Hormonal and Storage Metabolic Regulation of Germination in Toona sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Seed Cultivation During the Initial Stages of Germination
2.3. Seed Water Content and Respiration Rate
2.4. Assessment of Starch, Soluble Sugar, Soluble Protein, and Fat Contents
2.4.1. Determination of Starch and Soluble Sugar Content
2.4.2. Determination of Soluble Protein Content
2.4.3. Determination of Fat Content
2.5. Assessment of Metabolic Enzyme Activity (Amylase, Protease, Acid Phosphatase)
2.5.1. Determination of Amylase Activity
2.5.2. Determination of Protease Activity
2.5.3. Determination of ACP Activity
2.6. Assessment of Key Oxidative Pathway Enzyme Activity (PGI, MDH, G-6-PDH, and 6-PGDH)
2.6.1. Determination of Phosphohexose Isomerase (PGI) Activity
2.6.2. Determination of Malate Dehydrogenase (MDH) Activity
2.6.3. Determination of Glucose-6-Phosphate Dehydrogenase, and 6-Phosphogluconate Dehydrogenase (G-6-PDH and 6 PGDH) Activity
2.7. Assessment of Endogenous Hormones (GA3, ZR, IAA, ABA)
2.8. Transmission Electron Microscopy
2.9. Statistical Analysis
3. Results
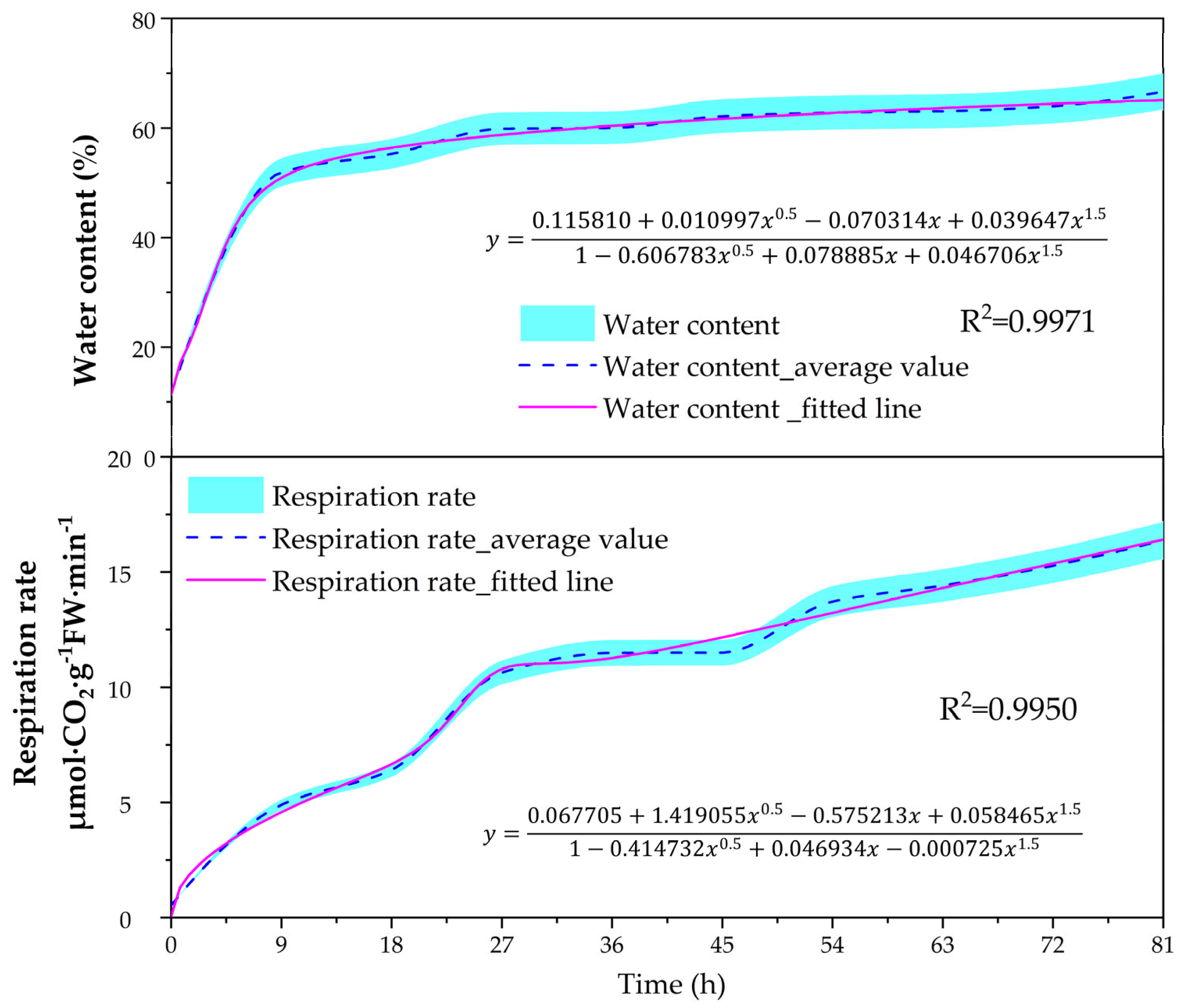
3.1. Changes in Water Content and Respiration Rate of Seeds
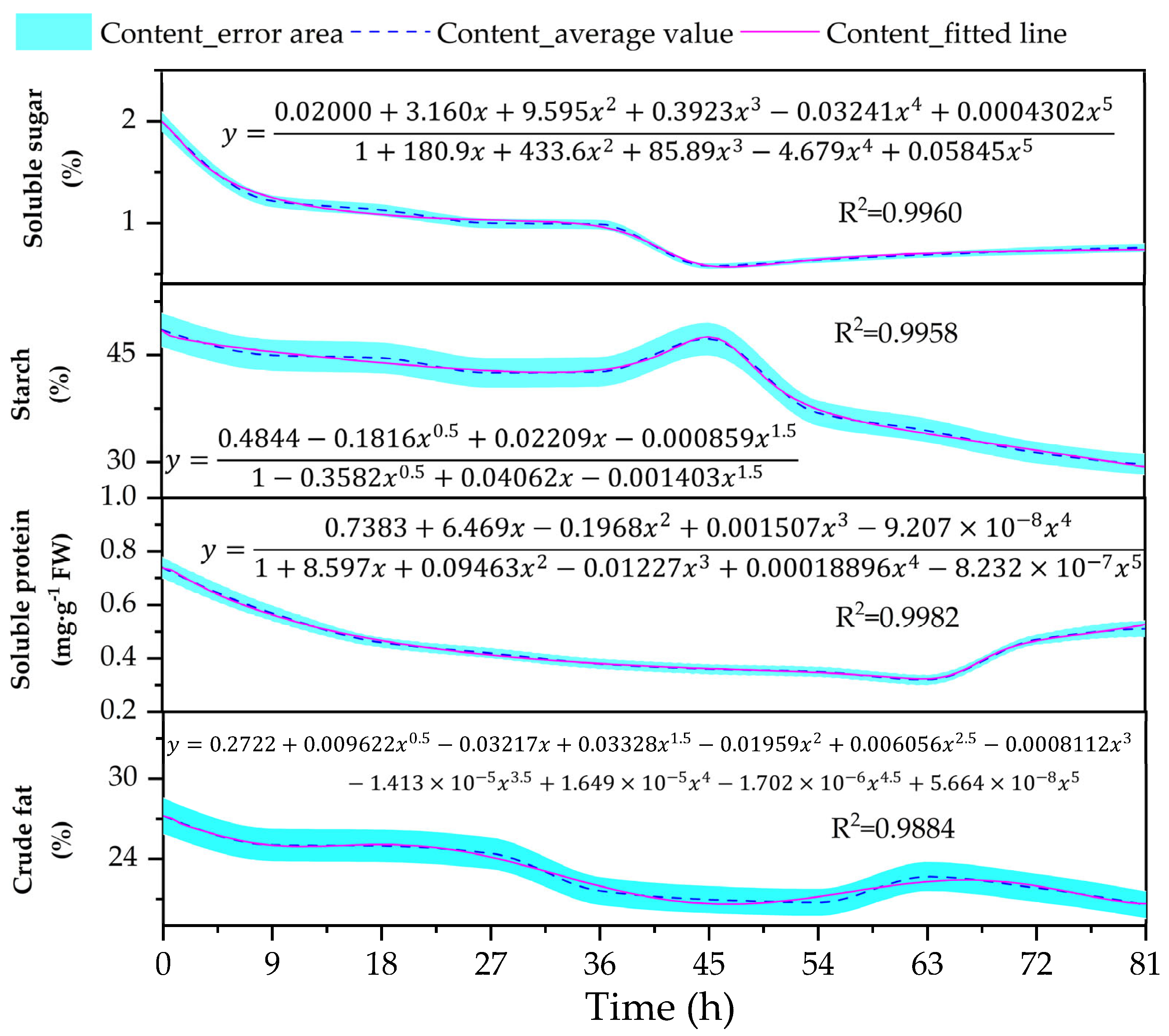
3.2. Changes in the Content of Reserve Materials (Soluble Sugar, Starch, Soluble Protein, and Crude Fat) in the Embryos
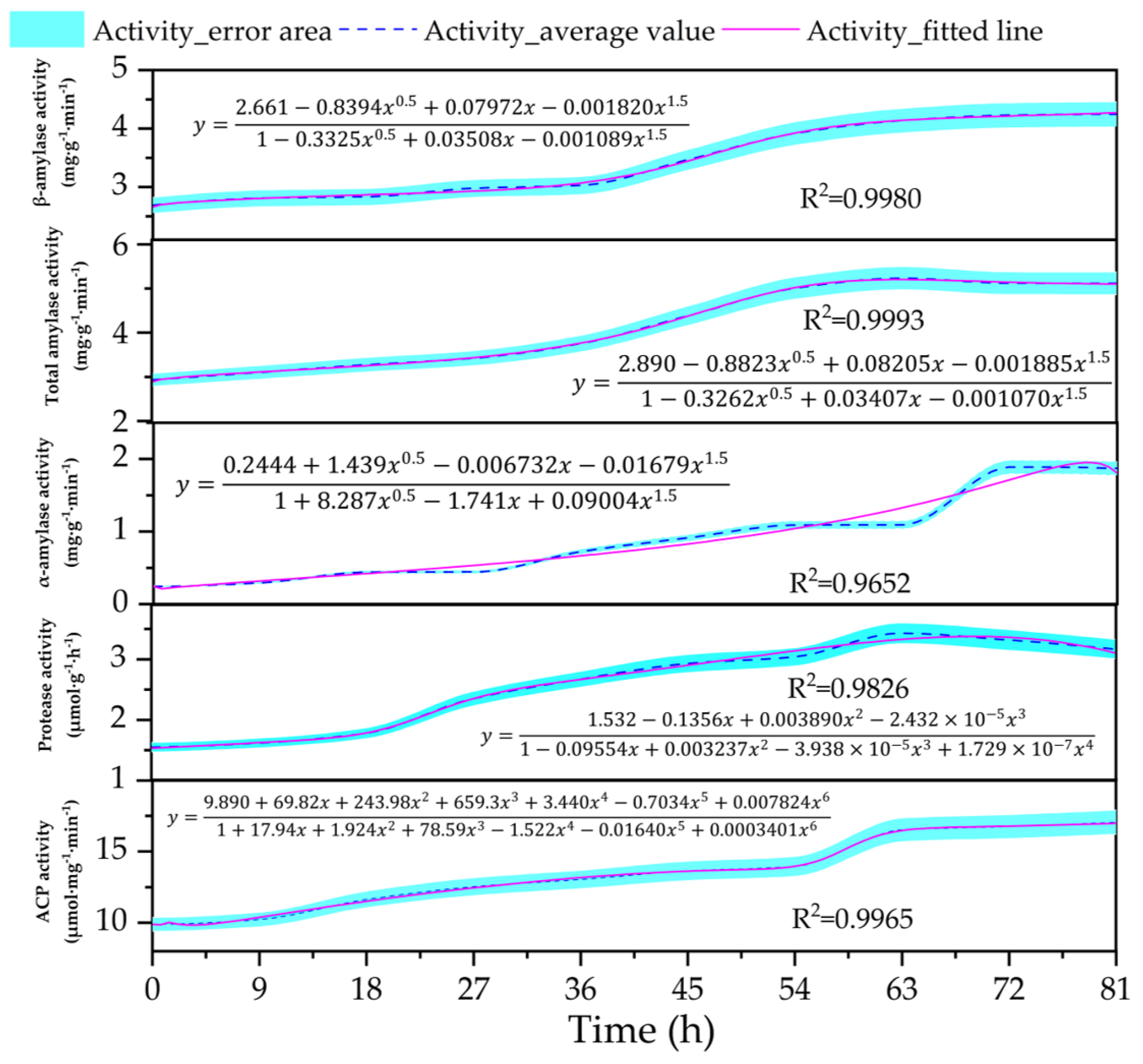
3.3. Changes in the Content of Metabolic Enzyme Activity (Amylase, Protease, Acid Phosphatase) in the Embryos
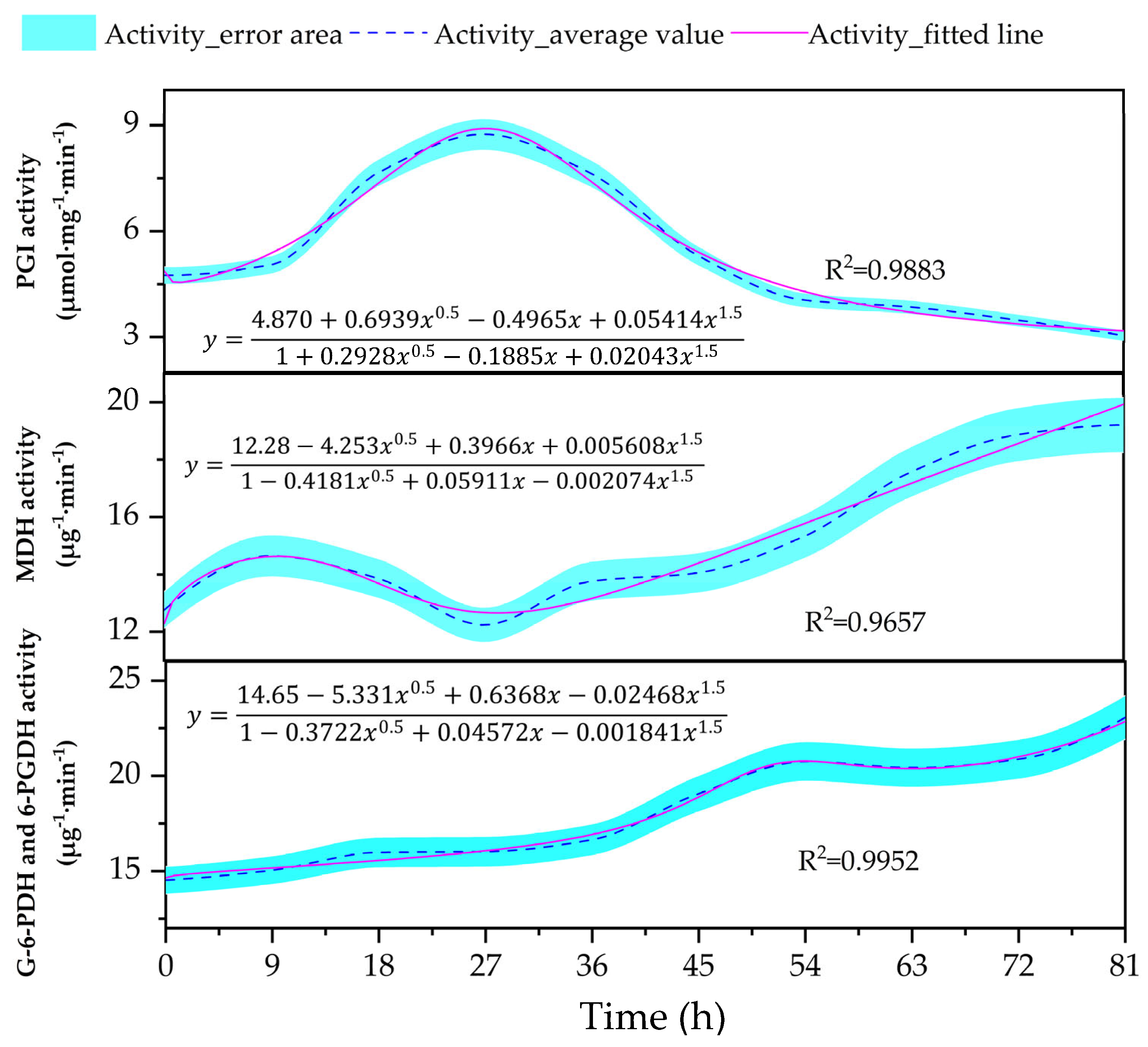
3.4. Changes in the Content of Key Oxidative Pathway Enzyme Activity (PGI, MDH, G-6-PDH, and 6-PGDH) in the Embryos
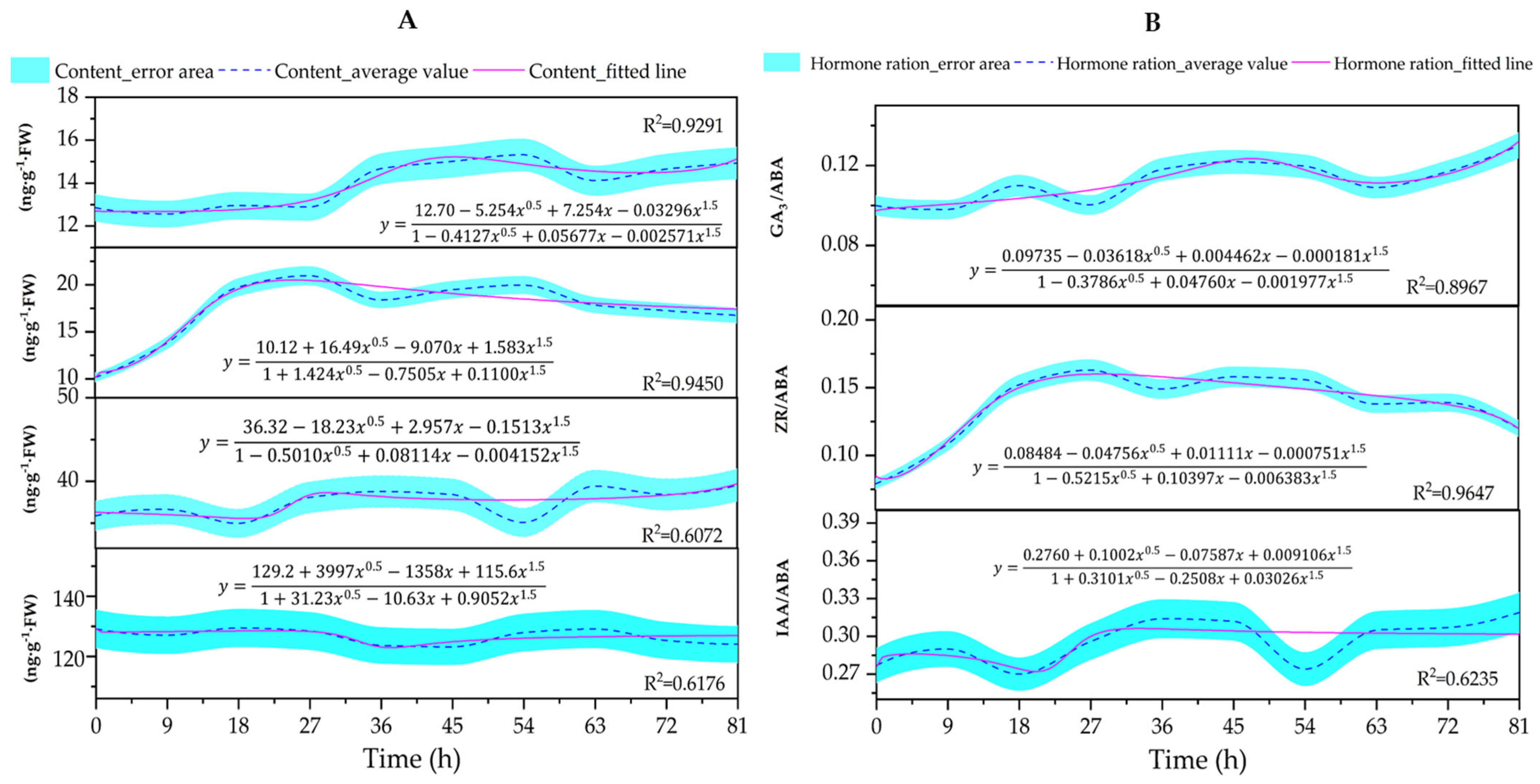
3.5. Changes in the Content of Endogenous Hormones (GA3, ZR, IAA, and ABA) and Ratios (GA3/ABA, IAA/ABA, and ZR/ABA) in the Embryos
3.6. Morphological Changes in the Contents of Cotyledon Cells
4. Discussion
4.1. Changes in Seed Water Content and Respiration Rate
4.2. Changes in Reserve Materials Within Embryos
4.3. Changes in the Activity of Hydrolytic Enzymes Within Embryos
4.4. Changes in the Activities of Key Enzymes of Oxidative Pathways Within Embryos
4.5. Changes in Endogenous Hormone Content in Embryos
4.6. Ultrastructural Changes in Cotyledon Cell Contents
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, C.J.; Wang, Q.; Lin, C.W. Progress on the pharmacological effects of Toona sinensis leaves. China Pharm. 2011, 22, 277–280. [Google Scholar]
- Zhao, Q.; Zhong, X.-L.; Zhu, S.-H.; Wang, K.; Tan, G.-F.; Meng, P.-H.; Zhang, J. Research advances in Toona sinensis, a traditional Chinese medicinal plant and popular vegetable in China. Diversity 2022, 14, 572. [Google Scholar] [CrossRef]
- Li, W.Z.; Ding, J.X.; Zhang, X.P.; Tian, J.Z. Advances in chemical composition, extraction methods and bioactivity of Toona sinensis. J. Pharm. Res. 2012, 31, 37–39. [Google Scholar]
- Yang, M.; Zhong, G.Y.; Gao, Y.H.; Lv, J.C.; Wang, R.Q.; Hang, R.Q.; Huang, P.; Li, H.X. Current status and research advances in propagation techniques of Toona sinensis. Bull. Agric. Sci. Technol. 2021, 11, 217–220. [Google Scholar]
- Fang, J.-Y.; Zhu, Y.; Wang, C.-Y.; Ye, K.-K.; Gao, W.-D.; Zhang, Z.-H.; Yan, J.-J.; Li, Q.-M. Physiological and biochemical changes of Toona sinensis seeds during artificial aging. For. Res. 2020, 33, 163–169. [Google Scholar]
- Hou, J.; Wang, D.; Su, P.; Ding, S.; Wu, L. In vitro large-scale propagation and genetic fidelity of Toona sinensis (Juss.) M. Roem., an economically important vegetable tree. Plant Cell Tissue Organ. Cult. 2022, 151, 275–291. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic processes during seed germination. Adv. Seed Biol. 2017, 2017, 141–166. [Google Scholar]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Taylor, K.A. A modification of the phenol/sulfuric acid assay for total carbohydrates giving more comparable absorbances. Appl. Biochem. Biotechnol. 1995, 53, 207–214. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F. Seed Science Laboratory Manual; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Younessi-Hamzekhanlu, M.; Izadi-Darbandi, A.; Malboobi, M.A.; Ebrahimi, M.; Abdipour, M.; Sparvoli, F.; Paolo, D. Agrobacterium rhizogenes transformed soybeans with AtPAP18 gene show enhanced phosphorus uptake and biomass production. Biotechnol. Biotechnol. Equip. 2018, 32, 865–873. [Google Scholar] [CrossRef]
- Zhang, S.L. Effects of Salicylic Acid on Respiratory Pathway of Huang-Guan Pear; Agricultural University of Heibei: Baoding, China, 2008. [Google Scholar]
- Brown, A.; Wray, J. Correlated changes of some enzyme activities and cofactor and substrate contents of pea cotyledon tissue during germination. Biochem. J. 1968, 108, 437–444. [Google Scholar] [CrossRef]
- Bergmeyer, H. Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1956. [Google Scholar]
- Zhao, J.; Li, G.; Yi, G.-X.; Wang, B.-M.; Deng, A.-X.; Nan, T.-G.; Li, Z.-H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79–85. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Du, M.; Eneji, A.E.; Wang, B.; Duan, L.; Li, Z.; Tian, X. Mechanism of phytohormone involvement in feedback regulation of cotton leaf senescence induced by potassium deficiency. J. Exp. Bot. 2012, 63, 5887–5901. [Google Scholar] [CrossRef] [PubMed]
- He, Z. Guidance to Experiment on Chemical Control in Crop Plants; Beijing Agricultural University Publishers: Beijing, China, 1993; pp. 60–68. [Google Scholar]
- Szczerba, A.; Płażek, A.; Pastuszak, J.; Kopeć, P.; Hornyák, M.; Dubert, F. Effect of low temperature on germination, growth, and seed yield of four soybean (Glycine max L.) cultivars. Agronomy 2021, 11, 800. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Long, W.; Yao, X.H.; Lv, L.Y.; Wang, K.L. An analysis of seed traits and endogenous hormone levels after seed soakings in Camellia oleifera. J. Nanjing For. Univ. 2020, 44, 148–156. [Google Scholar]
- Nie, X.Y.; Bai, F.H.; Li, Z.; Zhang, S.S.; Chu, H.; Cai, S.J.; Li, C.H.; Xu, T.L. Study on the seed biological characteristics of two types of Gentiana plant seed in Qinghai. J. Qinghai Univ. 2024, 42, 27–33. [Google Scholar]
- Araújo, P.C.; Torres, S.B.; Benedito, C.P.; Paiva, E.P.d. Condicionamento fisiológico e vigor de sementes de maxixe. Rev. Bras. Sementes 2011, 33, 482–489. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Bai, Y.; Xie, Y.; Li, L.; Mu, S.; Gao, J. Transcriptome analysis of energy supply process during seed germination in Phyllostachys edulis. Plant Mol. Biol. Report. 2023, 41, 489–511. [Google Scholar] [CrossRef]
- Bewley, J.; Black, M. Seeds: Physiology of Germination and Development; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Batool, M.; El-Badri, A.M.; Wang, C.; Mohamed, I.A.; Wang, Z.; Khatab, A.; Bashir, F.; Xu, Z.; Wang, J.; Kuai, J. The role of storage reserves and their mobilization during seed germination under drought stress conditions of rapeseed cultivars with high and low oli contents. Crop Environ. 2022, 1, 231–240. [Google Scholar] [CrossRef]
- Chen, Y.-S.; David Ho, T.-H.; Liu, L.; Lee, D.H.; Lee, C.-H.; Chen, Y.-R.; Lin, S.-Y.; Lu, C.-A.; Yu, S.-M. Sugar starvation-regulated MYBS2 and 14-3-3 protein interactions enhance plant growth, stress tolerance, and grain weight in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 21925–21935. [Google Scholar] [CrossRef]
- Zhu, M.; Zang, Y.; Zhang, X.; Shang, S.; Xue, S.; Chen, J.; Tang, X. Insights into the regulation of energy metabolism during the seed-to-seedling transition in marine angiosperm Zostera marina L.: Integrated metabolomic and transcriptomic analysis. Front. Plant Sci. 2023, 14, 1130292. [Google Scholar] [CrossRef]
- Gianinetti, A.; Finocchiaro, F.; Bagnaresi, P.; Zechini, A.; Faccioli, P.; Cattivelli, L.; Valè, G.; Biselli, C. Seed dormancy involves a transcriptional program that supports early plastid functionality during imbibition. Plants 2018, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Galland, M.; Huguet, R.; Arc, E.; Cueff, G.; Job, D.; Rajjou, L. Dynamic proteomics emphasizes the importance of selective mRNA translation and protein turnover during Arabidopsis seed germination. Mol. Cell. Proteom. 2014, 13, 252–268. [Google Scholar] [CrossRef]
- Sharma, S.; Gambhir, S.; Munshi, S.K. Changes in lipid and carbohydrate composition of germinating soybean seeds under different storage conditions. Asian J. Plant Sci. 2007, 6, 502–507. [Google Scholar]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Penfield, S.; Graham, S.; Graham, I. Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem. Soc. Trans. 2005, 33, 380–383. [Google Scholar] [CrossRef]
- Joshi, R. Role of enzymes in seed germination. Int. J. Creat. Res. Thoughts 2018, 6, 1481–1485. [Google Scholar]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The rice alpha-amylase, conserved regulator of seed maturation and germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Itoh, S.; Saitoh, T.; Ikeda, A.; Tashiro, T.; Nagato, Y. Characterization of β-amylase and its deficiency in various rice cultivars. Theor. Appl. Genet. 1999, 98, 32–38. [Google Scholar] [CrossRef]
- Li, Y.-J.; Hu, Q.-P. Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase. Open Life Sci. 2020, 15, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gao, X.; Wu, Y. Key Metabolite differences between Korean Pine (Pinus koraiensis) seeds with primary physiological dormancy and no-dormancy. Front. Plant Sci. 2021, 12, 767108. [Google Scholar] [CrossRef]
- Botha, F.; Potgieter, G.; Botha, A.-M. Respiratory metabolism and gene expression during seed germination. Plant Growth Regul. 1992, 11, 211–224. [Google Scholar] [CrossRef]
- Wu, Y.; Bao, W.Q.; Hu, H.; Zhou, Y.J.; Shen, Y.B. Effects of H2SO4 and Gibberellin A3 on Dormancy Release of Tilia miqueliana Seeds. J. Plant Growth Regul. 2022, 41, 796–807. [Google Scholar] [CrossRef]
- Gong, D.; He, F.; Liu, J.; Zhang, C.; Wang, Y.; Tian, S.; Sun, C.; Zhang, X. Understanding of hormonal regulation in rice seed germination. Life 2022, 12, 1021. [Google Scholar] [CrossRef]
- Guo, H.; Li, J.; Liu, Y.; Fernández-Pascual, E. Lipid metabolism during seed germination of Pistacia chinensis and its response to gibberellic acid. Plant Physiol. Biochem. 2025, 219, 109371. [Google Scholar] [CrossRef]
- Chen, L.P. Studies on the Changes of Physiological and Biochemical Characteristics of Pinus tabuliformis Seeds During Initial Germinating Stages; Nanjin Forestry University: Nanjing, China, 2009. [Google Scholar]
- Liu, J.; Qiu, S.; Xue, T.; Yuan, Y. Physiology and transcriptome of Sapindus mukorossi seeds at different germination stages. Genomics 2024, 116, 110822. [Google Scholar] [CrossRef]
- Shun, G.Z.; Xiao, S.H. Abscisic Acid and Seed Dormancy. Plant Physiol. Commun. 2004, 40, 115–120. [Google Scholar]
- Gao, Y.; Zhu, M.; Wang, H.; Li, S. Dynamic changes to endogenous germination inhibitors in Cercis chinensis seeds during dormancy release. HortScience 2021, 56, 557–562. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, J.; Xu, F.; Chu, J.F.; Yan, C.Y.; Schläppi, M.R.; Wang, Y.P.; Chu, C.C. Expression Patterns of ABA and GA Metabolism Genes and Hormone Levels during Rice Seed Development and Imbibition: A Comparison of Dormant and Non-Dormant Rice Cultivars. J. Genet. Genom. 2014, 41, 327–338. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Z.; Gao, J.; Gong, Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014, 79, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, S.; Xue, T.; Yuan, Y. Physiology and transcriptome of Eucommia ulmoides seeds at different germination stages. Plant Signal. Behav. 2024, 19, 2329487. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yan, X.; Li, T.H.; Li, Y.L.; Zhang, Y.B.; Xing, L.; Guo, S.Y. Physiological responses of seed dormancy and germination to cold stratification in Phoebe hui Cheng ex Yang. Plant Sci. J. 2022, 40, 398–407. [Google Scholar]
- Tuan, P.A.; Rohit, K.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular Mechanisms Underlying Abscisic Acid/Gibberellin Balance in the Control of Seed Dormancy and Germination in Cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef]
- Yaish, M.W.; Ashraf, E.K.; Tong, Z.; Beatty, P.H.; Good, A.G.; Yong-Mei, B.; Rothstein, S.J.; Copenhaver, G.P. The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice. PLoS Genet. 2010, 6, e1001098. [Google Scholar] [CrossRef]
- Yan, F.; Wei, T.; Yang, C.; Yang, Y.; Luo, Z.; Jiang, Y. Combined Analysis of Untargeted Metabolomics and Transcriptomics Revealed Seed Germination and Seedling Establishment in Zelkova schneideriana. Genes 2024, 15, 488. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, L.; Tian, C.Y.; Huang, Z.Y. Germination dimorphism in Suaeda acuminata: A new combination of dormancy types for heteromorphic seeds. South. Afr. J. Bot. 2012, 78, 270–275. [Google Scholar] [CrossRef]
- Si, Q.; Ma, Y.; Zang, D. The causes of dormancy and the changes of endogenous hormone content in Cephalotaxus sinensis seeds. Agric. Sci. 2016, 7, 834. [Google Scholar]
- Ge, C.H.; Ji, Q.L.; Liu, Y.P.; Wang, X.H. The Changes of Protein Bodies’ Ultrastructure in Cell of Seeds of Populus euphratic Oliv in the Process of Storage. Seed 2007, 5, 27–29. [Google Scholar]
- Qin, Z.; Wang, T.; Zhao, Y.; Ma, C.; Shao, Q. Molecular Machinery of Lipid Droplet Degradation and Turnover in Plants. Int. J. Mol. Sci. 2023, 24, 16039. [Google Scholar] [CrossRef]
- Dong, J.H.; Cui, D.C.; Shao, Z.Z.; Jin, L.F. Ultrastructural Changes and Localization of Acid Phosphatase in Scutellar Epi Thelium of Coix lacryma-jobi During Germination. Acta Biol. Exp. Sin. 1996, 29, 71–79. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, Z.; Wu, Y.; Shen, Y. Hormonal and Storage Metabolic Regulation of Germination in Toona sinensis. Horticulturae 2025, 11, 685. https://doi.org/10.3390/horticulturae11060685
Liu L, Wang Z, Wu Y, Shen Y. Hormonal and Storage Metabolic Regulation of Germination in Toona sinensis. Horticulturae. 2025; 11(6):685. https://doi.org/10.3390/horticulturae11060685
Chicago/Turabian StyleLiu, Linyue, Zhiyuan Wang, Yu Wu, and Yongbao Shen. 2025. "Hormonal and Storage Metabolic Regulation of Germination in Toona sinensis" Horticulturae 11, no. 6: 685. https://doi.org/10.3390/horticulturae11060685
APA StyleLiu, L., Wang, Z., Wu, Y., & Shen, Y. (2025). Hormonal and Storage Metabolic Regulation of Germination in Toona sinensis. Horticulturae, 11(6), 685. https://doi.org/10.3390/horticulturae11060685





