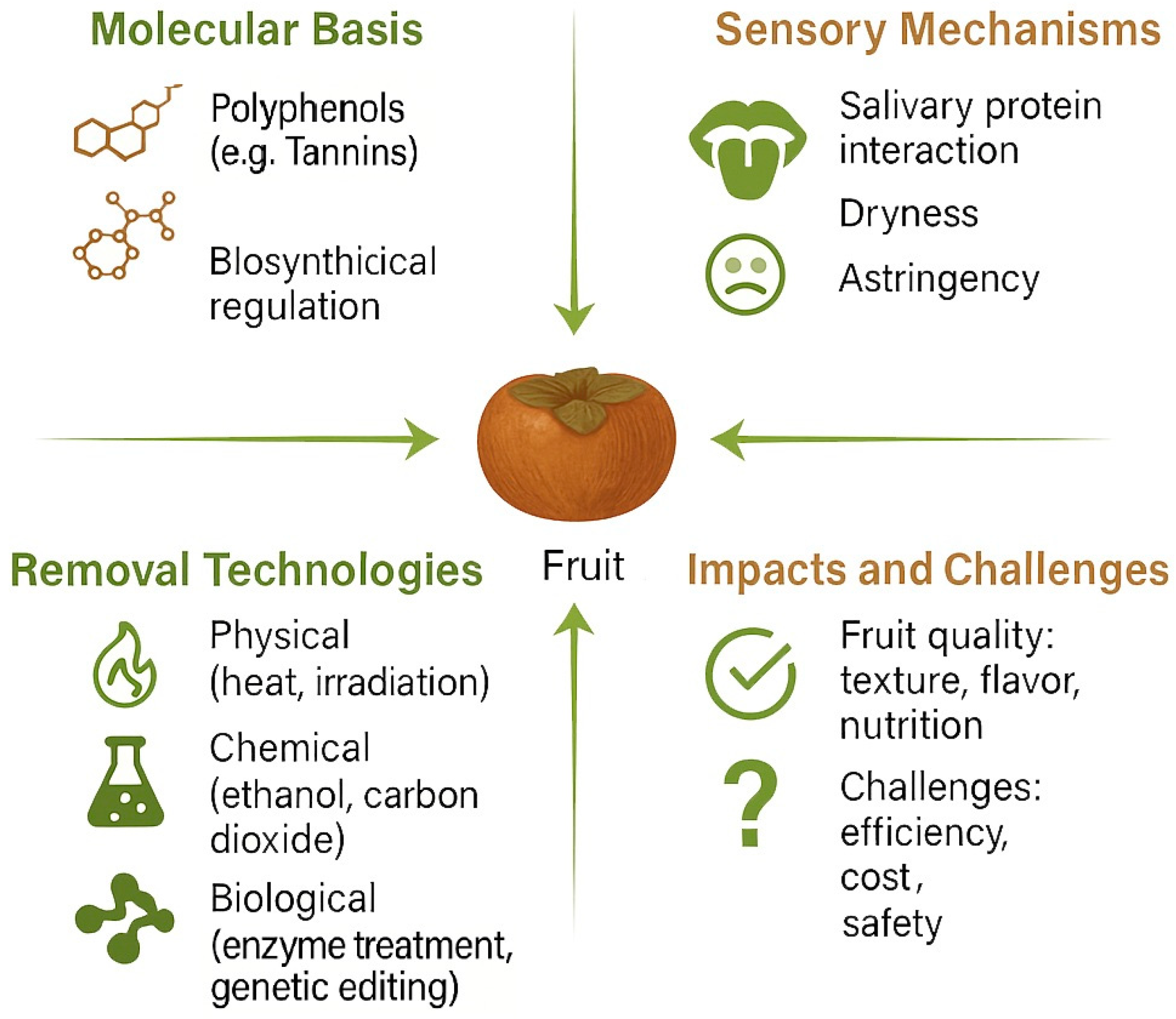
Fruit Astringency: Mechanisms, Technologies, and Future Directions
Abstract
1. Introduction
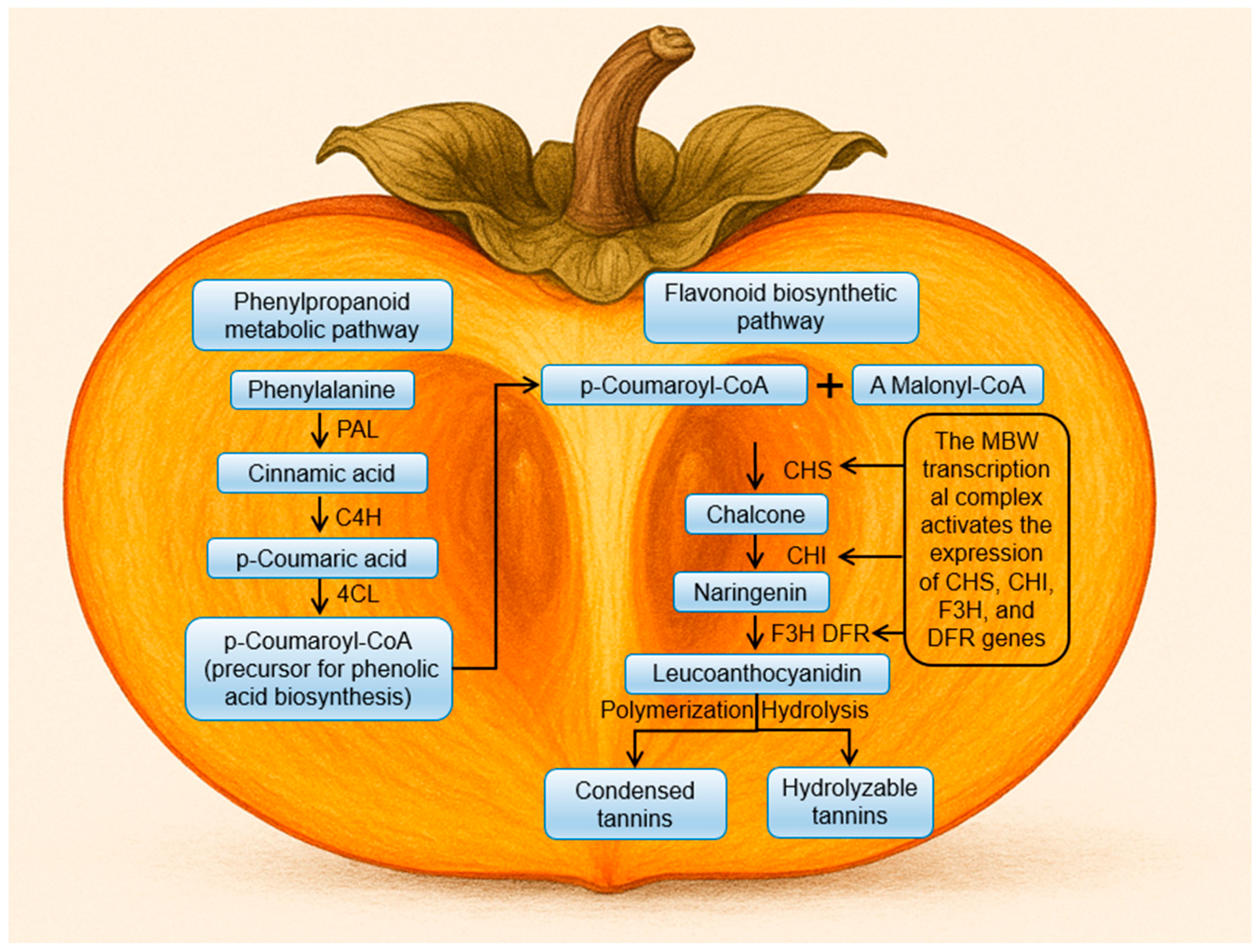
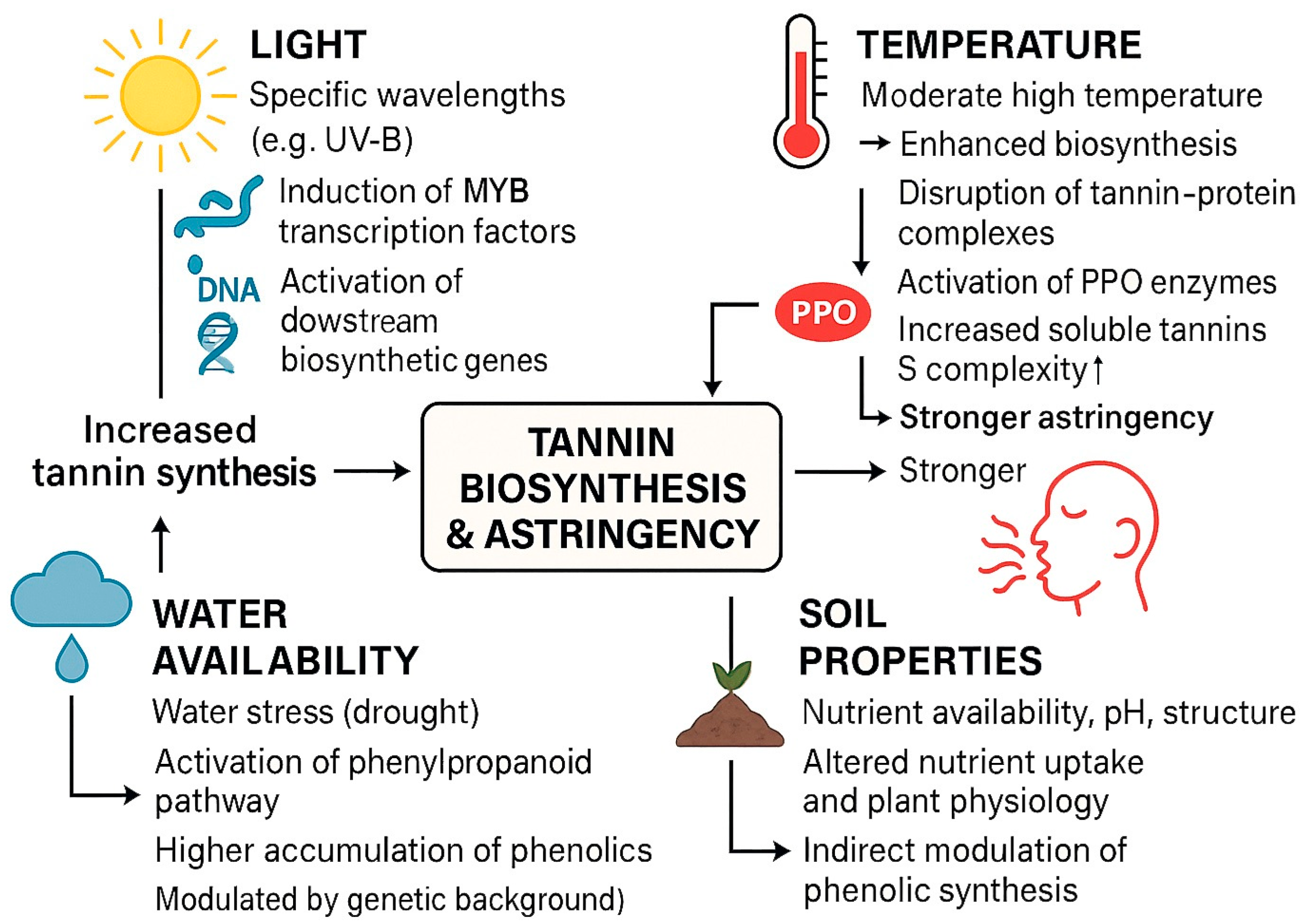
2. Formation of Fruit Astringency: Biosynthesis, Accumulation, and Environmental Regulation
2.1. Biosynthesis and Accumulation of Phenolic Compounds
2.2. Influence of Environmental Factors on Fruit Astringency Formation
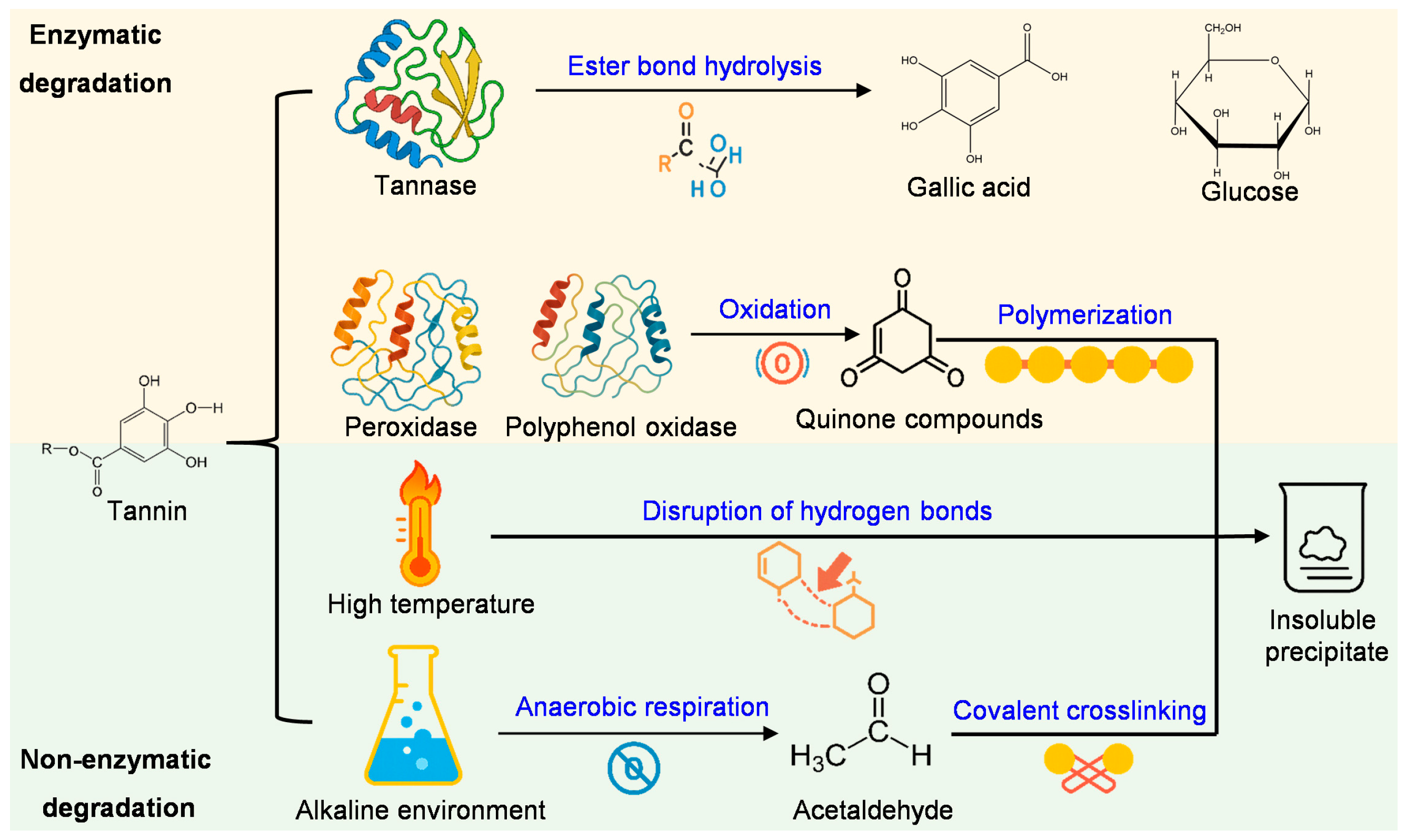
3. Mechanisms of Fruit De-Astringency (Astringency Removal)
3.1. Degradation Mechanisms of Tannins
3.2. Transformation and Complexation of Phenolic Compounds
4. Quality Effects of De-Astringency
4.1. Color Changes
4.2. Texture Changes
4.3. Flavor Changes
4.4. Nutritional Component Changes
5. Fruit De-Astringency Technologies: Principles and Applications
6. Current Applications and Challenges of Fruit De-Astringency Technologies
6.1. Application Practices in Different Fruits
6.2. Challenges in De-Astringency Technologies
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors Influencing Their Sensory Properties and Their Effects on Food and Beverage Preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Gomez-Carneros, C. Bitter Taste, Phytonutrients, and the Consumer: A Review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, R. The Influence of Stage of Ripeness on Consumer Perceptions Regarding Selected Aspects of Non-Astringent Asian Persimmons (Diospyros Kaki Thunb). Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2024. [Google Scholar] [CrossRef]
- Stiletto, A.; Trestini, S. Factors behind Consumers’ Choices for Healthy Fruits: A Review of Pomegranate and Its Food Derivatives. Agric. Food Econ. 2021, 9, 31. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Gomes Reis, M.; Maggs, R.; Hort, J. Understanding Consumer Acceptability of Verjuice, Its Potential Applications and Sensory and Chemical Drivers of Liking. Food Res. Int. 2024, 188, 114480. [Google Scholar] [CrossRef]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A Review on Astringency and Bitterness Perception of Tannins in Wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Cosme, F. Chemical and Sensory Characteristics of Fruit Juice and Fruit Fermented Beverages and Their Consumer Acceptance. Beverages 2022, 8, 33. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial Properties of Red Wine Polyphenols: Astringency and Bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L. (Eds.) Fennema’s Food Chemistry, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-37291-4. [Google Scholar]
- Canon, F.; Paté, F.; Cheynier, V.; Sarni-Manchado, P.; Giuliani, A.; Pérez, J.; Durand, D.; Li, J.; Cabane, B. Aggregation of the Salivary Proline-Rich Protein IB5 in the Presence of the Tannin EgCG. Langmuir ACS J. Surf. Colloids 2013, 29, 1926–1937. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, X.; Zhang, J.; Ju, Y.; Que, Z.; Liao, X.; Lao, F.; Fang, Y.; Ma, T. Letting Wine Polyphenols Functional: Estimation of Wine Polyphenols Bioaccessibility under Different Drinking Amount and Drinking Patterns. Food Res. Int. 2020, 127, 108704. [Google Scholar] [CrossRef]
- Herderich, M.J.; Smith, P.A. Analysis of Grape and Wine Tannins: Methods, Applications and Challenges. Aust. J. Grape Wine Res. 2005, 11, 205–214. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and Hydrolysable Tannins as Antioxidants Influencing the Health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Antoniou, C.; Rouphael, Y.; Graziani, G.; Kyratzis, A. Mapping the Primary and Secondary Metabolomes of Carob (Ceratonia siliqua L.) Fruit and Its Postharvest Antioxidant Potential at Critical Stages of Ripening. Antioxidants 2021, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.D.T.; Zhang, J.; Seididamyeh, M.; Srivarathan, S.; Netzel, M.E.; Sivakumar, D.; Sultanbawa, Y. Hydrolysable Tannins, Physicochemical Properties, and Antioxidant Property of Wild-Harvested Terminalia Ferdinandiana (Exell) Fruit at Different Maturity Stages. Front. Nutr. 2022, 9, 961679. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between Polyphenols and Polysaccharides: Mechanisms and Consequences in Food Processing and Digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Maugeri, A.; Lombardo, G.E.; Cirmi, S.; Süntar, I.; Barreca, D.; Laganà, G.; Navarra, M. Pharmacology and Toxicology of Tannins. Arch. Toxicol. 2022, 96, 1257–1277. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health Effects, Sources, Utilization and Safety of Tannins: A Critical Review. Toxin Rev. 2021, 40, 432–444. [Google Scholar] [CrossRef]
- Melo, L.F.M.d.; Aquino-Martins, V.G.d.Q.; Silva, A.P.d.; Oliveira Rocha, H.A.; Scortecci, K.C. Biological and Pharmacological Aspects of Tannins and Potential Biotechnological Applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S310–S329. [Google Scholar] [CrossRef]
- Chung, H.-S.; Kim, H.-S.; Lee, Y.-G.; Seong, J.-H. Effect of Deastringency Treatment of Intact Persimmon Fruits on the Quality of Fresh-Cut Persimmons. Food Chem. 2015, 166, 192–197. [Google Scholar] [CrossRef]
- Novillo, P.; Salvador, A.; Magalhaes, T.; Besada, C. Deastringency Treatment with CO2 Induces Oxidative Stress in Persimmon Fruit. Postharvest Biol. Technol. 2014, 92, 16–22. [Google Scholar] [CrossRef]
- Fathi-Najafabadi, A.; Salvador, A.; Navarro, P.; Gil, R.; Besada, C. Effect of Temperature during and Immediately after CO2-Deastringency Treatment on Internal Flesh Browning after Cold Storage of Persimmon Fruit. Sci. Hortic. 2020, 268, 109363. [Google Scholar] [CrossRef]
- Ding, Y.; Shen, X.; Ding, Y.; Zhang, P.; Zhu, Q.; Wang, Y.; Zhang, Q.; Luo, Z.; Yang, Y.; Du, X.; et al. A Comprehensive Transcriptomic and Metabolomic Map Reveals the Molecular Mechanism of Persimmon Fruit Deastringency upon 40 °C Warm Water Treatment. Postharvest Biol. Technol. 2025, 220, 113313. [Google Scholar] [CrossRef]
- Brahem, M.; Renard, C.M.G.C.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and Quantification of Fruit Phenolic Compounds of European and Tunisian Pear Cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, L.; Ren, X.; Li, X. Persimmon Peel Deastringency by CO2 and Ethanol Combination: Product Quality and Polyphenols Bioavailability. J. Food Process. Preserv. 2018, 42, e13665. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Z.; Liang, X.; Tu, C.; Khalifa, I.; Wang, C.; Zhu, Y.; Chen, H.; Hu, L.; Li, C. Unlocking the Potential of Persimmons: A Comprehensive Review on Emerging Technologies for Post-Harvest Challenges, Processing Innovations, and Prospective Applications. Food Chem. 2024, 459, 140344. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins—A Final Frontier in Flavonoid Research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and Metabolism of Phenolic Compounds. In Fruit and Vegetable Phytochemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 115–124. ISBN 978-1-119-15804-2. [Google Scholar]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. ISBN 978-3-642-22144-6. [Google Scholar]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-Y.; Sharma, S.B.; Paiva, N.L.; Ferreira, D.; Dixon, R.A. Role of Anthocyanidin Reductase, Encoded by BANYULS in Plant Flavonoid Biosynthesis. Science 2003, 299, 396–399. [Google Scholar] [CrossRef]
- Tanner, G.J.; Francki, K.T.; Abrahams, S.; Watson, J.M.; Larkin, P.J.; Ashton, A.R. Proanthocyanidin Biosynthesis in Plants. Purification of Legume Leucoanthocyanidin Reductase and Molecular Cloning of Its cDNA. J. Biol. Chem. 2003, 278, 31647–31656. [Google Scholar] [CrossRef]
- Akagi, T.; Katayama-Ikegami, A.; Yonemori, K. Proanthocyanidin Biosynthesis of Persimmon (Diospyros kaki Thunb.) Fruit. Sci. Hortic. 2011, 130, 373–380. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional Control of Flavonoid Biosynthesis by MYB–bHLH–WDR Complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef]
- Wang, R.; Shi, X.; Li, K.; Bunker, A.; Li, C. Activity and Potential Mechanisms of Action of Persimmon Tannins According to Their Structures: A Review. Int. J. Biol. Macromol. 2023, 242, 125120. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin Synthesis and Expression of Genes Encoding Leucoanthocyanidin Reductase and Anthocyanidin Reductase in Developing Grape Berries and Grapevine Leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Yu, K.; Jun, J.H.; Duan, C.; Dixon, R.A. VvLAR1 and VvLAR2 Are Bifunctional Enzymes for Proanthocyanidin Biosynthesis in Grapevine. Plant Physiol. 2019, 180, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Bontpart, T.; Ferrero, M.; Khater, F.; Marlin, T.; Vialet, S.; Vallverdù-Queralt, A.; Pinasseau, L.; Ageorges, A.; Cheynier, V.; Terrier, N. Focus on Putative Serine Carboxypeptidase-like Acyltransferases in Grapevine. Plant Physiol. Biochem. PPB 2018, 130, 356–366. [Google Scholar] [CrossRef]
- Yao, S.; Liu, Y.; Zhuang, J.; Zhao, Y.; Dai, X.; Jiang, C.; Wang, Z.; Jiang, X.; Zhang, S.; Qian, Y.; et al. Insights into Acylation Mechanisms: Co-Expression of Serine Carboxypeptidase-like Acyltransferases and Their Non-Catalytic Companion Paralogs. Plant J. Cell Mol. Biol. 2022, 111, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Khater, F.; Fournand, D.; Vialet, S.; Meudec, E.; Cheynier, V.; Terrier, N. Identification and Functional Characterization of cDNAs Coding for Hydroxybenzoate/Hydroxycinnamate Glucosyltransferases Co-Expressed with Genes Related to Proanthocyanidin Biosynthesis. J. Exp. Bot. 2012, 63, 1201–1214. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Sun, C.; Wu, X.; Chen, W.; Luo, Z.; Xu, L.; Zhang, Q. DkDTX1/MATE1 Mediates the Accumulation of Proanthocyanidin and Affects Astringency in Persimmon. Plant Cell Environ. 2024, 47, 5205–5219. [Google Scholar] [CrossRef]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.-P.; Mérillon, J.-M.; Hamdi, S. Characterization of a Grapevine R2R3-MYB Transcription Factor That Regulates the Phenylpropanoid Pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Vialet, S.; Guiraud, J.-L.; Torregrosa, L.; Bertrand, Y.; Cheynier, V.; This, P.; Terrier, N. A Negative MYB Regulator of Proanthocyanidin Accumulation, Identified through Expression Quantitative Locus Mapping in the Grape Berry. New Phytol. 2014, 201, 795–809. [Google Scholar] [CrossRef]
- Shi, X.; Cao, S.; Wang, X.; Huang, S.; Wang, Y.; Liu, Z.; Liu, W.; Leng, X.; Peng, Y.; Wang, N.; et al. The Complete Reference Genome for Grapevine (Vitis vinifera L.) Genetics and Breeding. Hortic. Res. 2023, 10, uhad061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, K.; Li, H.; Kuang, Y.; Liang, Z. Biography of Vitis Genomics: Recent Advances and Prospective. Hortic. Res. 2024, 11, uhae128. [Google Scholar] [CrossRef]
- Ren, C.; Liu, Y.; Guo, Y.; Duan, W.; Fan, P.; Li, S.; Liang, Z. Optimizing the CRISPR/Cas9 System for Genome Editing in Grape by Using Grape Promoters. Hortic. Res. 2021, 8, 52. [Google Scholar] [CrossRef]
- Ren, C.; Gathunga, E.K.; Li, X.; Li, H.; Kong, J.; Dai, Z.; Liang, Z. Efficient Genome Editing in Grapevine Using CRISPR/LbCas12a System. Mol. Hortic. 2023, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, S.; Farooq, U.; Shafi, A.; Akram, K.; Murtaza, M.A.; Kausar, T.; Siddique, F. Chemistry and Functionality of Bioactive Compounds Present in Persimmon. J. Chem. 2016, 2016, 3424025. [Google Scholar] [CrossRef]
- Imperato, F. (Ed.) Phytochemistry: Advances in Research; Research Signpost: Trivandrum, India, 2006; ISBN 978-81-308-0034-9. [Google Scholar]
- Kishor, P.B.K.; Guddimalli, R.; Kulkarni, J.; Singam, P.; Somanaboina, A.K.; Nandimandalam, T.; Patil, S.; Polavarapu, R.; Suravajhala, P.; Sreenivasulu, N.; et al. Impact of Climate Change on Altered Fruit Quality with Organoleptic, Health Benefit, and Nutritional Attributes. J. Agric. Food Chem. 2023, 71, 17510–17527. [Google Scholar] [CrossRef]
- Jenkins, G.I. Signal Transduction in Responses to UV-B Radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef]
- Xu, F.; Gao, X.; Xi, Z.; Zhang, H.; Peng, X.; Wang, Z.; Wang, T.; Meng, Y. Application of Exogenous 24-Epibrassinolide Enhances Proanthocyanidin Biosynthesis in Vitis Vinifera ‘Cabernet Sauvignon’ Berry Skin. Plant Growth Regul. 2015, 75, 741–750. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.K.; Strid, A. UV-B Exposure, ROS, and Stress: Inseparable Companions or Loosely Linked Associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Guy, C.L. Cold Acclimation and Freezing Stress Tolerance: Role of Protein Metabolism. Annu. Rev. Plant Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Ding, Y.; Bi, J.; Chen, J.; Chen, Q.; Morozova, K.; Scampicchio, M.; Zhou, M. The Occurring of Astringency during Persimmon Pulp Drying and Its Correlation with Tannin Derivatives. J. Food Compos. Anal. 2024, 133, 106386. [Google Scholar] [CrossRef]
- Edagi, F.K.; Sestari, I.; Terra, F.A.M.; Chiou, D.G.; Kluge, R.A.; Antoniolli, L.R. Effect of Ripening Stage on Astringency Removal of “Rama Forte” Persimmon. Acta Hortic. 2009, 269–274. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Tavarini, S.; Gil, M.I.; Tomas-Barberan, F.A.; Buendia, B.; Remorini, D.; Massai, R.; Degl’Innocenti, E.; Guidi, L. Effects of Water Stress and Rootstocks on Fruit Phenolic Composition and Physical/Chemical Quality in Suncrest Peach. Ann. Appl. Biol. 2011, 158, 226–233. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press, Inc.: Cambridge, MA, USA, 1995; ISBN 978-0-12-425060-4. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants. Trends Plant Ence 1995, 7. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the Plant-Soil System. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef]
- Xia, Y.; Feng, J.; Zhang, H.; Xiong, D.; Kong, L.; Seviour, R.; Kong, Y. Effects of Soil pH on the Growth, Soil Nutrient Composition, and Rhizosphere Microbiome of Ageratina Adenophora. PeerJ 2024, 12, e17231. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.; Zhong, X.; Chen, X.; Zhang, P.; Deng, Z. Cloning, Purification and Characterization of a Novel Thermostable Recombinant Tannase from Galactobacillus Timonensis. Enzym. Microb. Technol. 2025, 184, 110575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Sreekumar, R.; Sabu, A. Tannase and Its Applications in Food Processing. In Green Bio-Processes: Enzymes in Industrial Food Processing; Parameswaran, B., Varjani, S., Raveendran, S., Eds.; Springer: Singapore, 2019; pp. 357–381. ISBN 978-981-13-3263-0. [Google Scholar]
- Mahmoud, A.E.; Fathy, S.A.; Rashad, M.M.; Ezz, M.K.; Mohammed, A.T. Purification and Characterization of a Novel Tannase Produced by Kluyveromyces Marxianus Using Olive Pomace as Solid Support, and Its Promising Role in Gallic Acid Production. Int. J. Biol. Macromol. 2018, 107, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.; Esteban-Torres, M.; Mancheño, J.M.; de Las Rivas, B.; Muñoz, R. Tannin Degradation by a Novel Tannase Enzyme Present in Some Lactobacillus Plantarum Strains. Appl. Environ. Microbiol. 2014, 80, 2991–2997. [Google Scholar] [CrossRef]
- Yao, W.; Yao, L.; Wang, Z.-E.; Song, X.; Liang, Z. Efficient Photoresponsive One-Dimensional Covalent Organic Framework as Oxidase-like Enzyme for Ultrasensitive Detection of Antioxidants. Talanta 2025, 286, 127519. [Google Scholar] [CrossRef]
- Dantas, T.B.H.; Ferraz, A.C.d.O.; Honório, S.L.; Lima, G.P.P. Polyphenoloxidase Activity in Peaches after Vibration. Eng. Agríc. 2013, 33, 312. [Google Scholar] [CrossRef]
- Novillo, P.; Salvador, A.; Llorca, E.; Hernando, I.; Besada, C. Effect of CO2 Deastringency Treatment on Flesh Disorders Induced by Mechanical Damage in Persimmon. Biochemical and Microstructural Studies. Food Chem. 2014, 145, 454–463. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Onda, M.; Takahashi, T. Effect of Heating Methods on Astringency Recurrence, Syneresis, and Physical Properties of Persimmon Paste. J. Food Sci. Technol. 2021, 58, 4616–4625. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Shimizu, T.; Fujii, Y.; Fushimi, T.; Calabrese, V. Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules 2024, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Liao, H.-J.; Hung, C.-C. Functional, Thermal and Structural Properties of Green Banana Flour (Cv. Giant Cavendish) by De-Astringency, Enzymatic and Hydrothermal Treatments. Plant Foods Hum. Nutr. 2023, 78, 52–60. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary Protein-Phenolic Interactions: Characterization, Biochemical-Physiological Consequences, and Potential Food Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, A.E.; Candir, E.; Toplu, C.; Yildiz, E. Effect of Hot Water Treatment on Astringency Removal in Persimmon Cultivars. Int. J. Fruit Sci. 2020, 20, S557–S569. [Google Scholar] [CrossRef]
- Huang, R.; Xie, X.; Xu, C. Utilization of Egg White Powders to Mitigate the Astringency of Aronia Berry Juice and Produce Protein-Proanthocyanidin Aggregates with Enhanced Stability during Digestion. Food Chem. 2025, 464, 141748. [Google Scholar] [CrossRef] [PubMed]
- González, C.M.; Hernando, I.; Moraga, G. Influence of Ripening Stage and De-Astringency Treatment on the Production of Dehydrated Persimmon Snacks. J. Sci. Food Agric. 2021, 101, 603–612. [Google Scholar] [CrossRef]
- González, C.M.; Gil, R.; Moraga, G.; Salvador, A. Natural Drying of Astringent and Non-Astringent Persimmon “Rojo Brillante”. Drying Kinetics and Physico-Chemical Properties. Foods 2021, 10, 647. [Google Scholar] [CrossRef]
- Han, W.; Cao, K.; Diao, S.; Sun, P.; Li, H.; Mai, Y.; Suo, Y.; Fu, J. Characterization of Browning during CO2 Deastringency Treatment in Astringent Persimmon Fruit. J. Food Meas. Charact. 2022, 16, 2273–2281. [Google Scholar] [CrossRef]
- Matsuo, T.; Itoo, S. A Model Experiment for De-Astringency of Persimmon Fruit with High Carbon Dioxide Treatment: In Vitro Gelation of Kaki-Tannin by Reacting with Acetaldehyde. Agric. Biol. Chem. 1982, 46, 683–689. [Google Scholar] [CrossRef]
- Dheeraj; Srivastava, A.; Mishra, A. Mitigation of Cashew Apple Fruits Astringency. Environ. Sustain. 2023, 6, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G. Quantitative and Sensory Aspects of Flavor of Tomato and Other Vegertables and Fruits. Flavor Sci. Sensib. Princ. Tech. 1993, 259–286. [Google Scholar]
- Young, H.; Paterson, V.J. Characterisation of Bound Flavour Components in Kiwifruit. J. Sci. Food Agric. 1995, 68, 257–260. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, X.; Li, X.; Yang, S.; Ferguson, I.B.; Chen, K. Lipoxygenase Gene Expression in Ripening Kiwifruit in Relation to Ethylene and Aroma Production. J. Agric. Food Chem. 2009, 57, 2875–2881. [Google Scholar] [CrossRef]
- Jin, R.; Wu, W.; Liu, X.; Chen, K.; Yin, X. Dof Transcription Factors Are Involved in High CO2 Induced Persimmon Fruit Deastringency. Horticulturae 2022, 8, 643. [Google Scholar] [CrossRef]
- Taira, S.; Itamura, H.; Abe, K.; Watanabe, S. Comparison of the Characteristics of Removal of Astringency in Two Japanese Persimmon Cultivars, ‘Denkuro’ and ‘Hiratanenashi’. J. Jpn. Soc. Hortic. Sci. 1989, 58, 319–325. [Google Scholar] [CrossRef][Green Version]
- Guan, C.; Chen, L.; Chen, W.; Mo, R.; Zhang, Q.; Du, X.; Liu, J.; Luo, Z. SSAP Analysis Reveals Candidate Genes Associated with Deastringency in Persimmon (Diospyros kaki Thunb.) Treated with 40 °C Water. Tree Genet. Genomes 2015, 11, 20. [Google Scholar] [CrossRef]
- Novillo, P.; Salvador, A.; Navarro, P.; Besada, C. Sensitivity of Astringent and Non-Astringent Persimmon Cultivars to Flesh Disorders Induced by Mechanical Damage. Acta Hortic. 2015, 605–610. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Liang, J.; Ou, M.; Chen, J.; Li, G. Extraction, Purification and Anti-Radiation Activity of Persimmon Tannin from Diospyros kaki L.f. J. Environ. Radioact. 2016, 162–163, 182–188. [Google Scholar] [CrossRef]
- Kim, B.-O.; Cha, W.-S.; Ahn, D.-H.; Cho, Y.-J. The change on cell wall composition and physiological characteristic of astringent persimmon fruits by gamma irradiation. Food Sci. Preserv. 2015, 22, 512–519. [Google Scholar] [CrossRef]
- Cancino-Vázquez, R.; Salvador-Figueroa, M.; Hernández-Ortiz, E.; Grajales-Conesa, J.; Vázquez-Ovando, A. Gamma Irradiation of Mango ‘Ataulfo’ at Low Dose: Effect on Texture, Taste, and Odor Fruit. Food Sci. Technol. Res. 2020, 26, 59–64. [Google Scholar] [CrossRef]
- Xia, T.; Shi, S.; Wan, X. Impact of Ultrasonic-Assisted Extraction on the Chemical and Sensory Quality of Tea Infusion. J. Food Eng. 2006, 74, 557–560. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Yang, K.; Qi, Y.; Zhang, J.; Fan, M.; Wei, X. Optimization of Ultrasonic-Assisted Extraction of Antioxidant Tannin from Young Astringent Persimmon (Diospyros kaki L.) Using Response Surface Methodology. J. Food Process. Preserv. 2018, 42, e13657. [Google Scholar] [CrossRef]
- Maldonado, R.; Molina-Garcia, A.D.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. High CO2 Atmosphere Modulating the Phenolic Response Associated with Cell Adhesion and Hardening of Annona Cherimola Fruit Stored at Chilling Temperature. J. Agric. Food Chem. 2002, 50, 7564–7569. [Google Scholar] [CrossRef]
- Bibi, N.; Chaudry, M.A.; Khan, F.; Ali, Z.; Sattar, A. Phenolics and Physico-Chemical Characteristics of Persimmon during Post-Harvest Storage. Food/Nahrung 2001, 45, 82–86. [Google Scholar] [CrossRef]
- Bibi, N.; Khattak, A.B. Effect of Modified Atmosphere on Methanol Extractable Phenolics of Persimmon Modified Atmosphere Effect on Persommon Phenolics. Int. J. Food Sci. Technol. 2007, 42, 185–189. [Google Scholar] [CrossRef]
- Pesis, E.; Ben-Arie, R. Carbon Dioxide Assimilation during Postharvest Removal of Astringency from Persimmon Fruit. Physiol. Plant. 1986, 67, 644–648. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L.; Chen, L. Bioaccessibility of Total Phenolic Concentration and Antioxidant Capacity of Pomegranate Fruit Juice and Marc After in vitro Digestion. Acta Hortic. 2015, 285–290. [Google Scholar] [CrossRef]
- Chung, H.-S.; Kim, D.-H.; Kim, H.-S.; Lee, Y.-G.; Seong, J.-H.; Youn, K.-S.; Moon, K.-D. Quality Comparison of Dried Slices Processed from Whole Persimmons Treated with Different Deastringency Methods. Food Sci. Biotechnol. 2017, 26, 401–407. [Google Scholar] [CrossRef]
- Rozana, R.; Tantalu, L. Produksi Kesemek Non-Astrigensi Dengan Perlakuan Hot Water Treatment Dan Aplikasi Koh. J. Food Technol. Agroind. 2019, 1, 26–35. [Google Scholar] [CrossRef]
- Li, Y.R.; Zhang, H.Y.; Wang, A.M.; Sima, Z.W. Research and Design on the Control System of the Deastringency of Fresh Persimmon. Adv. Mater. Res. 2011, 328–330, 2000–2003. [Google Scholar] [CrossRef]
- Ahmed, J. Green Banana Processing, Products and Functional Properties. In Handbook of Banana Production, Postharvest Science, Processing Technology, and Nutrition; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 141–168. ISBN 978-1-119-52826-5. [Google Scholar]
- Liao, H.-J.; Hung, C.-C. Chemical Composition and in Vitro Starch Digestibility of Green Banana (Cv. Giant Cavendish) Flour and Its Derived Autoclaved/Debranched Powder. LWT-Food Sci. Technol. 2015, 64, 639–644. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Alebidi, A.I.; Al-Obeed, R.S.; Soliman, S.S. Preharvest Ethephon Spray on Fruit Quality and Increasing the Rate of Ripening of Date Palm Fruit (Phoenix dactylifera L.) Cv. Helali. Prog. Nutr. 2017, 19, 97–103. [Google Scholar] [CrossRef]
- Pesis, E. The Role of the Anaerobic Metabolites, Acetaldehyde and Ethanol, in Fruit Ripening, Enhancement of Fruit Quality and Fruit Deterioration. Postharvest Biol. Technol. 2005, 37, 1–19. [Google Scholar] [CrossRef]
- Ketsa, S.; Warrington, I.J. Anthocyanin Physiology and Biochemistry in Fleshy Fruit Species: Mangosteen as a Model. Crop Sci. 2024, 64, 1987–2013. [Google Scholar] [CrossRef]
- Igiebor, F.A.; Odozi, E.B.; Ikhajiagbe, B. Chemical-Based Fruit Ripening and the Implications for Ecosystem Health and Safety. In One Health Implications of Agrochemicals and their Sustainable Alternatives; Ogwu, M.C., Chibueze Izah, S., Eds.; Springer Nature: Singapore, 2023; pp. 335–353. ISBN 978-981-99-3439-3. [Google Scholar]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food Phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Zou, B.; Wu, J.; Yu, Y.; Xiao, G.; Xu, Y. Evolution of the Antioxidant Capacity and Phenolic Contents of Persimmon during Fermentation. Food Sci. Biotechnol. 2017, 26, 563–571. [Google Scholar] [CrossRef]
- Taskin, M. Co-Production of Tannase and Pectinase by Free and Immobilized Cells of the Yeast Rhodotorula Glutinis MP-10 Isolated from Tannin-Rich Persimmon (Diospyros kaki L.) Fruits. Bioprocess Biosyst. Eng. 2013, 36, 165–172. [Google Scholar] [CrossRef]
- Vaquero, I.; Marcobal, Á.; Muñoz, R. Tannase Activity by Lactic Acid Bacteria Isolated from Grape Must and Wine. Int. J. Food Microbiol. 2004, 96, 199–204. [Google Scholar] [CrossRef]
- Aguilar, C.N.; Rodríguez, R.; Gutiérrez-Sánchez, G.; Augur, C.; Favela-Torres, E.; Prado-Barragan, L.A.; Ramírez-Coronel, A.; Contreras-Esquivel, J.C. Microbial Tannases: Advances and Perspectives. Appl. Microbiol. Biotechnol. 2007, 76, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Taira, S.; Ono, M.; Matsumoto, N. Reduction of Persimmon Astringency by Complex Formation between Pectin and Tannins. Postharvest Biol. Technol. 1997, 12, 265–271. [Google Scholar] [CrossRef]
- Hao, S. Study on Enzymatic Deastringency of Hovenia dulcis and Formulation Optimization of Compound Beverage with Green Tea. Storage Process 2023, 23, 42–48. [Google Scholar]
- Amorim, C.; Antoniolli, L.R.; Orsi, B.; Kluge, R.A. Advances in Metabolism and Genetic Control of Astringency in Persimmon (Diospyros kaki Thunb.) Fruit: A Review. Sci. Hortic. 2023, 308, 111561. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, M.; Xu, L.; Zhang, Q.; Zhou, C.; Hu, X.; Luo, Z. Recent Advances in Natural Deastringency and Genetic Improvement of Chinese PCNA Persimmon (Diospyros kaki). Horticulturae 2023, 9, 1273. [Google Scholar] [CrossRef]
- Constabel, C.P. Molecular Controls of Proanthocyanidin Synthesis and Structure: Prospects for Genetic Engineering in Crop Plants. J. Agric. Food Chem. 2018, 66, 9882–9888. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Wang, M.; Zhang, Y.; Ruan, X.; Zhang, Q.; Luo, Z.; Yang, Y. DkWRKY Interacts with Pyruvate Kinase Gene DkPK1 and Promotes Natural Deastringency in C-PCNA Persimmon. Plant Sci. Int. J. Exp. Plant Biol. 2020, 290, 110285. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef]
- Dederer, H.-G.; Hamburger, D. (Eds.) Regulation of Genome Editing in Plant Biotechnology: A Comparative Analysis of Regulatory Frameworks of Selected Countries and the EU; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-17118-6. [Google Scholar]
- Kato-Nitta, N.; Maeda, T.; Inagaki, Y.; Tachikawa, M. Expert and Public Perceptions of Gene-Edited Crops: Attitude Changes in Relation to Scientific Knowledge. Palgrave Commun. 2019, 5, 137. [Google Scholar] [CrossRef]
- Funk, C. The New Food Fights: U.S. Public Divides Over Food Science; Pew Research Center: Washington, DC, USA, 2016. [Google Scholar]
- Zhang, S.; Shen, J.; Li, D.; Cheng, Y. Strategies in the Delivery of Cas9 Ribonucleoprotein for CRISPR/Cas9 Genome Editing. Theranostics 2021, 11, 614–648. [Google Scholar] [CrossRef]
- Smyth, S.J. Canadian Regulatory Perspectives on Genome Engineered Crops. GM Crops Food 2017, 8, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Novillo, P.; Gil, R.; Besada, C.; Salvador, A. Astringency Removal of “Rojo Brillante” Persimmon by Combining CO2 and Ethanol Application. Acta Hortic. 2015, 599–604. [Google Scholar] [CrossRef]
- Vilhena, N.Q.; Tessmer, M.A.; Hernando, I.; Kluge, R.A.; Quiles, A.; Salvador, A. Structural Changes Caused by CO2 or Ethanol Deastringency Treatments in Cold-Stored ‘Giombo’ Persimmon. Agronomy 2022, 12, 2464. [Google Scholar] [CrossRef]
- Chen, W.; Xiong, Y.; Xu, L.; Zhang, Q.; Luo, Z. An Integrated Analysis Based on Transcriptome and Proteome Reveals Deastringency-Related Genes in CPCNA Persimmon. Sci. Rep. 2017, 7, 44671. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.; Filho, E.G.A.; Garruti, D.S.; Bender, R.J.; Antoniolli, L.R. Predictive Modeling and Correlation between the Sensory and Physicochemical Attributes in ‘Rama Forte’ Astringent Persimmon. Sci. Hortic. 2024, 338, 113753. [Google Scholar] [CrossRef]
- Husnayain, N.; Adi, P.; Mulyani, R.; Tsai, S.-Y.; Chang, C.-K.; Punthi, F.; Yudhistira, B.; Cheng, K.-C.; Hsieh, C.-W. Active Packaging of Chitosan-Casein Phosphopeptide Modified Plasma–Treated LDPE for CO2 Regulation to Delay Texture Softening and Maintain Quality of Fresh-Cut Slice Persimmon During Storage. Food Bioprocess Technol. 2025, 18, 5532–5548. [Google Scholar] [CrossRef]
- Marsilio, V.; Campestre, C.; Lanza, B. Phenolic Compounds Change during California-Style Ripe Olive Processing. Food Chem. 2001, 74, 55–60. [Google Scholar] [CrossRef]
- Watkins, C.B. The Use of 1-Methylcyclopropene (1-MCP) on Fruits and Vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Conte, P.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Piga, A. Table Olives: An Overview on Effects of Processing on Nutritional and Sensory Quality. Foods 2020, 9, 514. [Google Scholar] [CrossRef]
- Chinprahast, N.; Siripatrawan, U.; Leerahawong, A.; Traiananwuttikul, K. Effects of Blanching and Vacuum Impregnation on Physicochemical and Sensory Properties of Indian Gooseberry (Phyllanthus emblica L.). J. Food Process. Preserv. 2013, 37, 57–65. [Google Scholar] [CrossRef]
- Salvador, A.; Arnal, L.; Besada, C.; Larrea, V.; Quiles, A.; Pérez-Munuera, I. Physiological and Structural Changes during Ripening and Deastringency Treatment of Persimmon Fruit Cv. ‘Rojo Brillante’. Postharvest Biol. Technol. 2007, 46, 181–188. [Google Scholar] [CrossRef]
- Yan, T.; Zhao, Y.; Jiang, Z.; Chen, J. Acetaldehyde Induces Cytotoxicity via Triggering Mitochondrial Dysfunction and Overactive Mitophagy. Mol. Neurobiol. 2022, 59, 3933–3946. [Google Scholar] [CrossRef]
- Boeckx, J.; Pols, S.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Regulation of the Central Carbon Metabolism in Apple Fruit Exposed to Postharvest Low-Oxygen Stress. Front. Plant Sci. 2019, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Benkeblia, N. Physiological and Biochemical Response of Tropical Fruits to Hypoxia/Anoxia. Front. Plant Sci. 2021, 12, 670803. [Google Scholar] [CrossRef]
- Goto, Y.; Watanabe, O. Development of Inhibitory Technique for Astringency Recurrence in Astringent Persimmon Fruit. Nippon Shokuhin Kagaku Kogaku Kaishi 2010, 57, 220–223. [Google Scholar] [CrossRef]
- Habashi, R.; Hacham, Y.; Dhakarey, R.; Matityahu, I.; Holland, D.; Tian, L.; Amir, R. Elucidating the Role of Shikimate Dehydrogenase in Controlling the Production of Anthocyanins and Hydrolysable Tannins in the Outer Peels of Pomegranate. BMC Plant Biol. 2019, 19, 476. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; McGhie, T.K.; Andre, C.M.; Hellens, R.P.; Allan, A.C. Transcriptional Analysis of Apple Fruit Proanthocyanidin Biosynthesis. J. Exp. Bot. 2012, 63, 5437–5450. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Win, N.M.; Mang, H.; Cho, Y.-J.; Jung, H.-Y.; Kang, I.-K. Effects of 1-Methylcyclopropene Treatment on Fruit Quality during Cold Storage in Apple Cultivars Grown in Korea. Horticulturae 2021, 7, 338. [Google Scholar] [CrossRef]
- Munera, S.; Aleixos, N.; Besada, C.; Gómez-Sanchis, J.; Salvador, A.; Cubero, S.; Talens, P.; Blasco, J. Discrimination of Astringent and Deastringed Hard ‘Rojo Brillante’ Persimmon Fruit Using a Sensory Threshold by Means of Hyperspectral Imaging. J. Food Eng. 2019, 263, 173–180. [Google Scholar] [CrossRef]
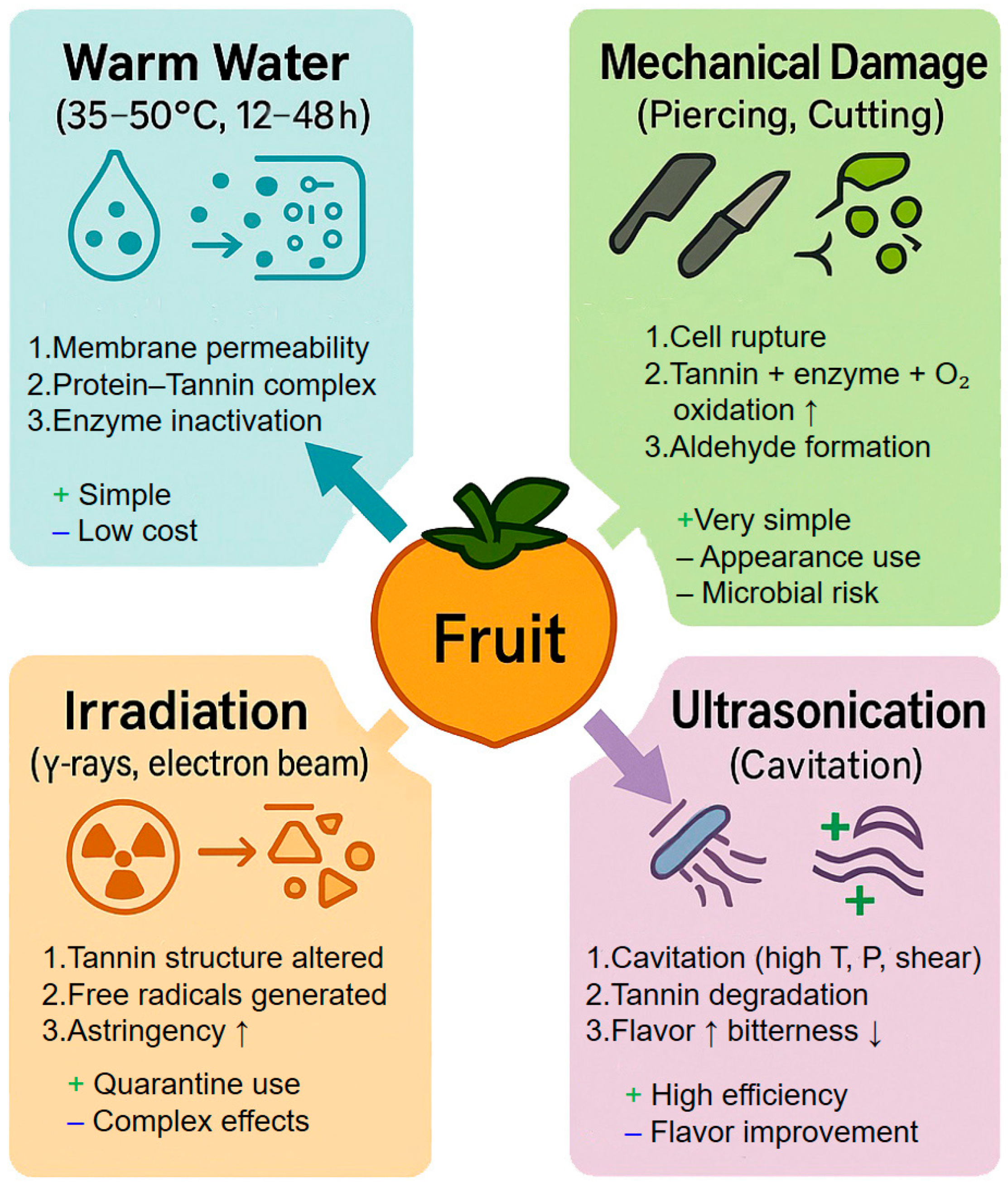
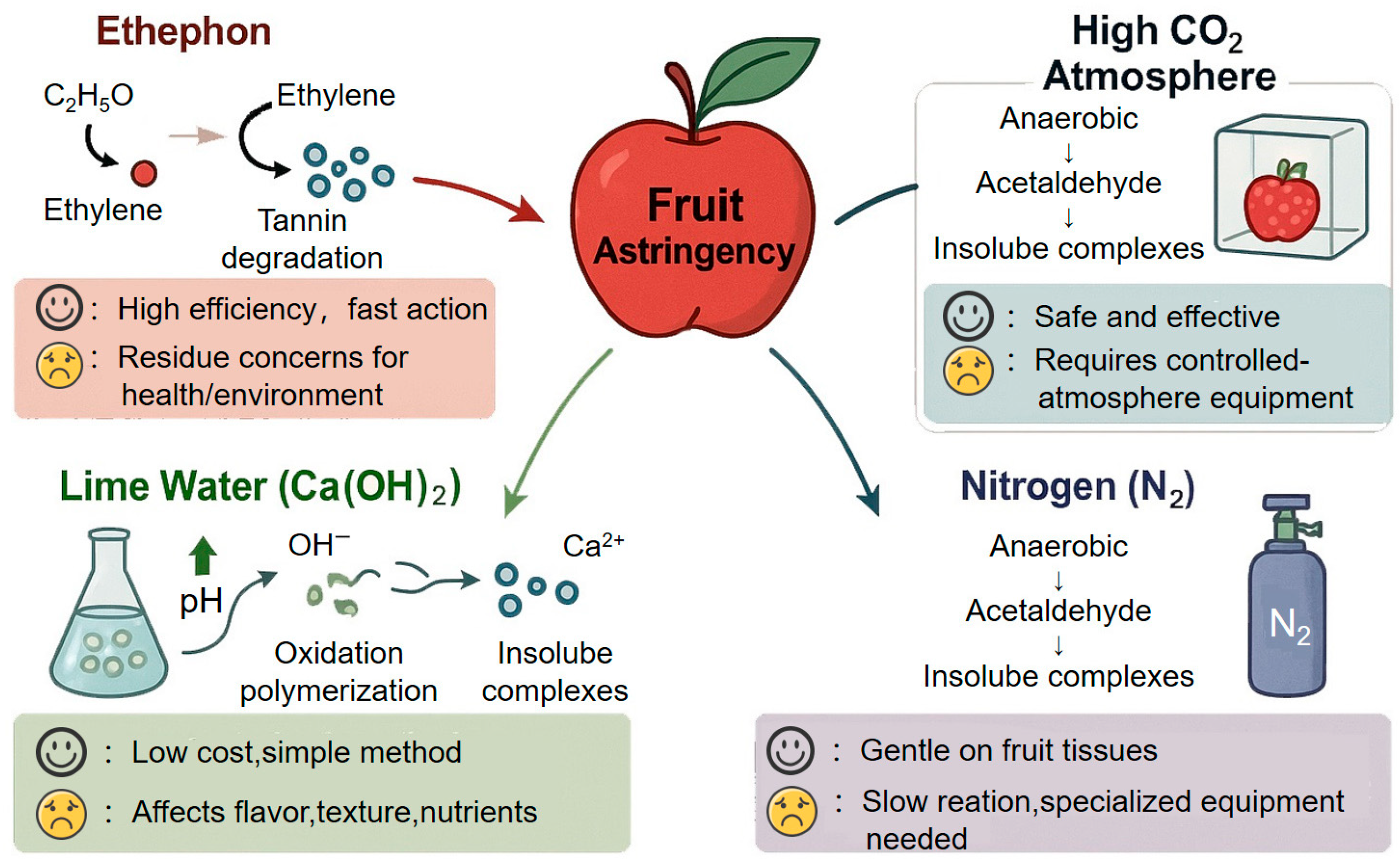
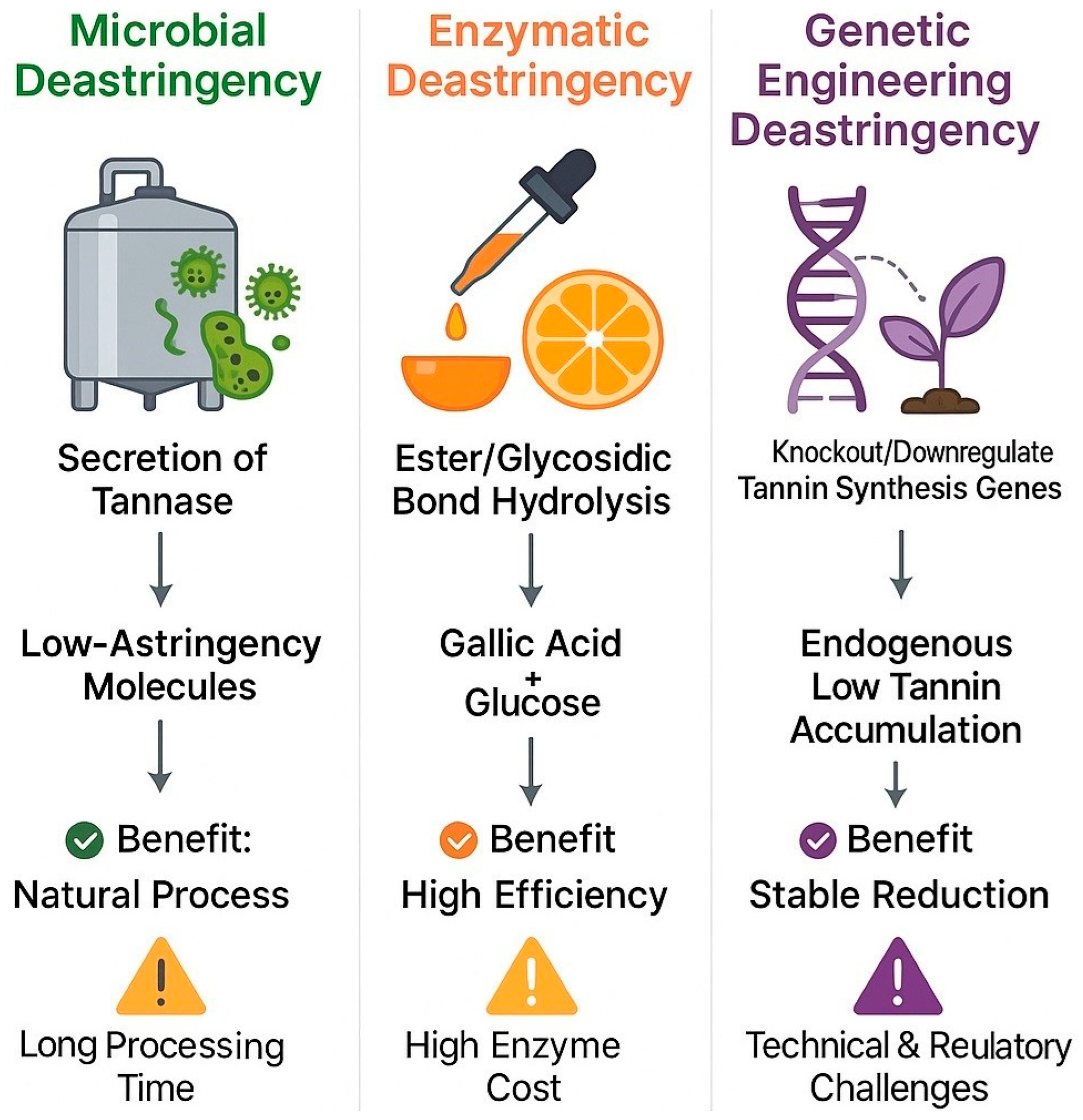
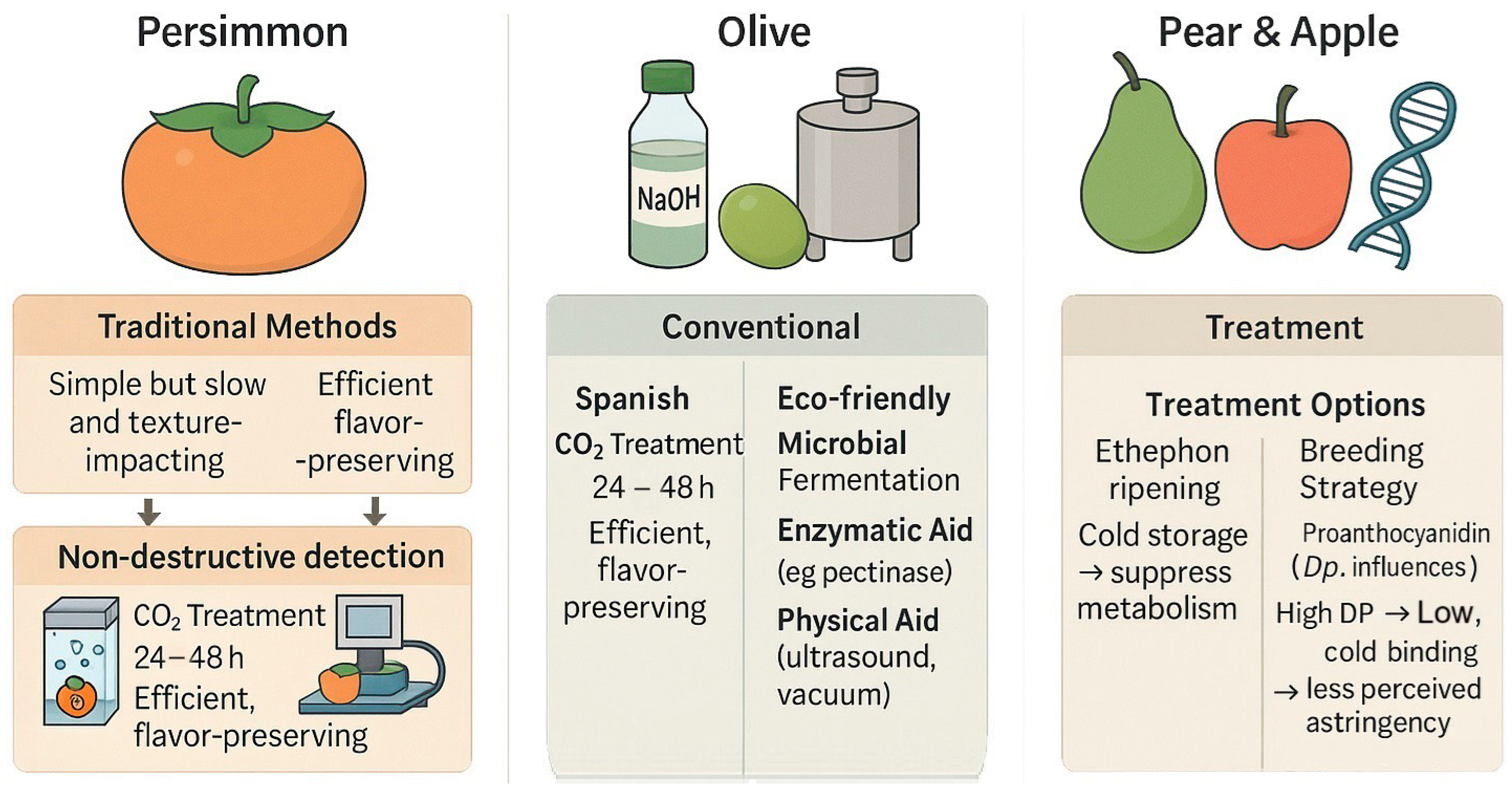
| Transformation Mechanism | Oxidation Reaction | Polymerization Reaction | Condensation/Complexation Reaction |
|---|---|---|---|
| Key Conditions/Triggers | O2, Polyphenol Oxidase (PPO) | Alkaline pH > 9, Ca(OH)2 | Proteins (e.g., ovalbumin) |
| Mode of Action | Phenols → Quinones → Insoluble polymers (browning) | Tannin cross-linking into high-MW polymers | Tannins bind proteins via hydrophobic interactions |
| Target Compounds | Soluble tannins (e.g., catechins) | Condensed tannins | Proanthocyanidins (PAs) and proteins |
| Example Result/Effect | ↓ Phenols 30–50%, ↓ Astringency 60% | ↓ SCT from 15.76 → 9.04 mg CE/g (↓ 42.6%) | ↓ TPC 35%, ↓ TPAC 66.7%, ↓ Astringency score 7.75 → 4.25 |
| Applicable De-astringency Methods | Mechanical damage | Lime-water treatment | Warm-water de-astringency/protein-addition de-astringency |
| References | [24,76] | [28] | [28,77,81] |
| Quality Attribute | Change Trend/Manifestation | Mechanism/Cause | Examples and Methods | Key References |
|---|---|---|---|---|
| Color | Peel turns orange-red; ↓ L*, b*, C*; warm water reduces browning | Phenolic oxidation/polymerization; PPO/enzymatic browning | Warm water: Best for color CO2: Maintains pigments | [82,83,84] |
| Texture | ↓ Firmness; softening especially with heat; enzyme/microbial milder | Cell wall breakdown; pectin loss; acetaldehyde disrupts structure | Warm water: High softening Enzyme: Minor effect | [22,85,86] |
| Flavor | ↓ Astringency improves taste; ethanol may add off-flavor; CO2 retains original aroma | Tannin reduction; aroma compound shift; ethanol threshold effects | Alcohol: Risk of off-flavor CO2: Preserves aroma Ethephon: Less effective | [27,88,89] |
| Nutritional Components | ↓ Total phenolic content, antioxidants; some methods retain Vit C, acids | Tannin degradation/insolubilization; vitamin loss from heat or oxidation | CO2: Keeps Vit C, loses polyphenols Enzyme: Gentle on nutrients | [87,89,93] |
| Method Type | Specific Method | Principle | Tannin Reduction | Treatment Time | Cost Level | Advantages | Disadvantages | Applicable Fruits |
|---|---|---|---|---|---|---|---|---|
| Physical | Warm Water | Disrupts cells, promotes tannin precipitation | ~96.5% | 5 h | Low | Simple, low-cost | Slow, softens texture | Persimmon, Olive |
| Mechanical Damage | Stimulates tannin oxidation via injury | Not significant (CO2-assisted) | Minutes | High | Simple | Affects appearance/storage | Persimmon, Apple | |
| Irradiation | Breaks tannin molecules via radiation | ~20% | Minutes | High | High efficiency | Expensive, may affect quality | Persimmon, Grape | |
| Ultrasound | Cavitation enhances tannin degradation | 10–30% | 10–30 min | High | Efficient | High equipment cost | Persimmon, Olive | |
| Chemical | Alcohol | Ethanol induces tannin polymerization | 85–90% | 24–36 h | Low | Efficient | Alters flavor/aroma | Persimmon, Pear |
| Ethephon | Ethylene accelerates ripening and metabolism | 80–90% | 24–48 h | Very Low | Efficient, effective | Residue and safety concerns | Persimmon, Pear | |
| Lime Water | Alkalinity + Ca2+ promote oxidation/polymerization | 70–80% | 24–48 h | Very Low | Extremely cheap | May affect taste/nutrition | Persimmon, Olive | |
| Carbon Dioxide | CO2 induces acetaldehyde → tannin polymerization | ~91.6% | 24–36 h | Medium | Efficient, green | High equipment cost | Persimmon, Apple | |
| Nitrogen | Anaerobiosis triggers acetaldehyde-mediated polymerization | 50–55% | 2 h | Low–Medium | Efficient, mild | Requires inert atmosphere | Persimmon, Grape | |
| Biological | Microbial | Microbial enzymes metabolize/degrade tannins | 35–40% | ~3.5 months | Medium | Eco-friendly, preserves quality | Slow, requires fermentation control | Persimmon, Tea leaves |
| Enzymatic | Exogenous enzymes (e.g., tannase) degrade tannins | 50–80% | 4–12 h | Low–Medium | High specificity, mild conditions | Expensive enzymes | Persimmon, Juice | |
| Genetic Engineering | Gene edits regulate tannin synthesis/metabolism | 80–90% | 3+ years (breeding) | Very High | Long-lasting, no postharvest treatment | High cost, technical/regulatory barriers | Persimmon, Apple |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zheng, M.; Li, X.; Song, K.; Shi, D. Fruit Astringency: Mechanisms, Technologies, and Future Directions. Horticulturae 2025, 11, 699. https://doi.org/10.3390/horticulturae11060699
Zhao W, Zheng M, Li X, Song K, Shi D. Fruit Astringency: Mechanisms, Technologies, and Future Directions. Horticulturae. 2025; 11(6):699. https://doi.org/10.3390/horticulturae11060699
Chicago/Turabian StyleZhao, Wanru, Meizhu Zheng, Xue Li, Kai Song, and Dongfang Shi. 2025. "Fruit Astringency: Mechanisms, Technologies, and Future Directions" Horticulturae 11, no. 6: 699. https://doi.org/10.3390/horticulturae11060699
APA StyleZhao, W., Zheng, M., Li, X., Song, K., & Shi, D. (2025). Fruit Astringency: Mechanisms, Technologies, and Future Directions. Horticulturae, 11(6), 699. https://doi.org/10.3390/horticulturae11060699






