Environmental and Phytohormonal Factors Regulating Anthocyanin Biosynthesis in Fruits
Abstract
1. Introduction
2. Environmental Factors
2.1. Light
2.2. Temperature
2.3. Mineral Nutrients
2.4. Drought and Salt Stress
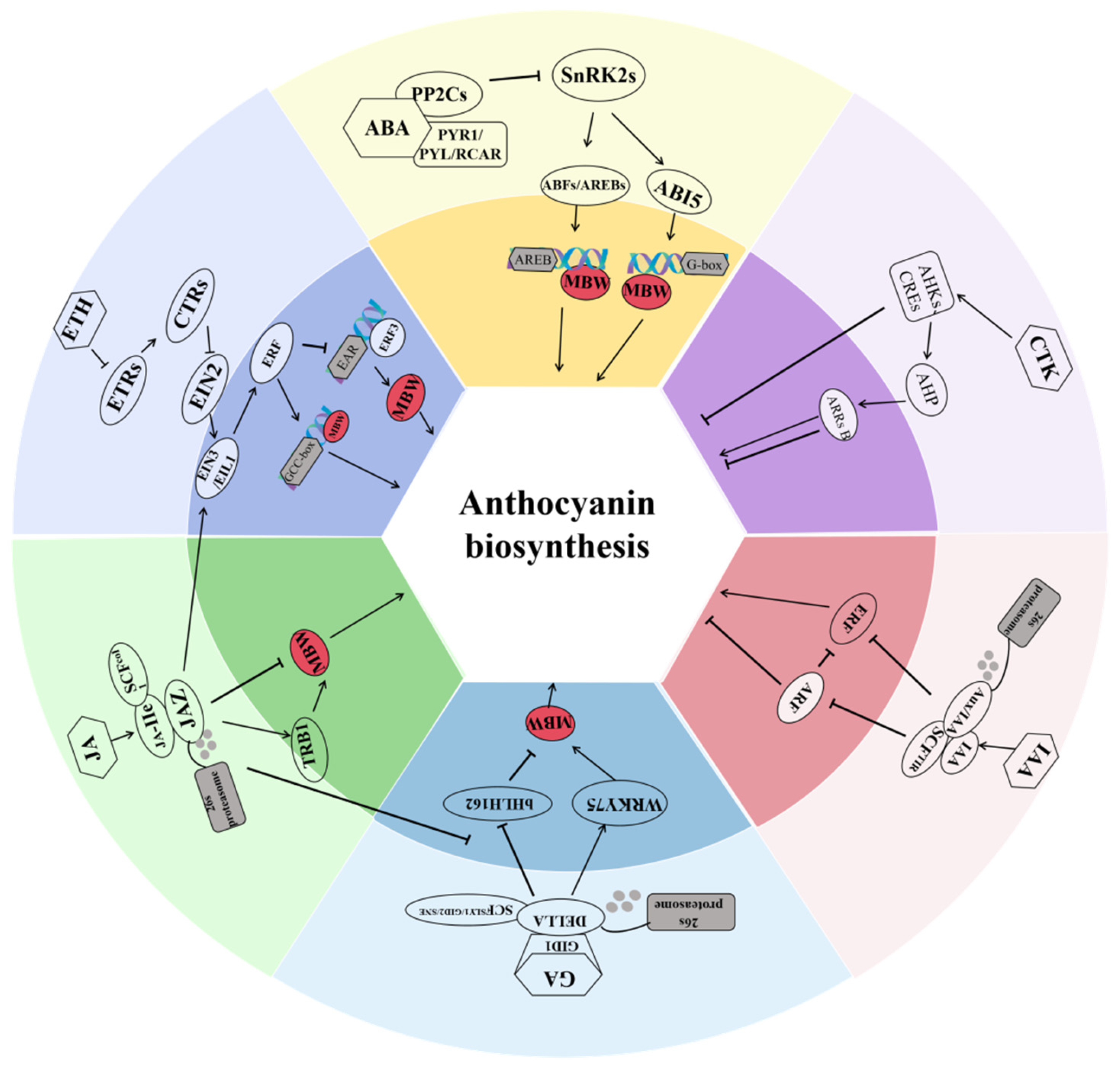
3. Phytohormones
3.1. Abscisic Acid
3.2. Ethylene
3.3. Jasmonate
3.4. Gibberellins
3.5. Auxin
3.6. Cytokinin
4. Interactions Between Environmental Factors and Phytohormones
4.1. Light and Temperature
4.2. Light and Phytohormones
4.3. Temperature and Phytohormones
4.4. Drought, Low-Nitrogen Stress, and Phytohormones
| Species | Environment | Phytohormone | Regulators | Regulated Genes/Enzymes | Anthocyanin Biosynthesis | Reference |
|---|---|---|---|---|---|---|
| Apple (Malus domestica) | Light | Ethylene | LNC610 | ACO1 | Inhibit | [147] |
| Light | JA | ERF109 WER | CHS, UFGT, bHLH3 | Promotion | [21] | |
| Light, Low Temperature | GA | HY5 | DFR | Promotion | [153] | |
| Plum (Prunus salicina Lindl.) | Low Temperature | Ethylene | ERF1B, MYB10.1, bHLH3 | UFGT | Promotion | [155] |
| Peach (Prunus persica (L.) Batsch) | Low Temperature | JA | BBX32, ZAT5 | MYB10.1 | Promotion | [49] |
| Grapevine (Vitis vinifera) | Drought | ABA | MYBA1, miR156b, SBP8/13 | MYC1, MYBA1, WD40 | Promotion | [159] |
| High Temperature | ABA | VvARF3 | VvFHY3 | Inhibition | [151] | |
| High Temperature | CTK | VvMYB44-1 | VvLOG8- VvCKX4 | Inhibition | [152] | |
| Sweet Cherry (Prunus avium L.) | High Temperature | ABA | CYP707A, AOG | Promotion | [150] |
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Costa, D.; Galvão, A.M.; Di Paolo, R.E.; Freitas, A.A.; Lima, J.C.; Quina, F.H.; Maçanita, A.L. Photochemistry of the hemiketal form of anthocyanins and its potential role in plant protection from UV-B radiation. Tetrahedron 2015, 71, 3157–3162. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal. Behav. 2015, 10, e1027850. [Google Scholar] [CrossRef]
- Lu, X.; Li, J.; Wang, B.; Qin, S. Computational Insights into the Radical Scavenging Activity and Xanthine Oxidase Inhibition of the Five Anthocyanins Derived from Grape Skin. Antioxidants 2024, 13, 1117. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, B.; Guo, Y.; Choung, S. Preventive effect of Vaccinium uliginosum L. extract and its fractions on age-related macular degeneration and its action mechanisms. Arch. Pharm. Res. 2016, 39, 21–32. [Google Scholar] [CrossRef]
- Jo, K.; Bae, G.Y.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K. An Anthocyanin-Enriched Extract from Vaccinium uliginosum Improves Signs of Skin Aging in UVB-Induced Photodamage. Antioxidants 2020, 9, 844. [Google Scholar] [CrossRef]
- Shi, N.; Chen, X.; Chen, T. Anthocyanins in Colorectal Cancer Prevention Review. Antioxidants 2021, 10, 1600. [Google Scholar] [CrossRef]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and Biochemistry of Anthocyanin Biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Zhu, L.; Chang, P.; Li, L.; Zhang, L. Identification and Characterization of MYB-bHLH-WD40 Regulatory Complex Members Controlling Anthocyanidin Biosynthesis in Blueberry Fruits Development. Genes 2019, 10, 496. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, S.; Xu, H.; Allan, A.C.; Zhang, X.; Fan, L.; Chen, L.; Su, J.; Shu, Q.; Li, K. The interaction of MYB, bHLH and WD40 transcription factors in red pear (Pyrus pyrifolia) peel. Plant Mol. Biol. 2021, 106, 407–417. [Google Scholar] [CrossRef]
- Lin-Wang, K.; Bolitho, K.; Grafton, K.; Kortstee, A.; Karunairetnam, S.; McGhie, T.K.; Espley, R.V.; Hellens, R.P.; Allan, A.C. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol. 2010, 10, 50. [Google Scholar] [CrossRef]
- Telias, A.; Lin-Wang, K.; Stevenson, D.E.; Cooney, J.M.; Hellens, R.P.; Allan, A.C.; Hoover, E.E.; Bradeen, J.M. Apple skin patterning is associated with differential expression of MYB10. BMC Plant Biol. 2011, 11, 93. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, B.; Tan, Z.; Song, B.; Cao, H.; Zong, C. RNA sequencing and anthocyanin synthesis-related genes expression analyses in white-fruited Vaccinium uliginosum. BMC Genom. 2018, 19, 5351. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Li, M.; Wang, J.; Yang, Y.; Zhang, X.; Yan, G.; Zhang, H.; Liu, J.; Zhang, K. The R2R3 MYB transcription factor PavMYB 10.1 involves in anthocyanin biosynthesis and determines fruit skin colour in sweet cherry (Prunus avium L.). Plant Biotechnol. J. 2016, 14, 2120–2133. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Zhao, L.; Li, C.; Yu, J.; Li, T.; Yang, W.; Zhang, S.; Su, H.; Wang, L. A novel NAC transcription factor, MdNAC42, regulates anthocyanin accumulation in red-fleshed apple by interacting with MdMYB10. Tree Physiol. 2020, 40, 413–423. [Google Scholar] [CrossRef]
- Jaakola, L.; Poole, M.; Jones, M.O.; Kämäräinen-Karppinen, T.; Koskimäki, J.J.; Hohtola, A.; Häggman, H.; Fraser, P.D.; Manning, K.; King, G.J.; et al. A SQUAMOSA MADS Box Gene Involved in the Regulation of Anthocyanin Accumulation in Bilberry Fruits. Plant Physiol. 2010, 153, 1619–1629. [Google Scholar] [CrossRef]
- An, J.P.; Yao, J.F.; Xu, R.R.; You, C.X.; Wang, X.F.; Hao, Y.J. Apple bZIP transcription factor MdbZIP44 regulates abscisic acid-promoted anthocyanin accumulation. Plant Cell Environ. 2018, 41, 2678–2692. [Google Scholar] [CrossRef]
- Cong, L.; Qu, Y.; Sha, G.; Zhang, S.; Ma, Y.; Chen, M.; Zhai, R.; Yang, C.; Xu, L.; Wang, Z. PbWRKY75 promotes anthocyanin synthesis by activating PbDFR, PbUFGT, and PbMYB10b in pear. Physiol. Plant. 2021, 173, 1841–1849. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Wu, Y.; Zheng, L.; Zhang, G. Regulatory Mechanisms of Anthocyanin Biosynthesis in Apple and Pear. Int. J. Mol. Sci. 2021, 22, 8441. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Yang, M.; Liang, Z.; Qi, M.; Dong, Y.; Xu, Y.; Lin, X.; Li, L. The action of RED light: Specific elevation of pelargonidin-based anthocyanin through ABA-related pathway in strawberry. Postharvest Biol. Technol. 2022, 186, 111835. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Zhang, M.; Wu, T.; Song, T.; Yao, Y.; Zhang, J.; Tian, J. MdWER interacts withMdERF109 andMdJAZ2 to mediate methyl jasmonate- and light-induced anthocyanin biosynthesis in apple fruit. Plant J. 2024, 118, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Liu, J.; Lin, S.; Wang, J.; Lin, W.; Xu, W. GA-DELLA pathway is involved in regulation of nitrogen deficiency-induced anthocyanin accumulation. Plant Cell Rep. 2017, 36, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Saure, M.C. External control of anthocyanin formation in apple. Sci. Hortic. 1990, 42, 181–218. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Wang, S.; Li, W.; Liu, D.; Guo, X.; Qu, B. Transcriptomic analysis of bagging-treated ‘Pingguo’ pear shows that MYB4-like1, MYB4-like2, MYB1R1 and WDR involved in anthocyanin biosynthesis are up-regulated in fruit peels in response to light. Sci. Hortic. 2019, 244, 428–434. [Google Scholar] [CrossRef]
- Miah, M.S.; Farcuh, M. Reflective groundcovers promote anthocyanin content and advance fruit maturity of ‘Evercrisp’ apples grown in the Mid-Atlantic US. Front. Plant Sci. 2024, 15, 1478498. [Google Scholar] [CrossRef]
- Weber, S.; Damerow, L.; Kunz, A.; Blanke, M. Anthocyanin synthesis and light utilisation can be enhanced by reflective mulch—Visualisation of light penetration into a tree canopy. J. Plant Physiol. 2019, 233, 52–57. [Google Scholar] [CrossRef]
- Roosta, H.R.; Bikdeloo, M.; Ghorbanpour, M. The growth, nutrient uptake and fruit quality in four strawberry cultivars under different Spectra of LED supplemental light. BMC Plant Biol. 2024, 24, 179. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Liu, Z.; Zheng, T.; Dong, T.; Jin, H.; Jia, H.; Fang, J. Transcriptomic and Metabolomic Profiling Reveals the Effect of LED Light Quality on Fruit Ripening and Anthocyanin Accumulation in Cabernet Sauvignon Grape. Front. Nutr. 2021, 8, 790697. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Peng, X.; Sun, B.; Wang, X.; Tang, H. Characterization of anthocyanin and proanthocyanidin biosynthesis in two strawberry genotypes during fruit development in response to different light qualities. J. Photochem. Photobiol. B Biol. 2018, 186, 225–231. [Google Scholar] [CrossRef]
- Kokalj, D.; Zlatić, E.; Cigić, B.; Kobav, M.B.; Vidrih, R. Postharvest flavonol and anthocyanin accumulation in three apple cultivars in response to blue-light-emitting diode light. Sci. Hortic. 2019, 257, 108711. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, S.; Wang, X.; Han, F.; Luo, H.; Liu, Z.; Kang, C. The red/far-red light photoreceptor FvePhyB regulates tissue elongation and anthocyanin accumulation in woodland strawberry. Hortic. Res. 2023, 10, uhad232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, L.; Wang, Y.; Zhang, L.; Xu, S.; Wang, X.; He, W.; Zhang, Y.; Lin, Y.; Wang, Y.; et al. The blue light signal transduction module FaCRY1-FaCOP1-FaHY5 regulates anthocyanin accumulation in cultivated strawberry. Front. Plant Sci. 2023, 14, 1144273. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Bai, S.; Ni, J.; Yang, Q.; Zhao, Y.; Teng, Y. The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’ pear. Planta 2018, 248, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Meng, J.; Zhang, S.; Chi, R.; Wang, C.; Wang, D.; Li, H. The UV-B-Induced Transcription Factor HY5 Regulated Anthocyanin Biosynthesis in Zanthoxylum bungeanum. Int. J. Mol. Sci. 2022, 23, 2651. [Google Scholar] [CrossRef]
- Fang, H.; Dong, Y.; Yue, X.; Hu, J.; Jiang, S.; Xu, H.; Wang, Y.; Su, M.; Zhang, J.; Zhang, Z.; et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019, 42, 2090–2104. [Google Scholar] [CrossRef]
- Reay, P.F.; Lancaster, J.E. Accumulation of anthocyanins and quercetin glycosides in ‘Gala’ and ‘Royal Gala’ apple fruit skin with UV-B–Visible irradiation: Modifying effects of fruit maturity, fruit side, and temperature. Sci. Hortic. 2001, 90, 57–68. [Google Scholar] [CrossRef]
- Ma, A.; Wang, D.; Lu, H.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. LcCOP1 and LcHY5 control the suppression and induction of anthocyanin accumulation in bagging and debagging litchi fruit pericarp. Sci. Hortic. 2021, 287, 110281. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, J.; Cai, Y.; Yang, Q.; Zhang, Y.; Ogutu, C.O.; Liu, J.; Zhao, Y.; Wang, F.; He, H.; et al. PpHYH is responsible for light-induced anthocyanin accumulation in fruit peel of Prunus persica. Tree Physiol. 2022, 42, 1662–1677. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Yin, L.; Ni, J.; Yang, Q.; Yan, X.; Yang, F.; Guo, X.; Li, H.; Teng, Y. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019, 100, 1208–1223. [Google Scholar] [CrossRef]
- Liu, H.; Su, J.; Zhu, Y.; Yao, G.; Allan, A.C.; Ampomah-Dwamena, C.; Shu, Q.; Lin-Wang, K.; Zhang, S.; Wu, J. The involvement of PybZIPa in light-induced anthocyanin accumulation via the activation of PyUFGT through binding to tandem G-boxes in its promoter. Hortic. Res. 2019, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Wang, S.; Li, C.; Zhang, Z.; Wang, N.; Li, B.; Chen, X. Ultraviolet-B-induced MdWRKY71-L expression regulates anthocyanin synthesis in apple. Environ. Exp. Bot. 2022, 201, 105000. [Google Scholar] [CrossRef]
- Alabd, A.; Ahmad, M.; Zhang, X.; Gao, Y.; Peng, L.; Zhang, L.; Ni, J.; Bai, S.; Teng, Y. Light-responsive transcription factor PpWRKY44 induces anthocyanin accumulation by regulating PpMYB10 expression in pear. Hortic. Res. 2022, 9, uhac199. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Sun, W.; Sun, Y.; Li, J.; Zhang, J.; Wu, T.; Song, T.; Yao, Y.; Tian, J. MPK6-mediated HY5 phosphorylation regulateslight-induced anthocyanin accumulation in apple fruit. Plant Biotechnol. J. 2023, 21, 283–301. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Zhang, D.; Li, H.; Li, P.; Ma, F. Reactive oxygen species produced via plasma membrane NADPH oxidase regulate anthocyanin synthesis in apple peel. Planta 2014, 240, 1023–1035. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Hu, W.; Sun, B.; Chen, Q.; Tang, H. Anthocyanin accumulation and related gene expression affected by low temperature during strawberry coloration. Acta Physiol. Plant. 2018, 40, 192. [Google Scholar] [CrossRef]
- Ryu, S.; Han, J.H.; Cho, J.G.; Jeong, J.H.; Lee, S.K.; Lee, H.J. High temperature at veraison inhibits anthocyanin biosynthesis in berry skins during ripening in ‘Kyoho’ grapevines. Plant Physiol. Biochem. 2020, 157, 219–228. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, G.; Zhang, W.; Goltsev, V.; Sun, S.; Wang, J.; Li, P.; Ma, F. Anthocyanin concentration depends on the counterbalance between its synthesis and degradation in plum fruit at high temperature. Sci. Rep. 2017, 7, 7684. [Google Scholar] [CrossRef]
- Dou, F.; Phillip, F.O.; Liu, H. Combined Metabolome and Transcriptome Analysis Revealed the Accumulation of Anthocyanins in Grape Berry (Vitis vinifera L.) under High-Temperature Stress. Plants 2024, 13, 2394. [Google Scholar] [CrossRef]
- Wei, B.; Li, M.; Jia, X.; Zhang, P.; Li, J. Metabolomics and transcriptomics reveal molecular mechanisms of anthocyanin accumulation in ‘Nanhong’ pear (Pyrus ussuriensis) peel at different temperatures. Sci. Hortic. 2024, 328, 112898. [Google Scholar] [CrossRef]
- Bu, Y.; Wang, S.; Li, C.; Fang, Y.; Zhang, Y.; Li, Q.; Wang, H.; Chen, X.; Feng, S. Transcriptome Analysis of Apples in High-Temperature Treatments Reveals a Role of MdLBD37 in the Inhibition of Anthocyanin Accumulation. Int. J. Mol. Sci. 2022, 23, 3766. [Google Scholar] [CrossRef] [PubMed]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; Incà, E.D.; Tornielli, G.B.; Filippetti, I. The grapevine VviPrx31 peroxidase as a candidate gene involved in anthocyanin degradation in ripening berries under high temperature. J. Plant Res. 2016, 129, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Yakushiji, H.; Sato, A. Postharvest light irradiation and appropriate temperature treatment increase anthocyanin accumulation in grape berry skin. Postharvest Biol. Technol. 2019, 147, 89–99. [Google Scholar] [CrossRef]
- Habibi, F.; García-Pastor, M.E.; Puente-Moreno, J.; Garrido-Auñón, F.; Serrano, M.; Valero, D. Anthocyanin in blood oranges: A review on postharvest approaches for its enhancement and preservation. Crit. Rev. Food Sci. 2023, 63, 12089–12101. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Y.; Zhang, X.; Chen, M.; Wu, T.; Zhang, J.; Xing, Y.; Tian, J.; Yao, Y. ROS1 promotes low temperature-induced anthocyanin accumulation in apple by demethylating the promoter of anthocyanin-associated genes. Hortic. Res. 2022, 9, uhac7. [Google Scholar] [CrossRef]
- Zou, S.; Zhuo, M.; Abbas, F.; Zeng, R.; Hu, G.; Wang, H.; Huang, X. ROS- and CBF- mediated pathways are involved in chlorophyll degradation and anthocyanin accumulation enhanced by cool temperatures in ripening litchi fruits. Postharvest Biol. Technol. 2024, 212, 11288. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Shi, Y.; Lv, S.; Zhu, C.; Xu, C.; Zhang, B.; Allan, A.C.; Grierson, D.; Chen, K. The R2R3 MYB Ruby1 is activated by two cold responsive ethylene response factors, via the retrotransposon in its promoter, to positively regulate anthocyanin biosynthesis in citrus. Plant J. 2024, 119, 1433–1448. [Google Scholar] [CrossRef]
- Huang, D.; Xue, L.; Lu, Y.; Liu, M.; Lin-Wang, K.; Allan, A.C.; Zhang, B.; Chen, K.; Xu, C. PpBBX32 and PpZAT5 modulate temperature-dependent and tissue-specific anthocyanin accumulation in peach fruit. Hortic. Res. 2024, 11, uhae212. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, R.; Wang, S.; Gao, Y.; Wang, L.; Yang, S.; Zhang, X.; Yu, W.; Wu, X.; Li, K.; et al. PpZAT5 suppresses the expression of a B-box gene PpBBX18 to inhibit anthocyanin biosynthesis in the fruit peel of red pear. Front. Plant Sci. 2022, 13, 3389. [Google Scholar] [CrossRef]
- Mao, W.; Han, Y.; Chen, Y.; Sun, M.; Feng, Q.; Li, L.; Liu, L.; Zhang, K.; Wei, L.; Han, Z.; et al. Low temperature inhibits anthocyanin accumulation in strawberry fruit by activating FvMAPK3-induced phosphorylation of FvMYB10 and degradation of Chalcone Synthase 1. Plant Cell 2022, 34, 1226–1249. [Google Scholar] [CrossRef]
- Jatana, B.S.; Kitchens, C.; Ray, C.; Gerard, P.; Tharayil, N. Chemical Forms of Nitrogen Fertilizers Differentially Influence the Content and Composition of Aroma Volatiles and Phytonutrients in Strawberry Fruits. J. Agr. Food Chem. 2025, 73, 6241–6252. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jia, X.; Huo, L.; Che, R.; Gong, X.; Wang, P.; Ma, F. MdATG18a overexpression improves tolerance to nitrogen deficiency and regulates anthocyanin accumulation through increased autophagy in transgenic apple. Plant Cell Environ. 2018, 41, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, X.; Wang, Y.; Xu, J.; Jiang, S.; Zhang, Y. MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals. Int. J. Mol. Sci. 2022, 23, 7755. [Google Scholar] [CrossRef] [PubMed]
- Soubeyrand, E.; Basteau, C.; Hilbert, G.; van Leeuwen, C.; Delrot, S.; Gomès, E. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Chen, G.; Yang, G.; Ming, M.; Zhang, S.; Xue, Z.; Han, C.; Li, J.; Wu, J. Transcriptome Analysis Identified PyNAC42 as a Positive Regulator of Anthocyanin Biosynthesis Induced by Nitrogen Deficiency in Pear (Pyrus spp.). Horticulturae 2024, 10, 980. [Google Scholar] [CrossRef]
- Wang, X.; An, J.; Liu, X.; Su, L.; You, C.; Hao, Y. The Nitrate-Responsive Protein MdBT2 Regulates Anthocyanin Biosynthesis by Interacting with the MdMYB1 Transcription Factor. Plant Physiol. 2018, 178, 890–906. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.; Xu, H.; Jiang, S.; Fang, H.; Zhang, T.; Su, M.; Xu, L.; Zhang, Z.; Chen, X. Nitrogen Affects Anthocyanin Biosynthesis by Regulating MdLOB52 Downstream of MdARF19 in Callus Cultures of Red-Fleshed Apple (Malus sieversii f. niedzwetzkyana). J. Plant Growth Regul. 2018, 37, 719–729. [Google Scholar] [CrossRef]
- Ruiz, A.; Sanhueza, M.; Gómez, F.; Tereucán, G.; Valenzuela, T.; García, S.; Cornejo, P.; Hermosín Gutiérrez, I. Changes in the content of anthocyanins, flavonols, and antioxidant activity in Fragaria ananassa var. Camarosa fruits under traditional and organic fertilization. J. Sci. Food Agr. 2019, 99, 2404–2410. [Google Scholar]
- Li, H.; He, K.; Zhang, Z.; Hu, Y. Molecular mechanism of phosphorous signaling inducing anthocyanin accumulation in Arabidopsis. Plant Physiol. Biochem. 2023, 196, 121–129. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Wang, B.; Li, H.; Zhang, J.; Ma, Y.; Dai, H.; Wang, Y.; Zhang, Z. CRISPR/Cas9 targeted knockout FvPHO2 can increase phosphorus content and improve fruit quality of woodland strawberry. Sci. Hortic. 2023, 317, 112078. [Google Scholar] [CrossRef]
- An, J.P.; Li, H.L.; Liu, Z.Y.; Wang, D.R.; You, C.X.; Han, Y. The E3 ubiquitin ligase SINA1 and the protein kinase BIN2 cooperatively regulate PHR1 in apple anthocyanin biosynthesis. J. Integr. Plant Biol. 2023, 65, 2175–2193. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhao, X.; Xiao, Q.; Hu, W.; Wang, P.; Luo, Y.; Xia, H.; Lin, L.; Lv, X.; Liang, D.; et al. Identification of Key Genes Induced by Different Potassium Levels Provides Insight into the Formation of Fruit Quality in Grapes. Int. J. Mol. Sci. 2023, 24, 1218. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Song, Z.; Yu, B.; Li, Q.; Wang, D. Analysis of rabbiteye blueberry metabolomes and transcriptomes reveals mechanisms underlying potassium-induced anthocyanin production. Sci. Rep. 2025, 15, 757. [Google Scholar] [CrossRef] [PubMed]
- Fiaz, M.; Wang, C.; Zia Ul Haq, M.; Haider, M.S.; Zheng, T.; Mengqing, G.; Jia, H.; Jiu, S.; Fang, J. Molecular Evaluation of Kyoho Grape Leaf and Berry Characteristics Influenced by Different NPK Fertilizers. Plants 2021, 10, 1578. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; An, X.; Song, Z.; Zhu, Y.; Tan, Y.; Guo, X.; Wang, D. Effects of nitrogen, phosphorus and potassium formula fertilization on the yield and berry quality of blueberry. PLoS ONE 2023, 18, e283137. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, H.; Xue, L.; Nie, N.; Zhang, H.; Zhao, N.; He, S.; Liu, Q.; Gao, S.; Zhai, H. IbMYC2 Contributes to Salt and Drought Stress Tolerance via Modulating Anthocyanin Accumulation and ROS-Scavenging System in Sweet Potato. Int. J. Mol. Sci. 2024, 25, 2096. [Google Scholar] [CrossRef]
- An, J.P.; Zhang, X.W.; Bi, S.Q.; You, C.X.; Wang, X.F.; Hao, Y.J. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple. Plant J. 2020, 101, 573–589. [Google Scholar] [CrossRef]
- Wang, D.; Yang, K.; Wang, X.; Lin, X.; Rui, L.; Liu, H.; Liu, D.; You, C. Overexpression of MdZAT5, an C2H2-Type Zinc Finger Protein, Regulates Anthocyanin Accumulation and Salt Stress Response in Apple Calli and Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1897. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Chang, H.; Zhou, J.; Luo, Y.; Zhang, K.; Wang, B. Sweet cherry fruit miRNAs and effect of high CO2 on the profile associated with ripening. Planta 2019, 249, 1799–1810. [Google Scholar] [CrossRef]
- Liu, X.; Pei, Y.; Wang, C.; Zhu, D.; Cheng, F. Hydrogen sulfide, regulated by VvWRKY30, promotes berry color changes in grapevine cabernet sauvignon. Sci. Hortic. 2023, 309, 111605. [Google Scholar] [CrossRef]
- Oh, H.D.; Yu, D.J.; Chung, S.W.; Chea, S.; Lee, H.J. Abscisic acid stimulates anthocyanin accumulation in ‘Jersey’ highbush blueberry fruits during ripening. Food Chem. 2018, 244, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.; Roberto, S.R.; Ahmed, S.; Colombo, R.C.; Silvestre, J.P.; Koyama, R.; de Souza, R.T. Anthocyanin Accumulation and Color Development of ‘Benitaka’ Table Grape Subjected to Exogenous Abscisic Acid Application at Different Timings of Ripening. Agronomy 2019, 9, 164. [Google Scholar] [CrossRef]
- Olmedo, P.; Núñez-Lillo, G.; Ponce, E.; Alvaro, J.E.; Baños, J.; Carrera, E.; González-Fernández, J.J.; Hormaza, J.I.; Campos, D.; Chirinos, R.; et al. Metabolite profiling and hormone analysis of the synchronized exocarp-mesocarp development during ripening of cv. ‘Fuerte’ and ‘Hass’ avocado fruits. Food Biosci. 2024, 60, 104454. [Google Scholar] [CrossRef]
- Acevedo, O.; Ponce, C.; Arellano, M.; Multari, S.; Carrera, E.; Donoso, J.M.; Martens, S.; Kuhn, N.; Meisel, L.A. ABA Biosynthesis- and Signaling-Related Gene Expression Differences between Sweet Cherry Fruits Suggest Attenuation of ABA Pathway in Bicolored Cultivars. Plants 2023, 12, 2493. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, K.; Liu, L.; Zhang, K.; Yuan, H.; Liao, X.; Wang, Q.; Guo, X.; Li, F.; Li, T. A Role for PacMYBA in ABA-Regulated Anthocyanin Biosynthesis in Red-Colored Sweet Cherry cv. Hong Deng (Prunus avium L.). Plant Cell Physiol. 2014, 55, 862–880. [Google Scholar] [CrossRef]
- Li, G.; Zhao, J.; Qin, B.; Yin, Y.; An, W.; Mu, Z.; Cao, Y. ABA mediates development-dependent anthocyanin biosynthesis and fruit coloration in Lycium plants. BMC Plant Biol. 2019, 19, 317. [Google Scholar] [CrossRef]
- Chai, Y.; Jia, H.; Li, C.; Dong, Q.; Shen, Y. FaPYR1 is involved in strawberry fruit ripening. J. Exp. Bot. 2011, 62, 5079–5089. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Q.; Li, J.; Chen, Y.; Luo, M.; Li, H.; Wang, J.; Wu, Y.; Duan, S.; Wang, L.; et al. Characterization of the ABA Receptor VlPYL1 That Regulates Anthocyanin Accumulation in Grape Berry Skin. Front. Plant Sci. 2018, 9, 592. [Google Scholar] [CrossRef]
- Kondo, S.; Kunugi, K.; Saito, T.; Ohkawa, K.; Takeuchi, J.; Todoroki, Y.; Khewkhom, N.; Srilaong, V.; Phlaetita, W.; Setha, S. Inhibitors of abscisic acid synthesis or signaling affect anthocyanin synthesis and photoreceptors in grape berries. Sci. Hortic. 2024, 338, 113623. [Google Scholar] [CrossRef]
- Ali, A.; Pardo, J.M.; Yun, D. Desensitization of ABA-Signaling: The Swing From Activation to Degradation. Front. Plant Sci. 2020, 11, 379. [Google Scholar] [CrossRef]
- Hu, B.; Lai, B.; Wang, D.; Li, J.; Chen, L.; Qin, Y.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. Three LcABFs are Involved in the Regulation of Chlorophyll Degradation and Anthocyanin Biosynthesis During Fruit Ripening in Litchi chinensis. Plant Cell Physiol. 2019, 60, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, X.; Ma, S.; Fan, S.; Chi, F.; Song, Y. Molecular mechanism of abscisic acid signaling response factor VcbZIP55 to promote anthocyanin biosynthesis in blueberry (Vaccinium corymbosum). Plant Physiol. Biochem. 2024, 210, 108611. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Du, B.; Xiao, Y.; Zhang, X.; Turupu, M.; Yao, Q.; Wang, X.; Yan, Y.; Li, T. bZIP Transcription Factor PavbZIP6 Regulates Anthocyanin Accumulation by Increasing Abscisic Acid in Sweet Cherry. Int. J. Mol. Sci. 2024, 25, 10207. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, X.; Liu, Y.; Wang, X.; You, C.; Hao, Y. ABI5 regulates ABA-induced anthocyanin biosynthesis by modulating the MYB1-bHLH3 complex in apple. J. Exp. Bot. 2021, 72, 1460–1472. [Google Scholar] [CrossRef]
- Liu, W.; Mei, Z.; Yu, L.; Gu, T.; Li, Z.; Zou, Q.; Zhang, S.; Fang, H.; Wang, Y.; Zhang, Z.; et al. The ABA-induced NAC transcription factor MdNAC1 interacts with a bZIP-type transcription factor to promote anthocyanin synthesis in red-fleshed apples. Hortic. Res. 2023, 10, uhad049. [Google Scholar] [CrossRef]
- Lu, W.; Wei, X.; Han, X.; Chen, R.; Xiao, C.; Zheng, X.; Mao, L. Participation of FaTRAB1 Transcription Factor in the Regulation of FaMADS1 Involved in ABA-Dependent Ripening of Strawberry Fruit. Foods 2023, 12, 1802. [Google Scholar] [CrossRef]
- Chen, R.; Mao, L.; Guan, W.; Wei, X.; Huang, Z.; Wu, Y. ABA-mediated miR5290 promotes anthocyanin biosynthesis by inhibiting the expression of FaMADS1 in postharvest strawberry fruit. Postharvest Biol. Technol. 2022, 189, 111934. [Google Scholar] [CrossRef]
- Wang, F.; Sha, J.; Chen, Q.; Xu, X.; Zhu, Z.; Ge, S.; Jiang, Y. Exogenous Abscisic Acid Regulates Distribution of 13C and 15N and Anthocyanin Synthesis in ‘Red Fuji’ Apple Fruit Under High Nitrogen Supply. Front. Plant Sci. 2020, 10, 1738. [Google Scholar] [CrossRef]
- An, J.; Wang, X.; Li, Y.; Song, L.; Zhao, L.; You, C.; Hao, Y. EIN3-LIKE1, MYB1, and ETHYLENE RESPONSE FACTOR3 Act in a Regulatory Loop That Synergistically Modulates Ethylene Biosynthesis and Anthocyanin Accumulation. Plant Physiol. 2018, 178, 808–823. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, Y.; Yang, X.; Wei, C.; Guan, J. Ethylene Signal Is Involved in the Regulation of Anthocyanin Accumulation in Flesh of Postharvest Plums (Prunus salicina Lindl.). Plants 2023, 12, 893. [Google Scholar] [CrossRef]
- Ni, J.; Premathilake, A.T.; Gao, Y.; Yu, W.; Tao, R.; Teng, Y.; Bai, S. Ethylene-activated PpERF105 induces the expression of the repressor-type R2R3-MYB gene PpMYB140 to inhibit anthocyanin biosynthesis in red pear fruit. Plant J. 2021, 105, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene Biosynthesis and Signaling Networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yu, A.; Ji, X.; Mu, Q.; Salman Haider, M.; Wei, R.; Leng, X.; Fang, J. Transcriptome and metabolite integrated analysis reveals that exogenous ethylene controls berry ripening processes in grapevine. Food Res. Int. 2022, 155, 111084. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Zhai, L.; Cui, Y.; Tang, G.; Huo, J.; Li, X.; Bian, S. The miR156/SPL12 module orchestrates fruit colour change through directly regulating ethylene production pathway in blueberry. Plant Biotechnol. J. 2024, 22, 386–400. [Google Scholar] [CrossRef]
- Piya, S.; Binder, B.M.; Hewezi, T. Canonical and noncanonical ethylene signaling pathways that regulate Arabidopsis susceptibility to the cystnematode Heterodera schachtii. New Phytol. 2019, 221, 946–959. [Google Scholar] [CrossRef]
- Sun, H.; Hu, K.; Wei, S.; Yao, G.; Zhang, H. ETHYLENE RESPONSE FACTORS 4.1/4.2 with an EAR motif repress anthocyanin biosynthesis in red-skinned pears. Plant Physiol. 2023, 192, 1892–1912. [Google Scholar] [CrossRef]
- Li, J.; Ma, N.; An, Y.; Wang, L. FcMADS9 of fig regulates anthocyanin biosynthesis. Sci. Hortic. 2021, 278, 109820. [Google Scholar] [CrossRef]
- De Santis, D.; Bellincontro, A.; Forniti, R.; Botondi, R. Time of Postharvest Ethylene Treatments Affects Phenols, Anthocyanins, and Volatile Compounds of Cesanese Red Wine Grape. Foods 2021, 10, 322. [Google Scholar] [CrossRef]
- Costa, D.V.; Almeida, D.P.; Pintado, M. Effect of postharvest application of ethylene on the profile of phenolic acids and anthocyanins in three blueberry cultivars (Vaccinium corymbosum). J. Sci. Food Agr. 2018, 98, 5052–5061. [Google Scholar] [CrossRef]
- Reis, L.; Forney, C.F.; Jordan, M.; Munro Pennell, K.; Fillmore, S.; Schemberger, M.O.; Ayub, R.A. Metabolic Profile of Strawberry Fruit Ripened on the Plant Following Treatment With an Ethylene Elicitor or Inhibitor. Front. Plant Sci. 2020, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wen, X.; Tang, L. Effect of methyl jasmonic acid on peach fruit ripening progress. Sci. Hortic. 2017, 220, 206–213. [Google Scholar] [CrossRef]
- Tang, T.; Zhou, H.; Wang, L.; Zhao, J.; Ma, L.; Ling, J.; Li, G.; Huang, W.; Li, P.; Zhang, Y. Post-harvest Application of Methyl Jasmonate or Prohydrojasmon Affects Color Development and Anthocyanins Biosynthesis in Peach by Regulation of Sucrose Metabolism. Front. Nutr. 2022, 9, 871467. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Song, S.; Ren, Q.; Wu, D.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.; Xie, D. The Jasmonate-ZIM-Domain Proteins Interact with the WD-Repeat/bHLH/MYB Complexes to Regulate Jasmonate-Mediated Anthocyanin Accumulation and Trichome Initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814. [Google Scholar] [CrossRef]
- Delgado, L.; Zúñiga, P.; Figueroa, N.; Pastene, E.; Escobar-Sepúlveda, H.; Figueroa, P.; Garrido-Bigotes, A.; Figueroa, C. Application of a JA-Ile Biosynthesis Inhibitor to Methyl Jasmonate-Treated Strawberry Fruit Induces Upregulation of Specific MBW Complex-Related Genes and Accumulation of Proanthocyanidins. Molecules 2018, 23, 1433. [Google Scholar] [CrossRef]
- An, X.; Tian, Y.; Chen, K.; Liu, X.; Liu, D.; Xie, X.; Cheng, C.; Cong, P.; Hao, Y. MdMYB9 and MdMYB11 are Involved in the Regulation of the JA-Induced Biosynthesis of Anthocyanin and Proanthocyanidin in Apples. Plant Cell Physiol. 2015, 56, 650–660. [Google Scholar] [CrossRef]
- An, J.P.; Xu, R.R.; Liu, X.; Zhang, J.C.; Wang, X.F.; You, C.X.; Hao, Y.J. Jasmonate induces biosynthesis of anthocyanin and proanthocyanidin in apple by mediating the JAZ1-TRB1-MYB9 complex. Plant J. 2021, 106, 1414–1430. [Google Scholar] [CrossRef]
- Su, Z.; Wang, X.; Xuan, X.; Sheng, Z.; Jia, H.; Emal, N.; Liu, Z.; Zheng, T.; Wang, C.; Fang, J. Characterization and Action Mechanism Analysis of VvmiR156b/c/d-VvSPL9 Module Responding to Multiple-Hormone Signals in the Modulation of Grape Berry Color Formation. Foods 2021, 10, 896. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, Å.; Qian, M.; Li, J.; Li, Y.; Shen, J.; et al. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. J. 2020, 18, 1223–1240. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Wang, N.; Jiang, S.; Fang, H.; Zhang, Z.; Yang, G.; Wang, Y.; Su, M.; Xu, L.; et al. The ethylene response factor MdERF1B regulates anthocyanin and proanthocyanidin biosynthesis in apple. Plant Mol. Biol. 2018, 98, 205–218. [Google Scholar] [CrossRef]
- Qu, S.; Li, M.; Wang, G.; Zhu, S. Application of ABA and GA3 alleviated browning of litchi (Litchi chinensis Sonn.) via different strategies. Postharvest Biol. Technol. 2021, 181, 111672. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kamiya, Y. Gibberellin biosynthesis: Its regulation by endogenous and environmental signals. Plant Cell Physiol. 2000, 41, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Wang, Z.; Yang, C.; Lin-Wang, K.; Espley, R.; Liu, J.; Li, X.; Wu, Z.; Li, P.; Guan, Q.; et al. PbGA2ox8 induces vascular-related anthocyanin accumulation and contributes to red stripe formation on pear fruit. Hortic. Res. 2019, 6, 131–137. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Han, Y.; An, J. The RGL2a-TCP46-MYB1 module regulates GA-mediated anthocyanin biosynthesis in apple. Fruit Res. 2023, 3, 21. [Google Scholar] [CrossRef]
- An, J.; Zhang, X.; Li, H.; Wang, D.; You, C.; Han, Y. The E3 ubiquitin ligases SINA1 and SINA2 integrate with the protein kinase CIPK20 to regulate the stability of RGL2a, a positive regulator of anthocyanin biosynthesis. New Phytol. 2023, 239, 1332–1352. [Google Scholar] [CrossRef]
- An, J.P.; Xu, R.R.; Wang, X.N.; Zhang, X.W.; You, C.X.; Han, Y. MdbHLH162 connects the gibberellin and jasmonic acid signals to regulate anthocyanin biosynthesis in apple. J. Integr. Plant Biol. 2024, 66, 265–284. [Google Scholar] [CrossRef]
- Ji, X.; Zhao, L.; Liu, B.; Yuan, Y.; Han, Y.; You, C.; An, J. MdZFP7 integrates JA and GA signals via interaction with MdJAZ2 and MdRGL3a in regulating anthocyanin biosynthesis and undergoes degradation by the E3 ubiquitin ligase MdBRG3. J. Integr. Plant Biol. 2025, 67, 1339–1363. [Google Scholar] [CrossRef]
- Li, M.; Cheng, S.; Wang, Y.; Dong, Y. Improving Fruit Coloration, Quality Attributes, and Phenolics Content in ‘Rainier’ and ‘Bing’ Cherries by Gibberellic Acid Combined with Homobrassinolide. J. Plant Growth Regul. 2020, 39, 1130–1139. [Google Scholar] [CrossRef]
- Moro, L.; Hassimotto, N.M.A.; Purgatto, E. Postharvest Auxin and Methyl Jasmonate Effect on Anthocyanin Biosynthesis in Red Raspberry (Rubus idaeus L.). J. Plant Growth Regul. 2017, 36, 773–782. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, R.; Wang, N.; Yang, L.; Chen, X. Transcriptome profiling reveals auxin suppressed anthocyanin biosynthesis in red-fleshed apple callus (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. (Pctoc) 2015, 123, 389–404. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.; Xu, H.; Jiang, S.; Fang, H.; Su, M.; Zhang, Z.; Zhang, T.; Chen, X. Auxin regulates anthocyanin biosynthesis through the Aux/IAA-ARF signaling pathway in apple. Hortic. Res. 2018, 5, 1038. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Liu, Z.Y.; Wang, X.N.; Han, Y.; You, C.X.; An, J.P. E3 ubiquitin ligases SINA4 and SINA11 regulate anthocyanin biosynthesis by targeting the IAA29-ARF5-1-ERF3 module in apple. Plant Cell Environ. 2023, 46, 3902–3918. [Google Scholar] [CrossRef] [PubMed]
- Clayton-Cuch, D.; Yu, L.; Shirley, N.; Bradley, D.; Bulone, V.; Böttcher, C. Auxin Treatment Enhances Anthocyanin Production in the Non-Climacteric Sweet Cherry (Prunus avium L.). Int. J. Mol. Sci. 2021, 22, 10760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Wang, N.; Xu, H.; Qu, C.; Jiang, S.; Fang, H.; Su, M.; Zhang, Z.; Chen, X. MdMYBL2 helps regulate cytokinin-induced anthocyanin biosynthesis in red-fleshed apple (Malus sieversii f. niedzwetzkyana) callus. Funct. Plant Biol. 2019, 46, 187–196. [Google Scholar] [CrossRef]
- An, J.; Song, L.; Zhao, L.; You, C.; Wang, X.; Hao, Y. Effects of Overexpression of Apple Cytokinin Response Factor Gene MdCRF on Anthocyanins Accumulation and Salt Stress Tolerance. Sci. Agric. Sin. 2017, 50, 3196–3204. [Google Scholar]
- Ni, J.; Bai, S.; Gao, L.; Qian, M.; Zhong, L.; Teng, Y. Identification, classification, and transcription profiles of the B-type response regulator family in pear. PLoS ONE 2017, 12, e171523. [Google Scholar] [CrossRef]
- Rajput, R.; Tyagi, S.; Anchal, K.; Singh, S.; Laxmi, A.; Misra, P.; Pandey, A. Type-B response regulators MaRR_B9 and MaRR_B12 coordinate cytokinin-mediated negative regulation of anthocyanin biosynthesis in banana fruits. bioRxiv 2024, 2024–2028. [Google Scholar]
- Ji, X.; Wang, Y.; Zhang, R.; Wu, S.; An, M.; Li, M.; Wang, C.; Chen, X.; Zhang, Y.; Chen, X. Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. (Pctoc) 2015, 120, 325–337. [Google Scholar] [CrossRef]
- Hu, B.; Li, J.; Wang, D.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. Transcriptome profiling of Litchi chinensis pericarp in response to exogenous cytokinins and abscisic acid. Plant Growth Regul. 2018, 84, 437–450. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, B.; Bai, J.; Qian, M.; Shu, Q.; Su, J.; Teng, Y. Effects of high temperatures on UV-B/visible irradiation induced postharvest anthocyanin accumulation in ‘Yunhongli No.1‘ (Pyrus pyrifolia Nakai) pears. Sci. Hortic. 2012, 134, 53–59. [Google Scholar] [CrossRef]
- Fang, H.; Dong, Y.; Yue, X.; Chen, X.; He, N.; Hu, J.; Jiang, S.; Xu, H.; Wang, Y.; Su, M.; et al. MdCOL4 Interaction Mediates Crosstalk Between UV-B and High Temperature to Control Fruit Coloration in Apple. Plant Cell Physiol. 2019, 60, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Saito, T.; Honda, C.; Hatsuyama, Y.; Ito, A.; Moriguchi, T. An apple B-box protein, MdCOL11, is involved in UV-B- and temperature-induced anthocyanin biosynthesis. Planta 2014, 240, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Rodyoung, A.; Masuda, Y.; Tomiyama, H.; Saito, T.; Okawa, K.; Ohara, H.; Kondo, S. Effects of light emitting diode irradiation at night on abscisic acid metabolism and anthocyanin synthesis in grapes in different growing seasons. Plant Growth Regul. 2016, 79, 39–46. [Google Scholar] [CrossRef]
- Honda, C.; Iwanami, H.; Yoshimura, K. Effect of blue LED light irradiation on anthocyanin synthesis in the skin of detached apples. Int. Soc. Hortic. Sci. (ISHS) Leuven Belg. 2021, 1312, 235–242. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, X.; Niu, J.; Ma, F.; Li, P. Visible light regulates anthocyanin synthesis via malate dehydrogenases and the ethylene signaling pathway in plum (Prunus salicina L.). Physiol. Plant. 2021, 172, 1739–1749. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, K.; Sun, W.; Yang, T.; Wu, T.; Song, T.; Zhang, J.; Yao, Y.; Tian, J. A long noncoding RNA functions in high-light-induced anthocyanin accumulation in apple by activating ethylene synthesis. Plant Physiol. 2022, 189, 66–83. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Sun, Y.; Zhang, X.; Du, B.; Turupu, M.; Yao, Q.; Gai, S.; Tong, S.; Huang, J.; et al. Two B-box proteins, PavBBX6/9, positively regulate light-induced anthocyanin accumulation in sweet cherry. Plant Physiol. 2023, 192, 2030–2048. [Google Scholar] [CrossRef]
- Kadomura-Ishikawa, Y.; Miyawaki, K.; Takahashi, A.; Masuda, T.; Noji, S. Light and abscisic acid independently regulated FaMYB10 in Fragaria × ananassa fruit. Planta 2015, 241, 953–965. [Google Scholar] [CrossRef]
- Tan, Y.; Wen, B.; Xu, L.; Zong, X.; Sun, Y.; Wei, G.; Wei, H. High temperature inhibited the accumulation of anthocyanin by promoting ABA catabolism in sweet cherry fruits. Front. Plant Sci. 2023, 14, 1079292. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, W.; Yao, H.; Ali, Z.; Xiao, M.; Ma, Z.; Li, J.; Zhou, W.; Cui, J.; et al. VvFHY3 links auxin and endoplasmic reticulum stress to regulate grape anthocyanin biosynthesis at high temperatures. Plant Cell 2025, 37, koae303. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Li, C.; Wang, P.; Ren, Y.; Chen, J.; Liu, G.; Hakeem, A.; Liu, Y.; Shi, X.; Hou, T.; et al. The transcription factor VvMYB44-1 plays a role in reducing grapevine anthocyanin biosynthesis at high temperature. Plant Physiol. 2025, 197, kiae657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Liu, R.; Hao, H.; Bi, Y. Gibberellins negatively regulate low temperature-induced anthocyanin accumulation in a HY5/HYH-dependent manner. Plant Signal. Behav. 2011, 6, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sang, W.; Xu, R.; Cao, J. Alteration of flesh color and enhancement of bioactive substances via the stimulation of anthocyanin biosynthesis in ‘Friar’ plum fruit by low temperature and the removal. Food Chem. 2020, 310, 125862. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Y.; Wang, L.; Zhao, Z.; Cao, J.; Fu, D.; Jiang, W. PsERF1B-PsMYB10.1-PsbHLH3 module enhances anthocyanin biosynthesis in the flesh-reddening of amber-fleshed plum (cv. Friar) fruit in response to cold storage. Hortic. Res. 2023, 10, uhad91. [Google Scholar] [CrossRef]
- Chen, J.; Liu, F.; Ismail, B.B.; Wang, W.; Xu, E.; Pan, H.; Ye, X.; Liu, D.; Cheng, H. Effects of ethephon and low-temperature treatments on blood oranges (Citrus sinensis L. Osbeck): Anthocyanin accumulation and volatile profile changes during storage. Food Chem. 2022, 393, 133381. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Shang, H.; Meng, X. Effect of methyl jasmonate on the anthocyanin content and antioxidant activity of blueberries during cold storage. J. Sci. Food Agr. 2015, 95, 337–343. [Google Scholar] [CrossRef]
- Darwish, O.S.; Ali, M.R.; Khojah, E.; Samra, B.N.; Ramadan, K.M.A.; El-Mogy, M.M. Pre-Harvest Application of Salicylic Acid, Abscisic Acid, and Methyl Jasmonate Conserve Bioactive Compounds of Strawberry Fruits during Refrigerated Storage. Horticulturae 2021, 7, 568. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, M.; Feng, M.; Liu, G.; Torregrosa, L.; Tao, X.; Ren, R.; Fang, Y.; Zhang, Z.; Meng, J.; et al. miR156b-targeted VvSBP8/13 functions downstream of the abscisic acid signal to regulate anthocyanins biosynthesis in grapevine fruit under drought. Hortic. Res. 2024, 11, uhad29. [Google Scholar] [CrossRef]
- Hussein, A.S.; Ibrahim, R.A.; Eissa, M.A. Exogenous Pre-harvest Application of Abscisic and Jasmonic Acids Improves Fruit Quality by Enhancing Sugar Synthesis and Reducing Acidity in Pomegranate (Punica granatum L. cv. Wonderful). J. Soil Sci. Plant Nutr. 2023, 23, 2237–2246. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, L.; Chen, J.; Bao, X.; Zhang, D.; Liu, J.; Wang, W.; Wei, Y.; Zong, C. Environmental and Phytohormonal Factors Regulating Anthocyanin Biosynthesis in Fruits. Horticulturae 2025, 11, 681. https://doi.org/10.3390/horticulturae11060681
Kuang L, Chen J, Bao X, Zhang D, Liu J, Wang W, Wei Y, Zong C. Environmental and Phytohormonal Factors Regulating Anthocyanin Biosynthesis in Fruits. Horticulturae. 2025; 11(6):681. https://doi.org/10.3390/horticulturae11060681
Chicago/Turabian StyleKuang, Luodan, Jiazhuo Chen, Xiaoyu Bao, Dong Zhang, Jiaru Liu, Wei Wang, Yi Wei, and Chengwen Zong. 2025. "Environmental and Phytohormonal Factors Regulating Anthocyanin Biosynthesis in Fruits" Horticulturae 11, no. 6: 681. https://doi.org/10.3390/horticulturae11060681
APA StyleKuang, L., Chen, J., Bao, X., Zhang, D., Liu, J., Wang, W., Wei, Y., & Zong, C. (2025). Environmental and Phytohormonal Factors Regulating Anthocyanin Biosynthesis in Fruits. Horticulturae, 11(6), 681. https://doi.org/10.3390/horticulturae11060681






