Abstract
Onopordum nervosum subsp. platylepis Murb. is an Asteraceae species found in Tunisia, Algeria, and Libya. It has been studied for its potential use as a vegetable rennet alternative to animal-derived rennet, making it important to understand its germination characteristics. This species often shows low germinability due to dormancy, which limits its large-scale cultivation. In preliminary experiments, many factors were analyzed to evaluate the seed germination of this thistle including physical stratification with abrasive paper and high-temperature water, chemical treatments with hydrogen peroxide (H2O2) or sulfuric acid (H2SO4), and the exogenous use of the growth regulator gibberellic acid (GA3). Based on the obtained results and subsequent analysis, GA3 and cold stratification durations were selected for the second experiment, which used a full factorial RSM design with three levels for each factor. Additionally, the total polyphenol content (TPC), total flavonoid content (TFC), condensed tannin content (CTC), and DPPH assay were analyzed during the optimization process and for ungerminated seeds. The findings revealed that 14 days of cold stratification followed by 750 ppm GA3 was the most efficient method for breaking the dormancy of these thistle seeds. The highest TPC was recorded in ungerminated seeds. However, germinated Onopordum nervosum ssp. platylepis seeds showed higher levels of TFC and CTC (14.83 mg QE/g DW and 6.49 mg EC/g DW, respectively) compared to the non-germinated ones and demonstrated the greatest potency in inhibiting free radicals (DPPH EC50 = 0.018 mg/mL) at the identified optimal germination conditions. Ungerminated seeds indicated significant phenolic content (TPC) and a limited ability to reduce oxidants, which could explain their low germination percentage. Our findings on the seed germination and dormancy characteristics of this endemic thistle will aid in the protection and development of its germplasm.
1. Introduction
Onopordum nervosum subsp. platylepis Murb. (Asteraceae) is an endemic plant in Tunisia, Algeria, and Libya [1,2,3]. In Tunisia, this species grows particularly in the northern and central regions, with rare occurrences in the south [4]. This species is traditionally used in rural areas to produce raw cheese in Tunisia and has recently been the subject of research on the influence of genotype on milk clotting activity and chemical composition, suggesting that flowers of this thistle are a good source of milk-clotting agents and promote the plant’s potential as a cheese-making substitute for animal rennet [5]. Cultivating this plant for the production of novel thistle-based milk coagulants could offer a sustainable method to preserve biodiversity, ensure consistent flower availability, reduce reliance on animal rennet by providing a plant-based alternative that aligns with vegetarian and religious dietary practices, and support conservation efforts [5]. Furthermore, the flowers of this endemic thistle are recognized for their high phenolic content and antioxidant activity, making them a promising agri-food material in both spontaneous and cultivated plants [5,6]. Additionally, the impacts of different drying methodologies on the properties of Onopordum nervosum subsp. platylepis flowers have been investigated, leading to the development of new methods for preserving and using these bioactive compounds [6].
Onopordum nervosum is an annual, monocarpic, heliophilic species that flowers at the beginning of summer, even under the extreme summer conditions of the Mediterranean climate. This basiphilous plant generally prefers sandy-loamy and sandy-clayey-loamy soils [4], growing at the edges of roads and on rubble [1,7]. This thistle species is robust, reaching a height of 50–150 cm, with erect stems and pale green leaves. The flower head of this plant has a bristly appearance, containing brown achenes (seed + pericarp) and white feathery structures present in bunches at the tips of the seeds, forming the pappus. The seeds are brown and ovoid (flattened at the end). Achenes, which are three times shorter than their pappus, have a transversely wrinkled surface through which the seeds are widely spread [7,8].
Endemic plant species are particularly susceptible to the combined pressures of anthropogenic threats and natural selection. The Mediterranean climate zone is among the most globally affected regions by species decline [9]. Therefore, conservation biology is concerned with preserving plant species, especially rare or endemic ones, and the preservation and valorization of these plants have become a priority for sustainable exploitation. Onopordum alexandrinum, a species within the genus Onopordum, has dramatically declined and become fragmented in Egypt due to natural processes and human activities including climate change and habitat destruction [10]. Similarly, Onopordum nogalesii, an endemic plant from Fuerteventura in the Canary Islands (Macaronesian region, Spain), is critically endangered. The main threats to this species include grazing, collection, and habitat loss [10]. Ex situ conservation programs should be considered to supplement the preservation purposes of threatened species [11]. Since flowering species disperse their progeny through seeds, seed banking is the most efficient conservation approach to preserve biodiversity [11]. According to Baskin and Baskin [12], many wild plant seeds are dormant immediately after reaching maturity, and the same case has been observed with Onopordum nervosum seeds [13]. Testing the germination requirements of threatened species can and should be carried out using information obtained from germination tests [14,15]. In Tunisia, several species and groups of species are particularly threatened including medicinal, spontaneous, and cultivated species [16]. O. platylepis is a threatened species in this region [5,6,17] and requires continuous enhancement of cultivation techniques to ensure the long-term survival of this species [5]. These efforts are important to preserve the genetic diversity and ecological role of O. platylepis [5]. Advancing propagation and cultivation strategies for this species could enable a more effective reduction in threats from habitat loss and human activities. However, cultivation on a large scale, preservation, and valorization of this thistle in the food sector are difficult due to seed germination limitations.
The germination characteristics of a plant species can help us better understand a plant’s life cycle [18]. It determines the success of in situ and ex situ cultivations, ensuring survival of the population and agricultural production [19,20,21]. Therefore, understanding seed germination behavior is essential to develop effective ex situ conservation procedures, which have important industrial, economic, and societal impacts [22].
Germination is controlled by internal and external factors, and dormancy is induced in response to the environment’s impact on the mother plant [23]. The availability of oxygen and water, ambient temperature, and light conditions determine seed development and dormancy induction [20,24]. Drought, heat, cold, and salinity stress induce metabolic changes, biochemical, and physiological perturbations, leading to oxidative stress during the plant growth stages [25,26,27].
Dormancy ensures seed survival under unfavorable conditions and promotes germination when environmental conditions are favorable. It can be classified into three main types: physical, physiological, and morphological dormancy [12,28]. Identifying seed dormancy types is important for promoting germination under optimal conditions. Methods such as temperature variations, chemical treatments, and mechanical seed coat removal have been widely investigated to enhance seed germination [29,30]. In the Asteraceae family, many thistles develop cypselas with distinct germination patterns [31]. During winter, the presence of inhibitors such as abscisic acid (ABA) and phenolic compounds can lead to a gradual germination process in Onopordum nervosum [32]. Furthermore, previous studies indicate that seeds of Onopordum nervosum Boiss. have profound physiological dormancy, with ABA identified as a primary endogenous inhibitor [13].
Researchers have revealed that the exogenous application of plant growth hormones, particularly gibberellins (GA3), can effectively overcome seed dormancy in a wide range of plant species [13]. Specifically, in Onopordum nervosum, the findings strongly suggest that endogenous levels of GA3 and GA20 play an important role in releasing dormancy [33].
Many studies have examined phenolic compounds as a response mechanism for improving germination conditions [34,35]. Different phenolic compounds found in seeds could block respiration or other metabolic pathways, preventing their germination [36]. These compounds inhibit germination by reducing gas exchange, generating reactive oxygen species, and acting as antioxidants [37]. Additionally, some phenolic compounds, such as tannins, can precipitate proteins and enzymes involved in seed germination. Phenolic compounds are recognized for their involvement in the plants’ mechanisms to mitigate environmental stress and their capacity to defend seeds from predation and disease. It has been demonstrated that their effect as natural germination inhibitors is dependent on the concentration of the compound, the type of seed, and other environmental factors [38].
The conservation of plants requires a deeper understanding of their life cycle. Recently, the characteristics of germination of endemic and rare plant species have gained increasing relevance and importance [39]. In order to develop conservation guidelines for threatened or endemic plants, it is necessary to understand their biology and habitat characteristics [40]. To our knowledge, this is the first study aimed at understanding the germination and dormancy properties of endemic Onopordum nervosum ssp. platylepis seeds as well as improving the germination conditions using response surface methodology (RSM). RSM offers several advantages for optimizing germination conditions: it allows for the simultaneous evaluation of many factors affecting seed germination, reduces the number of experimental runs required, and helps identify interactions between variables [41]. This method also enables the development of predictive models for estimating the germination outcomes and improves reproducibility [41], making it a valuable tool for optimizing the germination conditions, supporting cultivation, and facilitating the conservation and utilization of O. platylepis. This study entailed evaluating the total phenolic content (TPC), total flavonoid content (TFC), and condensed tannin content (CTC) as well as antioxidant activities using the DPPH and FRAP tests. Tests for seed germination can be used to assess the ecology of seed germination in endemic and/or rare species [42], making the exploration of seed dormancy and the propagation of this endemic thistle important for future breeding programs and its valorization in the food sector.
2. Materials and Methods
2.1. Cypsela Collection
Bulk collections of O. platylepis were taken in late July and August 2020 from 50 randomly selected spontaneous plants, each separated by approximately 5 m, from a wild population located in Chott Meriem, Sousse, Tunisia (latitude N 35°91′6.738″, longitude E 10°56′2.078″) at a non-agricultural site. This region was chosen for seed collection because it lies within the natural distribution range of O. platylepis. The location is characterized by sandy-loamy soils and a Mediterranean climate with hot, dry summers and mild, wet winters, which may influence the seed germination patterns of this thistle. Additionally, an important number of individuals have been found in this area, providing a relevant population for this study. The identification of the species and plant material was conducted by Prof. Rabiaa Haouala, a botanist from ISA-CM at the University of Sousse.
Dried petals were removed from flower heads (Figure 1b) to recover the seeds (Figure 1c). Immediately after each collection, cypselas were manually cleaned, dried, and any defective ones were discarded. Then, after collection, the achenes were subjected immediately to different germination tests.
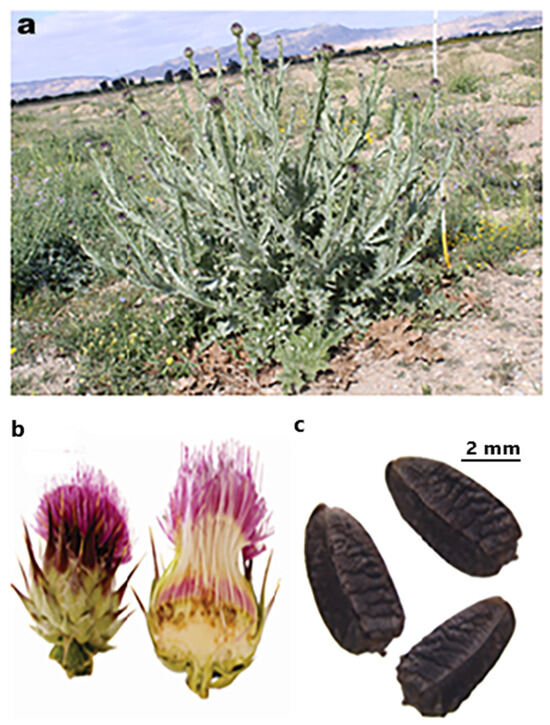
Figure 1.
Onopordum nervosum subsp. platylepis Murb. wild plant (a), flower and capitula (b), and seeds (Gx8) (c).
2.2. Seeds Water Uptake
To assess the permeability of achene coats of O. platylepis to water, 20 achenes collected were individually weighed using an electronic balance (Ohaus corp, Pine Brook, NJ, USA). Subsequently, every achene was set within a 5-cm plastic Petri dish containing two filter paper discs moistened with 4 mL of distilled water, and then subjected to a 10-day incubation period in darkness (Petri dishes were covered with aluminum) in an incubator at 25 °C.
To measure the moisture content of seeds during the incubation period, the mass of each individual achene was measured daily after being carefully blotted dry. The water content (WC) was then calculated based on the increase in weight compared to the achene’s pre-drying weight, using the equation established by Baskin et al. [43].
where Wn presents the weight after n days of imbibition and Wi is the initial weight before imbibition.
2.3. Process for Germination Tests
Seeds were soaked in water for 24 h, then sterilized with a 5% sodium hypochlorite solution containing Tween 20 for 10 min. Following sterilization, they were rinsed three times with distilled water. For the germination tests, three replications of 20 achenes were arranged in 9 cm diameter sterile Petri dishes that were lined with two layers of filter paper and moistened with distilled water for the control group or with the appropriate treatment solution for the experimental ones. These dishes were subsequently maintained in darkness at 25 °C in an incubator, with regular checks on the seeds’ humidity. Germination was assessed by the emergence of the radicle, and achenes were counted as germinated once the radicle was at least 1 mm in length. Then, the germination percentage (SGP%) and mean germination time (MGT) were recorded accordingly.
The seed germination percentage (SGP%) was calculated by using the following equation [44]:
where n represents the number of germinated seeds by the end of the experiment and N represents the total number of seeds
The mean germination time (MGT) was calculated by using the following equation [45]:
where t represents the time in days from the first day to the final day of the experiment (germination test), and n represents the total number of seeds germinated on day t.
2.3.1. Experiment 1
Before the optimization process, preliminary tests were conducted to investigate the breaking dormancy methods. Physical stratification was tested by either using abrasive paper (No. 200) or soaking seeds in water at a high temperature of 100 °C for 30, 60, and 120 s. The efficacy of the chemical treatment was tested using hydrogen peroxide (H2O2) or sulfuric acid (H2SO4) treatments for 5, 10, or 15 min. Furthermore, the exogenous use of a growth regulator, such as gibberellins (GA3), was evaluated using four concentrations: 250, 500, 750, and 1000 ppm (imbibition for 24 h). Then, the seeds were rinsed with distilled water. These concentrations were assessed due to the dormancy previously observed in Onopordum nervosum Bois seeds, which require potent stimuli for dormancy release [13]. Finally, at the end of the experiments, seed germination was recorded.
2.3.2. Experiment 2
Germination Test
Three replications of 20 seeds were exposed to cold stratification at 4 °C for 0, 7, and 14 days before sowing, then arranged in 9 cm Petri dishes between two pieces of Whatman No. 1 filter paper and moistened as required with the corresponding gibberellic (GA3) solution (500 ppm, 750 ppm, and 1000 ppm). These parameters were selected based on preliminary tests to evaluate their combined effects on breaking seed dormancy in O. platylepis, as cold stratification and gibberellic acid (GA3) play important roles in influencing dormancy, providing a comprehensive assessment of their effectiveness in promoting germination. Subsequently, the germination percentage and mean germination time were recorded.
Analysis of Bioactive Compounds
Non-germinated and germinated samples were dried, finely powdered, and subsequently sealed in plastic bags under vacuum, protected from light exposure, and stored at 4 °C until further analysis. Three aqueous extracts (1:100, w/v) were prepared from 1 g of O. platylepis seed powder. The mixture was subjected to continuous mixing and then maintained at 40 °C for 60 min. The extracts were then centrifuged at 8000 rpm for 40 min, followed by filtration. The filtered extracts were stored at 4 °C for future use.
- Determination of total phenol content (TPC)
The phenolic compound content (TPC) was measured using the Folin–Ciocalteu phenol reagent method as described by Singleton and Rossi [46] with some modifications. An aliquot (100 μL) of the filtered extract was mixed with distilled water (1 mL) and the Folin–Ciocalteu reagent (500 μL), and the mixture was stirred for 1 min. After adding a 20% sodium carbonate solution (1.5 mL), the mixture was incubated for 120 min in the dark at room temperature. The absorbance of the mixture was then measured at 760 nm in triplicate using a UV–Vis spectrophotometer (Macy model, UV-1300, China). The results were expressed as mg of gallic acid equivalents (GAE)/g of dry weight (DW) through a calibration curve with gallic acid.
- Determination of total flavonoid content (TFC)
The flavonoid content was evaluated according to the method described by Kim et al. [47] with some modifications. Briefly, the seed extract (0.25 mL) was mixed with distilled water (1.25 mL). Subsequently, a 5% sodium nitrite solution (75 μL) was added and mixed for 6 min, after which 10% aluminum chloride (150 μL) was added to the mixture. After 5 min, 1 N sodium hydroxide (0.5 mL) was added along with distilled water (775 μL) to reach a total volume of 3000 μL. The absorbance of the final mixture was then measured at 510 nm using a spectrophotometer (Macy model, UV-1300, Shanghai Aesthetic analysis Instrument Co., Ltd., Shanghai, China). The results were expressed in mg of quercetin equivalents (mg QE) per g of dry weight (DW).
- Determination of total condensed content (TCT)
The condensed tannin content (CTC) of the extracted samples was analyzed using the vanillin assay according to the modified method of Titto [48]. A 4% vanillin reagent (3000 μL) was added to 50 μL of the extract. A 4% vanillin reagent (3000 μL) was added to the mixture. Samples were incubated for 15 min at room temperature, and finally, the absorbance was recorded at 500 nm. The results were reported as milligrams of catechin equivalents (CE) per gram of dry weight (DW).
Analysis of Antioxidant Activities
- Determination of DPPH radical scavenging activity
The DPPH radical scavenging activity of the O. platylepis seeds was measured using the method described by Kim et al. [49]. A 0.1 mM DPPH solution was prepared with 100% methanol to assess the DPPH radical scavenging activity. Briefly, the seed extract (1 mL) with different concentrations was mixed with the DPPH solution (1 mL) and was allowed to stand in the dark for 30 min at room temperature. The absorbance of the mixture was then measured at 517 nm using a UV–Vis spectrophotometer (Macy model, UV-1300, China). Vitamin C was used as the positive control.
The calculation formula of the DPPH radical scavenging rate is as follows:
where A0 represents the absorbance of the control containing 1 mL of DPPH solution and 1 mL of distilled water. A1 is the absorbance of the tested samples. EC50 represents the value of the effective concentration that is graphically determined by logarithmic regression representing the antioxidant concentration needed to reduce DPPH by 50%.
- Determination of Iron Reduction Ability (FRAP)
The reducing antioxidant power of the seed extract was determined following the method described by Yildirim et al. (2001). Briefly, the seed extract (0.5 mL) at different concentrations was mixed with phosphate buffer (0.625 mL, 0.2 mM L−1, pH = 6.6) and 1% potassium ferric oxide [50]. After being thoroughly mixed, the mixture was rapidly cooled in a water bath at 50 °C for 30 min. Then, 10% trichloroacetic acid (0.625 mL) was added and mixed well. From the supernatant, 1.25 mL was carefully pipetted and mixed with distilled water (1.25 mL) and 0.1% ferric chloride (0.25 mL). The absorbance of the final solution was measured at 700 mm. The higher the absorbance at 700 nm, the stronger the reducing ability of the seed extract. Vitamin C was used as the positive control. The EC50 value, which represents the concentration that yields an absorbance of 0.5, was determined graphically.
2.4. Optimization of O. platylepis Germination Conditions Using Response Surface Methodology (RSM)
In order to improve the germination conditions of O. platylepis, full factorial response surface methodology (RSM) was used based on a two-factor and three-level design. The evaluated factors were gibberellin concentration and cold stratification duration. The maximum and minimal coded values were attributed to all factors each paired with its corresponding true value. Therefore, 0 was centrally coded, +1 was the highest, and −1 was the lowest value (Table 1).

Table 1.
Factors for optimizing the germination conditions of O. platylepis using RSM.
Fourteen experiments were carried out following the established experimental design, with a duplication of the experiment being considered. The measured responses were seed germination percentage (SGP%), germination time (GT%), phenolic composition (total phenolic content (TPC), total flavonoid content (TFC), and condensed tannin content (CTC)), and antioxidant activity (DPPH assay).
2.5. Statistical Analysis
For the preliminary germination tests, the effect of various factors on germination was subjected to an analysis of variance (ANOVA), and the means were separated based on the Duncan test (p ≤ 0.05) through SPSS software (IBM SPSS Statistics 23). The experimental design was generated using Statistica software (STATISTICA 12.5, TIBCO, Palo Alto, CA, USA) with two independent factors: cold stratification time (X1; 0, 7, or 14 days) and GA3 concentration (X2; 500, 750, and 1000 ppm). The concentrations of GA3 and the cold stratification periods were chosen as two independent factors based on preliminary experiments and subsequent analysis. Data obtained from responses were fitted into a second-order polynomial equation as shown below:
where Y is the response variable (seed germination percentage, germination time, TPC, TFC, CTC, DPPH), X1 and X2 are process variables (cold stratification time and GA3 concentration, respectively), β0 (intercept), β1 and β2 (linear terms) are regression coefficients β11 and β22 (quadratic terms), β12 (interaction term), and ε represents the error. The model was simplified by removing insignificant terms according to the backward elimination technique [51]. The graphics in this manuscript were plotted using Excel 2016 and Statistica software (STATISTICA 12.5, TIBCO, Palo Alto, CA, USA).
Y = β0 + β1X1 + β2X2 + β12X1X2 + β11X12 + β22X22 + ε
3. Results
3.1. Seeds Coat Permeability
The water uptake of the achenes increased slightly from the initiation of imbibition until day 8. Thereafter, the achenes did not show any additional increase in water content until the end of the experiment (Figure 2). Investigating the seed’s water content is the only method to assess the water permeability of seed coats. The cypselae of O. platylepis have a fairly thick pericarp, which may influence the water uptake and then the germination process.
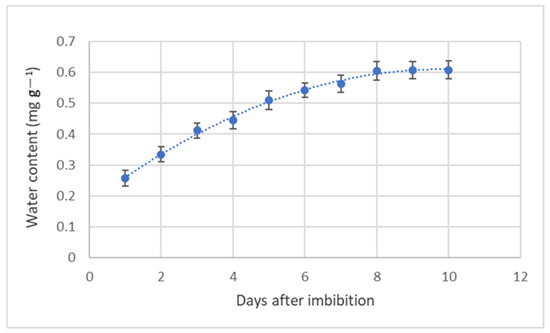
Figure 2.
Evolution of water imbibition during the germination process of O. platylepis seeds.
3.2. Experiment 1
Achenes treated with concentrated H2SO4 for 15 min showed germination percentages comparable to those found in the controls. However, the germination of achenes treated with 5-min H2O2 was comparable to the germination observed for those exposed to a 30-s immersion in water at 100 °C. A slight enhancement in germination was observed for achenes soaked in high-temperature water for 60 s. On the other hand, GA3-treated achenes reached significantly higher germination percentages compared to those found in the control and other treatments (p < 0.05; Figure 3). Despite this increase, the final germination percentage did not reach the optimal values. The experiment indicated the presence of a physiological dormancy.
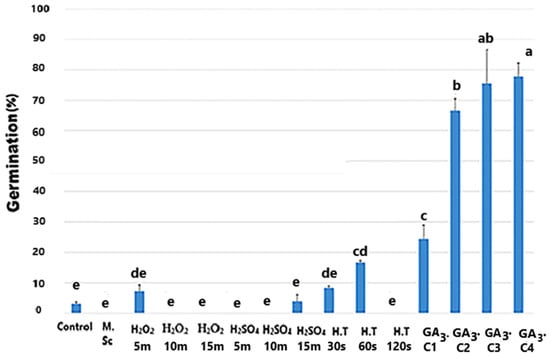
Figure 3.
Percent germination of O. platylepis achenes incubated after mechanical scarification (M. Sc), different soaking times for 5, 10, or 15 min in H2SO4 or H2O2 for 30, 60, or 120 s in hot water (H.T:100 °C) or after the exogenous application of different concentrations of gibberellic acid (GA3. C1, GA3. C2, GA3. C3, and GA3. C4, respectively, for GA3 250, 500, 750 and 1000 ppm). Each germination value is the mean ± SE of three replicates of 20 achenes each. T-bars indicate the SE. Different letters at the top of each bar indicate significant differences according to the Duncan test (p ≤ 0.05).
3.3. Experiment 2
3.3.1. Bioactive Compounds of Non-Germinated Seeds
The phenolic composition of the endemic Tunisian plant O. platylepis seeds is not well-documented. However, an evaluation of the phenolic composition of non-germinated O. platylepis seeds was conducted. Interestingly, the results showed an important content of total phenols (9.78 ± 0.48 mg GAE/g DW), a flavonoid content of 6.74 ± 0.31 mg QE/g DW, and an important condensed tannin content with a value of 4.35 mg CE/g DW.
3.3.2. Antioxidant Activity of Non-Germinated Seeds
Figure 4 shows the antioxidant capacity of the O. platylepis extract expressed in terms of DPPH radical inhibition (%) (Figure 4a) and ferric reducing antioxidant power (FRAP) (Figure 4b).
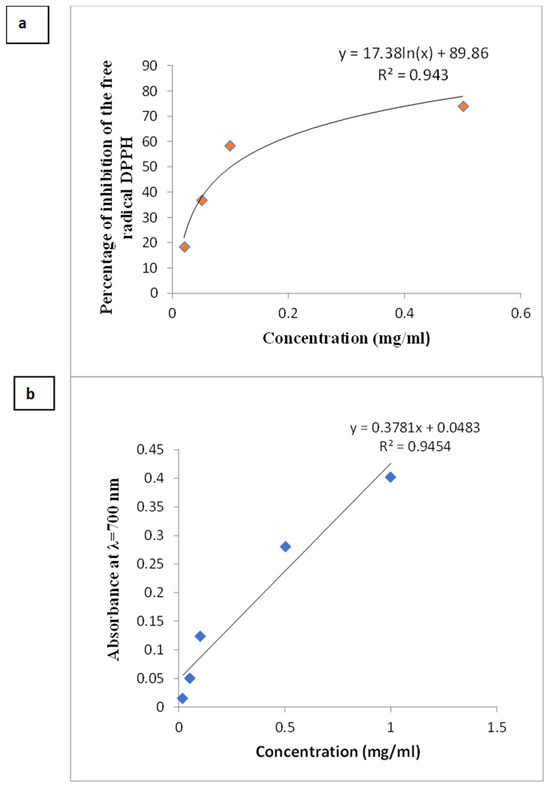
Figure 4.
Antioxidant activity of aqueous extracts of non-germinated O. platylepis seeds evaluated by DPPH radical scavenging activity (a) and the ferric reducing activity (FRAP) (b).
The non-germinated seed extract showed a DPPH⋅ scavenging ability with an EC50 of 0.10 mg/mL while the FRAP test revealed an iron reducing power with an EC50 of 1.19 mg/mL. Both tests showed EC50 values higher than those obtained with vitamin C (EC50c _DPPH = 8.1 ± 0.01 µg/mL; EC50c _FRAP = 0.05 ± 0.01 mg/mL). These findings suggest that the seeds of O. platylepis have a relatively limited capacity to reduce oxidants, which may contribute to their low germination percentage.
3.4. Optimization of Germination Conditions
3.4.1. Seed Germination
The results of the response surface method (RSM) optimization of the germination conditions for O. platylepis seeds are presented in Table 2, and the seed germination equation was determined (Equation (1)). The germination conditions seemed to be strongly influenced by the gibberellic acid (GA3) application and by the cold exposure of seeds at 4 °C. Using RSM, the optimal combinations of the GA3 concentrations and cold exposure durations to break dormancy and enhance seed germination could be determined. Results revealed the levels of each variable that yielded the best results, and the germination conditions were adjusted accordingly.

Table 2.
Analysis of the variance of the regression coefficients to the fitter polynomial equation for seed germination (SGP), mean germination time (MGT), total phenolic content (TPC), total flavonoid content (TFC), condensed tannin content (CTC), and DPPH test.
In order to adapt the model to the data, the key model response parameters, such as the p-value of each variable (X1, X2, X12, X22) or their interaction (X1X2), and regression coefficient (R2) value were assessed and are presented in Table 3.

Table 3.
ANOVA of responses (p value, R2) for the seed germination (SGP), mean germination time (MGT), total phenolic content (TPC), total flavonoid content (TFC), condensed tannin content (CTC), and DPPH radical scavenging activity.
The response surface plot (Figure 5a) clearly demonstrated that seed germination was significantly affected by the cold stratification time, GA3 concentration, and their interaction (p < 0.001). The R2 value of 0.98 indicates a good adjustment of the model to the experimental data (Table 3). Accordingly, the model was found to be suitable for the improvement of the germination conditions in O. platylepis seeds.
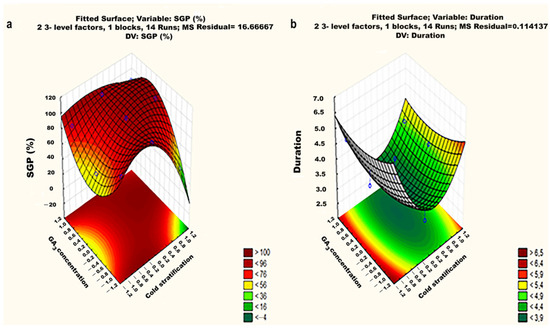
Figure 5.
Response surface methodology (RSM) showing the effect of cold exposure time and the GA3 concentration on the seed germination percentage (a) and mean germination time (duration) (b).
The seed germination of O. patylepis seeds increased significantly in correlation with concentrations of GA3, indicating a linear effect. Particularly, the optimal seed germination (100%) was observed when seeds were exposed to cold stratification (4 °C) for 14 days coupled with an application of 750 ppm GA3. The least successful seed germination percentage (51.6%) was recorded when the seeds were exposed to the same 14-day cold stratification but were exposed to a 500 ppm GA3 application.
Germination (%) = 84.07 + 22.22X12 + 18.33X2 + 27.5X1X2
3.4.2. Germination Time
Results showed that the mean germination time (duration) of these thistle seeds was significantly affected by the cold stratification factor (p = 0.0156) (Table 3). Experimental observations revealed a suitable fit to the model, as indicated by the coefficient of determination value (R2) = 0.88. The observed responses (Figure 5b) fell within a range of 4–6.6 days and revealed a gradual increase as the duration of the cold stratification factor was extended.
Based on the RSM results, the obtaining equation of germination time responses is as follows:
Mean Germination Time (days) = 4.96 − 0.77X1 − 1.27X12
3.4.3. Total Phenolic Content (TPC)
Based on the results generated by RSM, the model and the contour plot for TPC are shown in Equation (3) and Figure 6a, respectively, indicating a highly significant difference at p < 0.001, attributable to the effects of gibberellic acid (GA3) concentration and the duration of cold stratification conditions, respectively (Table 3). Experimental observations showed a good fit to the model, with determination coefficients (R2) = 0.99. Among the germination conditions tested, the highest TPC was observed in the germinated seeds with a treatment of 1000 ppm GA3 (18.42 ± 0.6 mg GAE/g DW), and the lowest content was obtained after cold stratification for 7 days and a treatment of 750 ppm of GA3 (6.52 ± 0.7 mg GAE/g DW). These values were respectively 1.8-fold higher and 1.4-fold lower than the TPC present in non-germinated Onopordum seeds. At the determined optimal germination conditions (14 days of cold stratification and a seed treatment of 750 ppm GA3), which resulted in 100% of germination of O. platylepis seeds, the TPC was measured at 6.68 ± 0.7 mg GAE/g DW.
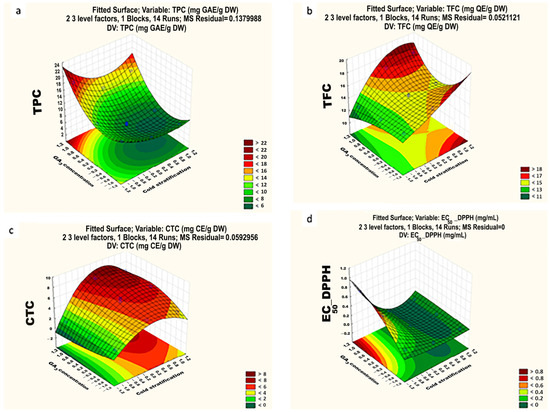
Figure 6.
Response surfaces and contour plots for TPC (total phenol content) (a), TFC (total flavonoid content) (b), CTC (condensed tannin content) (c), and antioxidant activity determined by the DPPH (d) assays in O. platylepis seeds exposed to different germination conditions (cold stratification time and applied GA3 concentration).
The TPC of the O. platylepis seeds varied greatly depending on the germination conditions. An increase in the applied concentration of GA3 correlated with a proportional rise in the synthesis of TPC. The intensified production of TPC as a result of seed treatment with 1000 ppm GA3 could potentially induce stress within the seeds.
TPC (mg GAE/g DW) = 10.672 − 3.94X1 − 2.39X12 + 6.67X2 − 3.83X22
3.4.4. Total Flavonoid Content (TFC)
The results of various germination conditions on total flavonoid content are shown in Table 2 and Figure 6b. Experimental observations displayed a good fit to the model with determination coefficients (R2) = 0.99.
Both cold stratification time and application of GA3, along with their interaction, had a significant effect on the seeds’ TFC (Table 3). However, the GA3 concentration had no significant effects at the linear level (p > 0.05) (Equation (4)). TFC values ranged from 12.62 ± 0.3 to 17.31 ± 0.4 mg QE/g DW. The measured TFC at the determined optimal germination conditions was found to be 14.83 ± 0.4 mg QE/g DW. This value was 2.2-fold higher than the TFC of non-germinated seeds.
TFC (mg QE/g DW) = 15.13 + 3.42X1 + 0.88X12 − 1.24X22
3.4.5. Total Condensed Tannins
The TCT varied greatly from 1.26 to 7.76 (mg CE/g DW) depending on the germination conditions (Table 2, Figure 6c). Both cold stratification duration and the application of GA3 as well as their combined effects had a highly significant effect on the TCT during the improvement in germination process (Table 3). The GA3 concentration had no significant effects at the quadratic level (p > 0.05). The R2 value of 0.99 indicates a good adjustment of the model to the experimental data (Table 3).
Results revealed that an increase in the applied concentration of GA3 significantly boosted the condensed tannin content. Moreover, the extension of the cold stratification time revealed a quadratic effect on the production of these products. The obtained CTC under the determined optimal germination conditions, which enabled 100% of seed germination, was found to be 6.49 ± 0.1 mg CE/g DW. This value indicates a 1.48-fold increase compared to the CTC of the non-germinated seeds.
Based on the RSM outputs, the regression model (Equation (5)) of the condensed tannin content responses is as follows:
CTC = 4.95 + 4.44X1 + 2.56X12 +1.6X2 + 1.68X1X2
3.4.6. Antioxidant Activity of Germinated Seeds
The regression model and the contour plot for the DPPH radical antioxidant activities of the germinated seed extract are shown in Equation (6) and Figure 6d. Experimental observations displayed a perfect fit to the model with determination coefficients (R2) = 1.
The duration of cold stratification, GA3 concentration as well as their interaction showed a high significant effect (p < 0.01, Table 3) on the antioxidant activity of O. platylepis while optimizing the germination conditions.
The DPPH· EC50 values for O. platylepis varied within the range from EC50 = 0.015 ± 0.002 to EC50 = 0.71 ± 0.08 mg/mL depending on the germination conditions. The most important DPPH activities were found when germination was performed at 500 ppm GA3 (with EC50 values of 0.16 ± 0.05, 0.015 ± 0.0001, and 0.0158 ± 0.0004 mg/mL, respectively, after 0, 7, and 14 days of cold stratification). These values were higher than the positive control and were approximately 6.4 times lower than the EC50 value obtained in the non-germinated O. platylepis seeds, except in cases where seeds were not exposed to cold stratification, in which the EC50 was slightly higher. Prolonged exposure to cold (4 °C) led to an increase in the DPPH radical scavenging activity, peaking after 7 days of cold stratification with an EC50 value of 0.015 ± 0.0001 mg/mL. However, the lowest antioxidant activity was observed in seeds treated with varying concentrations of GA3 without being exposed to cold stratification.
DPPH = 0.168 − 0.45X1 − 0.226X12 + 0.189X2 + 0.029X22 − 0.28X1X2
When the seed germination reached 100% under optimal conditions, the O. platylepis seeds indicated an important antioxidant activity with an EC50 = 0.018 ± 0.0001 mg/mL.
4. Discussion
Despite advanced knowledge in seed biology, overcoming dormancy remains a complex and challenging task, often varying significantly between species [52]. Seed water uptake is an essential prerequisite for germination; this step differs greatly between species depending on seed size, weight, permeability, and chemical composition [53]. In this study, the water uptake of the achenes increased slightly from the start of imbibition until day 8. After that, the water content of the achenes remained constant until the end of the experiment. This initial gradual increase in water uptake is important for germination, ensuring that seeds achieve the necessary hydration level to activate enzymatic and metabolic activities [54]. However, our germination results showed low germination percentages in the control group without treatment. This indicates that while water uptake is necessary, it is not sufficient alone to break the dormancy of Onopordum nervosum ssp. platylepis seeds.
A water-impermeable seed coat can induce physical dormancy. According to the published literature on dormancy breaking techniques, various methods like scarification and stratification through physical and chemical treatments have been extensively studied [30]. However, the responses of dormant O. platylepis cypsela to these treatments have demonstrated low final seed germination. Gibberellic acid (GA3) appears to be an effective growth regulator, indicating that seeds possess physiological dormancy.
Seed phenolic compounds are among the factors influencing germination. It is commonly known that dormant seeds contain enzymes and compounds essential to the initial activation of metabolic processes that occur during the first steps of seed germination [55]. Research has demonstrated that phenolic compounds exert an inhibitory effect on water uptake, respiratory activities, and therefore on germination processes [55,56]. This fact could provide insights into the important phenolic compound levels determined in the non-germinated seeds of O. platylepis. Kabtni et al. [57] demonstrated that in semi-arid regions, seeds present a substantial amount of TFC. Moreover, it has been demonstrated that during seed maturation, under challenging environmental conditions, an increased gene expression of enzymes associated with the biosynthetic pathways of flavonoids and procyanidins can be observed [58]. Condensed tannins, a specific subgroup of flavonoids, are recognized as highly bioactive secondary metabolites in plants, playing essential roles in defending against pathogens and predators as well as in physiological processes like seed maturation and dormancy [59]. Condensed tannin content leads to lignification, and the seed’s cell wall becomes less permeable, which can limit the water uptake and gas flow needed for germination process. Additionally, tannins have the potential to prolong the degradation process of seed coats when present in the soil [60]. Therefore, we can conclude that our endemic plant, adapted to the semi-arid climate, accumulates flavonoid and condensed tannin contents in the seeds as an adaptation strategy to the growth environment. Our findings provide further evidence in support of the hypothesis that the seed composition of O. platylepis might contribute to the species’ ecological success through direct and indirect defense mechanisms against biotic and abiotic stress by affecting the germination process.
Several factors, such as genetic background, growth location, and maturity stage, significantly influence the phenolic composition of a plant [61,62]. Cynara cardunculus L. is a plant that belongs to the same family (Asteraceae) as O. platylepis. The methanolic extract of its seeds showed a significant total phenolic content of 14.33 mg GAE/g DW. Additionally, these seeds contained a notable flavonoid content of 9.78 mg EC/g DW and a condensed tannin content of 2.00 mg EC/g DW [63]. Khaldi et al. [64] investigated both the wild and cultivated Tunisian cardoon varieties, revealing significant variation in the total phenolic content of the methanolic extracts, ranging from 23.25 to 15.04 mg GAE g-1 DW, and a total flavonoid content of 8.93 mg CE g-1 DW was found in the methanolic extracts from wild cardoon seeds. Differences in the phenolic compound content can be explained by the difference in extraction solvents, plant species, and plant growth conditions [61,65]. Researchers have demonstrated the biological and potential chemical composition of cardoon seeds, highlighting the multifaceted possibilities associated with the valorization of these seeds [62]. Typically, major changes in metabolism occur during the seed life cycle, from high water requirements in the early stages to extensive dehydration during maturation. During drying and sequent hydration, reactive oxygen species (ROS) are accumulated, and antioxidant compounds are produced to ensure seed survival [66]. The antioxidant activity of O. platylepis seeds can be correlated with the presence of phenolic compounds, mostly because the high value of TPC indicates high DPPH antiradical capacity. Our findings align with previous results reported by Khaldi et al. [64], demonstrating that the seeds of wild cardoons (Cynara cardunculus L.) provided high DPPH quenching activity, higher than that of the other plant organs. Researchers have reported that the antioxidant activity plays a role in modulating the balance of the gibberellic acid and abscisic acid ratio in the stimulation of dormancy release.
Different tests are continuously being studied to improve seed germination. Methods like cold stratification, physical abrasion, hot water, hydrogen peroxide, or sulfuric acid treatment are commonly employed due to their ability to enhance seed germination efficiency within a short time frame [29,30]. Studies have shown that the exogenous application of growth regulators, mainly gibberellins (GA3), effectively breaks the seed dormancy of many species [13,33].
Regardless of the extensive understanding of seed biology, overcoming dormancy continues to be a challenging task [67]. Our findings showed that the responses of these wild plant seeds, which reveal dormancy at the time of dispersal, are dependent on the specific GA3 concentration and the exposure duration to cold (4 °C). The response surface (RSM) plans obtained showed that the optimal conditions for an optimal seed germination of O. platylepis seeds were 14 days of exposure to cold (4 °C) and an exogenous application of GA3 at 750 ppm. These conditions led to a seed germination of 100% being achieved within 5 days. Determining the germination ability relies not only on the final germination percentage attained but also on the rapidity of the process and its temporal evolution (germination time) [68]. These two parameters combined are often used to determine the success of a treatment for breaking dormancy [69]. The high R2 values indicated the robustness of the analyzed variables and that they were reliable with high values closer to 1, signifying a better fit model [70]. This finding signifies that the model used was assumed to be appropriate for improving the germination conditions for O. platylepis. Hopkins and Gravatt [71] reported similar promotional impacts of cold stratification and gibberellic acid treatment on seed germination. Cold stratification is a conventional technique employed to overcome dormancy [72]. During this process, the concentration of abscisic acid (ABA) rises in newly matured seeds, and subsequently declines after warm or cold stratification. Concurrently, the levels of gibberellins (GA1, GA3, GA4, or GA7) increase following cold stratification [72,73].
Increased levels of endogenous gibberellic acid have been reported to improve the germination potentialities of seeds of different genera of Onopordum nervosum Boiss [66]. Pérez-García and Duran [13] demonstrated that the application of exogenous gibberellic acid (GA3) improved seed germination within two populations of O. nervosum. The study reported a positive correlation between the concentration of GA3 and seed germination. This is in agreement with the results of the present investigation. However, this study evaluated the germination characteristics of O. platylepis at one site. Consequently, future research involving additional sites could enhance our understanding of how different factors influence the seed germination of this thistle, aiding in its cultivation and protection.
Numerous studies have been carried out to enhance the germination conditions through assessing phenolic compounds and biological activities as responses [35]. All response variables, except TPC, at the optimal germination conditions in O. platylepis seeds were found to be significantly higher than in the non-germinated ones. At these conditions, the TPC was observed to be relatively low compared to the non-germinated seeds. This could be attributed to the fact that some phenolic compounds at lower levels had the potential to generate controlled quantities of ROS, which may help in the initiating steps of the germination processes. Seed germination, involving the dynamic and complex flow of phytochemicals and nutrients, depends on several intrinsic and extrinsic factors. Recently, reactive oxygen species (ROS) have been considered among these factors [74]. Consequently, comparing the bioactive compounds and antioxidant activity of different plants during seed germination seems to be difficult because of these different factors. Among these factors, temperature can modulate phenol biosynthesis. Seed adaptation to temperature variation induces synergistic biochemical compositions [75]. Regarding antioxidant activity, it should be noted that germination resulted in an improvement in the antioxidant potential of O. platylepis seeds. This fact is in agreement with a study conducted by Cáceres et al. [51] on rice seeds. Indeed, this potential is important if the cold exposure duration exceeds 7 days. This effect can be attributed to a higher accumulation of antioxidant compounds with the ability to scavenge peroxyl radicals.
5. Conclusions
According to the results obtained in this study, a germination protocol can be recommended for O. platylepis seeds. The improvement of the germination process for this endemic thistle using response surface methodology showed that the optimal conditions were 14 days of cold stratification and 750 ppm of exogenous gibberellic acid application. These conditions resulted in a germination percentage of 100% within 5 days, increasing the total flavonoid content and condensed tannins, and improving the capacity to inhibit free radicals. However, using abrasive paper, high-temperature water, and chemical treatments using hydrogen peroxide (H2O2) or sulfuric acid (H2SO4) did not significantly increase seed germination, suggesting that dormancy is physiological rather than physical in nature. Furthermore, the results showed important phenolic compounds in the non-germinated seeds, which could precipitate proteins and enzymes involved in seed germination. This endemic thistle faces threats in Tunisia; therefore, ex situ conservation strategies including seed propagation are essential to preserve the biodiversity of this rare species and protect it from extinction. Our findings could provide a basis for future research aiming to enhance the seed germination of O. platylepis and potentially help in its cultivation and valorization in the food sector.
Author Contributions
N.D.: Conceptualization, methodology, investigation, writing—original draft preparation; R.K.: Investigation, methodology, writing—review and editing; S.A.B.: Investigation, writing—review and editing; I.E.: Conceptualization, software, writing—review and editing; L.N.: Methodology, resources; F.H.: Supervision, funding acquisition, writing—review and editing; A.M.A.: Resources, writing—review and editing; H.A.A.: Resources, writing—review and editing; M.K.: Methodology, resources; B.A.M.D.: Supervision, validation, methodology, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RG23096).
Data Availability Statement
The original contributions presented in the study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Neffati, M.; Ghrabi Gammar, Z.; Akrimi, N.; Henehi, B. Endemic plants of Tunisia. Flora Mediterr. 1999, 9, 163–174. [Google Scholar]
- El Oualidi, J.; Khamar, H.; Fennane, M.; Ibn Tattou, M.; Chauvet, S.; Taleb, M.S. Checklist des Endémiques et Spécimens Types de la Florevasculaire de l’Afrique du Nord; Institut Scientifique, Universite Mohammed V-Agdal: Rabat, Morocco, 2012; Volume 25, pp. 1–189. [Google Scholar]
- Dobignard, A.A.; Chatelain, C.A.; Fisher, M.C.; Orso, J.C.; Jeanmonod, D.C. Index synonymique de la flore d’Afrique du Nord. In Dicotyledoneae: Acanthaceae-Asteraceae; Conservatoire et Jardin botaniques de la Ville de Genève: Geneva, Switzerland, 2011; Volume 2. [Google Scholar]
- Hachicha, S.F.; Barrek, S.; Skanji, T.; Ghrabi, Z.G.; Zarrouk, H. Composition chimique de l’huile des graines d’Onopordom nervosum subsp. Platylepis Murb (Astéracées). J. Soc. Chim. Tunis. 2007, 9, 23–28. [Google Scholar]
- Kouki, R.; Essaidi, I.; Annabi, K.; Dhen, N.; Haouala, F.; Alhudhaibi, A.M.; Alrudayni, H.A.; Bziouech, S.A.; Ayari, O.; Al Mohandes Dridi, B. Chemical Composition, Antioxidant Activity, and Milk-Clotting Activity of Aqueous Extracts from Leaves, Stems, and Flowers of Three Tunisian Ecotypes of Spontaneous and Cultivated Onopordum nervosum ssp. platylepis Murb.: A Potential Novel Vegetable Rennet Option. Agronomy 2024, 14, 987. [Google Scholar] [CrossRef]
- Essaidi, I.; Dhen, N.; Lassoued, G.; Kouki, R.; Haouala, F.; Alhudhaibi, A.M.; Alrudayni, H.A.; Dridi Almohandes, B. Onopordum nervosum ssp. platylepis Flowers as a Promising Source of Antioxidant and Clotting Milk Agents: Behavior of Spontaneous and Cultivated Plants under Different Drying Methodologies. Processes 2023, 11, 2962. [Google Scholar] [CrossRef]
- Pottier-Alapetite, G.A. Flore de la Tunisie: Angiospermes-Dicotylédones-Gamopétales; Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et le Ministère de l’Agriculture: Tunis, Tunisia, 1981. [Google Scholar]
- Zarembo, E.V.; Boyko, E.V. Carpology of some East Asian Cardueae (Asteraceae). In Anales del Jardín Botánico de Madrid; CSIC Press: Madrid, Spain, 2008; Volume 65, pp. 129–134. [Google Scholar]
- Sala, O.A.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Yahia, A.; Mashaly, I.; El-Bana, M.; Rizk, R.; El-Sherbeny, G. Intraspecific Variations in Functional and Molecular Traits of Near-Endemic Onopordum alexandrinum Boiss. in Natural and Anthropogenic Habitats along the Western Mediterranean Coast of Egypt: Implications for Conservation. Plants 2020, 9, 1041. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Pérez-Garcia, F.; Duran, J.M. The effect of gibberellic acid on germination of Onopordum nervosum Boiss. seeds. Seed Sci. Technol. 1990, 18, 83–88. [Google Scholar]
- Godefroid, S.; Van de Vyver, A.; Vanderborght, T. Germination capacity and viability of threatened species collections in seed banks. Biodivers. Conserv. 2010, 19, 1365–1383. [Google Scholar] [CrossRef]
- Corli, A.; Orsenigo, S.; Porro, F.; Rossi, G.; Lodetti, S.; Mondoni, A. Germination niche of co-occurring threatened native and alien species: A case study in Lindernia procumbens and L. dubia. Plant Ecol. 2024, 225, 725–729. [Google Scholar] [CrossRef]
- Ben Mariam, E. Biodiversity in Tunisia (Fauna, Flora): Threats and Solutions. 2023. Available online: https://www.researchgate.net/publication/375552581_Biodiversity_in_Tunisia_Fauna_Flora_threats_and_solutions (accessed on 5 September 2024).
- Brahim, N.E.M.B.; Chaabane, A.; Toumi, L.; Sebei, H. Les plantes rares de la Tunisie septentrionale et centrale. In Annales de l’Inrat; National Institute of Agricultural Research of Tunisia: Tunis, Tunisia, 2014; Volume 389, pp. 1–18. [Google Scholar]
- Francisco-Ortega, J.; Santos-Guerra, A.; Kim, S.C.; Crawford, D.J. Plant genetic diversity in the Canary Islands: A conservation perspective. Am. J. Bot. 2000, 87, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.; Kumar, P.P. Regulation of Seed Germination: The Involvement of Multiple Forces Exerted via Gibberellic Acid Signaling. Mol. Plant 2018, 12, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Chen, Z. The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Yang, L.E.; Peng, D.L.; Li, Z.M.; Huang, L.; Yang, J.; Sun, H. Cold stratification, temperature, light, GA3, and KNO3 effects on seed germination of Primula beesiana from Yunnan, China. Plant Divers. 2020, 42, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.; Jurado, E.; Himenez-Bremont, J.F. Breaking seed dormancy in specially protected Turbinicarpus lophophoroides and Turbinicarpus pseudopectinatus (Cactaceae). Plant Species Biol. 2008, 23, 43–46. [Google Scholar] [CrossRef]
- Topham, A.T.; Taylor, R.E.; Yan, D.; Nambara, E.; Johnston, I.G.; Bassel, G.W. Temperature variability is integrated by a spatially embedded decision-making center to break dormancy in Arabidopsis seeds. Proc. Natl. Acad. Sci. USA 2017, 114, 6629–6634. [Google Scholar] [CrossRef]
- Corbineau, F. Oxygen, a key signalling factor in the control of seed germination and dormancy. Seed Sci. Res. 2022, 32, 126–136. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef]
- Khan, M.N.; Fu, C.; Li, J.; Tao, Y.; Li, Y.; Hu, J.; Chen, H.; Khan, Z.; Wu, H.; Li, Z. Seed nanopriming: How do nanomaterials improve seed tolerance to salinity and drought? Chemosphere 2023, 310, 136911. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Dixon, K.W.; Silveira FA, O.; Chapman, T.D.i.; Sacco, A.; Mondoni, A.; Turner, S.R.; Cross, A.T. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28, S256–S265. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Cavers, P.B. Variation in germination response within Scotch thistle, Onopordum acanthium L., populations matured under greenhouse and field conditions. Écoscience 2000, 7, 57–65. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, S.; Sharma, S.S. Seed germination behavior of some medicinal plants of Lahaul and Spiticoldd desert (Himachal Pradesh): Implications for conservation and cultivation. Curr. Sci. 2006, 90, 1113–1118. [Google Scholar]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia; Inkata Press: Melbourne, Australia, 1992; pp. 342–344. [Google Scholar]
- Gálvez, C.; Bermejo, J.H. Life cycle and adaptive strategies of three non-annual, ruderal Cardueae: Onopordum nervosum, Carthamus arborescens, and Cirsium scabrum. Plant Syst. Evol. 1990, 171, 117–128. [Google Scholar] [CrossRef]
- Pérez, C.; Pérez-García, F.; Fernández, H.; Revilla, M.A. The levels of GA3 and GA20 may be associated with dormancy release in Onopordum nervosum seeds. Plant Growth Reg. 2002, 38, 141–143. [Google Scholar] [CrossRef]
- Ruiz-Hernández, A.A.; Cárdenas-López, J.L.; Cortez-Rocha, M.O.; González-Aguilar, G.A.; Robles-Sánchez, R.M. Optimization of germination of white sorghum by response surface methodology for preparing porridges with biological potential. CyTA J. Food 2021, 19, 49–55. [Google Scholar] [CrossRef]
- Perales-Sánchez, J.X.; Reyes-Moreno, C.; Gómez-Favela, M.A.; Milán-Carrillo, J.; Cuevas-Rodríguez, E.O.; Valdez-Ortiz, A.; Gutiérrez-Dorado, R. Increasing the antioxidant activity, total phenolic and flavonoid contents by optimizing the germination conditions of amaranth seeds. Plant Foods Hum. Nutr. 2014, 69, 196–202. [Google Scholar] [CrossRef]
- Moussavi, S.R.; Rezaei, M.; Mousavi, A. A General Overview on Seed Dormancy and Methods of Breaking It. Adv. Environ. Biol. 2011, 5, 3333–3337. [Google Scholar]
- Ogawa, K.; Iwabuchi, M. A Mechanism for Promoting the Germination of Zinnia elegans Seeds by Hydrogen Peroxide. Plant Cell Physiol. 2001, 42, 286–291. [Google Scholar] [CrossRef]
- Inácio, M.C.; Moraes, I.M.; Mendonça, P.C.; Morel LJ, F.; França, S.C.; Bertoni, B.W.; Pereira AM, S. Phenolic Compounds Influence Seed Dormancy of Palicourea rigida H.B.K. (Rubiaceae), a Medicinal Plant of the Brazilian Savannah. Am.J. Plant Sci. 2013, 4, 129–133. [Google Scholar] [CrossRef][Green Version]
- Yücel, G.; Erken, K. Optimal germination methods, ornamental plant features, and ex situ conservation of endemic Campanula grandis Fisch and CA Mey. J. Environ. Eng. Landsc. Manag. 2023, 31, 132–141. [Google Scholar] [CrossRef]
- Ayele, T.B.; Gailing, O.; Finkeldey, R. Assessment and integration of genetic, morphological and demographic variation in Hageniaabyssinica (Bruce) J.F. Gmel to guide its conservation. J. Nat. Conserv. 2011, 19, 8–17. [Google Scholar] [CrossRef]
- Olabinjo, O.O. Response Surface Techniques as an Inevitable Tool in Optimization Process. In Response Surface Methods-Theory, Applications and Optimization Techniques; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Jang, G.H.; Chung, J.M.; Rhie, Y.H.; Lee, S.Y. Seed Dormancy Class and Ecophysiological Features of Veronicastrum sibiricum (L.) Pennell (Scrophulariaceae) Native to the Korea Peninsula. Plants 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Askin, C.C.; Kin, C.C.; Zackrisson, O.; Askim, J.M. Role of warm stratification in promoting germination of seeds of Empetrum hermaphroditum (Empetraceae), a circumboreal species with a stony endocarp. Am. J. Bot 2002, 89, 486–493. [Google Scholar] [CrossRef]
- Maghdouri, M.; Ghasemnezhad, M.; Rabiei, B.; Golmohammadi, M.; Atak, A. Optimizing Seed Germination and Seedling Growth in Different Kiwifruit Genotypes. Horticulturae 2021, 7, 314. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of phenolics and their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Tiitto, R.J. Phenolic constituents in the leaves of northemwiliows methods for the the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Kim, B.R.; Park, S.S.; Youn, G.J.; Kwak, Y.J.; Kim, M.J. Characteristics of Sunsik, a cereal-based readyto-drink Korean beverage, with added germinated wheat and herbal plant extract. Foods 2020, 9, 1654. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, P.J.; Martínez-Villaluenga, C.; Amigo, L.; Frias, J. Maximising the phytochemical content and antioxidant activity of Ecuadorian brown rice sprouts through optimal germination conditions. Food Chem. 2014, 152, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An Updated Overview on the Regulation of Seed Germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Street, R.A.; Van Staden, J. Germination and seedling growth requirements for propagation of Dioscorea dregeana (Kunth) Dur. and Schinz—A tuberous medicinal plant. S. Afr. J. Bot. 2007, 73, 131–137. [Google Scholar] [CrossRef]
- Vidak, M.; Lazarević, B.; Javornik, T.; Šatović, Z.; Carović-Stanko, K. Seed Water Absorption, Germination, Emergence and Seedling Phenotypic Characterization of the Common Bean Landraces Differing in Seed Size and Color. Seeds 2022, 1, 324–339. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Sidari, M. The effect of phenols on respiratory enzymes in seed germination Respiratory enzyme activities during germination of Pinus laricio seeds treated with phenols extracted from different forest soils. Plant Growth Regul. 2001, 35, 31–35. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Souto, X.C.; Gonzlez, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Kabtni, S.; Sdouga, D.; BettaibRebey, I.; Save, M.; Trifi-Farah, N.; Fauconnier, M.L.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 8293. [Google Scholar] [CrossRef]
- MacGregor, D.R.; Kendall, S.L.; Florance, H.; Fedi, F.; Moore, K.; Paszkiewicz, K.; Smirnoff, N.; Penfield, S. Seed production temperature regulation of primary dormancy occurs through control of seed coat phenylpropanoid metabolism. New Phytol. 2015, 205, 642–652. [Google Scholar] [CrossRef]
- Televiciute, D.; Taraseviciene, Z.; Danilcenko, H.; Barcauskaite, K.; Kandaraite, M.; Paulauskiene, A. Changes in chemical composition of germinated leguminous under abiotic stress conditions. Food Sci. Technol. 2020, 40, 415–421. [Google Scholar] [CrossRef]
- Caldas, G.V.; Blair, M.W. Inheritance of seed condensed tannins and their relationship with seed-coat color and pattern genes in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 119, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Ferreira, I. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostic, M.; Soković, M.; Santos-Buelga, C.; Isabel CF, R.; Barros, L. Seasonal variation in bioactive properties and phenolic composition of cardoon (Cynara cardunculus var. altilis) bracts. Food Chem. 2021, 336, 127744. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. Comptes Rendus Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, S.; Chaouachi, F.; Ksouri, R.; Gazzah, M. Polyphenolic composition in different organs of Tunisia populations of Cynara cardunculus L. and their antioxidant activity. J. Food Nutr. Res. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef]
- Huarte, H.R.; Puglia, G.D.; Varisco, D.; Pappalardo, H.; Calderaro, P.; Toscano, V.; Raccuia, S.A. Effect of reactive oxygen species on germination of Cynara cardunculus (L.) cultivars. Acta Hortic. 2020, 1284, 33–40. [Google Scholar] [CrossRef]
- Norden, N.; Daws, M.I.; Antoine, C.; Gonzalez, M.A.; Garwood, N.C.; Chave, J. The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Funct. Ecol. 2009, 23, 203–210. [Google Scholar] [CrossRef]
- Blakesley, D.; Elliott, S.; Kuarak, C.; Navakitbumrung, P.; Zangkum, S.; Anusarnsunthorn, V. Propagating framework tree species to restore seasonally dry tropical forest: Implications of seasonal seed dispersal and dormancy. For. Ecol. Manag. 2002, 164, 31–38. [Google Scholar] [CrossRef]
- Aung, T.; Kim, S.J.; Eun, J.B. A hybrid RSM-ANN-GA approach on disper of extraction conditions for bioactive component-rich laver (Porphyra dentata) extract. Food Chem. 2022, 366, 130689. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.A.; Gravatt, D.A. Effects of cold stratification and hormones on seed germination of Sarracenia alata. Tex. J. Sci. 2019, 71, 7. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chien, C.T.; Baskin, J.M.; Baskin, C.C. Storage behavior and changes in 461 concentrations of abscisic acid and gibberellins during dormancy break and germination in 462 seeds of Phellodendron amurense var. wilsonii (Rutaceae). Tree Physiol. 2010, 30, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chou, S.H.; Tsai, C.C.; Hsu, W.Y.; Baskin, C.C.; Baskin, J.M.; Chien, C.T.; Kuo-Huang, L.L. Effects of moist cold stratification on germination, plant growth regulators, metabolites and embryo ultrastructure in seeds of Acer morrisonense (Sapindaceae). Plant Physiol. Biochem. 2015, 94, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Lan, Q.; Pritchard, H.W.; Xue, H.; Wang, X. Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol. Biochem. 2016, 109, 406–415. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Modi, A.T.; Mohammed, F. The effect of temperature in moringa seed phytochemical compounds and carbohydrate mobilization. S. Afr. J. Bot. 2016, 102, 190–196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).