2017, 54, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A. Conservación de Dos Variedades de Arándano Alto En Condiciones de Frío Convencional. Rev. FCA UNCuyo 2004, 36, 53–62. [Google Scholar]
- Saltveit, M.E. Respiratory Metabolism; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132784. [Google Scholar]
- Hancock, J.; Callow, P.; Serçe, S.; Hanson, E.; Beaudry, R. Effect of Cultivar, Controlled Atmosphere Storage, and Fruit Ripeness on the Long-Term Storage of Highbush Blueberries. Horttechnology 2008, 18, 199–205. [Google Scholar] [CrossRef]
- Smrke, T.; Cvelbar Weber, N.; Razinger, J.; Medic, A.; Veberic, R.; Hudina, M.; Jakopic, J. Short-Term Storage in a Modified Atmosphere Affects the Chemical Profile of Blueberry (Vaccinium corymbosum L.) Fruit. Horticulturae 2024, 10, 194. [Google Scholar] [CrossRef]
- Kritzinger, H. Seasonal Patterns in Carbohydrates and Macro Nutrients in Southern Highbush Blueberry Plants. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2014; p. 189. [Google Scholar]
- Retamales, J.B.; Hancock, J.F. Blueberries; Cabi: Wallingford, UK, 2018; Volume 27, ISBN 1780647263. [Google Scholar]
- Paul, V.; Pandey, R.; Srivastava, G.C. The Fading Distinctions between Classical Patterns of Ripening in Climacteric and Non-Climacteric Fruit and the Ubiquity of Ethylene-An Overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef]
- Chen, H.; Cao, S.; Fang, X.; Mu, H.; Yang, H.; Wang, X.; Xu, Q.; Gao, H. Changes in Fruit Firmness, Cell Wall Composition and Cell Wall Degrading Enzymes in Postharvest Blueberries during Storage. Sci. Hortic. 2015, 188, 44–48. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Jiang, A.; Xiu, Z.; Liao, J.; Yang, X.; Guan, Y.; Saren, G.; Feng, K. Effect of Ethanol Treatment on the Quality and Volatiles Production of Blueberries after Harvest. J. Sci. Food Agric. 2019, 99, 6296–6306. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.M.; Moreno, E.; García-Mas, J.; Nicolai, B.; Lammertyn, J.; Monforte, A.J.; Fernández-Trujillo, J.P. Climacteric or Non-Climacteric Behavior in Melon Fruit. 1. Aroma Volatiles. Postharvest Biol. Technol. 2008, 49, 27–37. [Google Scholar] [CrossRef]
- Yuan, F.; Yan, J.; Yan, X.; Liu, H.; Pan, S. Comparative Transcriptome Analysis of Genes Involved in Volatile Compound Synthesis in Blueberries (Vaccinium Virgatum) during Postharvest Storage. Postharvest Biol. Technol. 2020, 170, 111327. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue Light-Emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Liu, Z.; Zheng, T.; Dong, T.; Jin, H.; Jia, H.; Fang, J. Transcriptomic and Metabolomic Profiling Reveals the Effect of LED Light Quality on Fruit Ripening and Anthocyanin Accumulation in Cabernet Sauvignon Grape. Front. Nutr. 2021, 8, 790697. [Google Scholar] [CrossRef]
- Jakopic, J.; Veberic, R.; Stampar, F. The Effect of Reflective Foil and Hail Nets on the Lighting, Color and Anthocyanins of “Fuji” Apple. Sci. Hortic. 2007, 115, 40–46. [Google Scholar] [CrossRef]
- Appel, H.M. Phenolics in Ecological Interactions: The Importance of Oxidation. J. Chem. Ecol. 1993, 19, 1521–1552. [Google Scholar] [CrossRef] [PubMed]
- Selahle, M.K.; Sivakumar, D.; Soundy, P. Effect of Photo-Selective Nettings on Post-Harvest Quality and Bioactive Compounds in Selected Tomato Cultivars. J. Sci. Food Agric. 2014, 94, 2187–2195. [Google Scholar] [CrossRef]
- Lauria, G.; Lo Piccolo, E.; Ceccanti, C.; Paoli, L.; Giordani, T.; Guidi, L.; Malorgio, F.; Massai, R.; Nali, C.; Pellegrini, E.; et al. Supplemental Red Light More than Other Wavebands Activates Antioxidant Defenses in Greenhouse-Cultivated Fragaria × Ananassa Var. Elsanta Plants. Sci. Hortic. 2023, 321, 112319. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of Red and Blue Light on Anthocyanin Accumulation and Differential Gene Expression in Strawberry (Fragaria × Ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yan, J.; Pan, S.; Yuan, F. Changes of the Aroma Composition and Other Quality Traits of Blueberry “Garden Blue” during the Cold Storage and Subsequent Shelf Life. Foods 2020, 9, 1223. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, J.; Luo, C.; Rehman, L.; Li, X.; Xie, X. Advances in Detecting Fruit Aroma Compounds by Combining Chromatography and Spectrometry. J. Sci. Food Agric. 2023, 103, 4755–4766. [Google Scholar] [CrossRef]
- Dias, R.P.; Johnson, T.A.; Ferrão, L.F.V.; Munoz, P.R.; de la Mata, A.P.; Harynuk, J.J. Improved Sample Storage, Preparation and Extraction of Blueberry Aroma Volatile Organic Compounds for Gas Chromatography. J. Chromatogr. Open 2023, 3, 100075. [Google Scholar] [CrossRef]

| 2022 | ||||||
|---|---|---|---|---|---|---|
| Treatment | Storage Duration | L* | C* | h° | Firmness (N) | Total Soluble Solids (°Brix) |
| Black net | Harvest | 30.76 ± 2.18 b AB | 1.41 ± 0.55 b B | 291.8 ± 21.35 A | 0.22 ± 0.09 AB | 11.43 ± 1.30 bc |
| Red net | 33.66 ± 1.92 a | 2.55 ± 0.58 a AB | 278.6 ± 10.53 | 0.20 ± 0.07 | 10.97 ± 1.07 c | |
| Yellow net | 31.32 ± 2.72 ab A | 1.69 ± 0.49 b A | 290.5 ± 23.35 A | 0.29 ± 0.15 | 11.73 ± 0.68 bc | |
| Exclusion net | 31.78 ± 2.69 ab | 1.59 ± 0.71 b AB | 266.8 ± 62.55 AB | 0.22 ± 0.09 | 13.43 ± 1.62 a | |
| Control | 32.00 ± 2.22 ab AB | 1.98 ± 0.53 ab AB | 276.1 ± 10.68 B | 0.23 ± 0.11 | 12.39 ± 1.13 ab | |
| Significance | * | *** | NS | NS | *** | |
| Black net | 18 days | 40.58 ± 20.72 A | 3.27 ± 2.81 A | 233.8 ± 8.92 b B | 0.31 ± 0.11 A | 11.22 ± 0.92 b |
| Red net | 37.45 ± 17.70 | 2.97 ± 2.38 A | 274.3 ± 7.90 ab | 0.25 ± 0.06 | 11.63 ± 0.95 ab | |
| Yellow net | 30.91 ± 2.76 A | 1.90 ± 0.92 A | 281.5 ± 21.7 a A | 0.24 ± 0.05 | 11.99 ± 1.69 ab | |
| Exclusion net | 35.65 ± 18.39 | 2.57 ± 2.63 A | 295.8 ± 24.3 a AB | 0.23 ± 0.08 | 12.81 ± 1.93 a | |
| Control | 38.19 ± 17.30 A | 3.14 ± 2.28 A | 276.7 ± 9.34 ab B | 0.24 ± 0.09 | 12.37 ± 1.37 ab | |
| Significance | NS | NS | ** | NS | * | |
| Black net | 29 days | 26.92 ± 2.78 bc B | 1.51 ± 0.97 B | 302.8 ± 28.76 a A | 0.24 ± 0.11 AB | 11.55 ± 1.48 |
| Red net | 28.48 ± 1.79 abc | 1.67 ± 0.82 BC | 303.5 ± 79.33 a | 0.22 ± 0.08 | 11.25 ± 1.19 | |
| Yellow net | 26.37 ± 2.41 c B | 1.27 ± 1.05 AB | 290.8 ± 25.57 b B | 0.33 ± 0.14 | 11.31 ± 1.63 | |
| Exclusion net | 29.19 ± 2.34 ab | 1.51 ± 0.47 AB | 286.4 ± 17.40 b B | 0.30 ± 0.14 | 12.27 ± 2.11 | |
| Control | 29.46 ± 1.89 a B | 1.36 ± 0.59 BC | 288.9 ± 23.77 b B | 0.32 ± 0.15 | 12.09 ± 1.32 | |
| Significance | *** | NS | ** | NS | NS | |
| Black net | 37 days | 30.60 ± 1.86 AB | 0.73 ± 0.37 b B | 308.1 ± 15.92 ab A | 0.20 ± 0.07 B | 11.29 ± 1.12 |
| Red net | 31.88 ± 2.28 | 1.20 ± 0.31 a C | 289.4 ± 14.62 b | 0.28 ± 0.06 | 11.02 ± 1.34 | |
| Yellow net | 29.78 ± 1.23 A | 0.87 ± 0.28 b B | 299.6 ± 15.29 b A | 0.22 ± 0.06 | 10.87 ± 1.19 | |
| Exclusion net | 30.35 ± 2.19 | 0.95 ± 0.37 ab B | 307.7 ± 27.83 ab A | 0.27 ± 0.07 | 11.73 ± 1.43 | |
| Control | 30.99 ± 1.93 AB | 0.75 ± 0.27 b C | 322.3 ± 26.84 a A | 0.25 ± 0.08 | 11.16 ± 1.59 | |
| Significance | NS | ** | ** | NS | NS | |
| Sign. black net | ** | *** | *** | * | NS | |
| Sign. red net | NS | ** | NS | NS | NS | |
| Sign. yellow net | *** | ** | *** | NS | NS | |
| Sign. exclusion net | NS | * | * | NS | NS | |
| Sign. control | * | *** | *** | NS | NS | |
| 2023 | ||||||
|---|---|---|---|---|---|---|
| Treatment | Storage Duration | L* | C* | h° | Firmness (N) | Total Soluble Solids (°Brix) |
| Black net | Harvest | 31.60 ± 2.18 A | 2.59 ± 0.61 | 296.3 ± 12.78 B | 0.16 ± 0.02 a A | 10.80 ± 1.06 |
| Red net | 32.21 ± 1.89 | 2.44 ± 0.46 B | 285.2 ± 16.55 C | 0.13 ± 0.03 bc AB | 10.57 ± 1.75 A | |
| Yellow net | 31.69 ± 2.56 | 2.41 ± 0.65 B | 284.4 ± 17.08 B | 0.12 ± 0.03 c B | 10.46 ± 1.68 A | |
| Exclusion net | 33.27 ± 2.22 A | 2.65 ± 0.94 | 299.6 ± 25.68 | 0.16 ± 0.05 a AB | 9.98 ± 0.77 | |
| Significance | NS | NS | NS | ** | NS | |
| Black net | 7 days | 29.28 ± 1.32 b AB | 2.17 ± 0.80 | 317.0 ± 17.84 AB | 0.15 ± 0.00 b AB | 9.91 ± 1.21 |
| Red net | 31.58 ± 2.28 ab | 2.49 ± 0.82 AB | 325.2 ± 21.81 AB | 0.16 ± 0.05 b AB | 9.80 ± 1.00 AB | |
| Yellow net | 33.53 ± 1.93 a | 2.87 ± 0.65 AB | 319.7 ± 11.81 A | 0.21 ± 0.05 a A | 8.97 ± 1.06 B | |
| Exclusion net | 31.80 ± 2.83 ab AB | 3.50 ± 1.97 | 321.1 ± 25.04 | 0.17 ± 0.03 ab AB | 9.80 ± 0.99 | |
| Significance | ** | NS | NS | * | NS | |
| Black net | 14 days | 28.37 ± 2.35 b B | 1.81 ± 1.11 | 307.4 ± 27.50 AB | 0.14 ± 0.04 AB | 10.50 ± 1.26 |
| Red net | 31.56 ± 2.80 a | 2.56 ± 0.54 AB | 306.7 ± 26.84 BC | 0.14 ± 0.06 AB | 9.47 ± 1.42 AB | |
| Yellow net | 32.47 ± 1.74 a | 2.92 ± 1.06 AB | 308.0 ± 27.88 A | 0.18 ± 0.06 A | 9.27 ± 0.96 AB | |
| Exclusion net | 31.12 ± 0.86 a AB | 3.17 ± 1.61 | 311.0 ± 32.93 | 0.18 ± 0.06 A | 9.88 ± 0.82 | |
| Significance | ** | NS | NS | NS | NS | |
| Black net | 21 days | 28.29 ± 2.91 b B | 2.64 ± 1.74 | 331.0 ± 24.97 A | 0.17± 0.05 A | 9.71 ± 1.72 a |
| Red net | 32.51 ± 2.89 a | 3.88 ± 1.44 A | 345.0 ± 12.76 A | 0.17 ± 0.03 A | 8.38 ± 0.20 b B | |
| Yellow net | 32.12 ± 1.62 a | 3.32 ± 1.93 AB | 323.4 ± 12.71 A | 0.16 ± 0.03 AB | 8.38 ± 0.78 b B | |
| Exclusion net | 30.41 ± 1.60 ab B | 2.44 ± 0.85 | 317.8 ± 28.18 | 0.16 ± 0.03 AB | 9.63 ± 0.73 ab | |
| Significance | ** | NS | NS | NS | ** | |
| Black net | 28 days | 29.13 ± 1.89 AB | 2.58 ± 1.16 ab | 327.0 ± 22.92 A | 0.12 ± 0.04 B | 9.59 ± 0.82 |
| Red net | 30.69 ± 3.45 | 3.07 ± 1.83 ab AB | 328.9 ± 22.44 AB | 0.11 ± 0.03 B | 9.02 ± 0.97 B | |
| Yellow net | 31.59 ± 1.76 | 3.91 ± 1.27 a A | 330.3 ± 19.44 A | 0.12 ± 0.04 B | 8.64 ± 0.73 B | |
| Exclusion net | 31.22 ± 0.74 AB | 2.10 ± 0.55 b | 312.1 ± 26.34 | 0.11 ± 0.03 B | 9.39 ± 0.39 | |
| Significance | NS | * | NS | NS | NS | |
| Sign. black net | ** | NS | ** | * | NS | |
| Sign. red net | NS | * | *** | * | ** | |
| Sign. yellow net | NS | * | *** | *** | *** | |
| Sign. exclusion net | ** | NS | NS | * | NS | |
| Treatment | Storage Duration | Sucrose | Glucose | Fructose | Total |
|---|---|---|---|---|---|
| Black net | Harvest | 7.34 ± 0.38 c B | 33.75 ± 1.74 a B | 32.64 ± 1.53 a AB | 73.73 ± 3.07 a C |
| Red net | 9.46 ± 0.48 b C | 28.60 ± 0.99 b C | 27.95 ± 1.47 b C | 66.00 ± 2.44 b C | |
| Yellow net | 10.96 ± 0.20 a BC | 24.80 ± 0.69 c C | 24.18 ± 0.81 c B | 59.94 ± 1.40 c C | |
| Exclusion net | 9.95 ± 0.34 b CD | 27.70 ± 0.79 b D | 27.13 ± 1.08 b C | 64.87 ± 1.86 b D | |
| Significance | *** | *** | *** | *** | |
| Black net | 7 days | 10.62 ± 0.96 A | 32.41 ± 1.40 B | 31.31 ± 2.46 ab B | 74.34 ± 3.54 BC |
| Red net | 12.35 ± 0.77 A | 30.63 ± 1.73 BC | 28.89 ± 3.21 b BC | 71.87 ± 4.91 BC | |
| Yellow net | 10.63 ± 1.06 BC | 31.14 ± 0.37 A | 29.95 ± 0.37 b A | 71.73 ± 1.30 AB | |
| Exclusion net | 11.32 ± 0.42 AB | 32.22 ± 1.92 BC | 36.00 ± 0.78 a A | 79.54 ± 1.35 AB | |
| Significance | NS | NS | * | NS | |
| Black net | 14 days | 12.04 ± 0.89 A | 37.84 ± 1.81 a A | 35.23 ± 0.99 a A | 85.11 ± 3.17 a A |
| Red net | 11.66 ± 0.89 AB | 34.66 ± 1.96 ab A | 37.43 ± 1.90 a A | 83.75 ± 0.92 a A | |
| Yellow net | 12.67 ± 0.46 A | 31.43 ± 0.85 b A | 30.66 ± 0.82 b A | 74.76 ± 1.34 b A | |
| Exclusion net | 12.31 ± 0.48 A | 36.34 ± 0.77 a A | 34.82 ± 0.95 a A | 83.47 ± 1.55 a A | |
| Significance | NS | ** | ** | *** | |
| Black net | 21 days | 11.65 ± 0.49 ab A | 35.32 ± 0.66 a AB | 34.42 ± 0.88 a AB | 81.34 ± 1.85 a AB |
| Red net | 10.42 ± 0.14 c BC | 32.88 ± 1.03 b AB | 33.95 ± 1.26 a AB | 77.24 ± 2.12 a AB | |
| Yellow net | 11.88 ± 0.35 a AB | 29.08 ± 0.75 c B | 30.37 ± 1.21 b A | 71.32 ± 2.27 b AB | |
| Exclusion net | 10.67 ± 0.64 bc B | 33.51 ± 0.27 ab B | 33.61 ± 1.15 a A | 77.78 ± 0.46 a BC | |
| Significance | ** | *** | ** | ** | |
| Black net | 28 days | 8.68 ± 0.30 b B | 33.52 ± 0.40 a B | 35.36 ± 0.24 a A | 77.56 ± 0.73 a BC |
| Red net | 8.95 ± 0.43 b C | 31.76 ± 1.68 ab ABC | 33.62 ± 1.71 a AB | 74.33 ± 3.25 ab B | |
| Yellow net | 10.30 ± 0.81 a C | 27.88 ± 0.48 c B | 29.51 ± 0.40 b A | 67.69 ± 1.55 c B | |
| Exclusion net | 9.42 ± 0.17 ab C | 29.79 ± 0.96 bc CD | 30.87 ± 0.78 b B | 70.08 ± 1.57 bc D | |
| Significance | ** | *** | *** | ** | |
| Sign. black net | *** | *** | ** | *** | |
| Sign. red net | *** | ** | *** | *** | |
| Sign. yellow net | * | *** | *** | *** | |
| Sign. exclusion net | ** | *** | *** | *** |
| Treatment | Storage Duration | Citric Acid | Tartaric Acid | Malic Acid | Shikimic Acid | Total |
|---|---|---|---|---|---|---|
| Black net | Harvest | 8.07 ± 0.62 c B | 0.65 ± 0.07 B | 0.79 ± 0.04 c CD | 0.04 ± 0.004 | 9.55 ± 0.66 c D |
| Red net | 9.32 ± 0.29 b AB | 0.68 ± 0.07 | 0.87 ± 0.05 bc | 0.04 ± 0.005 | 10.92 ± 0.40 b AB | |
| Yellow net | 11.36 ± 0.43 a | 0.62 ± 0.17 B | 0.96 ± 0.08 ab C | 0.04 ± 0.005 | 12.98 ± 0.55 a | |
| Exclusion net | 11.02 ± 0.79 a A | 0.73 ± 0.05 | 1.02 ± 0.10 a A | 0.04 ± 0.005 | 12.80 ± 0.92 a A | |
| Significance | *** | NS | *** | NS | *** | |
| Black net | 7 days | 9.72 ± 0.91 A | 0.71 ± 0.10 AB | 0.71 ± 0.05 c D | 0.04 ± 0.006 | 11.18 ± 1.05 BC |
| Red net | 10.59 ± 0.58 A | 0.74 ± 0.05 | 0.94 ± 0.09 ab | 0.04 ± 0.004 | 12.31 ± 0.46 A | |
| Yellow net | 11.09 ± 1.09 | 0.85 ± 0.11 AB | 1.05 ± 0.09 a BC | 0.03 ± 0.002 | 13.02 ± 1.18 | |
| Exclusion net | 10.07 ± 0.46 AB | 0.66 ± 0.04 | 0.81 ± 0.06 bc B | 0.04 ± 0.003 | 11.57 ± 0.51 AB | |
| Significance | NS | NS | ** | NS | NS | |
| Black net | 14 days | 11.24 ± 0.66 A | 0.74 ± 0.12 AB | 0.87 ± 0.09 b BC | 0.04 ± 0.006 | 12.89 ± 0.68 A |
| Red net | 10.68 ± 1.25 A | 0.73 ± 0.13 | 0.94 ± 0.09 b | 0.04 ± 0.002 | 12.40 ± 1.38 A | |
| Yellow net | 11.61 ± 0.53 | 0.85 ± 0.12 AB | 1.15 ± 0.04 a AB | 0.04 ± 0.001 | 13.66 ± 0.61 | |
| Exclusion net | 10.76 ± 0.35 A | 0.69 ± 0.02 | 0.96 ± 0.03 b AB | 0.04 ± 0.002 | 12.44 ± 0.35 A | |
| Significance | NS | NS | ** | NS | NS | |
| Black net | 21 days | 10.39 ± 0.18 b A | 0.75 ± 0.05 b AB | 0.98 ± 0.06 b AB | 0.03 ± 0.003 | 12.16 ± 0.29 b AB |
| Red net | 8.89 ± 0.57 c B | 0.58 ± 0.04 b | 0.80 ± 0.04 c | 0.04 ± 0.004 | 10.31 ± 0.61 c B | |
| Yellow net | 11.66 ± 0.58 a | 1.16 ± 0.29 a A | 1.17 ± 0.02 a AB | 0.04 ± 0.002 | 14.04 ± 0.87 a | |
| Exclusion net | 9.03 ± 0.48 c B | 0.66 ± 0.06 b | 1.02 ± 0.07 b A | 0.04 ± 0.003 | 10.75 ± 0.37 bc B | |
| Significance | *** | ** | *** | NS | *** | |
| Black net | 28 days | 7.58 ± 0.11 b B | 0.90 ± 0.03 A | 1.08 ± 0.03 a A | 0.04 ± 0.003 | 9.60 ± 0.12 b CD |
| Red net | 8.44 ± 0.35 b B | 0.66 ± 0.03 | 0.93 ± 0.06 b | 0.04 ± 0.003 | 10.07 ± 0.43 b B | |
| Yellow net | 10.45 ± 0.70 a | 0.77 ± 0.12 AB | 1.22 ± 0.05 a A | 0.04 ± 0.003 | 12.47 ± 0.76 a | |
| Exclusion net | 9.91 ± 0.26 a AB | 0.85 ± 0.16 | 1.10 ± 0.07 a A | 0.03 ± 0.003 | 11.90 ± 0.48 a AB | |
| Significance | *** | NS | ** | NS | *** | |
| Sign. black net | *** | ** | ** | NS | ** | |
| Sign. red net | ** | NS | NS | NS | ** | |
| Sign. yellow net | NS | *** | ** | NS | NS | |
| Sign. exclusion net | *** | NS | *** | NS | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grohar, M.C.; Indihar, E.; Burin, T.; Veberic, R.; Jakopic, J.; Smrke, T. Storage Morphological and Biochemical Performance of Highbush Blueberries (Vaccinium corymbosum L.) Grown Under Photoselective Nets. Horticulturae 2025, 11, 713. https://doi.org/10.3390/horticulturae11070713
Grohar MC, Indihar E, Burin T, Veberic R, Jakopic J, Smrke T. Storage Morphological and Biochemical Performance of Highbush Blueberries (Vaccinium corymbosum L.) Grown Under Photoselective Nets. Horticulturae. 2025; 11(7):713. https://doi.org/10.3390/horticulturae11070713
Chicago/Turabian StyleGrohar, Mariana Cecilia, Eva Indihar, Tea Burin, Robert Veberic, Jerneja Jakopic, and Tina Smrke. 2025. "Storage Morphological and Biochemical Performance of Highbush Blueberries (Vaccinium corymbosum L.) Grown Under Photoselective Nets" Horticulturae 11, no. 7: 713. https://doi.org/10.3390/horticulturae11070713
APA StyleGrohar, M. C., Indihar, E., Burin, T., Veberic, R., Jakopic, J., & Smrke, T. (2025). Storage Morphological and Biochemical Performance of Highbush Blueberries (Vaccinium corymbosum L.) Grown Under Photoselective Nets. Horticulturae, 11(7), 713. https://doi.org/10.3390/horticulturae11070713








