Abstract
γ-aminobutyric acid (GABA) is a four-carbon non-protein amino acid, with many regulatory effects in humans. It aids in regulating blood glucose levels and pressure and is widely recognized for its ability to promote cognitive balance through the alleviation of stress and improvements in sleep quality. The GABA content of longan pulp is higher than that of many other fruits and vegetables; however, much is still unknown about GABA’s biosynthesis in longan. In this study, we found that the GABA content of ‘Baoshi No. 1’ (BS1) pulp was significantly higher than that of ‘Chunxiang’ (CX) pulp. The GAD activity was higher in BS1 pulp than CX pulp, while there was no significant difference in the GABA-T activity. Additionally, five GAD genes were identified in longan, and an analysis of their transcriptional levels showed that only the expression level of DlGAD3 corresponded to the GABA content and GAD activity. DlGAD3 was localized in the cytoplasm, and its transient overexpression promoted an increase in the GABA content in Nicotiana benthamiana leaves. Overall, our results show that DlGAD3 is able to promote the accumulation of GABA and may play a major role in its biosynthesis in longan pulp.
1. Introduction
Longan (Dimocarpus longan Lour.), an evergreen species within the Sapindaceae family, grows in tropical and subtropical regions and is extensively cultivated in southern China and Southeast Asian countries []. The longan fruit has extremely high economic and nutritional value [] and is a famous example of “Medical Food Homology” in traditional Chinese medicine []. It has been recorded in the Compendium of Materia Medica (Ben Cao Gang Mu in Chinese) by Li Shizhen as an herbal medicine []. Several studies have shown that longan contains many bioactive products that have memory-enhancing effects and that are able to treat amnesia, insomnia, neurasthenia, heart palpitations, and fatigue [,].
GABA, a ubiquitous four-carbon non-proteinogenic amino acid widely found in plants and animals, is a bypass product of the tricarboxylic acid (TCA) cycle []. It is involved in many physiological processes in plants, including gene expression, cell wall modifications, interactions during signal transduction, responses to abiotic and biotic stress, and the regulation of plant development and carbon and nitrogen metabolism [,]. GABA can be used to improve the quality of fruits. For example, the use of exogenous GABA can increase the sugar (glucose, fructose, and sucrose) content of tomatoes by regulating gene expression []. Spraying pomegranates with GABA can increase their total phenolic and anthocyanin concentrations []. GABA is an inhibitory neurotransmitter in the human brain [] and exerts several regulatory effects, such as relieving insomnia and regulating blood glucose levels and pressure, meaning that oral GABA intake can induce relaxation and diminish anxiety [,,]. Enriching the GABA content in litchi juice could help to regulate the gut flora, thus ameliorating obesity [].
The GABA shunt and polyamine metabolism regulate GABA biosynthesis []. In plants, GABA metabolism and biosynthesis are mainly regulated by the GABA shunt [] (Figure 1), in which glutamate decarboxylase (GAD, EC 4.1.1.15) is a rate-limiting enzyme during []. GAD catalyzes the irreversible conversion of glutamate to GABA in the cytoplasm. Subsequently, GABA is transported into mitochondria and converted into succinic semialdehyde (SSA) by GABA transaminase (GABA-T). The conversion of SSA into succinate is catalyzed by semialdehyde dehydrogenase (SSADH), the enzyme activity of which depends on the presence of NAD+. Succinate then enters the TCA cycle [,].
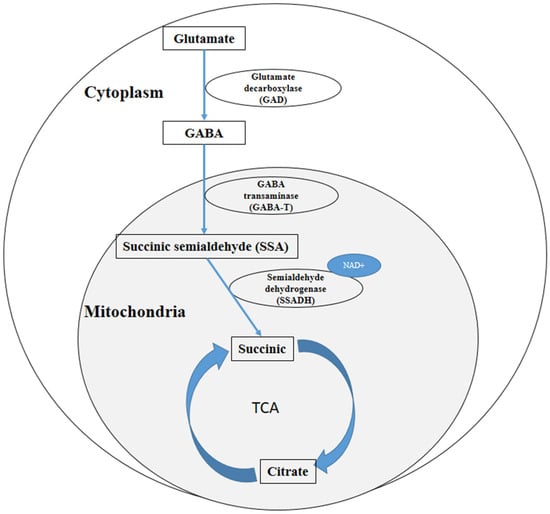
Figure 1.
Overview of the metabolic pathway of GABA in plants.
The GAD gene has been cloned and studied in many species, such as Arabidopsis [], tomatoes [], wheat [], and cotton []. Five members (GAD1–5) of the GAD gene family have been discovered in Arabidopsis thaliana []. The expression of GAD shows tissue specificity, and different GADs perform diverse functions and respond to different stimuli. AtGAD1 (At5G17330) is mainly expressed in the roots, while AtGAD2 (At1G65960) plays a key role in the leaves and shoots [,,,,]. In rice, OsGAD4 shows the highest expression among the five OsGAD genes under certain stress conditions []. Furthermore, the overexpression of SlGAD2 and SlGAD3 has the potential to promote GABA biosynthesis in tomato fruits [].
High-value compounds in longan, including GABA, have gradually gained attention []. The GABA content in longan is higher than that in many other fruits and vegetables, ranging from 51.48 mg/100 g to 180.42 mg/100 g [,]. Research has been conducted on the GAD gene family in longan during early somatic embryogenesis []; however, the key GAD gene for GABA biosynthesis in longan pulp remains unclear. In this study, we determined the differences in the GABA content and GAD and GABA-T activity between two longan cultivars. We identified five GAD genes and one GABA-T gene in longan through gene family analysis. Subsequently, we investigated the expression of the GADs and GABA-T through an RT-qPCR and determined that DlGAD3 plays a key role in GABA biosynthesis in longan pulp. The subcellular localization of DlGAD3 was found to be in the cytoplasm, and the transient transformation of DlGAD3 significantly promoted GABA production in Nicotiana benthamiana leaves. Our findings reveal a mechanism of GABA biosynthesis in longan pulp and provide a theoretical basis for further study.
2. Materials and Methods
2.1. Plant Materials
‘Baoshi No. 1’ (BS1), a hybrid of ‘Dongbao No. 9’ × ‘Shixia’, is known for its early maturation and high quality. ‘Chunxiang’ (CX), which flowers easily and produces a high yield, is a hybrid of ‘Dongbao No. 9’ × ‘Wanxiang’. BS1 and CX fruits were obtained from the Longan Resource Nurseries, part of the National Fruit Gene Pool (Fuzhou). The pulp was cut into pieces and mixed, with three biological replicates performed for each cultivar. All the collected samples were immediately frozen in liquid nitrogen and stored at −80 °C until further analysis.
2.2. Samples Preparation of Metabolomics
The samples underwent vacuum freeze-drying using a lyophilizer (Scientz-100F, SCIENTZ, Ningbo, China). Before the process, the cold trap was defrosted, and the baffles and compressor filter screens were cleaned with alcohol. The samples were pretreated for freeze-drying, and the freeze-dryer was pre-cooled for three hours. Once the shelf temperature reached −50 °C, the samples, which had been stored at −80 °C, were transferred to the shelf using dry ice. Following this initial protocol, the samples were freeze-dried for 65 h under a vacuum, during which the shelf temperature was gradually increased from −45 °C to 25 °C to achieve stable, dry conditions through water sublimation. The room temperature was maintained at approximately 25 °C. The samples were ground into a fine powder (MM 400, Retsch, Haan, Germany). The grinding jar was air-dried using a blower and immersed in liquid nitrogen for 1–2 min until the bubbling ceased. Each sample was placed in a clean grinding jar to prevent direct contact and minimize heat transfer. A steel ball was added to each jar, and the jars were tightly sealed. The samples were ground at 30 Hz for 30 s, and the resulting powder was transferred to labeled Eppendorf tubes. A portion of the sample powder (50 mg) was mixed with 1200 µL of a pre-cooled 70% methanolic aqueous solution at −20 °C. The mixture was vortexed for 30 s every 30 min, and this process was repeated six times. After centrifugation at 12,000 rpm for 3 min, the supernatant was aspirated and filtered through a microporous membrane (with a 0.22 μm pore size). These samples were then subjected to metabolomic analysis.
2.3. Metabolomics Analysis
The resulting sample extracts were analyzed using a UPLC-ESI-MS/MS system (UPLC, ExionLC™ AD, https://sciex.com.cn/, (accessed on 23 December 2024); MS, Applied Biosystems 4500 Q TRAP, https://sciex.com.cn/, (accessed on 23 December 2024)). The analytical conditions have been described previously by Zhu et al. (2024) [] and Liu et al. (2023) []. The metabolites identified using widely targeted metabolomics were characterized using a self-built metabolic database and secondary mass spectrometry data from Metware Biotechnology, Ltd. (Wuhan, China). There were three biological replicates each for BS1 and CX: BS1-1, BS1-2, BS1-3, CX-1, CX-2, and CX-3.
2.4. Measurement of GABA Content
The GABA content was measured in μmol/g using a γ-Aminobutyric Acid (GABA) Content Assay Kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) following the manufacturer’s protocol.
2.5. Determination of GAD and GABA-T Activities
The GAD activity, expressed in units per gram of the sample (U/g), was quantified using a GAD Activity Assay Kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) following the manufacturer’s instructions. The GABA-T activity, expressed in units per gram of the sample (µg/h/g), was assayed using a dedicated GABA Transaminase Activity Assay Kit (Suzhou Grace Biotechnology Co., Ltd., Suzhou, China) following the manufacturer’s protocol.
2.6. RT-qPCR Analysis
The total RNA was isolated from the pulp of BS1 and CX using an RNAprep Pure Plant Plus Kit (TIANGEN, Beijing, China) following the manufacturer’s instructions, and cDNA was synthesized utilizing the HiScript IV RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China). The qPCR was performed using the Hieff UNICON® advanced qPCR SYBR Master Mix (Yeasen Biotech cat. 11185ES03) in an LC480 (Roche, Mannheim, Germany). The PCR reaction system (with a total volume of 20 µL) contained 10 µL of the PCR Master Mix, 1 µL of the cDNA template, 0.4 µL of each primer (10 μM), and 8.2 µL of ddH2O. The two-step amplification was implemented according to the manufacturer’s protocol as follows: an initial denaturation step at 95 °C for 2 min, followed by 45 cycles of 95 °C for 10 s and 60 °C for 30 s, and a final melt curve analysis (gradient dissociation) to assess the amplification specificity. DlActB was used as the reference gene [], and the primer sequences used are provided in Supplemental Table S2. The relative gene expression was calculated using the 2−ΔΔCt method [].
2.7. Identification of GAD in Longan and Construction of Phylogenetic Tree
The full-length protein sequences of five GAD genes (AT1G65960, AT5G17330, AT2G02010, AT2G02000, and AT3G17760) were downloaded from the TAIR database (https://www.arabidopsis.org/, (accessed on 16 December 2024)). The blast program and the five published Arabidopsis GAD sequences were used to identify all the candidate GADs in longan, and the genome of the D. longan cultivar ‘Jidanben’ was used as a reference (http://www.sapindaceae.com/, (accessed on 16 December 2024)) []. Simultaneously, we analyzed and downloaded the GADs of other Sapindaceae species [], including Litchi chinensis, Nephelium lappaceum, Xanthoceras sorbifolium, Sapindus mukorossi, Acer yangbiense, and Cardiospermum halicacabum. These sequences were used to construct a phylogenetic tree using the neighbor-joining (NJ) method, and 1000 bootstrap replicates were used with the default parameters. These protein sequences are provided in Supplemental Table S3.
2.8. Transient Expression Analysis and Subcellular Localization
To verify the gene function of DlGAD3, we constructed its expression vector, pSAK227-DlGAD3-GFP, as follows: the full-length DlGAD3 gene was cloned from the cDNA templates of BS1 fruit. Subsequently, the PCR product was purified and inserted into the HindIII/EcoRI-digested pSAK277-GFP vector backbone, and pSAK227-DlGAD3-GFP was generated through homologous recombination using the ClonExpress Ultra One Step Cloning Kit (C115-01, Vazyme, Nanjing, China) following the manufacturer’s instructions. The expression vector was transformed into Agrobacterium tumefaciens (GV3101) using the heat shock method and cultured on an LB agar medium at 28 °C for 3 days. A single colony was picked and cultured overnight at 28 °C, and the agrobacterium were collected through centrifugation to infect tobacco leaves. Nicotiana benthamiana leaves were infected with the Agrobacterium using an infection buffer solution (10 mM of MgCl2, 10 mM of MES, pH 5.7, 100 μM of acetosyringone), and the GFP signals were detected using a fluorescence microscope (DM4B, Leica, Wetzlar, Germany). Subcellular localization was analyzed in infiltrated leaves at 3 days post-infiltration, and the leaf samples were collected for the measurement of GABA content. The 35S promoter-driven GFP was used as a subcellular localization positive control. The primer sequences we used are provided in Table S2.
2.9. Statistical Analysis
The data were analyzed using IBM SPSS Statistics software, version 27.0 (IBM Corporation, Armonk, USA). The significance levels were tested using Student’s t-test, and *, **, and *** represent significance levels of p < 0.05, p < 0.01, and p < 0.001, respectively. The results are represented as the mean ± the standard error (SE).
3. Results
3.1. The Metabolomics Profile of the Two Longan Cultivars
Metabolome analysis was performed on the longan pulp (Figure 2A) to study the differences in metabolites between the two cultivars. The repeatability and reliability of the metabolome analysis were determined based on the overlap of the total ion current (TIC) curves for the different samples (Figure S1). A total of 1296 metabolites were detected using the UPLC-MS/MS platform (Table S1), and a category analysis revealed that the primary metabolites in the longan pulp were amino acids and derivatives (18.21%), followed by flavonoids (14.81%), phenolic acids (12.5%), lipids (11.11%), organic acids (7.71%), alkaloids (5.94%), saccharides (5.71%), nucleotides and derivatives (5.48%), terpenoids (5.09%), lignans and coumarins (4.78%), tannins (1.54%), quinones (0.39%), and others (6.71%) (Figure 2B). A hierarchical clustering heat map analysis was used to visualize the pattern of metabolite accumulation in these samples (Figure 2C). The metabolites in the pulp of the two longan cultivars were significantly different, and the biological replicates of each variety clustered together, showing the distinct metabolic characteristics of these cultivars.

Figure 2.
Analysis of metabolic characteristics of pulp from two longan cultivars using pie chart and heat map. (A) BS1 and CX longan fruit. (B) Classes of metabolites identified in longan pulp. (C) Clustering heat map of all metabolites.
3.2. Differences in GABA Content Between Two Longan Pulps
According to a metabolomics analysis, amino acids and derivatives are abundant in longan. A total of 236 amino acids and derivatives were identified, and the GABA levels differed significantly between the two longan cultivars (Figure 3A). The GABA content in BS1 was significantly higher than that in CX (Figure 3B), consistent with the results of the metabolomics analysis.
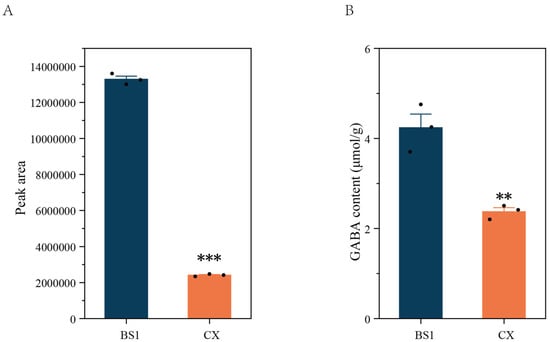
Figure 3.
Results for GABA from metabolomics analysis (A) and determination of GABA content (B) in longan pulp. Data are represented as mean ± standard error (SE) from three biological replicate assays, and significance levels were determined using Student’s t-test. ** p < 0.01; *** p < 0.001.
3.3. Differences in GAD and GABA-T Enzyme Activities Between Two Longan Pulps
The enzyme activities of GAD and GABA-T were measured to investigate the effect of the GABA shunt on the GABA content. The activity of GAD in BS1 was higher than that in CX (Figure 4A). However, no significant difference was observed in GABA-T activity between the two longan pulps (Figure 4B), showing that the trends in the GAD enzyme activity and GABA content were consistent.
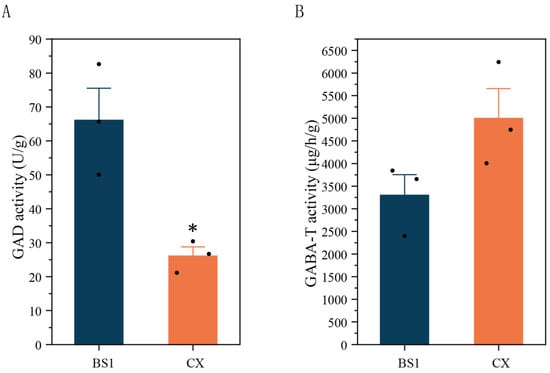
Figure 4.
Activities of GAD (A) and GABA-T (B) in longan pulp. Data are presented as mean ± standard error (SE) from three biological replicate assays, and significance levels were determined using Student’s t-test. * p < 0.05.
3.4. Identification of GAD and Construction of Phylogenetic Tree for DlGADs
To confirm GAD’s function in longan pulp, five GAD genes were detected through a genome-wide analysis: D.long035428.01 (GAD1), D.long027052.01 (DlGAD3), D.long027049.01 (DlGAD3-1), D.long027050.01 (DlGAD3-2), and D.long030275.01 (DlGAD5). Using MEGA-X, a phylogenetic tree of the GAD gene family was constructed (Figure 5) comprising the GAD families of eight species: Dimocarpus longan, Arabidopsis thaliana, Litchi chinensis, Nephelium lappaceum, Xanthoceras sorbifolium, Sapindus mukorossi, Acer yangbiense, and Cardiospermum halicacabum. All the species belonged to the Sapindaceae family except for Arabidopsis. The GAD genes of longan were grouped into three groups: Group I, Group II, and Group III. DlGAD3, DlGAD3-1, and DlGAD3-2 were clustered in Group I with AtGAD2, AtGAD3, and AtGAD4. DlGAD1 was clustered in Group II with AtGAD1, and DlGAD5 was clustered in Group III with AtGAD5. This observation suggests that different members of the GAD family have different functions in longan.
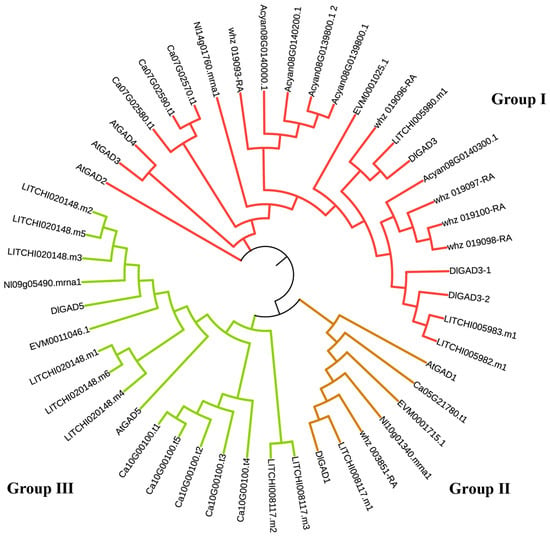
Figure 5.
Phylogenetic tree of the GAD gene families. Different branch colors represent different groups.
3.5. Gene Expression of GAD and GABA-T in Longan Pulp
The GAD and GABA-T genes (D.long033926.01) were retrieved using the genome of ‘Jidanben’ as a reference, and we analyzed the differences in the transcriptional profiles of the two longan pulps. Notably, the transcription of DlGAD3-1 and DlGAD3-2 was not detected in either cultivar. As shown in Figure 6A, DlGAD3 exhibited significantly higher expression in the pulp of BS1 compared to CX, while the expression of DlGAD1, DlGAD5, and DlGABA-T showed no significant differences (Figure 6B–D). These findings show that the differential expression of DlGAD3 may have led to the significant differences observed in the GABA content between the two longan pulps.
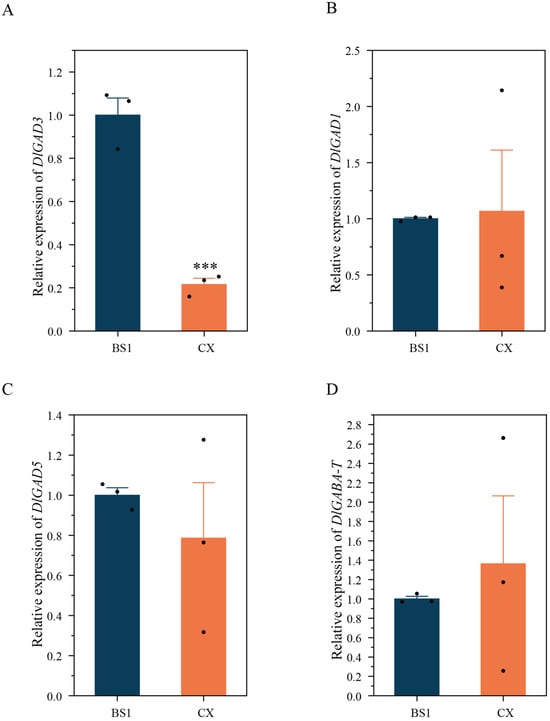
Figure 6.
Analysis of GADs and GABA-T expression in longan pulp. (A) Relative expression of DlGAD3; (B) Relative expression of DlGAD1; (C) Relative expression of DlGAD5; (D) Relative expression of GABA-T. Data are presented as mean ± standard error (SE) from three biological replicates, and significance levels were determined using Student’s t-test. *** p < 0.001. All gene expression data were normalized to BS1, with its expression level set as 1.
3.6. The Subcellular Localization and Functional Analysis of the DlGAD3 Gene
DlGAD3 was cloned and fused with a GFP tag in an overexpression vector to assess its subcellular localization. GFP signals were detected in both the cytoplasm and nucleus in the positive control (GFP-tag); however, they were only detected in the cytoplasm when the GFP tag was fused with DlGAD3 (Figure 7A), indicating that DlGAD3 is localized in the cytoplasm. The GABA content was significantly higher than that in the control (empty vector) when DlGAD3 was overexpressed in Nicotiana benthamiana leaves (Figure 7B).

Figure 7.
Functional analysis of DlGAD3. (A). Subcellular localization of DlGAD3 in Nicotiana benthamiana leaves. Scale = 50 μm. (B). GABA content. Data are presented as mean ± standard error (SE) from four biological replicates, and significance levels were determined using Student’s t-test. ** p < 0.01.
4. Discussion
Longan is a high-grade fruit in China and is valuable in traditional Chinese medicine [], where it is considered an example of ‘Medical Food Homology’ []. Longan possesses high nutritional and medicinal value. It exhibits some medicinal properties, including anti-oxidant, immunomodulatory, anti-cancer, anti-osteoporotic, prebiotic, memory-enhancing, and anxiolytic effects []. In this study, we presented the results from a metabolite analysis of two longan cultivars using UPLC-MS/MS. A total of 1296 metabolites were detected and categorized into 13 groups. The top three metabolic pathways were those for amino acids and derivatives (18.21%), flavonoids (14.81%), and phenolic acids (12.5%). Longan pulp is rich in amino acids and derivatives, which may contribute to its taste and flavor []. Amino acids are the building blocks of protein, and functional amino acids, such as arginine, glutamate, glutamine, and glycine, have distinct properties [,]. GABA is a functional amino acid and possesses physiological functions, such as regulating the blood pressure, nervous system, and hormone secretion, enhancing liver and kidney function, boosting immunity, preventing cancer, and providing anti-aging effects in mammals [,]. It is widely recognized that GABA can promote cognitive balance by alleviating stress and improving sleep quality []. In plants, the physiological and biochemical functions of GABA have been widely studied and reported. GABA has metabolic roles and signaling functions [] and is involved in regulating energy metabolism, the plant’s development and pH, the carbon/nitrogen (C/N) balance, and the defense system [,]. Longan produces fruit with a high GABA content, ranging from 51.48 mg/100 g to 180.42 mg/100 g [,]. In this study, we found that the GABA content of BS1 was higher than that of CX (Figure 3), providing a basis for studying GABA biosynthesis in longan.
Some treatments may induce changes in the enzyme activity of GAD and GABA-T to affect the synthesis of GABA; for example, CO2 treatments may cause a decline in the GABA concentration and glutamate decarboxylase activity in longan fruit []. In tomato fruit, UV-C treatment may increase the gene expression and activity of GAD, inhibit the expression and activity of GABA-T, and promote the biosynthesis of GABA []. CO2 treatment may also enhance the activity of GAD and promote the production of GABA in the postharvest fruit of some tomato varieties []. Ca2+ treatment may enhance the endogenous GABA content and upregulate the GAD enzyme activity in pear fruit [], and the use of exogenous 5-aminolevulinic acid may promote an increase in the GAD enzyme activity, thereby increasing the GABA content in tomato fruit []. These findings suggest that GAD is a key gene involved in GABA biosynthesis.
Arabidopsis thaliana possesses five members of the GAD gene family []. The expression of GAD genes exhibits tissue-specific characteristics in plants: AtGAD1, AtGAD2, and AtGAD4 are mainly expressed in the roots and leaves of Arabidopsis thaliana in the vegetative stage, and AtGAD5 is expressed in the pollen. The expression of AtGAD3 has been detected in anthers and embryos []. The GABA content has been found to decrease when the expression of GhGAD6 is reduced [], while the overexpression of BoGAD5 promoted an increase in the GABA content in broccoli sprouts []. PpGAD2 was found to be the key gene for the biosynthesis of GABA in pear flesh []. In this study, the GAD enzyme activity and GABA content were significantly higher in BS1 than CX (Figure 3 and Figure 4A), suggesting that the differential activity of GAD may underlie the difference in the GABA content between the two longan cultivars. Therefore, we analyzed the expression levels for the GAD gene family (Figure 5). The expression of DlGAD1 and DlGAD5 did not differ significantly, and the transcription of DlGAD3-1 and DlGAD3-2 was not detected in either cultivar. The expression of DlGAD3 in BS1 was significantly higher than that in CX (Figure 6A). We also found that the overexpression of DlGAD3 could promote GABA production in Nicotiana benthamiana leaves (Figure 7B). These results indicate that DlGAD3 may play a key role in the biosynthesis of GABA in longan pulp. However, reports have suggested that DlGAD5 cannot promote GABA production in longan embryogenic calluses, which may be due to their transient conversion efficiency []. This indicates that more research is needed on the role of GAD in GABA biosynthesis in longan. In one study, the expression of SlGABA-T1 led to a decrease in GABA during the tomato ripening period []. However, in our investigation, the GABA-T enzyme activity and the expression of DlGABA-T did not differ significantly between the two cultivars (Figure 4B and Figure 6D), indicating that GABA-T is not the cause of the difference in the GABA content between the two cultivars.
5. Conclusions
In summary, the DlGAD3 gene may promote GABA biosynthesis in longan pulp. Through a metabolomics analysis, we found that the GABA content in BS1 was significantly higher than that in CX. Subsequently, we found that the enzyme activity of GAD and the expression of DlGAD3 in BS1 were significantly higher than those in CX. A functional analysis of DlGAD3 showed its localization in the cytoplasm and its ability to increase the GABA content in Nicotiana benthamiana leaves through transient overexpression. Collectively, these findings demonstrate that DlGAD3 is able to promote GABA accumulation and underlies the differences in the GABA content between BS1 and CX.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11060686/s1: Figure S1: The TIC maps from QC samples mass spectrometry; Table S1: UPLC-MS/MS-based metabolite identification and quantification in longan pulp; Table S2: Primers used for quantitative real-time PCR and vector construction; Table S3: Proteins sequences of GADs used in this study for phylogenetic tree.
Author Contributions
Conceptualization, C.D., J.J. and S.Z.; formal analysis, W.S. and Z.Z.; funding acquisition, C.D., J.J. and S.Z.; investigation, W.W., T.Z. and Q.X.; methodology, Y.C. and T.Z.; project administration, J.J. and S.Z.; resources, Q.X. and Y.Z.; software, Y.C., Z.Z. and Y.Z.; supervision, J.J. and S.Z.; visualization, W.W.; writing—original draft, C.D. and W.W.; writing—review and editing, W.S. and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Funding information: Fujian Basic Research Project of Provincial Public Welfare Research Institutes: 2022R1028002; China Agriculture Research System-Litchi and Longan (No.CARS-32-24); the Financial Special Project of the Fujian Academy of Agricultural Sciences (2023), and the Technology Innovation Team Program of the Fujian Academy of Agricultural Sciences (CXTD2021004-1).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GAD | glutamate decarboxylase |
| BS1 | Baoshi No. 1 |
| CX | Chunxiang |
| GABA | γ-aminobutyric acid |
| TCA | tricarboxylic acid |
| SSA | succinic semialdehyde |
| GABA-T | GABA transaminase |
| SSADH | semialdehyde dehydrogenase |
References
- Xu, X.; Zhang, C.; Lai, C.; Zhang, Z.; Wu, J.; Su, Q.; Gan, Y.; Zhang, Z.; Chen, Y.; Guo, R.; et al. Genome-Wide Identification and Expression Analysis of Bx Involved in Benzoxazinoids Biosynthesis Revealed the Roles of DIMBOA during Early Somatic Embryogenesis in Dimocarpus longan Lour. Plants 2024, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, H.; Tang, J.; Lin, M.; Hung, Y.C.; Lin, H. Effects of acidic electrolyzed water treatment on storability, quality attributes and nutritive properties of longan fruit during storage. Food Chem. 2020, 320, 126641. [Google Scholar] [CrossRef]
- Tan, S.; Ke, Z.; Zhou, C.; Luo, Y.; Ding, X.; Luo, G.; Li, W.; Shi, S. Polyphenol Profile, Antioxidant Activity, and Hypolipidemic Effect of Longan Byproducts. Molecules 2023, 28, 2083. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; He, X.M.; Sun, J.; Li, C.B.; Li, L.; Sheng, J.F.; Xin, M.; Li, Z.C.; Zheng, F.J.; Liu, G.M.; et al. Polyphenols and Alkaloids in Byproducts of Longan Fruits (Dimocarpus Longan Lour.) and Their Bioactivities. Molecules 2019, 24, 1186. [Google Scholar] [CrossRef]
- Park, S.J.; Park, D.H.; Kim, D.H.; Lee, S.; Yoon, B.H.; Jung, W.Y.; Lee, K.T.; Cheong, J.H.; Ryu, J.H. The memory-enhancing effects of Euphoria longan fruit extract in mice. J. Ethnopharmacol. 2010, 128, 160–165. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Ho, C.-T.; Bai, N. Phytochemical constituents and biological activities of longan (Dimocarpus longan Lour.) fruit: A review. Food Sci. Hum. Wellness 2020, 9, 95–102. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, X.; Xiao, Q.; Wu, X.; Tian, Q.; Ma, W.; Shoaib, N.; Liu, Y.; Zhao, H.; Feng, Z.; et al. Advances in Plant GABA Research: Biological Functions, Synthesis Mechanisms and Regulatory Pathways. Plants 2024, 13, 2891. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Fariduddin, Q. Deciphering the enigmatic role of gamma-aminobutyric acid (GABA) in plants: Synthesis, transport, regulation, signaling, and biological roles in interaction with growth regulators and abiotic stresses. Plant Physiol. Biochem. 2024, 208, 108502. [Google Scholar] [CrossRef]
- Wu, X.; Huo, R.; Yuan, D.; Zhao, L.; Kang, X.; Gong, B.; Lü, G.; Gao, H. Exogenous GABA improves tomato fruit quality by contributing to regulation of the metabolism of amino acids, organic acids and sugars. Sci. Hortic. 2024, 338, 113750. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Guillén, F.; Martínez-Romero, D.; Carrión-Antoli, A.; Valero, D.; Serrano, M. γ-Aminobutyric acid treatments of pomegranate trees increase crop yield and fruit quality at harvest. Sci. Hortic. 2023, 309, 111633. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Fu, C.X.; Chu, Y.H.; Chen, Q.; Wang, H.; Wei, D.X.; Yao, Y.F. Selectively Probing the Magnetic Resonance Signals of γ-Aminobutyric Acid in Human Brains In Vivo. J. Magn. Reson. Imaging 2024, 59, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Heli, Z.; Hongyu, C.; Dapeng, B.; Yee Shin, T.; Yejun, Z.; Xi, Z.; Yingying, W. Recent advances of γ-aminobutyric acid: Physiological and immunity function, enrichment, and metabolic pathway. Front. Nutr. 2022, 9, 1076223. [Google Scholar] [CrossRef] [PubMed]
- Arora, I.; Mal, P.; Arora, P.; Paul, A.; Kumar, M. GABAergic implications in anxiety and related disorders. Biochem. Biophys. Res. Commun. 2024, 724, 150218. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of γ-Aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Wang, D.W.; Deng, Y.N.; Zhao, L.; Wang, K.; Wu, D.M.; Hu, Z.Y.; Liu, X.W. GABA and fermented litchi juice enriched with GABA promote the beneficial effects in ameliorating obesity by regulating the gut microbiota in HFD-induced mice. Food Funct. 2023, 14, 8170–8185. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193–194, 130–135. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, C.; Zhang, H.; Chen, S.; Wang, X.; Gan, L. Endogenous γ-Aminobutyric Acid Accumulation Enhances Salinity Tolerance in Rice. Plants 2024, 13, 2750. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Mao, L.; Ying, T. Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold-stored banana fruit. Food Chem. 2016, 197, 333–339. [Google Scholar] [CrossRef]
- Zik, M.; Arazi, T.; Snedden, W.A.; Fromm, H. Two isoforms of glutamate decarboxylase in Arabidopsis are regulated by calcium/calmodulin and differ in organ distribution. Plant Mol. Biol. 1998, 37, 967–975. [Google Scholar] [CrossRef]
- Akihiro, T.; Koike, S.; Tani, R.; Tominaga, T.; Watanabe, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Ashihara, H.; Matsukura, C.; et al. Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 2008, 49, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraan, N.A.; Sartawe, F.A.-b.; Qaryouti, M.M. Characterization of γ-aminobutyric acid metabolism and oxidative damage in wheat (Triticum aestivum L.) seedlings under salt and osmotic stress. J. Plant Physiol. 2013, 170, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, Y.; Cui, A.; Sun, L.; Han, M.; Wang, J.; Rui, C.; Lei, Y.; Liu, X.; Xu, N.; et al. Genome-wide identification of GAD family genes suggests GhGAD6 functionally respond to Cd2+ stress in cotton. Front. Genet. 2022, 13, 965058. [Google Scholar] [CrossRef]
- Scholz, S.S.; Reichelt, M.; Mekonnen, D.W.; Ludewig, F.; Mithöfer, A. Insect Herbivory-Elicited GABA Accumulation in Plants is a Wound-Induced, Direct, Systemic, and Jasmonate-Independent Defense Response. Front. Plant Sci. 2015, 6, 1128. [Google Scholar] [CrossRef] [PubMed]
- Benidickson, K.H.; Raytek, L.M.; Hoover, G.J.; Flaherty, E.J.; Shelp, B.J.; Snedden, W.A.; Plaxton, W.C. Glutamate decarboxylase-1 is essential for efficient acclimation of Arabidopsis thaliana to nutritional phosphorus deprivation. New Phytol. 2023, 240, 2372–2385. [Google Scholar] [CrossRef]
- Bouché, N.; Fait, A.; Zik, M.; Fromm, H. The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol. Biol. 2004, 55, 315–325. [Google Scholar] [CrossRef]
- Xu, B.; Feng, X.; Piechatzek, A.; Zhang, S.; Konrad, K.R.; Kromdijk, J.; Hedrich, R.; Gilliham, M. The GABA shunt contributes to ROS homeostasis in guard cells of Arabidopsis. New Phytol. 2023, 241, 73–81. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Kulsum, U.; Moniruzzaman, M.; Yasuda, N.; Akama, K. Truncation of the calmodulin binding domain in rice glutamate decarboxylase 4 (OsGAD4) leads to accumulation of γ-aminobutyric acid and confers abiotic stress tolerance in rice seedlings. Mol. Breed. 2024, 44, 21. [Google Scholar] [CrossRef]
- Takayama, M.; Koike, S.; Kusano, M.; Matsukura, C.; Saito, K.; Ariizumi, T.; Ezura, H. Tomato Glutamate Decarboxylase GenesSlGAD2andSlGAD3Play Key Roles in Regulating γ-Aminobutyric Acid Levels in Tomato (Solanum lycopersicum). Plant Cell Physiol. 2015, 56, 1533–1545. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, K.; Liu, X.; Hu, Z.; Zhao, L. Potential of longan (Dimocarpus longan Lour.) in functional food: A review of molecular mechanism-directing health benefit properties. Food Chem. 2024, 437, 137812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ndeurumio, K.H.; Zhao, L.; Hu, Z. Impact of Precooling and Controlled-Atmosphere Storage on gamma-Aminobutyric Acid (GABA) Accumulation in Longan (Dimocarpus longan Lour.) Fruit. J. Agric. Food Chem. 2016, 64, 6443–6450. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Jiang, F.; Huang, A.P.; Gao, H.Y.; Deng, C.J.; Chen, X.P.; Zheng, J.G. Evaluating of γ-aminobutyric Acid in Different Genotypes from Longan (Dimocarpus longan Lour.) peel. Fujian Fruits 2008, 3, 23–27. [Google Scholar]
- Lan, S.; Zhai, T.; Zhang, X.; Xu, L.; Gao, J.; Lai, C.; Chen, Y.; Lai, Z.; Lin, Y. Genome-wide identification and expression analysis of the GAD family reveal their involved in embryogenesis and hormones responses in Dimocarpus longan Lour. Gene 2024, 927, 148698. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wu, Y.; Li, C.; Li, X.; Li, K.; Yang, W.; Liang, Y.; Lu, J.; Zhang, L.; Lu, P.; et al. Metabolomics and quantitative descriptive analysis reveal the relationship between metabolites and taste attributes of flowers in two types of albino tea cultivars. LWT 2024, 199, 116074. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Jiang, X.; Du, B.; Wang, Q.; Ma, Y.; Liu, M.; Mao, Y.; Yang, J.; Li, F.; et al. Uncovering nutritional metabolites and candidate genes involved in flavonoid metabolism in Houttuynia cordata through combined metabolomic and transcriptomic analyses. Plant Physiol. Biochem. 2023, 203, 108059. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lai, Z.X. Reference gene selection for qPCR analysis during somatic embryogenesis in longan tree. Plant Sci. 2010, 178, 359–365. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Priyam, A.; Woodcroft, B.J.; Rai, V.; Moghul, I.; Munagala, A.; Ter, F.; Chowdhary, H.; Pieniak, I.; Maynard, L.J.; Gibbins, M.A.; et al. Sequenceserver: A Modern Graphical User Interface for Custom BLAST Databases. Mol. Biol. Evol. 2019, 36, 2922–2924. [Google Scholar] [CrossRef]
- Huang, G.J.; Wang, B.S.; Lin, W.C.; Huang, S.S.; Lee, C.Y.; Yen, M.T.; Huang, M.H. Antioxidant and Anti-Inflammatory Properties of Longan (Dimocarpus longan Lour.) Pericarp. Evid. Based Complement Altern. Med. 2012, 2012, 709483. [Google Scholar] [CrossRef]
- Yang, M.; Yan, T.; Yu, M.; Kang, J.; Gao, R.; Wang, P.; Zhang, Y.; Zhang, H.; Shi, L. Advances in understanding of health-promoting benefits of medicine and food homology using analysis of gut microbiota and metabolomics. Food Front. 2020, 1, 398–419. [Google Scholar] [CrossRef]
- Lai, T.; Shuai, L.; Han, D.; Lai, Z.; Du, X.; Guo, X.; Hu, W.; Wu, Z.; Luo, T. Comparative metabolomics reveals differences in primary and secondary metabolites between “Shixia” and “Chuliang” longan (Dimocarpus longan Lour.) pulp. Food Sci. Nutr. 2021, 9, 5785–5799. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Riechman, S.E.; Fluckey, J.D.; Wu, G. Interorgan Metabolism of Amino Acids in Human Health and Disease. Adv. Exp. Med. Biol. 2021, 1332, 129–149. [Google Scholar] [CrossRef]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, L.; Li, H.; Chen, Q.; Li, N.; Li, J.; Zhao, Z.; Xiao, D.; Tang, T.; Bi, C.; et al. Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): A review. PeerJ 2024, 12, e18712. [Google Scholar] [CrossRef]
- Rema Shree, A.B.; Anju, P.; Moothedath, I. Gamma amino butyric acid accumulation in medicinal plants without stress. Anc. Sci. Life 2014, 34, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, U.; Nohmi, T.; Sagane, R.; Hai, J.; Ohbayashi, K.; Miyazaki, M.; Yamatsu, A.; Kim, M.; Iwasaki, Y. Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves. Nutrients 2022, 14, 2492. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Z.; Zhang, N.; Liang, Y.; Gong, Z.; Wang, J.; Ditta, A.; Sang, Z.; Wang, J.; Li, X. Identification and function analysis of GABA branch three gene families in the cotton related to abiotic stresses. BMC Plant Biol. 2024, 24, 57. [Google Scholar] [CrossRef]
- Yan, L.; Zheng, H.H.; Liu, W.; Liu, C.H.; Jin, T.; Liu, S.; Zheng, L. UV-C treatment enhances organic acids and GABA accumulation in tomato fruits during storage. Food Chem. 2021, 338, 128126. [Google Scholar] [CrossRef]
- Deewatthanawong, R.; Nock, J.F.; Watkins, C.B. γ-Aminobutyric acid (GABA) accumulation in four strawberry cultivars in response to elevated CO2 storage. Postharvest. Biol. Technol. 2010, 57, 92–96. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Zhou, X.; Wei, B.; Zhao, Y.; Ji, S. Calcium Treatment Alleviates Pericarp Browning of ‘Nanguo’ Pears by Regulating the GABA Shunt After Cold Storage. Front. Plant Sci. 2020, 11, 580986. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Wang, J.W.; He, Y.M.; Feng, J.F.; Li, J.L.; Shang, X.P.; Wu, Y.; Yu, J.H.; Tang, Z.Q.; Xie, J.M. Exogenous ALA applied on different plant parts promotes tomato fruit quality and GABA synthesis. Front. Nutr. 2025, 11, 1520634. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.X.; Xu, X.W.; Liu, Y.; Zhang, Y.; Yang, L.Y.; Zhang, S.Q.; Xu, J. Induction of γ-aminobutyric acid plays a positive role to Arabidopsis resistance against Pseudomonas syringae. J. Integr. Plant Biol. 2020, 62, 1797–1812. [Google Scholar] [CrossRef]
- Jiao, W.H.; Wei, Q.L.; Chen, J.H.; Cao, S.F.; Luo, M.; Qian, Y.A.; Chen, Y.; Wei, Y.Y.; Shao, X.F.; Xu, F. Identification analysis of GAD gene family, and the role of BoGAD 5 in GABA enrichment in broccoli sprouts. Plant Growth Regul. 2024, 104, 1643–1655. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Liu, J.; Liu, D.H.; Wang, C.L. The ?-Aminobutyric Acid (GABA) Synthesis Gene Regulates the Resistance to Water Core-Induced Hypoxia Stress for Pear Fruits. Agronomy 2023, 13, 1062. [Google Scholar] [CrossRef]
- Koike, S.; Matsukura, C.; Takayama, M.; Asamizu, E.; Ezura, H. Suppression of γ-Aminobutyric Acid (GABA) Transaminases Induces Prominent GABA Accumulation, Dwarfism and Infertility in the Tomato (Solanum lycopersicum L.). Plant Cell Physiol. 2013, 54, 793–807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).