The Sustainability of Rosa rugosa Thunb. Under Climate Change Conditions: A Study of Morphological Variability in Urban Areas
Abstract
1. Introduction
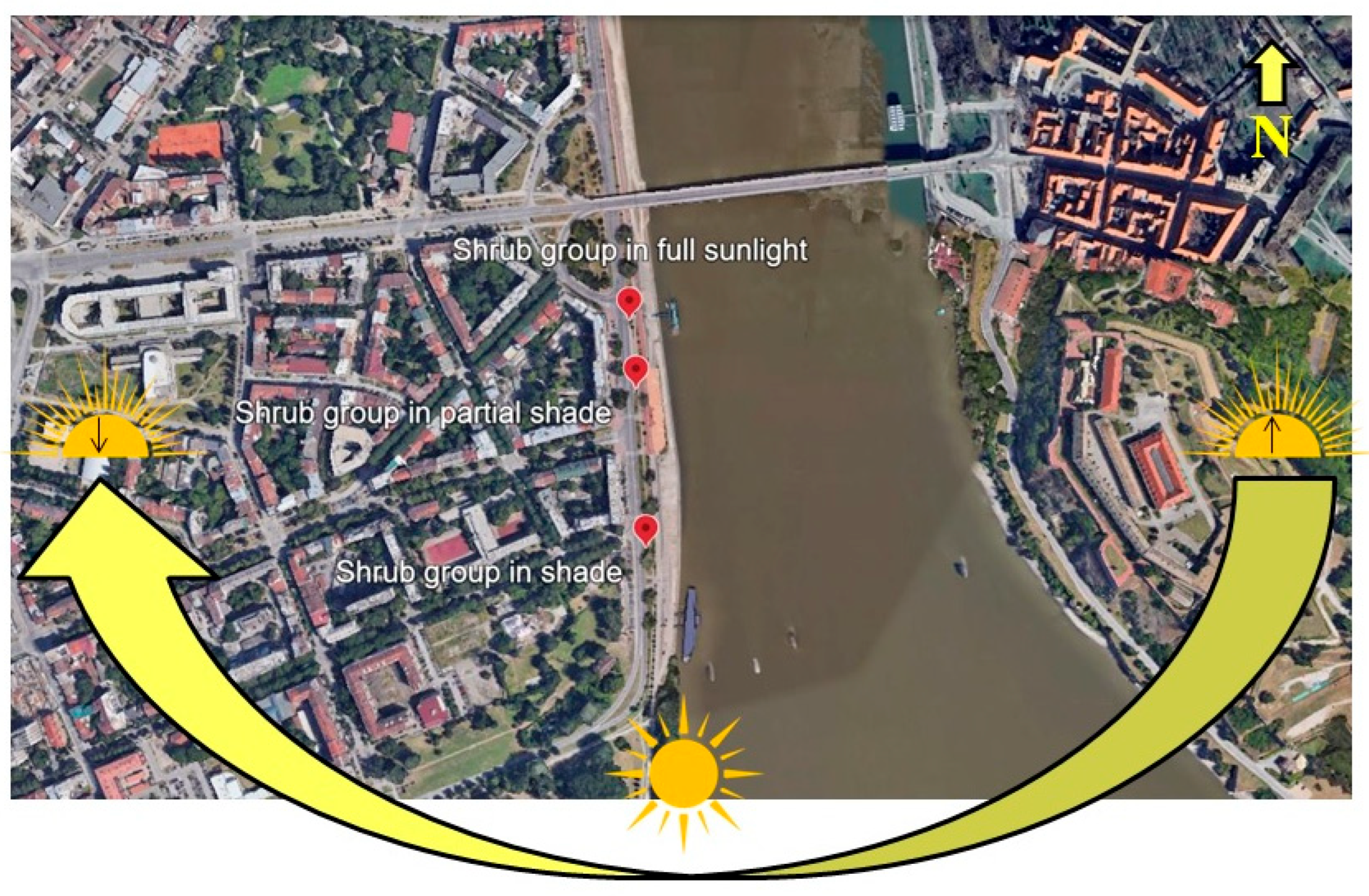
- Investigate the impact of different light conditions (full sun, partial shade, and shade) on the growth habit and fruit morphology of R. rugosa in urban environments.
- Analyze the adaptability of R. rugosa in different spatial contexts—beneath tree canopies, near trees, and in open spaces—focusing on morphological and bioecological characteristics.
- Assess its suitability for solitary versus combined planting with trees, considering light availability and spatial positioning.
2. Materials and Methods
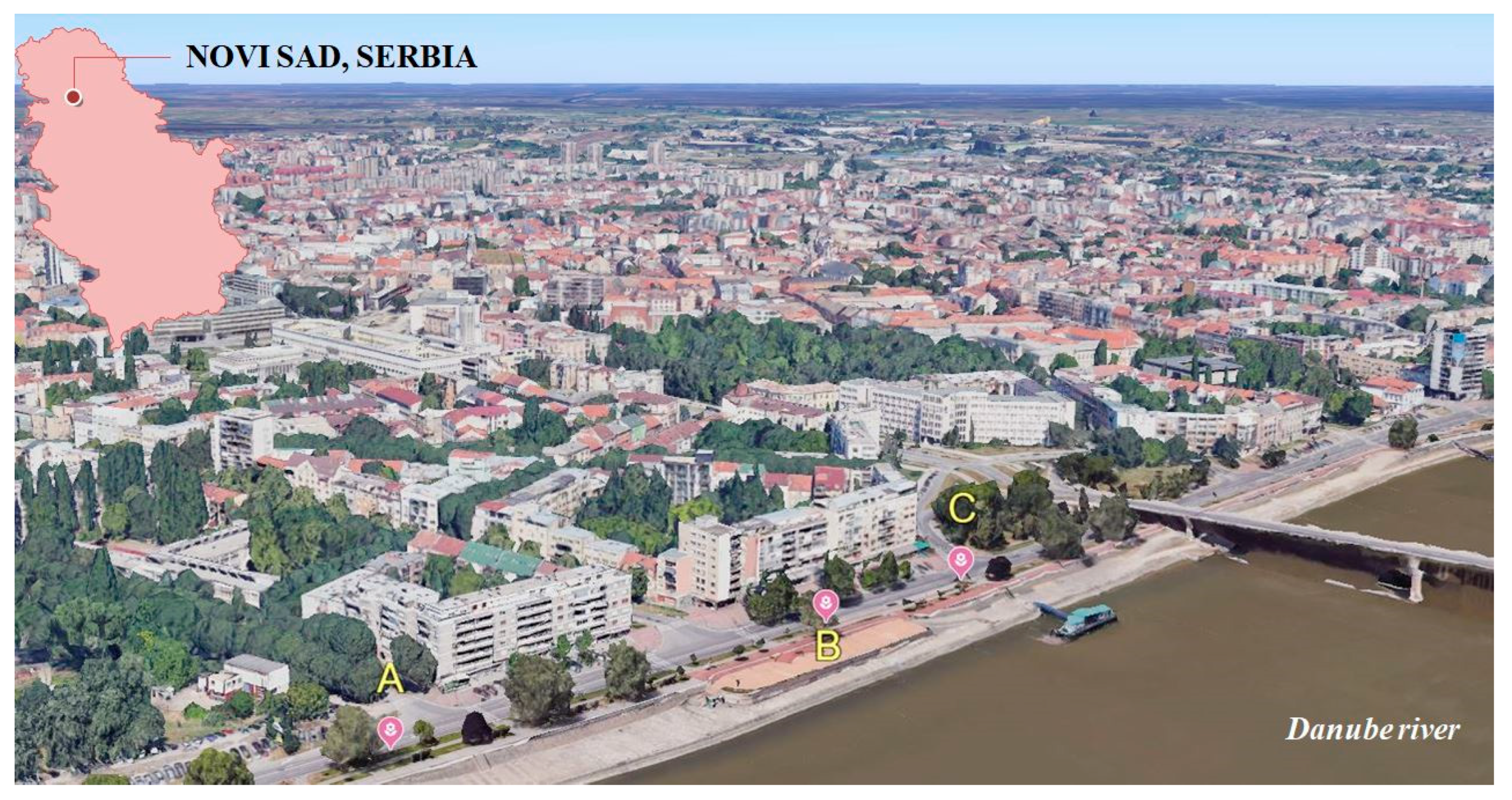
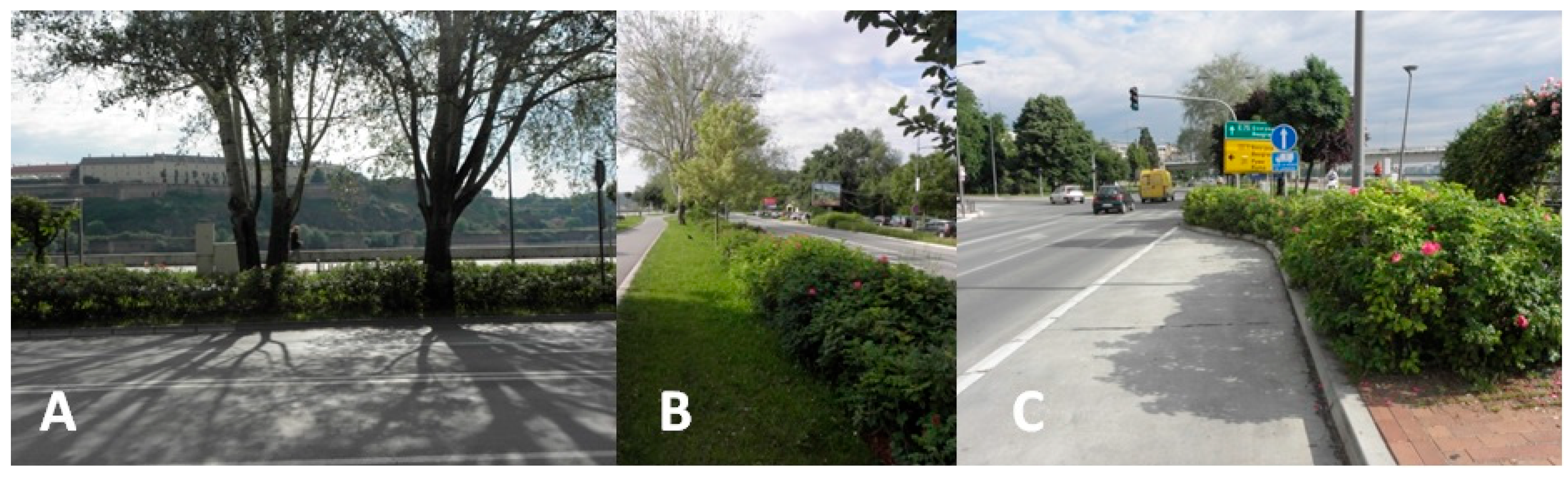
2.1. Study Area
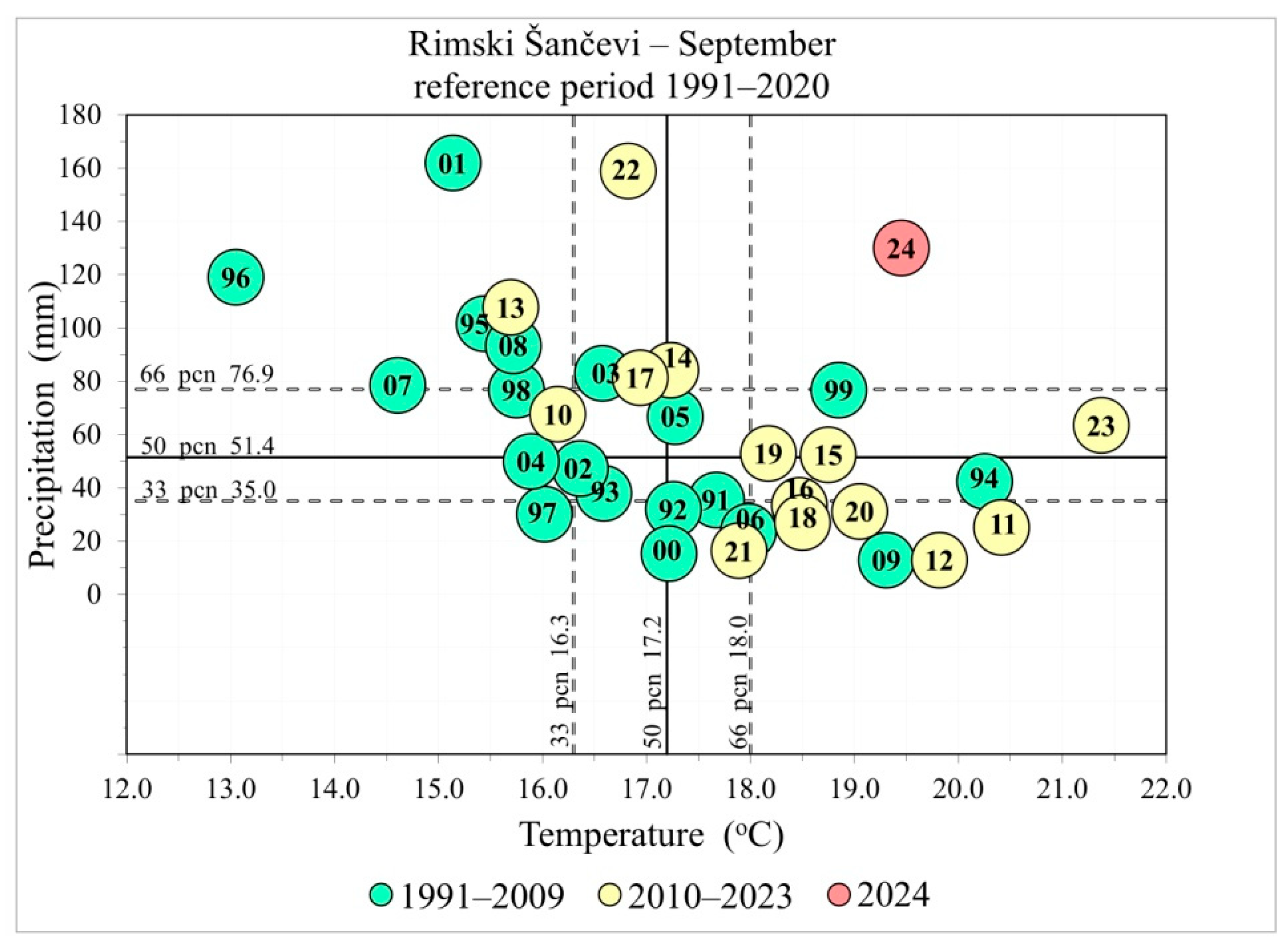
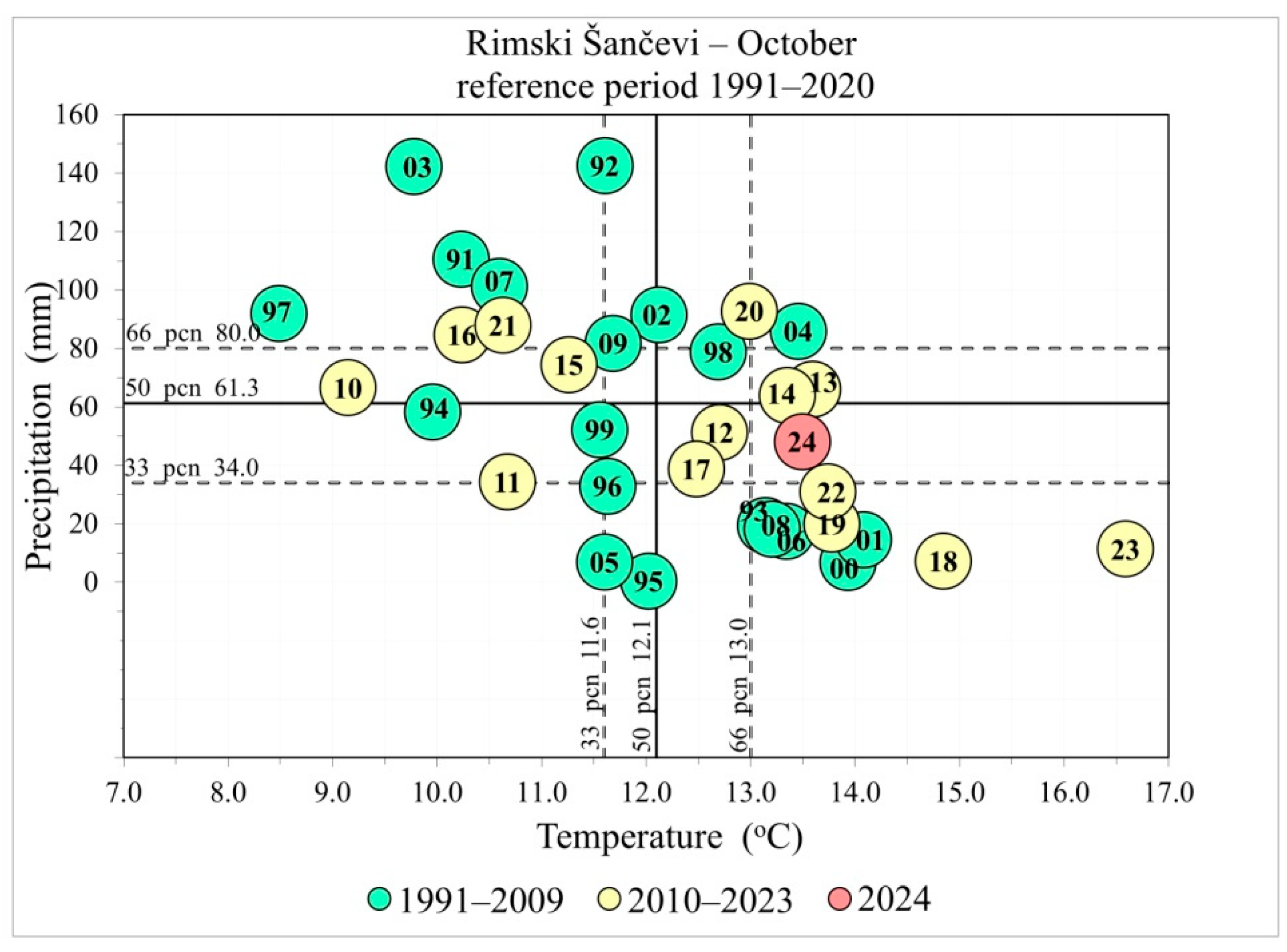
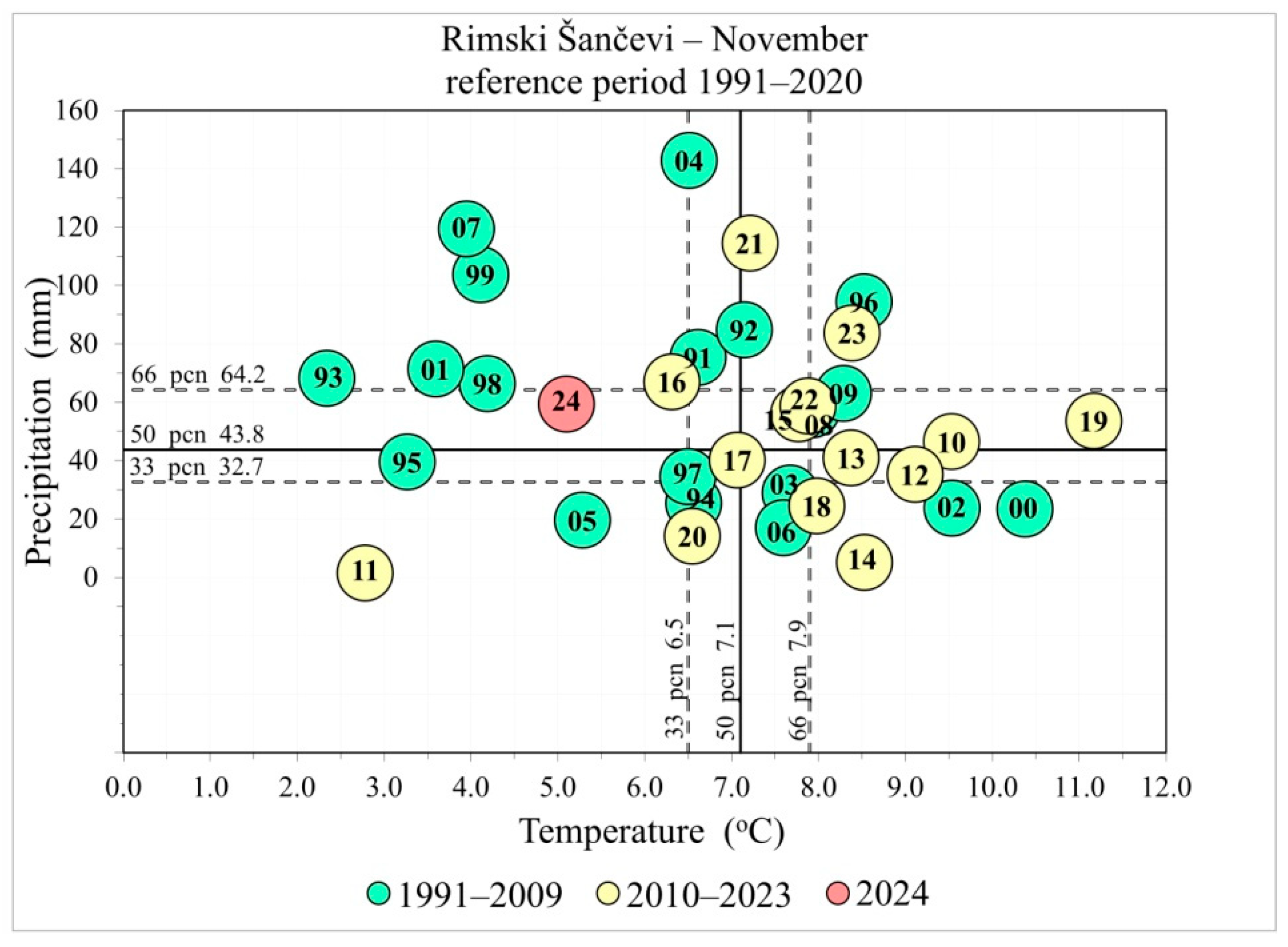
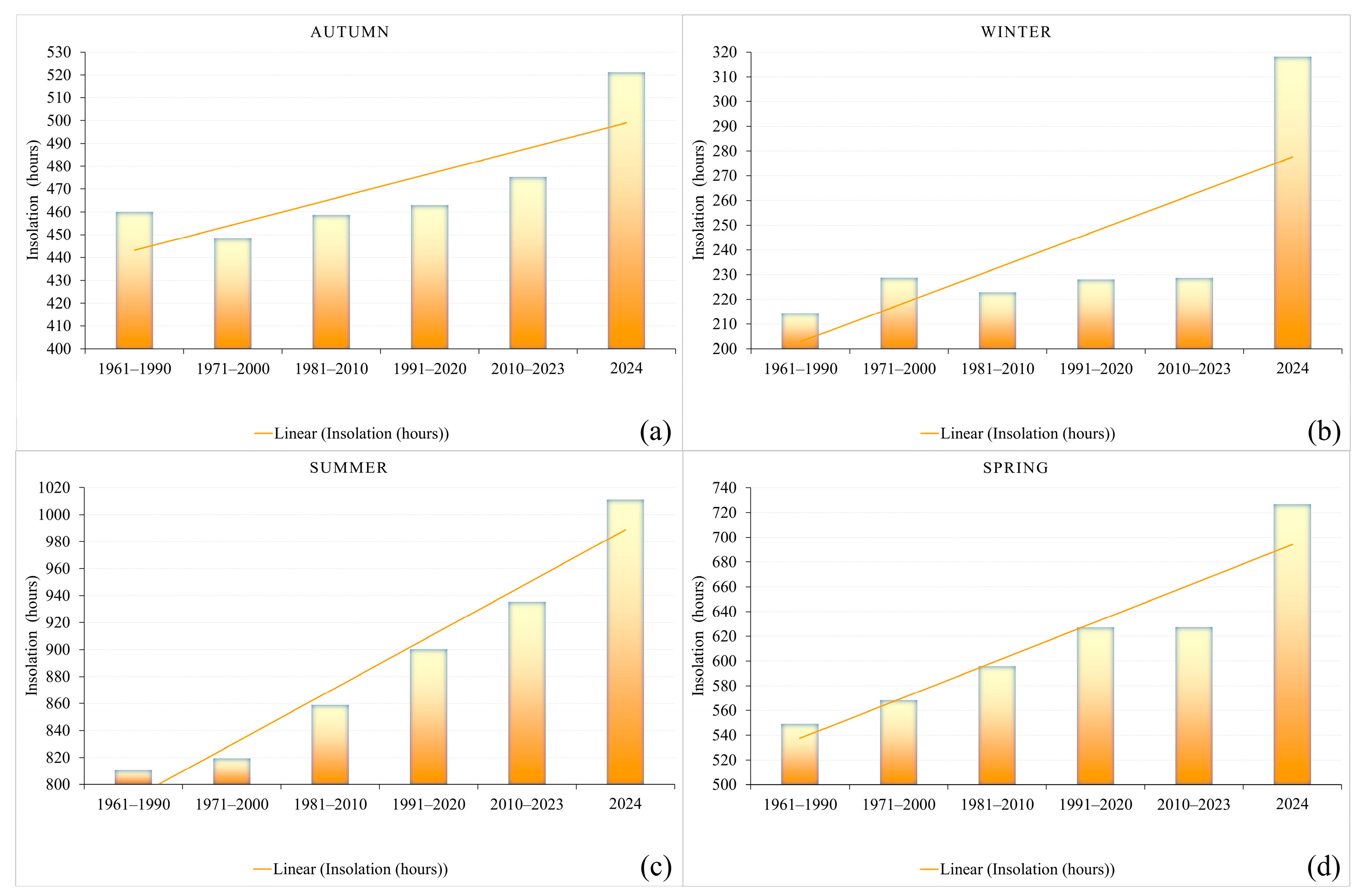
2.2. Climatic Data
2.3. Plant Material
2.4. Processing of Data
3. Results
3.1. Climatic Predictors
3.2. Bioecological Parameters of R. rugosa. Groups
3.3. Morphometric Characteristics of R. rugosa Fruits
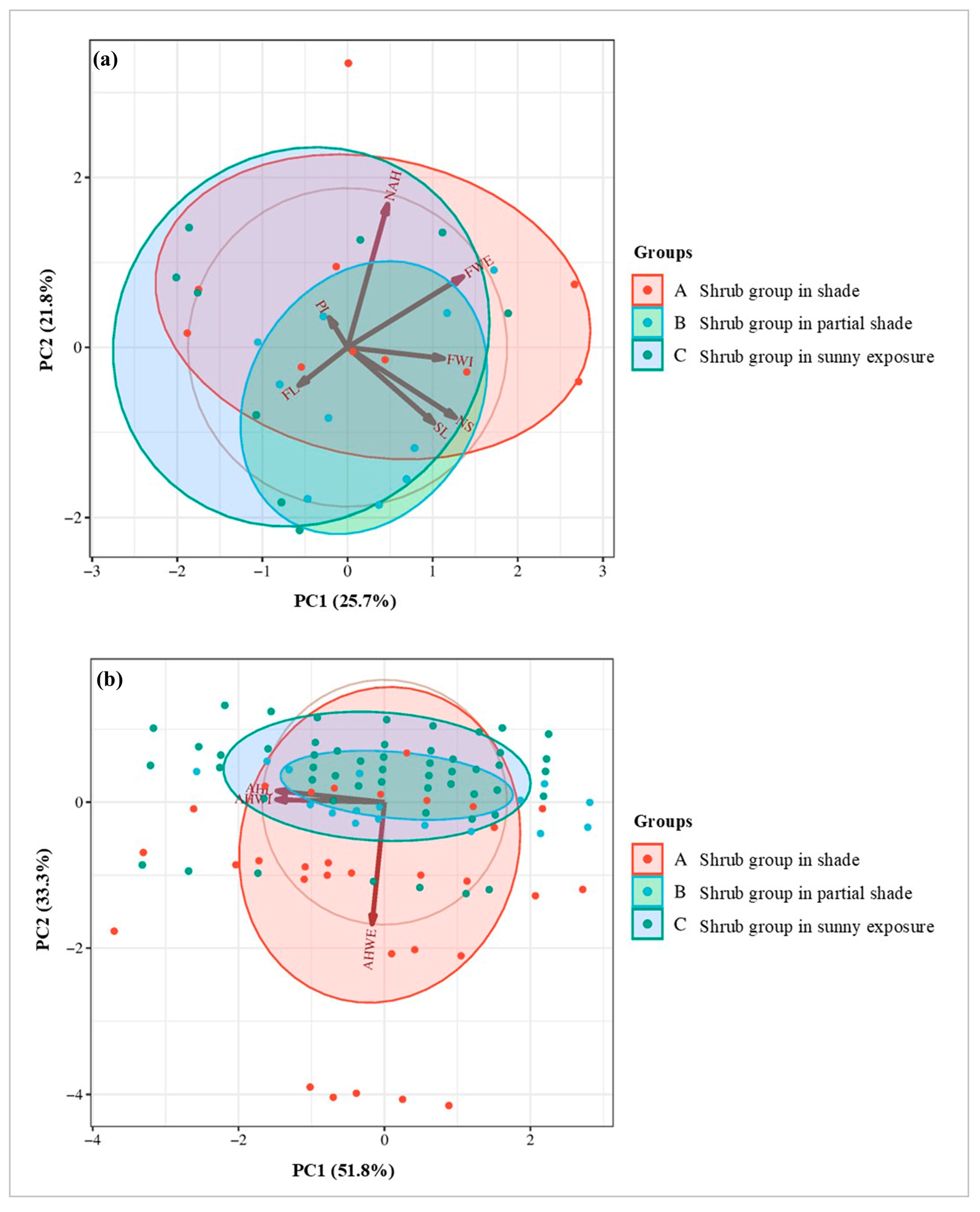
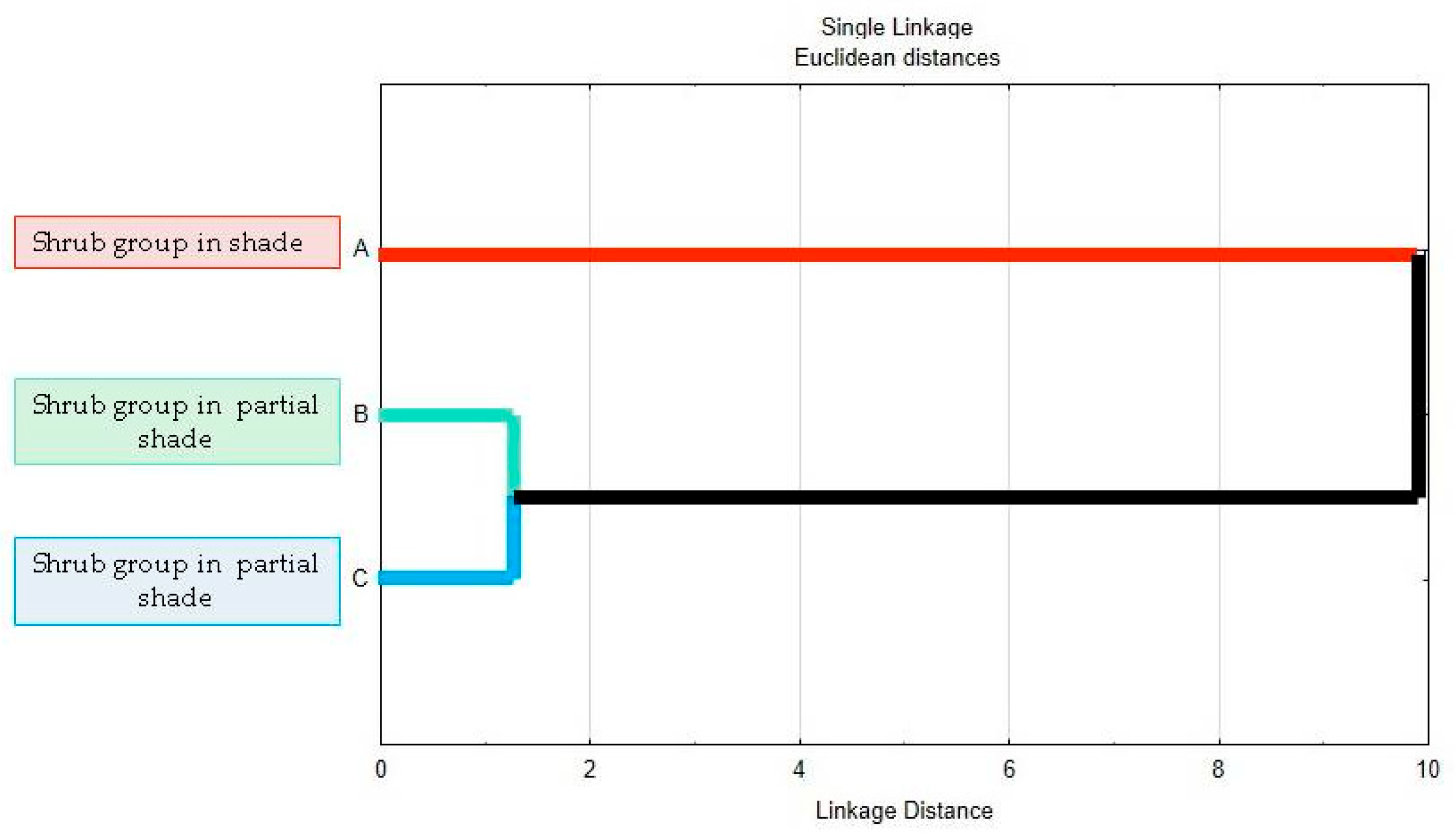
3.4. Multivariate Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NP | Number of plants per group |
| SA | Shrub area |
| SH | Shrub height |
| DD | Degree of damage |
| V | Vitality |
| D | Decorativeness |
| DNPS | Distance from the nearest paved surface |
| DBP | Distance from the bicycle path |
| DNT | Distance from the nearest tree |
| WGB | Width of the green belt |
| TH | Tree height |
| HFB | Height to the first branches |
| DBH | Diameter at breast height |
| CW | Crown width |
| FWE | Fruit weight |
| FL | Fruit length |
| FWI | Fruit width |
| PL | Petiole length |
| SL | Sepal length |
| NS | Number of sepals |
| NAH | Number of achenes in the hypanthium |
| ET | Extent of trichomes |
| CT | Color of trichomes |
References
- Buchwald, W.; Zieliński, J.; Mścisz, A.; Adamczak, A.; Mrozikiewicz, P.M. Current state and prospects for research on fruit roses in Poland. Herba Pol. 2007, 53, 85–92. [Google Scholar]
- Fatrcová-Šramková, K.; Brindza, J.; Ivanišová, E.; Juríková, T.; Schwarzová, M.; Horčinová Sedláčková, V.; Grygorieva, O. Morphological and antiradical characteristics of Rugosa rose (Rosa rugosa Thunb.) fruits canned in different kind of honeys and in beverages prepared from honey. Slovak J. Food Sci. 2019, 13, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ribotta, S.; Liccari, F.; Muggia, L.; Pallavicini, A.; Bagnolini, F.; Tordoni, E.; Bacaro, G. Invasion at the edge: The case of Rosa rugosa (Rosaceae) in Italy. Diversity 2021, 13, 645. [Google Scholar] [CrossRef]
- Zhang, S.; Isermann, M.; Gan, W.; Breed, M. Invasive Rosa rugosa populations outperform native populations, but some populations have greater invasive potential than others. Sci. Rep. 2018, 8, 5735. [Google Scholar] [CrossRef]
- Jørgensen, R.H.; Kollmann, J. Invasion of coastal dunes by the alien shrub Rosa rugosa is associated with roads, tracks and houses. Flora-Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 289–297. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Bruun, H.H. Rosa Rugosa Thunb. ex Murray. J. Ecol. 2005, 93, 441–470. [Google Scholar] [CrossRef]
- Šilić, Č. Ukrasno Drveće i Grmlje; IP “Svjetlost”, Zavod za udžbenike i Nastavna Sredstva: Sarajevo, Bosnia; Zavod za udžbenike i nastavna sredstva: Beograd, Serbia, 1990. [Google Scholar]
- Idžojtić, M. Dendrologija—Cvijet, Češer, Plod, Sjeme; Sveučilište u Zagrebu, Šumarski fakultet: Zagreb, Croatia, 2013. [Google Scholar]
- Li, L.; Zhu, H.; Ju, Y.; Lv, Z.; Qian, C.; Zhang, C.; Lu, Y.; Wang, J.; Li, W. Comparison of microstructure and physiological response of the leaves of six Rosa rugosa genotypes under drought stress. Ornam. Plant Res. 2024, 4, e016. [Google Scholar] [CrossRef]
- Thurn, M.; Lamb, E.; Eshenaur, B. Disease and Insect Resistant Ornamental Plants; New York State Integrated Pest Management Program: Geneva, NY, USA, 2019; Available online: https://ecommons.cornell.edu/server/api/core/bitstreams/a3bdfe68-8a30-4aeb-a871-931d597e25cb/content (accessed on 20 January 2025).
- Calzoni, G.L.; Antognoni, F.; Pari, E.; Fonti, P.; Gnes, A.; Speranza, A. Active biomonitoring of heavy metal pollution using Rosa rugosa plants. Environ. Pollut. 2007, 149, 239–245. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, X.Z.; Liu, R.F.; Wu, L.L.; Li, J.A. Analysis of Plant Growth and Flower Aromatic Composition in Chinese Rosa rugosa Cultivars Under Cadmium Stress. Horticulturae 2025, 11, 214. [Google Scholar] [CrossRef]
- Kunc, N.; Mikulič-Petkovšek, M.; Hudina, M.; Bavcon, J.; Vreš, B.; Osterc, G.; Ravnjak, B. Autochthonous Rose Hybrid Rosa pendulina × spinosissima Overshines Main Genotype Rosa pendulina in the Biochemical Characteristics of Their Hips. Horticulturae 2022, 8, 669. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Vaitkevičienė, N.; Levickienė, D.; Bunevičienė, K. Effect of Harvesting in Different Ripening Stages on the Content of the Mineral Elements of Rosehip (Rosa spp.) Fruit Flesh. Horticulturae 2022, 8, 467. [Google Scholar] [CrossRef]
- Najda, A.; Buczkowska, H. Morphological and chemical characteristic of fruits of selected Rosa sp. Mod. Phytomorphol. 2013, 3, 99–103. [Google Scholar] [CrossRef]
- Jiménez, S.; Jiménez-Moreno, N.; Luquin, A.; Laguna, M.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Chemical composition of rosehips from different Rosa species: An alternative source of antioxidants for the food industry. Food Addit. Contam. Part A 2017, 34, 1121–1130. [Google Scholar] [CrossRef]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Al-Yafeai, A.; Bellstedt, P.; Böhm, V. Bioactive compounds and antioxidant capacity of Rosa rugosa depending on degree of ripeness. Antioxidants 2018, 7, 134. [Google Scholar] [CrossRef]
- Novák, J.; Skalický, M. Botanika II. Systém Rostlin (Botany II. Plant System); Česká Zemědělská Univerzita v Praze: Praha, Czech Republic, 2007; Volume 215. [Google Scholar]
- Olech, M.; Nowak, R.; Los, R.; Rzymowska, J.; Malm, A.; Chrusciel, K. Biological activity and composition of teas and tinctures prepared from Rosa rugosa Thunb. Open Life Sci. 2012, 7, 172–182. [Google Scholar] [CrossRef]
- Lu, J.; Wang, C.H. Medicinal components and pharmacological effects of Rosa rugosa. Rec. Nat. Prod. 2018, 12, 535–543. [Google Scholar] [CrossRef]
- Sulborska, A.; Weryszko-Chmielewska, E. Characteristics of the secretory structures in the flowers of Rosa rugosa Thunb. Acta Agrobot. 2014, 67, 13–24. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef]
- Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and antioxidant activity of extracts from rose fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef] [PubMed]
- Henker, H. Rosa. In Illustrierte Flora von Mitteleuropa; Hegi, G., Ed.; Parey Buchverlag. Bd. 4/2: Berlin, Germany, 2000; Band 4, Teil 2C. [Google Scholar]
- Deng, Y.M.; Li, C.C.; Shao, Q.S.; Ye, X.Q.; She, J.M. Differential responses of double petal and multi petal jasmine to shading: I. Photosynthetic characteristics and chloroplast ultrastructure. Plant Physiol. Biochem. 2012, 55, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Kelman, D.; Johns, K.L.; Wright, A.D. Correlation between leaf age, shade levels, and characteristic beneficial natural constituents of tea (Camellia sinensis) grown in Hawaii. Food Chem. 2012, 133, 707–714. [Google Scholar] [CrossRef]
- Zhao, D.; Hao, Z.; Tao, J. Effects of shade on plant growth and flower quality in the herbaceous peony (Paeonia lactiflora Pall.). Plant Physiol. Biochem. 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Hou, W.; Luo, Y.; Wang, X.; Chen, Q.; Sun, B.; Wang, Y.; Liu, Z.; Tang, H.; Zhang, Y. Effects of shading on plant growth, flower quality and photosynthetic capacity of Rosa hybrida. In Proceedings of the AIP Conference Proceedings, Offenburg, Germany, 26–28 September 2017; AIP Publishing: Melville, NY, USA, 2018. [Google Scholar]
- Stanton, K.M.; Weeks, S.S.; Dana, M.N.; Mickelbart, M.V. Light exposure and shade effects on growth, flowering, and leaf morphology of Spiraea alba Du Roi and Spiraea tomentosa L. HortScience 2010, 45, 1912–1916. [Google Scholar] [CrossRef]
- Peavey, M.; Scalisi, A.; Islam, M.S.; Goodwin, I. Fruit Position, Light Exposure and Fruit Surface Temperature Affect Colour Expression in a Dark-Red Apple Cultivar. Horticulturae 2024, 10, 725. [Google Scholar] [CrossRef]
- Živković, B.; Nejgerbauer, V.; Tanasijević, Đ.; Miljković, N.; Stojković, L.; Drezgić, P. Zemljišta Vojvodine; Institut za Poljoprivredna Istraživanja: Novi Sad, Serbia, 1972. [Google Scholar]
- Hulisz, P.; Charzyński, P.; Greinert, A. Urban soil resources of medium-sized cities in Poland: A comparative case study of Toruń and Zielona Góra. J. Soils Sediments 2018, 18, 358–372. [Google Scholar] [CrossRef]
- WMO. State of the Global Climate; WMO Provisional Report; WMO: Geneva, Switzerland, 2021; Available online: http://adaptecca.es/sites/default/files/documentos/estado_del_clima_global_2020._organizacion_meteorologica_mundial_2021.pdf (accessed on 3 April 2025).
- RHMS. Meteorologija/Klimatologija Godišnjaci. 2025. Available online: https://www.hidmet.gov.rs/ciril/meteorologija/klimatologija_godisnjaci.php (accessed on 3 April 2025).
- Tomić, Z. Šumarska Fitocenologija; Šumarski Fakultet Univerziteta u Beogradu: Beograd, Serbia, 2004; pp. 176–182. [Google Scholar]
- Mattheck, C.; Bethge, K.; Erb, D. Failure criteria for trees. Arboric. J. 1993, 17, 201–209. [Google Scholar] [CrossRef]
- Anastasijević, N. Podizanje i Negovanje Zelenih Površina; Šumarski fakultet Univerziteta u Beogradu: Beograd, Srbija, 2007. [Google Scholar]
- Morozova, G.; Debelay, I. Green infrastructure as a factor of the sustainable development of Khabarovsk. Econ. Reg. Ekon. Reg. 2018, 14, 562. [Google Scholar] [CrossRef]
- UPOV. Guidelines for the Conduct of Tests Distinctness, Uniformity and Stability-Rosa L.; International Union for The Protection of New Varieties of Plants: Geneva, Switzerland, 2010. [Google Scholar]
- Stilinović, S. Semenarstvo Šumskog i Ukrasnog Drveća i Žbunja; Šumarski Fakultet: Belgrade, Serbia, 1985. [Google Scholar]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Du, H.Q.; Fan, W.L.; Hu, J.G.; Mao, F.J.; Dong, H. Long-term trend in vegetation gross primary production, phenology and their relationships inferred from the FLUXNET data. J. Environ. Manag. 2019, 246, 605–616. [Google Scholar] [CrossRef]
- Reaumur, R.A.F. Observations du Thermomètre Faites à Paris Pendant l’ Année 1735 Comparée sa Veccelles Quiontété Faitessous la Ligne à l’ Ile de France, à Algere Tenquelques-Unes de nos îles de l’ Amérique; Académie Royale des Sciences: Paris, France, 1735; pp. 545–580. [Google Scholar]
- Penzar, I.; Penzar, B. Agrometeorologija; Školska Knjiga: Zagreb, Croatia, 2000; 228p. [Google Scholar]
- Piao, S.L.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cycles 2007, 21, 11. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, B.; Yang, Q.C.; Chen, G.S.; Yang, B.J.; Lu, L.L.; Shen, M.; Peng, Y.Y. Responses of net primary productivity to phenological dynamics in the Tibetan Plateau, China. Agric. For. Meteorol. 2017, 232, 235–246. [Google Scholar] [CrossRef]
- Büntgen, U.; Piermattei, A.; Krusic, P.J.; Esper, J.; Sparks, T.; Crivellaro, A. Plants in the UK flower a month earlier under recent warming. Proc. R. Soc. B 2022, 289, 20212456. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, Z.; Sun, W.; Li, S.; Han, F.; Huang, S.; Yu, C. The Relative Effects of Climate Change and Phenological Change on Net Primary Productivity Vary with Grassland Types on the Tibetan Plateau. Remote Sens. 2023, 15, 3733. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Asrar, G.R.; Mao, J.; Li, X.; Li, W. Response of Vegetation Phenology to Urbanization in the Conterminous United States. Glob. Change Biol. 2016, 23, 2818–2830. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.; Stanciu Buican, A.; Ionescu, M. The Influence of Temperature on Phenology of OrnamentalWoody Species in Urban Environment. Sci. Pap. Ser. B Hortic. 2020, 64, 61–67. [Google Scholar]
- Vuksanović, V.; Kovačević, B.; Kebert, M.; Pavlović, L.; Kesić, L.; Čukanović, J.; Orlović, S. In vitro selection of drought-tolerant white poplar clones based on antioxidant activities and osmoprotectant content. Front. Plant Sci. 2023, 14, 1280794. [Google Scholar] [CrossRef]
- Wei, L.; Li, J.; Yang, Y.; Zhu, M.; Zhao, M.; Yang, J.; Yang, Z.; Zhou, L.; Zhou, S.; Gong, J.; et al. Characterization and potential bioactivity of polyphenols of Rosa rugosa. Food Biosci. Part A 2022, 50, 102108. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Pecio, Ł.; Łoś, R.; Malm, A.; Rzymowska, J.; Oleszek, W. Multidirectional characterisation of chemical composition and health-promoting potential of Rosa rugosa hips. Nat. Prod. Res. 2017, 31, 667–671. [Google Scholar] [CrossRef]
- Cho, E.J.; Yokozawa, T.; Rhyu, D.Y.; Kim, S.C.; Shibahara, N.; Park, J.C. Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1,1-diphenyl-2-picrylhydrazyl radical. Phytomedicine 2003, 10, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, K.C.P.; Figueiredo, C.A.V.; Figueredo, T.B.; Freire, K.R.L.; Santos, F.A.R.; Alcantara-Neves, N.M.; Silva, T.M.S.; Piuvezam, M.R. Anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J. Ethnopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.Ž.; Sentić, I.M.; Čukanović, J.D.; Ljubojević, M.Ž.; Pušić, M.G. Influence of urban heat island on Tilia tomentosa Moench. blooming. Zb. Matice Srp. Za Prir. Nauk. 2021, 141, 21–33. [Google Scholar]
- Zipper, S.C.; Schatz, J.; Singh, A.; Kucharik, C.J.; Townsend, P.A.; Loheide, S.P. Urban heat island impacts on plant phenology: Intra-urban variability and response to land cover. Environ. Res. Lett. 2016, 11, 054023. [Google Scholar] [CrossRef]
- Xiong, L.; IshitaniI, M.; Zhu, J.K. Interaction of osmotic stress, temperature, and abscisic acid in the regulation of gene expression in Arabidopsis. Plant Physiol. 1999, 119, 205–212. [Google Scholar] [CrossRef]
- Tang, C.-S.; Shi, B.; Gao, L.; Daniles, J.L.; Jiang, H.-T.; Liu, C. Urbanization effect on soil temperature in Nanjing, China. Energy Build. 2011, 43, 3090. [Google Scholar] [CrossRef]
- Kravkaz Kuscu, I.; Cetin, M.; Yigit, N.; Savaci, G.; Sevik, H. Relationship between enzyme activity (urease-catalase) and nutrient element in soil use. Pol. J. Environ. Stud. 2018, 27, 2107–2112. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Jiang, B.; Wen, Z.; Yang, N.; Li, L. Tree survival and growth are impacted by increased surface temperature on paved land. Landsc. Urban Plan. 2017, 162, 68–79. [Google Scholar] [CrossRef]
- Kostić, S.; Čukanović, J.; Ljubojević, M.; Hiel, K.; Mladenović, E. Influence of an Urban Paved Environment on Tree Dimensions and Vitality Characteristics: A Case Study of Sycamore Maple (Acer pseudoplatanus L.). Pol. J. Environ. Stud. 2019, 28, 4247–4255. [Google Scholar] [CrossRef]
- Van den Bosch, M.; Sang, Å.O. Urban natural environments as nature-based solutions for improved public health—A systematic review of reviews. Environ. Res. 2017, 158, 373–384. [Google Scholar] [CrossRef]
- Vujcic, M.; Tomicevic-Dubljevic, J.; Zivojinovic, I.; Toskovic, O. Connection between urban green areas and visitors’ physical and mental well-being. Urban For. Urban Green. 2019, 40, 299–307. [Google Scholar] [CrossRef]
- Coventry, P.A.; Brown, J.V.E.; Pervin, J.; Brabyn, S.; Pateman, R.; Breedvelt, J.; Gilbody, S.; Stancliffe, R.; McEachan, R.; White, P.C.L. Nature-based outdoor activities for mental and physical health: Systematic review and meta-analysis. SSM-Popul. Health 2021, 16, 100934. [Google Scholar] [CrossRef] [PubMed]
- Žižlavská, N.; Mikita, T.; Patočka, Z. The Effects of Roadside Woody Vegetation on the Surface Temperature of Cycle Paths. Land 2021, 10, 483. [Google Scholar] [CrossRef]
- Moghbel, M.; Salim, R.E. Environmental benefits of green roofs on microclimate of Tehran with specific focus on air temperature, humidity and CO2 content. Urban Clim. 2017, 20, 46–58. [Google Scholar] [CrossRef]
- Nguyen, P.-Y.; Astell-Burt, T.; Rahimi-Ardabili, H.; Feng, X. Green Space Quality and Health: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11028. [Google Scholar] [CrossRef]
- Al-Yafeai, A.; Malarski, A.; Böhm, V. Characterization of carotenoids and vitamin E in R. rugosa and R. canina: Comparative analysis. Food Chem. 2018, 242, 435–442. [Google Scholar] [CrossRef]
- Osnato, M.; Ignacio, C.; Poonam, N.; Unai, C.; Soraya, P. Photoperiod control of plant growth: Flowering time genes beyond flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Rozbiany, P.M.K.; Taha, S.M. Response of Strawberry [Fragaria X ananassa Duch.] Flowering and Yield to Photoperiod. In Recent Studies on Strawberries; Kafkas, N.E., Ed.; IntechOpen: London, UK, 2023. [Google Scholar]
- Bacelar, E.; Pinto, T.; Anjos, R.; Morais, M.C.; Oliveira, I.; Vilela, A.; Cosme, F. Impacts of Climate Change and Mitigation Strategies for Some Abiotic and Biotic Constraints Influencing Fruit Growth and Quality. Plants 2024, 13, 1942. [Google Scholar] [CrossRef]

| Characteristics | Abbreviations | Scores |
|---|---|---|
| Plant: growth type | GT | 1—miniature, 2—dwarf, 3—bed, 4—shrub, 5—climber, 6—ground cover |
| Plant: growth habit | GH | 1—upright, 2—semi-upright, 3—intermediate, 4—moderately spreading, 5—strongly spreading |
| Plant: height | H | 1—very short, 2—short, 3—medium, 4—tall, 5—very tall |
| Stem: number of prickles | NP | 1—absent or very few, 2—few, 3—medium, 4—many, 5—very many |
| Prickles: predominant color | PC | 1—greenish, 2—yellowish, 3—reddish, 4—purplish |
| Average Air Temperatures (°C) | ||||
|---|---|---|---|---|
| Season | Spring | Summer | Autumn | Winter |
| Months Period | 3–5 | 6–8 | 9–11 | 12–2 |
| 91/2020 | 12.2 | 21.9 | 12.0 | 1.6 |
| 10/2023 | 12.5 | 22.9 | 12.9 | 2.6 |
| 2024 | 15.3 | 26.2 | 12.7 | 5.9 |
| Average maximum air temperatures (°C) | ||||
| 91/2020 | 18.1 | 28.2 | 17.9 | 5.4 |
| 10/2023 | 18.3 | 29.2 | 18.9 | 6.5 |
| 2024 | 21.7 | 33.1 | 18.9 | 11.0 |
| Average minimum air temperatures (°C) | ||||
| 91/2020 | 6.6 | 15.7 | 7.4 | −1.8 |
| 10/2023 | 6.8 | 16.4 | 8.2 | −0.8 |
| 2024 | 8.8 | 18.7 | 7.5 | 1.7 |
| Total and average precipitation amounts (mm) | ||||
| 91/2020 | 163.4 | 222.3 | 169.0 | 125.4 |
| 10/2023 | 186.2 | 204.3 | 156.5 | 137.5 |
| 2024 | 114.5 | 87.9 | 237.9 | 79.7 |
| Total and average sunshine duration (h) | ||||
| 91/2020 | 627.2 | 900.3 | 463.0 | 228.1 |
| 10/2023 | 627.3 | 935.5 | 475.2 | 228.7 |
| 2024 | 726.9 | 1011.3 | 521.2 | 318.2 |
| Number of summer days (Tmax ≥ 25 °C) | ||||
| 91/2020 | 14.9 | 71.7 | 16.8 | 0.0 |
| 10/2023 | 15.0 | 76.5 | 20.9 | 0.0 |
| 2024 | 32 | 87 | 21 | 0 |
| Number of tropical days (Tmax ≥ 30 °C) | ||||
| 91/2020 | 1.6 | 33.6 | 3.2 | 0.0 |
| 10/2023 | 1.4 | 41.0 | 5.7 | 0.0 |
| 2024 | 1 | 77 | 10 | 0 |
| Sun Exposure | Area (m2) | Number of Individuals | Height (m) | Degree of Damage | Vitality | Decorativeness | DNPS (m) | DPB (m) | WGB (m) | DNT (m) | Fruit Yield (1–5) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 52.5 | 116 | 0.6 | * | 5 | 5 | 0.5 | 2.5 | 4.5 | 0.3 | 5 |
| B | 13.5 | 15 | 0.8 | / | 5 | 5 | 0.5 | 0.5–3 | 2–2.5 | 4.2 | 5 |
| C | 10.5 | 14 | 0.5 | / | 5 | 5 | 0.5 | 2–3 | 3.5–4 | / | 5 |
| Exposure | A | B | C | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits | Mean ± Se | SD | Cv (%) | Mean ± Se | SD | Cv (%) | Mean ± Se | SD | Cv (%) | F | p ** |
| FWE | 1.41 ± 0.26 | 0.81 | 47.34 | 1.71 ± 0.11 | 0.37 | 25.98 | 1.38 ± 0.12 | 0.39 | 27.97 | 215.42 | 0.00 * |
| FL | 1.44 ± 0.12 | 0.40 | 27.62 | 1.56 ± 0.07 | 0.24 | 15.47 | 1.66 ± 0.07 | 0.24 | 14.26 | 797.06 | 0.00 * |
| FWI | 2.22 ± 0.11 | 0.35 | 15.72 | 2.27 ± 0.08 | 0.26 | 11.38 | 2.34 ± 0.09 | 0.29 | 14.28 | 1559.4 | 0.00 * |
| PL | 1.03 ± 0.08 | 0.27 | 25.91 | 1.1 ± 0.09 | 0.30 | 27.10 | 1.2 ± 0.15 | 0.49 | 40.45 | 280.26 | 0.00 * |
| SL | 1.65 ± 0.17 | 0.53 | 32.10 | 1.77 ± 0.10 | 0.33 | 18.46 | 1.3 ± 0.11 | 0.36 | 27.38 | 433.43 | 0.00 * |
| NS | 4.6 ± 0.22 | 0.70 | 15.20 | 4.7 ± 0.15 | 0.48 | 10.28 | 4.7 ± 0.15 | 0.48 | 10.28 | 2051.16 | 0.00 * |
| NAH | 74.7 ± 6.03 | 19.06 | 25.52 | 64.40 ± 4.75 | 15.04 | 23.36 | 64.8 ± 7.99 | 25.27 | 39.00 | 338.56 | 0.00 * |
| AHL | 4.80 ± 0.33 | 1.03 | 21.51 | 4.25 ± 0.27 | 0.85 | 20.18 | 5.05 ± 0.29 | 0.93 | 18.34 | 7.47 | 0.00 * |
| AHWI | 2.17 ± 0.21 | 0.66 | 30.67 | 2.2 ± 0.15 | 0.46 | 21.29 | 2.27 ± 0.20 | 0.63 | 27.63 | 4.17 | 0.00 * |
| AHWE | 0.17 ± 0.03 | 0.09 | 51.92 | 0.12 ± 0.05 | 0.01 | 15.21 | 0.11 ± 0.01 | 0.03 | 30.74 | 1.76 | 0.00 * |
| Contrast | Difference * | Standardized Difference | Critical Value | Pr > Diff | Alpha (Modified) | Significant | Lower Bound (95%) | Upper Bound (95%) | Lower Bound (95%) | Upper Bound (95%) |
|---|---|---|---|---|---|---|---|---|---|---|
| FWE (g) | ||||||||||
| A vs. C ** | 0.329 | 1.300 | 2.159 | 0.408 | 0.098 | No | −0.218 | 0.876 |  |  |
| A vs. B | 0.265 | 1.018 | 2.056 | 0.318 | 0.050 | No | −0.270 | 0.799 |  |  |
| B vs. C | 0.064 | 0.247 | 2.056 | 0.807 | 0.050 | No | −0.470 | 0.599 |  |  |
| FL (cm) | ||||||||||
| C vs. A | 0.220 | 1.603 | 2.159 | 0.262 | 0.098 | No | −0.076 | 0.516 |  |  |
| C vs. B | 0.093 | 0.662 | 2.056 | 0.514 | 0.050 | No | −0.196 | 0.383 |  |  |
| B vs. A | 0.127 | 0.898 | 2.056 | 0.377 | 0.050 | No | −0.163 | 0.416 |  |  |
| PL (cm) | ||||||||||
| C vs. A | 0.170 | 1.042 | 2.159 | 0.558 | 0.098 | No | −0.182 | 0.522 |  |  |
| C vs. B | 0.067 | 0.398 | 2.056 | 0.694 | 0.050 | No | −0.278 | 0.411 |  |  |
| B vs. A | 0.103 | 0.616 | 2.056 | 0.543 | 0.050 | No | −0.241 | 0.448 |  |  |
| FWI (cm) | ||||||||||
| B vs. C | 0.227 | 1.604 | 2.159 | 0.262 | 0.098 | No | −0.079 | 0.532 |  |  |
| B vs. A | 0.047 | 0.330 | 2.056 | 0.744 | 0.050 | No | −0.244 | 0.337 |  |  |
| A vs. C | 0.180 | 1.309 | 2.056 | 0.202 | 0.050 | No | −0.103 | 0.463 |  |  |
| SL (cm) | ||||||||||
| B vs. C | 0.444 | 2.308 | 2.159 | 0.072 | 0.098 | Yes | 0.029 | 0.860 |  | |
| B vs. A | 0.094 | 0.490 | 2.056 | 0.628 | 0.050 | No | −0.301 | 0.490 |  |  |
| A vs. C | 0.350 | 1.867 | 2.056 | 0.073 | 0.050 | No | −0.035 | 0.735 |  |  |
| NS | ||||||||||
| B vs. A | 0.100 | 0.391 | 2.159 | 0.919 | 0.098 | No | −0.452 | 0.652 |  |  |
| B vs. C | 0.033 | 0.127 | 2.056 | 0.900 | 0.050 | No | −0.507 | 0.573 |  |  |
| B vs. A | 0.067 | 0.254 | 2.056 | 0.802 | 0.050 | No | −0.473 | 0.607 |  |  |
| NAH | ||||||||||
| A vs. C | 9.900 | 1.102 | 2.159 | 0.521 | 0.098 | No | −9.502 | 29.302 |  |  |
| A vs. B | 7.811 | 0.846 | 2.056 | 0.405 | 0.050 | No | −11.164 | 26.786 |  |  |
| B vs. C | 2.089 | 0.226 | 2.056 | 0.823 | 0.050 | No | −16.886 | 21.064 |  |  |
| AHWE | ||||||||||
| A vs. C | 0.057 | 2.189 | 2.056 | 0.038 | 0.098 | Yes | 0.003 | 0.110 |  | |
| A vs. B | 0.049 | 1.941 | 2.056 | 0.063 | 0.050 | No | −0.003 | 0.101 |  |  |
| B vs. C | 0.008 | 0.300 | 2.056 | 0.767 | 0.050 | No | −0.046 | 0.061 |  |  |
| AHL (mm) | ||||||||||
| C vs. B | 0.430 | 2.772 | 2.072 | 0.016 | 0.098 | Yes | 0.109 | 0.751 |  | |
| C vs. A | 0.214 | 1.376 | 1.968 | 0.170 | 0.050 | No | −0.092 | 0.520 |  |  |
| A vs. B | 0.216 | 1.389 | 1.968 | 0.166 | 0.050 | No | −0.090 | 0.522 |  |  |
| AHWI (mm) | ||||||||||
| A vs. C | 0.052 | 0.496 | 2.072 | 0.873 | 0.098 | No | −0.166 | 0.271 |  |  |
| A vs. B | 0.042 | 0.401 | 1.968 | 0.689 | 0.050 | No | −0.165 | 0.250 |  |  |
| B vs. C | 0.010 | 0.095 | 1.968 | 0.924 | 0.050 | No | −0.197 | 0.217 |  |  |
| Group | FWE (g) | FL (cm) | PL (cm) | FWI (cm) | SL (cm) | NS | NAH | AHWE (g) | AHL (mm) | AHWI (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| B | 1.718 a* | 1.567 a | 1.133 a | 2.267 a | 1.744 a | 4.667 a | 66.889 a | 0.120 ab | 4.390 b | 2.190 a |
| A | 1.453 a | 1.440 a | 1.030 a | 2.220 a | 1.650 ab | 4.600 a | 74.700 a | 0.169 a | 4.606 ab | 2.232 a |
| C | 1.389 a | 1.660 a | 1.200 a | 2.040 a | 1.300 b | 4.700 a | 64.800 a | 0.112 b | 4.820 a | 2.180 a |
| Pr > F (Model) | 0.403 | 0.291 | 0.583 | 0.249 | 0.066 | 0.924 | 0.520 | 0.073 | 0.023 ** | 0.871 |
| Significant | No | No | No | No | No | No | No | No | Yes | No |
| Pr > F (Group) | 0.403 | 0.291 | 0.583 | 0.249 | 0.066 | 0.924 | 0.520 | 0.073 | 0.023 | 0.871 |
| Significant | No | No | No | No | No | No | No | No | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čukanović, J.; Đorđević, S.; Petrov, D.; Ocokoljić, M.; Kolarov, R.; Čurčić, M.; Ljubojević, M. The Sustainability of Rosa rugosa Thunb. Under Climate Change Conditions: A Study of Morphological Variability in Urban Areas. Horticulturae 2025, 11, 684. https://doi.org/10.3390/horticulturae11060684
Čukanović J, Đorđević S, Petrov D, Ocokoljić M, Kolarov R, Čurčić M, Ljubojević M. The Sustainability of Rosa rugosa Thunb. Under Climate Change Conditions: A Study of Morphological Variability in Urban Areas. Horticulturae. 2025; 11(6):684. https://doi.org/10.3390/horticulturae11060684
Chicago/Turabian StyleČukanović, Jelena, Sara Đorđević, Djurdja Petrov, Mirjana Ocokoljić, Radenka Kolarov, Milana Čurčić, and Mirjana Ljubojević. 2025. "The Sustainability of Rosa rugosa Thunb. Under Climate Change Conditions: A Study of Morphological Variability in Urban Areas" Horticulturae 11, no. 6: 684. https://doi.org/10.3390/horticulturae11060684
APA StyleČukanović, J., Đorđević, S., Petrov, D., Ocokoljić, M., Kolarov, R., Čurčić, M., & Ljubojević, M. (2025). The Sustainability of Rosa rugosa Thunb. Under Climate Change Conditions: A Study of Morphological Variability in Urban Areas. Horticulturae, 11(6), 684. https://doi.org/10.3390/horticulturae11060684












