Tissue-Specific Metabolic Changes During Postharvest Storage of Friariello Napoletano
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Reagents and Laboratory Materials
2.3. Soluble Sugars and Starch
2.4. Pigments, α-Tocopherol, Ascorbate, and Glucosinolates Determination
2.5. Soluble Proteins, Organic Acids, and Free Amino Acids
2.6. Statistical Analysis and Data Visualization
3. Results
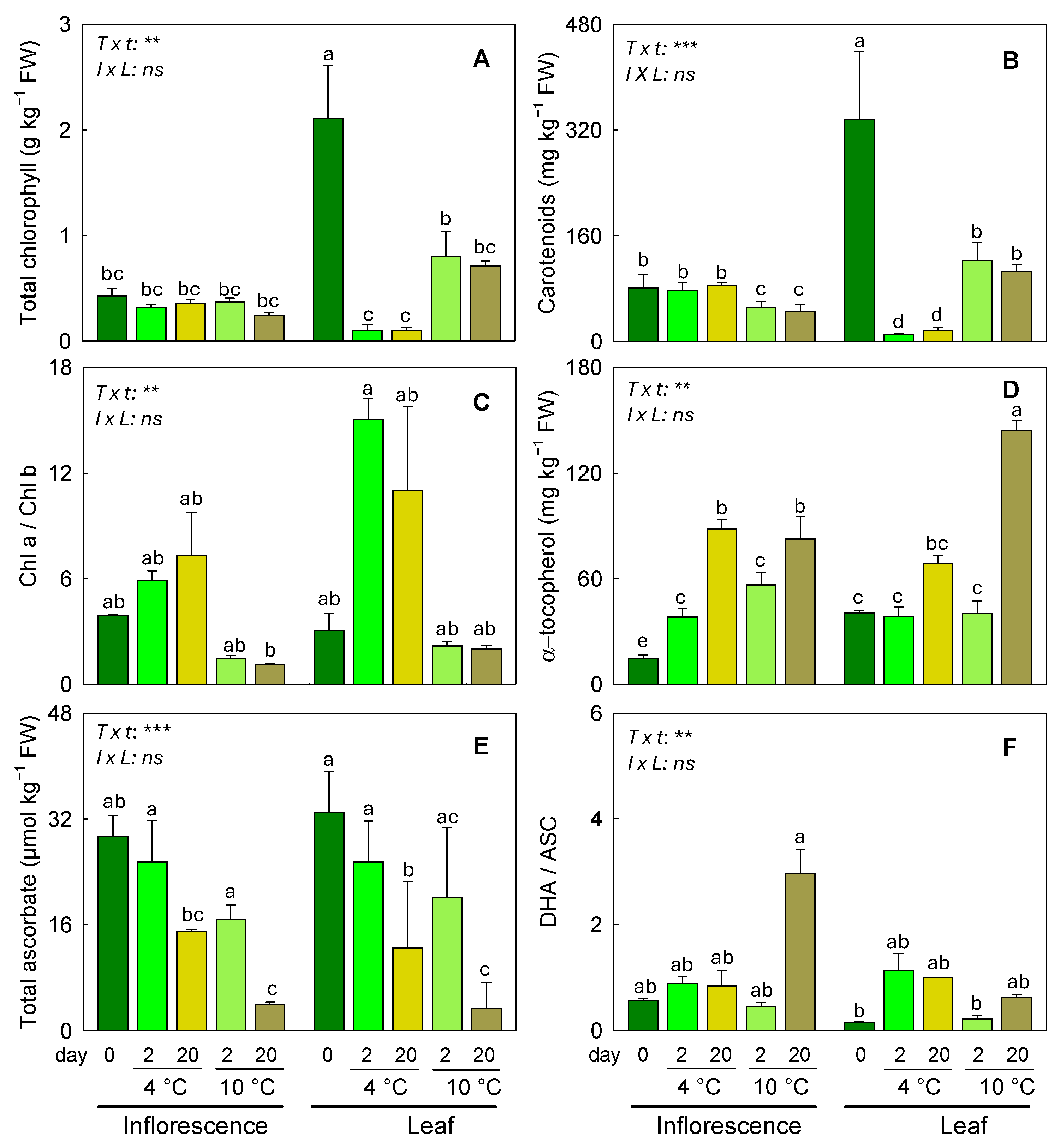
3.1. Responses of Pigments, α-Tocopherol, and Ascorbate to Different Storage Conditions
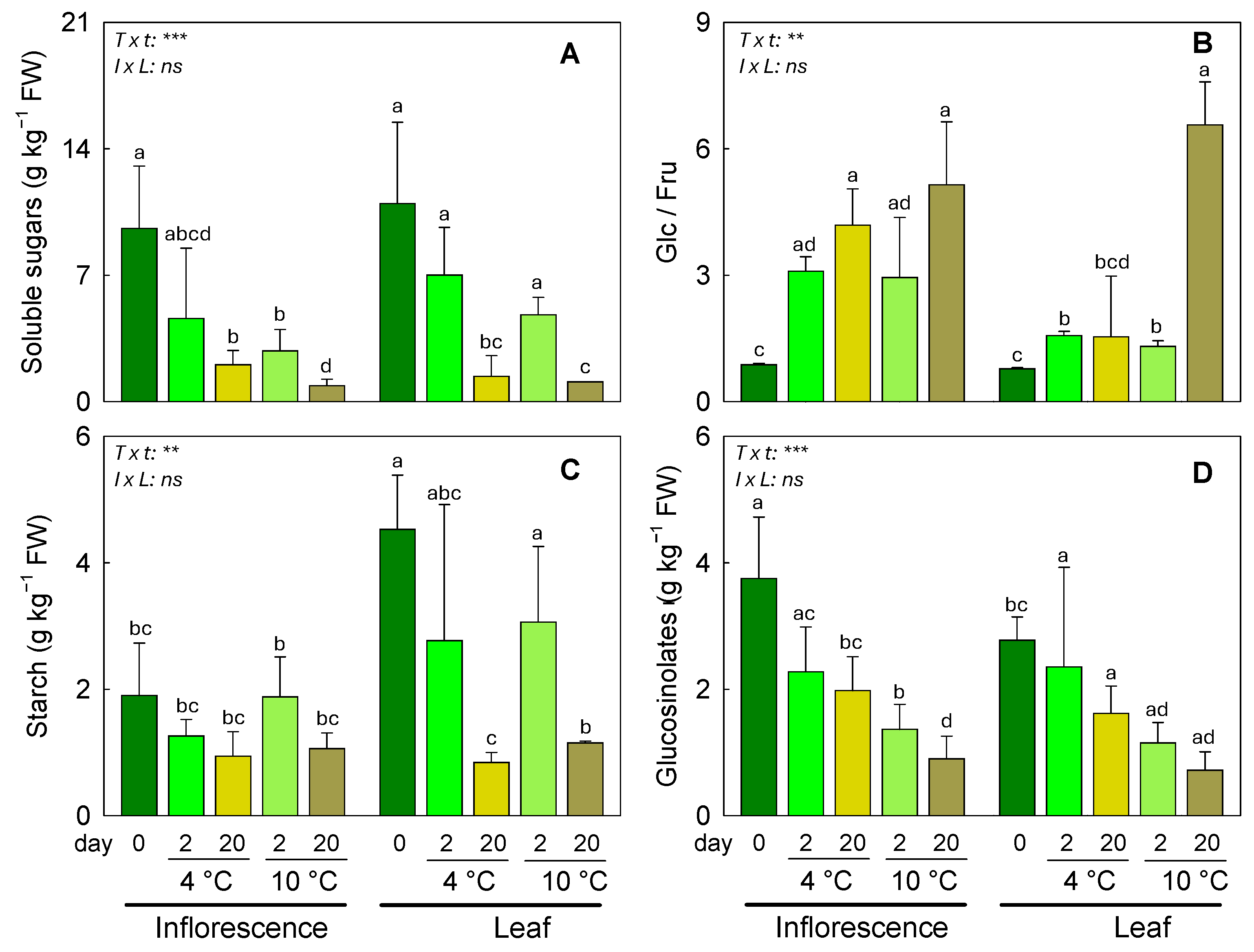
3.2. Responses of Soluble Sugars, Starch, and Glucosinolates to Different Postharvest Conditions
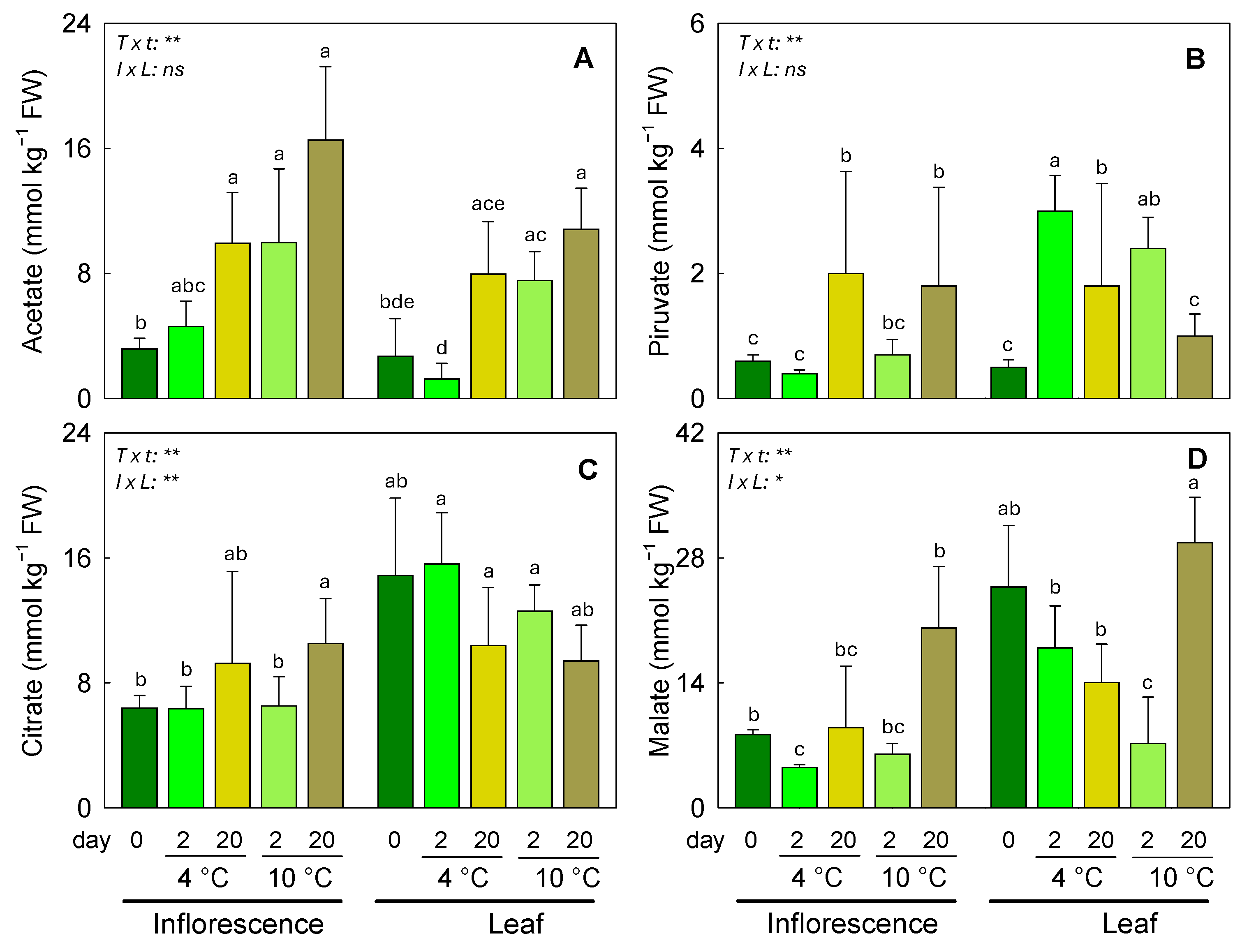
3.3. Responses of Organic Acids and Inorganic Anions to Different Postharvest Conditions
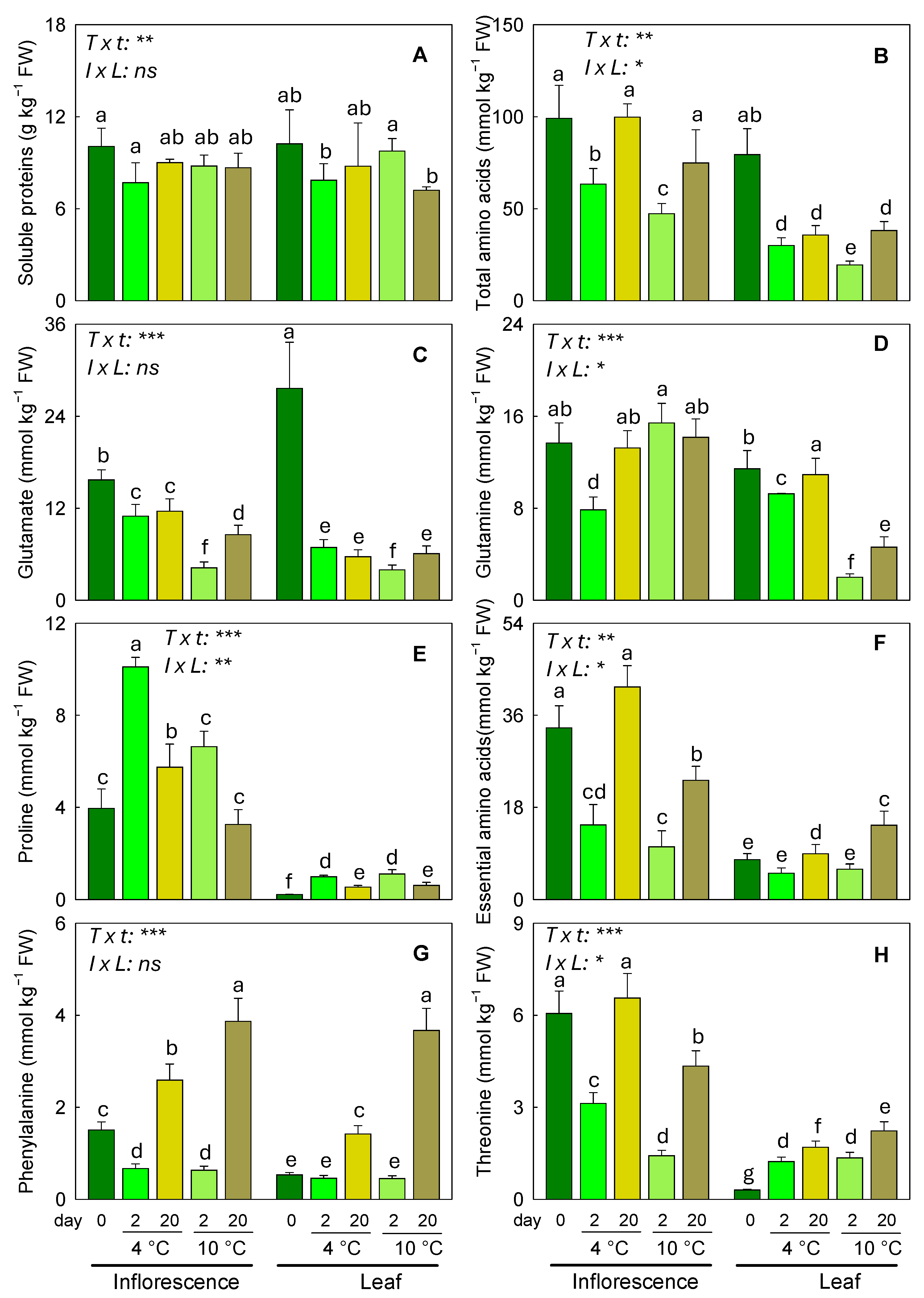
3.4. Protein and Amino Acid Response to Different Storage Conditions
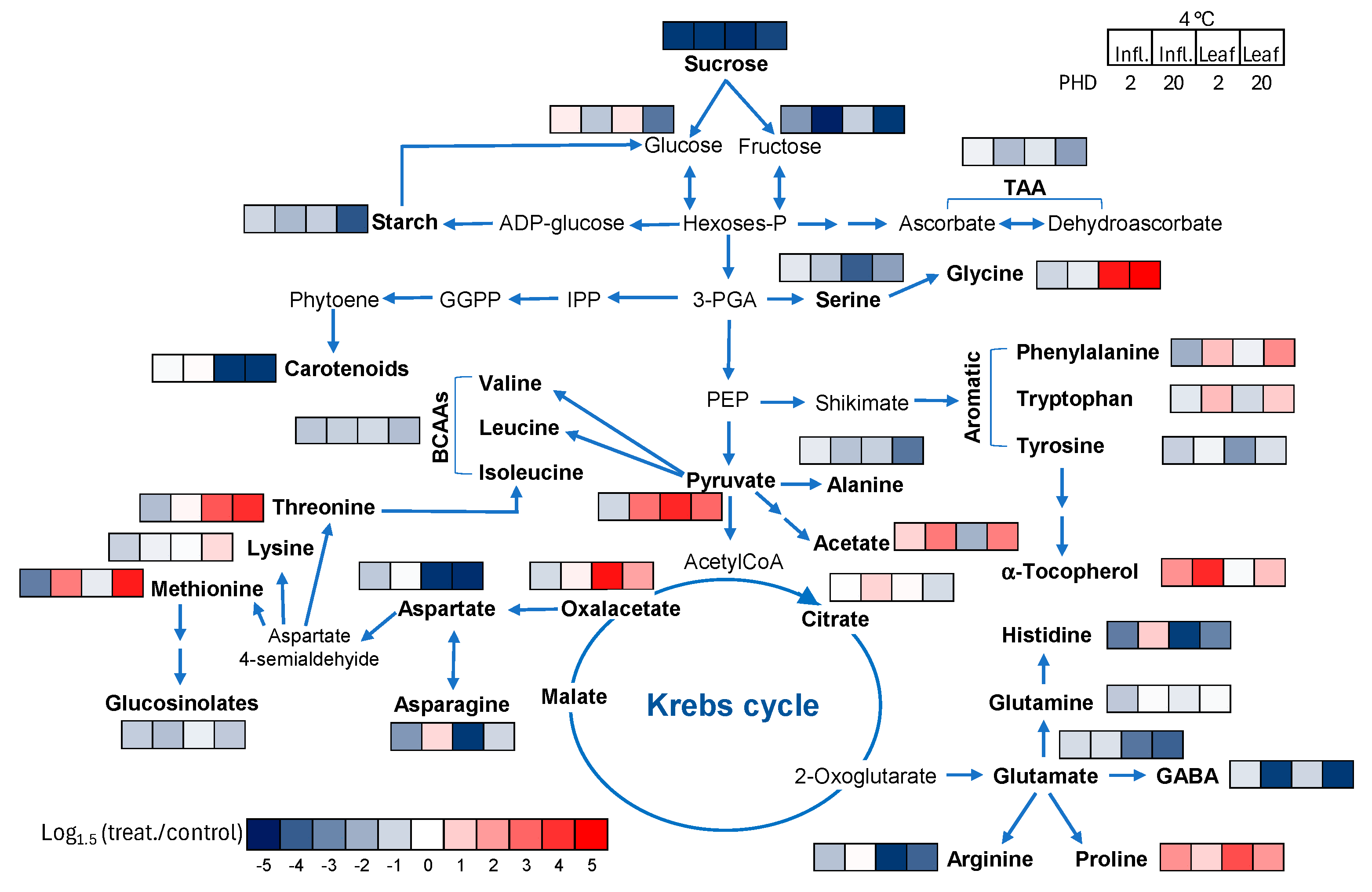
3.5. Heatmap Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbieri, G.; Pernice, R.; Maggio, A.; De Pascale, S.; Fogliano, V. Glucosinolates profile of Brassica rapa L. subsp. sylvestris L. Janch. var. esculenta Hort. Food Chem. 2008, 107, 1687–1691. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Pernice, R.; Fogliano, V.; Barbieri, G. Sulphur fertilization may improve the nutritional value of Brassica rapa L. subsp. sylvestris. Eur. J. Agron. 2007, 26, 418–424. [Google Scholar] [CrossRef]
- Conversa, G.; Bonasia, A.; Lazzizera, C.; Elia, A. Bio-physical, physiological, and nutritional aspects of ready-to-use cima di rapa (Brassica rapa L. subsp. sylvestris L. Janch. var. esculenta Hort.) as affected by conventional and organic growing systems and storage time. Sci. Hortic. 2016, 213, 76–86. [Google Scholar] [CrossRef]
- De Rosa, F. Il Broccolo di Rapa: Studio Orticolo; Tipi Ferrante: Naples, Italy, 1893; pp. 1–11, Extracted from: Riv. Agraria, 3(13); Available online: https://www.georgofili.it/biblioteca/scheda?id=36808&r=5768 (accessed on 1 June 2025).
- Angelini, F. Coltivazioni Erbacee. Volume 2: Piante da Organi Sotterranei, Piante Ortive, Piante da Foraggio, Piante Infestanti; SO.GRA.RO.: Rome, Italy, 1965; p. 831. Available online: https://opac.sbn.it/bid/PUV0005976 (accessed on 1 June 2025).
- Tesi, R. Principi di Orticoltura e Ortaggi d’Italia; Edagricole: Bologna, Italy, 1987; p. 340. ISBN 88-206-2629-2. [Google Scholar]
- Kole, C.; Wang, X. The Brassica Rapa Genome; Springer: Berlin, Germany, 2015; p. 169. Available online: https://link.springer.com/book/10.1007/978-3-662-47901-8 (accessed on 1 June 2025).
- ISTAT. Superfici e Produzioni Delle Principali Coltivazioni Agrarie e Floricole—Anno 2021; Italian National Institute of Statistics: Rome, Italy, 2021; Available online: https://www.istat.it/it/archivio/224146 (accessed on 19 January 2025).
- Mazzeo, R.; Morgese, A.; Sonnante, G.; Zuluaga, D.L.; Pavan, S.; Ricciardi, L.; Lotti, C. Genetic diversity in broccoli rabe (Brassica rapa L. subsp. sylvestris (L.) Janch.) from Southern Italy. Sci. Hortic. 2019, 253, 140–146. [Google Scholar] [CrossRef]
- Renna, M.; Rinaldi, V.A.; Gonnella, M. The Mediterranean diet between traditional foods and human health: The culinary example of Puglia (Southern Italy). Int. J. Gastron. Food Sci. 2015, 2, 63–71. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Rosa, E.A.S. Effect of post-harvest treatments on the level of glucosinolates in broccoli. J. Sci. Food Agric. 1999, 79, 1028–1032. [Google Scholar] [CrossRef]
- Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive compounds in Brassicaceae vegetables with a role in the prevention of chronic diseases. Molecules 2017, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Shezi, S.; Ngcobo, M.E.K.; Khanyile, N.; Ncama, K. Physio-metabolic mechanisms behind postharvest quality deterioration in broccoli (Brassica oleracea var. italica) and Swiss chard (Beta vulgaris L. var. cicla): A review. Plants 2024, 13, 3174. [Google Scholar] [CrossRef]
- Becker, T.M.; Juvik, J.A. The role of glucosinolate hydrolysis products from Brassica vegetable consumption in inducing antioxidant activity and reducing cancer incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; Moreno, D.A. Brassica foods as a dietary source of vitamin C: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1076–1091. [Google Scholar] [CrossRef]
- Providence Journal. Dark Leafy Rapini is a Nutritional All-Star. Available online: https://eu.providencejournal.com/story/lifestyle/food/recipes/2019/06/19/dark-leafy-rapini-is-nutritional-all-star/4879668007/ (accessed on 30 May 2025).
- Annunziata, M.G.; Attico, A.; Woodrow, P.; Oliva, M.A.; Fuggi, A.; Carillo, P. An improved fluorimetric HPLC method for quantifying tocopherols in Brassica rapa L. subsp. sylvestris after harvest. J. Food Compos. Anal. 2012, 27, 145–150. [Google Scholar] [CrossRef]
- Patil, M.; Sharma, S.; Sridhar, K.; Anurag, R.K.; Grover, K.; Dharni, K.; Mahajan, S.; Sharma, M. Effect of postharvest treatments and storage temperature on the physiological, nutritional, and shelf-life of broccoli (Brassica oleracea) microgreens. Sci. Hortic. 2024, 327, 112805. [Google Scholar] [CrossRef]
- de Chiara, M.L.V.; Cefola, M.; Pace, B.; Palumbo, M.; Amodio, M.L.; Colelli, G. Ready-to-use broccoli raab (Brassica rapa L. subsp. sylvestris) quality and volatilome as affected by packaging. Postharvest Biol. Technol. 2024, 213, 112961. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Ziane, N.; Salmieri, S.; Lacroix, M. Combined post-harvest treatments for improving quality and extending shelf-life of minimally processed broccoli florets (Brassica oleracea var. italica). Food Bioprocess Technol. 2018, 11, 84–95. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre-and post-harvest factors affecting glucosinolate content in broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef]
- Finger, F.L.; Endres, L.; Mosquim, P.R.; Puiatti, M. Physiological changes during postharvest senescence of broccoli. Pesqui. Agropecu. Bras. 1999, 34, 1455–1462. [Google Scholar] [CrossRef]
- Zdulski, J.A.; Rutkowski, K.P.; Konopacka, D. Strategies to extend the shelf life of fresh and minimally processed fruit and vegetables with edible coatings and modified atmosphere packaging. Appl. Sci. 2024, 14, 11074. [Google Scholar] [CrossRef]
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging postharvest technologies to enhance the shelf-life of fruit and vegetables: An overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef]
- Casajús, V.; Perini, M.; Ramos, R.; Lourenco, A.B.; Salinas, C.; Sánchez, E.; Fanello, D.; Civello, P.; Frezza, D.; Martínez, G. Harvesting at the end of the day extends postharvest life of kale (Brassica oleracea var. sabellica). Sci. Hortic. 2021, 276, 109757. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Liu, J.; Dong, X.; Zhang, H.; Jeong, B.R. Prolonged post-harvest preservation in lettuce (Lactuca sativa L.) by reducing water loss rate and chlorophyll degradation regulated through lighting direction-induced morphophysiological improvements. Plants 2024, 13, 2564. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Are You Storing Food Safely? Available online: https://www.fda.gov/consumers/consumer-updates/are-you-storing-food-safely (accessed on 28 May 2025).
- LG Electronics. Temperatura Frigorifero Ideale: Come Regolarla. Available online: https://www.lg.com/it/magazine/2021-02-temperatura-frigorifero-ideale-come-regolarla (accessed on 28 May 2025).
- Cantwell, M.; Suslow, T. Postharvest handling systems: Fresh-cut fruits and vegetables. In Postharvest Technology of Horticultural Crops; University of California: Davis, CA, USA, 2002; pp. 445–464. [Google Scholar]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Abdalla, N.A.; Taha, H.S.; Fári, M. Postharvest management of fruits and vegetables storage. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 15, pp. 65–152. [Google Scholar]
- Carillo, P.; Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; dell’Aversana, E.; D’Amelia, L.; Colla, G.; Caruso, G.; De Pascale, S.; Rouphael, Y. Sensory and functional quality characterization of protected designation of origin ‘Piennolo del Vesuvio’ cherry tomato landraces from Campania-Italy. Food Chem. 2019, 292, 166–175. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Carillo, P.; Fuggi, A.; Troccoli, A.; Woodrow, P. Metabolic profiling of cauliflower under traditional and reduced tillage systems. Aust. J. Crop Sci. 2013, 7, 1317–1323. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Vom Dorp, K.; Hölzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Hanson, A.D.; Dörmann, P. Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Clarkson, G.J.J.; Rothwell, S.D.; Fry, S.C. Novel insights into ascorbate retention and degradation during the washing and post-harvest storage of spinach and other salad leaves. Food Chem. 2017, 233, 237–246. [Google Scholar] [CrossRef]
- Watanabe, M.; Balazadeh, S.; Tohge, T.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.R.; Hoefgen, R. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiol. 2013, 162, 1290–1310. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Chrobok, D.; Law, S.R.; Brouwer, B.; Lindén, P.; Ziolkowska, A.; Liebsch, D.; Narsai, R.; Szal, B.; Moritz, T.; Rouhier, N.; et al. Dissecting the metabolic role of mitochondria during developmental leaf senescence. Plant Physiol. 2016, 172, 2132–2153. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Flaherty, E.J.; Shelp, B.J. γ-Aminobutyrate improves the postharvest marketability of horticultural commodities: Advances and prospects. Front. Plant Sci. 2022, 13, 884572. [Google Scholar] [CrossRef]
- Dellero, Y.; Clouet, V.; Marnet, N.; Pellizzaro, A.; Dechaumet, S.; Niogret, M.-F.; Bouchereau, A. Leaf status and environmental signals jointly regulate proline metabolism in winter oilseed rape. J. Exp. Bot. 2019, 71, 2098–2111. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Albayrak, F.Ö.; Vardar, F.; Gören-Sağlam, N. The role of GABA on programmed cell death and senescence in plants. In GABA in Plants; John Wiley & Sons Ltd.: London, UK, 2025; pp. 111–127. [Google Scholar]
- Li, W.; Zhang, H.; Li, X.; Zhang, F.; Liu, C.; Du, Y.; Gao, X.; Zhang, Z.; Zhang, X.; Hou, Z.; et al. Integrative metabolomic and transcriptomic analyses unveil nutrient remobilization events in leaf senescence of tobacco. Sci. Rep. 2017, 7, 12126. [Google Scholar]
- Wassermann, B.; Rybakova, D.; Müller, C.; Berg, G. Harnessing the microbiomes of Brassica vegetables for health issues. Sci. Rep. 2017, 7, 17649. [Google Scholar] [CrossRef]
- Guerrieri, R.; Cáliz, J.; Mattana, S.; Barceló, A.; Candela, M.; Elustondo, D.; Fortmann, H.; Hellsten, S.; Koenig, N.; Lindroos, A.-J.; et al. Substantial contribution of tree canopy nitrifiers to nitrogen fluxes in European forests. Nat. Geosci. 2024, 17, 130–136. [Google Scholar] [CrossRef]
- Guiboileau, A.; Sormani, R.; Meyer, C.; Masclaux-Daubresse, C. Senescence and death of plant organs: Nutrient recycling and developmental regulation. C. R. Biol. 2010, 333, 382–391. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Carillo, P.; Ferrante, A. Decoding the intricate metabolic and biochemical changes in plant senescence: A focus on chloroplasts and mitochondria. Ann. Bot. 2025, mcaf003. [Google Scholar] [CrossRef]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: Lessons from Arabidopsis to crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, G.M.; Annunziata, M.G.; Alberico, L.; Nicastro, R.; Woodrow, P.; Carillo, P. Tissue-Specific Metabolic Changes During Postharvest Storage of Friariello Napoletano. Horticulturae 2025, 11, 673. https://doi.org/10.3390/horticulturae11060673
Fusco GM, Annunziata MG, Alberico L, Nicastro R, Woodrow P, Carillo P. Tissue-Specific Metabolic Changes During Postharvest Storage of Friariello Napoletano. Horticulturae. 2025; 11(6):673. https://doi.org/10.3390/horticulturae11060673
Chicago/Turabian StyleFusco, Giovanna Marta, Maria Grazia Annunziata, Laura Alberico, Rosalinda Nicastro, Pasqualina Woodrow, and Petronia Carillo. 2025. "Tissue-Specific Metabolic Changes During Postharvest Storage of Friariello Napoletano" Horticulturae 11, no. 6: 673. https://doi.org/10.3390/horticulturae11060673
APA StyleFusco, G. M., Annunziata, M. G., Alberico, L., Nicastro, R., Woodrow, P., & Carillo, P. (2025). Tissue-Specific Metabolic Changes During Postharvest Storage of Friariello Napoletano. Horticulturae, 11(6), 673. https://doi.org/10.3390/horticulturae11060673











