Modulation of Biochemical Traits in Cold-Stored ‘Karaerik’ Grapes by Different Edible Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Grape Samples and Application Procedures
2.2. Evaluation of Weight Loss
2.3. Evaluation of Respiration Rate
2.4. Evaluation of External Ethylene
2.5. Evaluation of pH
2.6. Evaluation of Total Soluble Solids (TSS) and Maturity Index
2.7. Evaluation of Titratable Acidity (TA)
2.8. Evaluation of Color Measurements and Color Changes
2.9. Evaluation of Sugar Content
2.10. Evaluation of Total Phenolic Compound Content and Total Antioxidant Capacity
2.11. Evaluation of Organic Acid Content
2.12. Evaluation of Individual Phenolic Compounds
2.13. Statistical Analysis
3. Results
3.1. Weight Loss and Respiration Rate
3.2. pH, Titratable Acidity, and Total Soluble Solids
3.3. Color Parameters
3.4. Sugar and Acid Content
3.5. Antioxidant and Phenolic Compounds
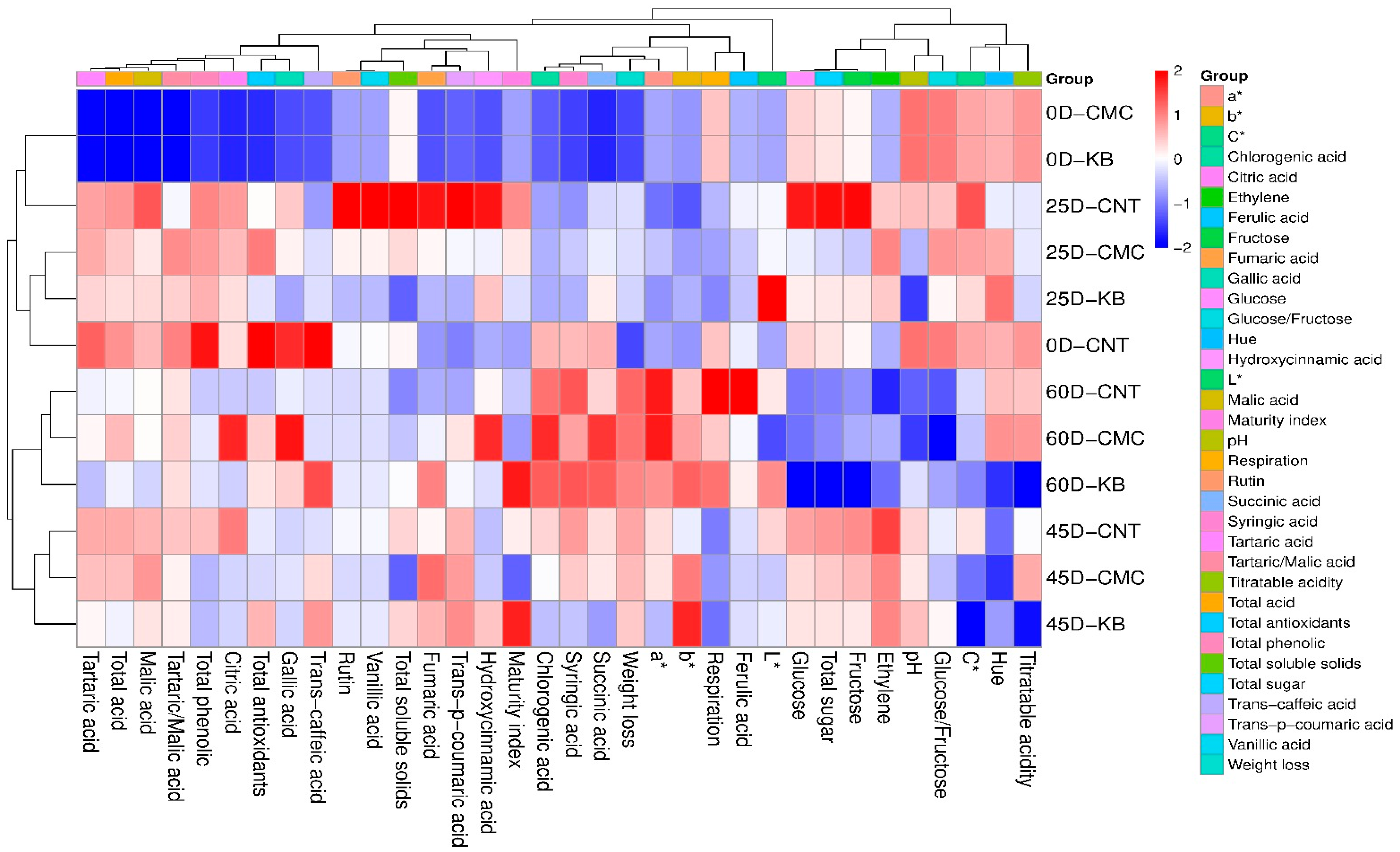
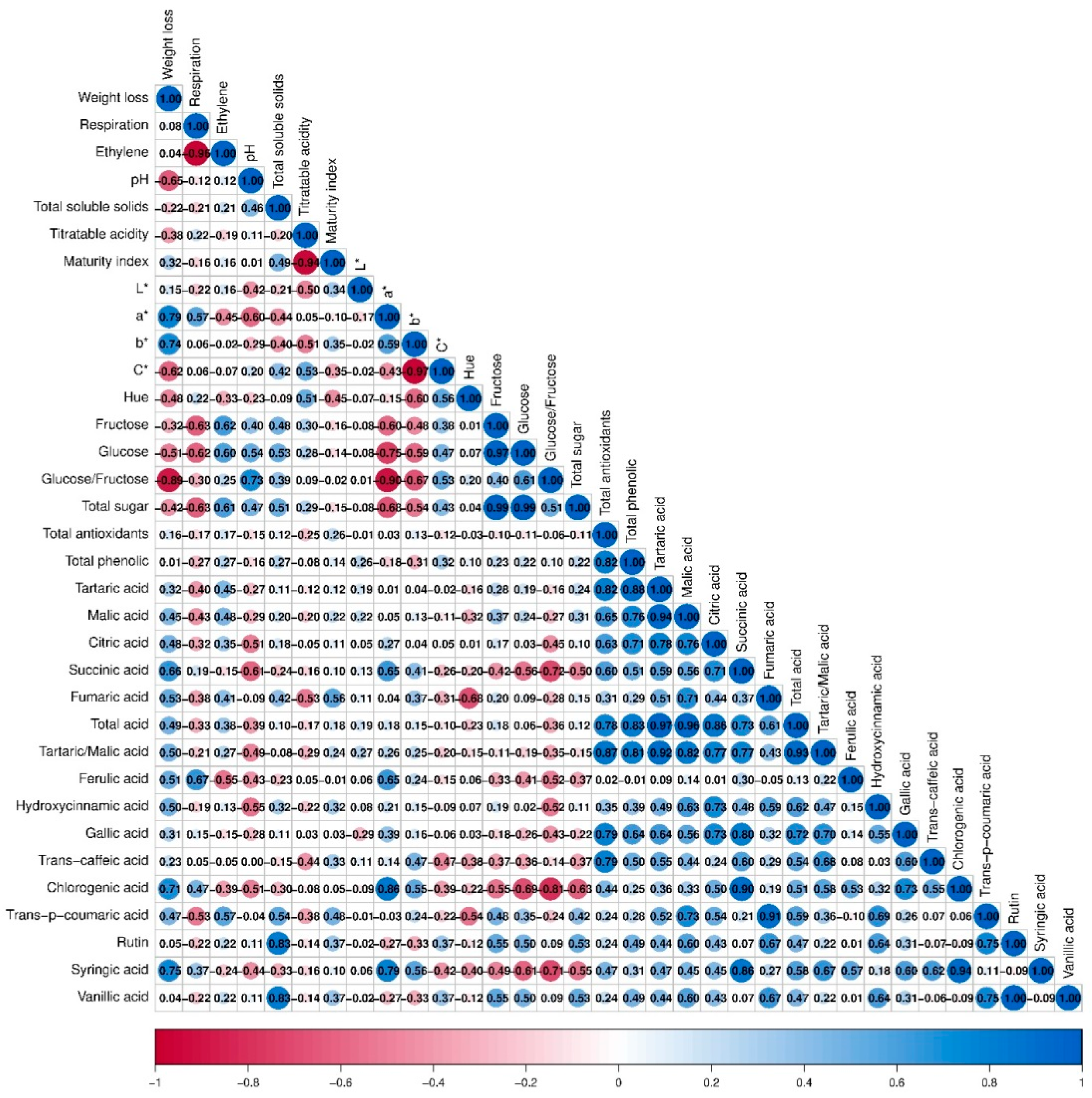
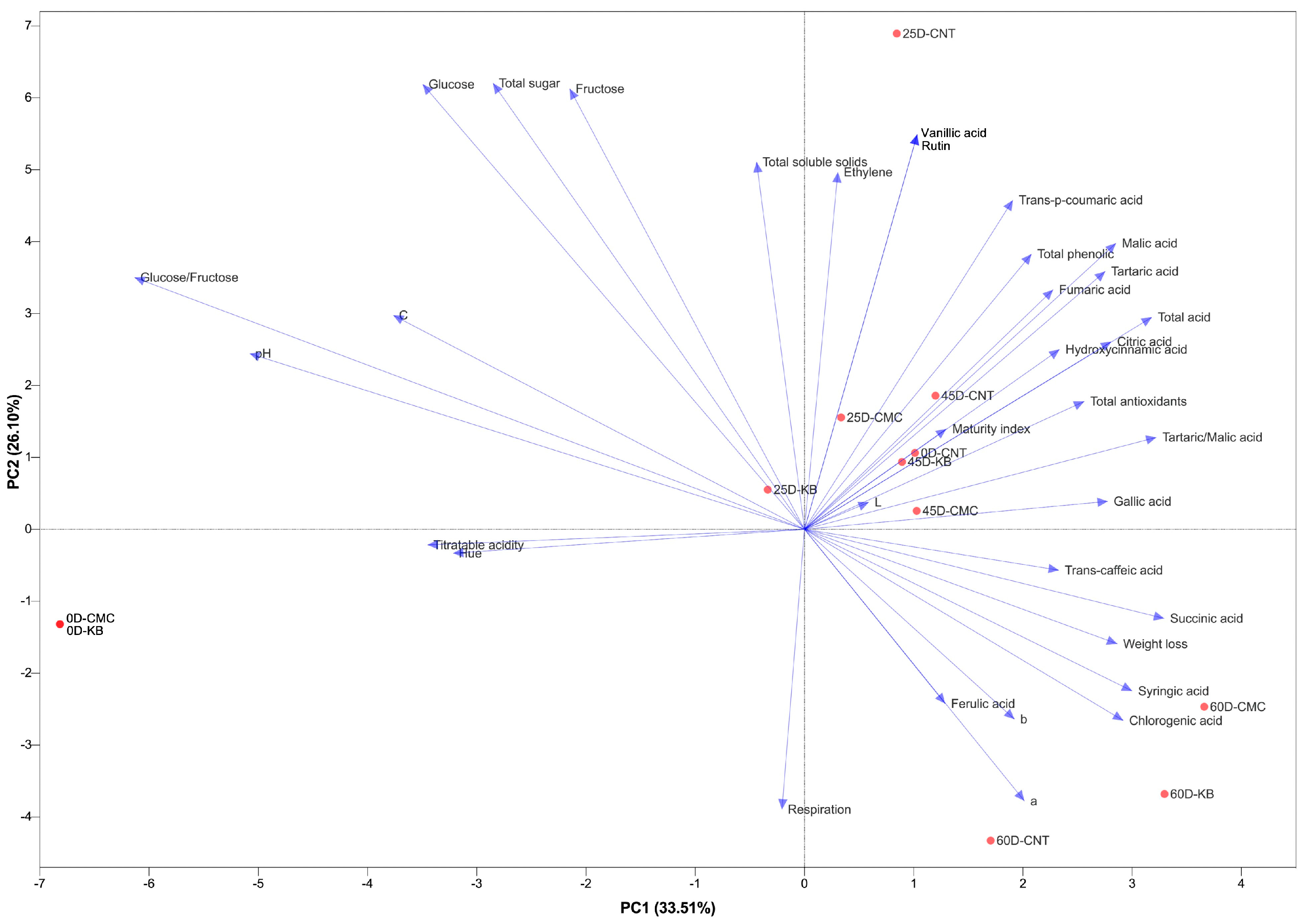
3.6. General Assessment
4. Discussion
4.1. Weight Loss and Respiration Rate
4.2. pH, Titratable Acidity, and Total Soluble Solids
4.3. Color Parameters
4.4. Sugar and Acid Content
4.5. Antioxidant and Phenolic Compounds
4.6. General Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaya, O.; Bozkurt, A.; Karakus, S.; Daler, S.; Yilmaz, T.; Turan, M. Essential Oils in Post-Harvest Disease Management: Metabolic Impact on Narince (Vitis vinifera L. cv) Grapes Against Botrytis cinerea. Physiol. Mol. Plant Pathol. 2024, 132, 102318. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Lichter, A.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Alchanatis, V.; Ostrovsky, V.; Lurie, S. Physical and visual properties of grape rachis as affected by water vapor pressure deficit. Postharvest Biol. Technol. 2011, 59, 25–33. [Google Scholar] [CrossRef]
- Al-Qurashi, A.D.; Awad, M.A. Postharvest chitosan treatment affects quality, antioxidant capacity, antioxidant compounds and enzymes activities of ‘El-Bayadi’table grapes after storage. Sci. Hortic. 2015, 197, 392–398. [Google Scholar] [CrossRef]
- Kaya, O.; Incesu, M.; Ates, F.; Keskin, N.; Verdugo-Vásquez, N.; Gutiérrez-Gamboa, G. Study of volatile organic compounds of two table grapes (cv. Italia and Bronx Seedless) along ripening in vines established in the Aegean region (Turkey). Plants 2022, 11, 1935. [Google Scholar] [CrossRef]
- Romero, I.; Vazquez-Hernandez, M.; Maestro-Gaitan, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Table grapes during postharvest storage: A review of the mechanisms implicated in the beneficial effects of treatments applied for quality retention. Int. J. Mol. Sci. 2020, 21, 9320. [Google Scholar] [CrossRef]
- Pandya, J.B. Post-Harvest Nutraceuticals: Edible Coatings for Enhanced Quality and Nutrition; Academic Guru Publishing House: Madhya Pradesh, India, 2025. [Google Scholar]
- de Aguiar, A.C.; Higuchi, M.T.; Yamashita, F.; Roberto, S.R. SO2-generating pads and packaging materials for postharvest conservation of table grapes: A review. Horticulturae 2023, 9, 724. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Lactic acid bacteria incorporated into edible coatings to control fungal growth and maintain postharvest quality of grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef]
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Morales, F.; Cabodevilla, A.; Pascual, I.; Urdiain, A. New Instrumentation in Grapevine Research: A Dual Respiration Prototype for Grape Berries and Whole Bunch. The Grape CO2/O2 Respiratory Quotient Revisited. Comput. Electron. Agric. 2024, 217, 108659. [Google Scholar] [CrossRef]
- Afifi, M.; Rezk, A.; Obenland, D.; El-Kereamy, A. Vineyard Light Manipulation and Silicon Enhance Ethylene-Induced Anthocyanin Accumulation in Red Table Grapes. Front. Plant Sci. 2023, 14, 1060377. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sun, J.; Cheng, J.; Yao, K.; Wu, X.; Zhou, X. Non-destructive Prediction of Total Soluble Solids and Titratable Acidity in Kyoho Grape Using Hyperspectral Imaging and Deep Learning Algorithm. Int. J. Food Sci. Technol. 2023, 58, 9–21. [Google Scholar] [CrossRef]
- Liu, H.F.; Wu, B.H.; Fan, P.G.; Li, S.H.; Li, L.S. Sugar and Acid Concentrations in 98 Grape Cultivars Analyzed by Principal Component Analysis. J. Sci. Food Agric. 2006, 86, 1526–1536. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The Phenolic Constituents of Prunus domestica. I. The Quantitative Analysis of Phenolic Constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Topalovic, A.; Mikulic-Petkovsek, M. Changes in Sugars, Organic Acids and Phenolics of Grape Berries of Cultivar Cardinal During Ripening. J. Food Agric. Environ. 2010, 8, 223–227. [Google Scholar]
- Rodriguez, F.I.; Esch, J.J.; Hall, A.E.; Binder, B.M.; Schaller, G.E.; Bleecker, A.B. A Copper Cofactor for the Ethylene Receptor ETR1 from Arabidopsis. Science 1999, 283, 996–998. [Google Scholar] [CrossRef]
- Unal, S.; Sabir, F.K.; Sabir, A. Aloe Vera Treatments Extend the Postharvest Life of Table Grapes by Delaying Weight Loss, Berry Softening, Rachis Browning, and Biochemical Changes. Erwerbs-Obstbau 2022, 64, 767–775. [Google Scholar] [CrossRef]
- Donat, A.; Sucu, S. The Effect of pre-harvest and post-harvest aloe vera gel treatments on fruit quality and Storage Performance of Table Grapes. Sci. Hortic. 2024, 331, 113117. [Google Scholar] [CrossRef]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Kaur, J.; Woodward, A. Water Loss: A Postharvest Quality Marker in Apple Storage. Food Bioprocess Technol. 2024, 17, 2155–2180. [Google Scholar] [CrossRef]
- Cui, H.; Abdel-Samie, M.A.S.; Lin, L. Novel Packaging Systems in Grape Storage—A Review. J. Food Process Eng. 2019, 42, e13162. [Google Scholar] [CrossRef]
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of Postharvest Water Loss in Fruits: Mechanisms, Influencing Factors, and Effective Control Strategies–A Comprehensive Review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Liu, X.; Du, M.; Tian, Y. Effect of polysaccharide derived from Osmunda japonica Thunb-incorporated carboxymethyl cellulose coatings on preservation of tomatoes. J. Food Process. Preserv. 2019, 43, e14239. [Google Scholar] [CrossRef]
- Eshghi, S.; Karimi, R.; Shiri, A.; Karami, M.; Moradi, M. The novel edible coating based on chitosan and gum ghatti to improve the quality and safety of ‘Rishbaba’table grape during cold storage. J. Food Meas. Charact. 2021, 15, 3683–3693. [Google Scholar] [CrossRef]
- Das, S.; Chaudhari, A.K.; Singh, V.K.; Dwivedy, A.K.; Dubey, N.K. Angelica Archangelica Essential Oil Loaded Chitosan Nanoemulsion as Edible Coating for Preservation of Table Grape Fruit Against Botrytis Cinerea Contamination and Storage Quality Deterioration. Postharvest Biol. Technol. 2023, 205, 112482. [Google Scholar] [CrossRef]
- Hassan, M.; Ali, S. Carboxymethyl Cellulose Coating Delays Quality Deterioration in Harvested Table Grapes During Cold and Ambient Storage Conditions. Prog. Org. Coat. 2025, 200, 109031. [Google Scholar] [CrossRef]
- Sun, M.; Yang, X.; Zhang, Y.; Wang, S.; Wong, M.W.; Ni, R.; Huang, D. Rapid and Visual Detection and Quantitation of Ethylene Released From Ripening Fruits: The New Use of Grubbs Catalyst. J. Agric. Food Chem. 2019, 67, 507–513. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Ji, H.; Sun, M.; Liu, T.; Wang, J.; Cao, H.; Zhu, Q. Ethylene biosynthesis and Signal Transduction During Ripening and softening in Non-Climacteric Fruits: An Overview. Front. Plant Sci. 2024, 15, 1368692. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-Abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef]
- Elhadef, K.; Chaari, M.; Akermi, S.; Hlima, H.B.; Ennouri, M.; Abdelkafi, S.; Agriopoulou, S.; Salahuddin, A.; Boulekbache-Makhlouf, L.; Mellouli, L.; et al. pH-sensitive films based on carboxymethyl cellulose/date pits anthocyanins: A convenient colorimetric indicator for beef meat freshness tracking. Food Biosci. 2024, 57, 103508. [Google Scholar] [CrossRef]
- Yekta, R.; Dabbagh Moghaddam, A.; Hosseini, H.; Sharifan, A.; Hadi, S.; Hosseini-Shokouh, S.J. Effect of using biodegradable film constituting red grape anthocyanins as a novel packaging on the qualitative attributes of emergency food bars during storage. Food Sci. Nutr. 2024, 12, 2702–2723. [Google Scholar] [CrossRef]
- Miglioranza, B.M.G.; Spinelli, F.R.; Stoffel, F.; Piemolini-Barreto, L.T. Biodegradable film for raisins packaging application: Evaluation of physico-chemical characteristics and antioxidant potential. Food Chem. 2021, 365, 130538. [Google Scholar] [CrossRef]
- Arnon, H.; Zaitsev, Y.; Porat, R.; Poverenov, E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol. Technol. 2014, 87, 21–26. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Barciela, P.; Carpena, M.; Prieto, M.A. Edible coatings as a natural packaging system to improve fruit and vegetable shelf life and quality. Foods 2023, 12, 3570. [Google Scholar] [CrossRef]
- Rolle, L.; Guidoni, S. Color and anthocyanin evaluation of red winegrapes by CIE L*, a*, b* parameters. Oeno One 2007, 41, 193–201. [Google Scholar] [CrossRef]
- Baldassi, C.; Lee, C.; Dossett, M.; Castellarin, S.D. High-throughput color determination of red raspberry puree and correlation of color parameters with total anthocyanins. Plant Methods 2024, 20, 78. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Marszałek, K.; Krzyżanowska, J.; Woźniak, Ł.; Skąpska, S. Kinetic modelling of polyphenol oxidase, peroxidase, pectin esterase, polygalacturonase, degradation of the main pigments and polyphenols in beetroot juice during high pressure carbon dioxide treatment. LWT-Food Sci. Technol. 2017, 85, 412–417. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2014, 65, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of edible coating in extension of fruit shelf life. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, Q.; Niu, B.; Liu, R.; Chen, H.; Xiao, S.; Wu, W.; Zhang, W.; Gao, H. The dual function of calcium ion in fruit edible coating: Regulating polymer internal crosslinking state and improving fruit postharvest quality. Food Chem. 2024, 447, 138952. [Google Scholar] [CrossRef] [PubMed]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolite changes during postharvest storage: Effects on fruit quality traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef]
- Ma, W.F.; Li, Y.B.; Nai, G.J.; Liang, G.P.; Ma, Z.H.; Chen, B.H.; Mao, J. Changes and Response Mechanism of Sugar and Organic Acids in Fruits Under Water Deficit Stress. PeerJ 2022, 10, e13691. [Google Scholar] [CrossRef]
- Eshghi, S.; Karimi, R.; Shiri, A.; Karami, M.; Moradi, M. Effects of polysaccharide-based coatings on postharvest storage life of grape: Measuring the changes in nutritional, antioxidant and phenolic compounds. J. Food Meas. Charact. 2022, 16, 1159–1170. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, W.K.; Wu, Q.C.; Yang, H.J. The release and catabolism of ferulic acid in plant cell wall by rumen microbes: A review. Anim. Nutr. 2022, 9, 335–344. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Liu, J.; Jiao, Z.; Zhang, Q.; Lv, Z.; Yang, W.; Chen, D.; Liu, H. Polysaccharide-Based Packaging Coatings and Films with Phenolic Compounds in Preservation of Fruits and Vegetables—A Review. Foods 2024, 13, 3896. [Google Scholar] [CrossRef]
- Kaya, O.; Karakus, S.; Ates, F.; Daler, S.; Hatterman-Valenti, H. Enhancing Royal grape quality through a three-year investigation of soil management practices and organic amendments on berry biochemistry. Chem. Biol. Technol. Agric. 2025, 12, 17. [Google Scholar] [CrossRef]
- Li, J.; Min, D.; Li, Z.; Fu, X.; Zhao, X.; Wang, J.; Zhang, X.; Li, F.; Li, X.; Guo, Y. Regulation of sugar metabolism by methyl jasmonate to improve the postharvest quality of tomato fruit. J. Plant Growth Regul. 2022, 41, 1615–1626. [Google Scholar] [CrossRef]
- Paixao, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Lattanzio, V. Bioactive polyphenols: Their role in quality and storability of fruit and vegetables. J. Appl. Bot. 2003, 77, 128–146. [Google Scholar]
- Karakus, S.; Atıcı, O.; Turan, M.; Kaya, O. Essential Oils Combat Botrytis Cinerea in Apples: Unveiling Sugar, Vitamin, and Antioxidant Dynamics. J. Food Meas. Charact. 2024, 18, 5590–5601. [Google Scholar] [CrossRef]
| NDS | T | WL | RESP | ETH | pH | TSS | TA | MI | L* | a* | b* | C* | Hue |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | CNT | 0.00 ± 0.00 | 36.69 ± 5.39 | 0.06 ± 0.03 | 3.26 ± 0.06 | 16.33 ± 1.52 | 0.76 ± 0.04 | 21.83 ± 3.22 | 33.30 ± 0.71 | 0.46 ± 0.38 | −2.36 ± 0.36 | 2.55 ± 0.32 | 276.02 ± 9.29 |
| CMC | 0.00 ± 0.00 | 36.69 ± 5.39 | 0.06 ± 0.03 | 3.26 ± 0.06 | 16.33 ± 1.52 | 0.76 ± 0.04 | 21.83 ± 3.22 | 33.30 ± 0.71 | 0.46 ± 0.38 | −2.36 ± 0.36 | 2.55 ± 0.32 | 276.02 ± 9.29 | |
| KB | 0.00 ± 0.00 | 36.69 ± 5.39 | 0.06 ± 0.03 | 3.26 ± 0.06 | 16.33 ± 1.52 | 0.76 ± 0.04 | 21.83 ± 3.22 | 33.30 ± 0.71 | 0.46 ± 0.38 | −2.36 ± 0.36 | 2.55 ± 0.32 | 276.02 ± 9.29 | |
| 25 | CNT | 4.50 ± 0.98 | 27.26 ± 3.31 | 0.08 ± 0.05 | 3.22 ± 0.06 | 17.77 ± 0.96 | 0.70 ± 0.09 | 25.74 ± 4.55 | 33.97 ± 0.31 | 0.36 ± 0.13 | −2.69 ± 0.21 | 2.89 ± 0.08 | 261.41 ± 12.83 |
| CMC | 4.45 ± 0.37 | 25.55 ± 5.71 | 0.09 ± 0.06 | 3.15 ± 0.11 | 16.47 ± 1.27 | 0.70 ± 0.09 | 24.02 ± 4.65 | 33.99 ± 0.42 | 0.52 ± 0.25 | −2.31 ± 0.34 | 2.58 ± 0.04 | 276.99 ± 12.99 | |
| KB | 4.23 ± 0.11 | 23.85 ± 4.55 | 0.08 ± 0.04 | 3.09 ± 0.09 | 15.60 ± 0.72 | 0.69 ± 0.12 | 23.03 ± 4.51 | 36.71 ± 0.68 | 0.42 ± 0.09 | −2.19 ± 0.22 | 2.35 ± 0.26 | 284.62 ± 2.31 | |
| 45 | CNT | 8.25 ± 1.28 | 23.20 ± 6.58 | 0.10 ± 0.06 | 3.21 ± 0.01 | 16.50 ± 1.08 | 0.71 ± 0.15 | 23.93 ± 5.86 | 34.40 ± 2.21 | 0.70 ± 0.30 | −1.85 ± 0.91 | 2.30 ± 0.64 | 242.92 ± 38.12 |
| CMC | 7.61 ± 1.08 | 24.91 ± 7.54 | 0.09 ± 0.07 | 3.20 ± 0.18 | 15.60 ± 0.44 | 0.75 ± 0.02 | 20.74 ± 0.94 | 33.58 ± 0.67 | 0.69 ± 0.26 | −1.01 ± 0.42 | 1.62 ± 0.19 | 234.13 ± 61.23 | |
| KB | 7.09 ± 0.75 | 22.38 ± 1.65 | 0.09 ± 0.05 | 3.22 ± 0.07 | 16.50 ± 1.51 | 0.60 ± 0.08 | 27.71 ± 4.59 | 33.88 ± 1.92 | 0.50 ± 0.07 | −0.54 ± 0.36 | 1.09 ± 0.06 | 249.80 ± 41.47 | |
| 60 | CNT | 10.01 ± 1.62 | 50.48 ± 11.22 | 0.04 ± 0.02 | 3.11 ± 0.08 | 15.77 ± 1.26 | 0.74 ± 0.24 | 22.60 ± 6.33 | 34.22 ± 2.56 | 1.08 ± 0.29 | −1.43 ± 0.24 | 2.04 ± 0.26 | 273.54 ± 34.89 |
| CMC | 9.68 ± 1.13 | 36.35 ± 8.12 | 0.06 ± 0.03 | 3.09 ± 0.07 | 16.03 ± 1.50 | 0.76 ± 0.14 | 21.79 ± 6.68 | 32.58 ± 2.25 | 1.08 ± 0.18 | −1.22 ± 0.38 | 1.96 ± 0.13 | 280.30 ± 29.76 | |
| KB | 9.13 ± 1.15 | 42.32 ± 11.22 | 0.05 ± 0.01 | 3.17 ± 0.04 | 16.30 ± 0.85 | 0.59 ± 0.02 | 27.78 ± 1.85 | 34.99 ± 1.77 | 0.85 ± 0.65 | −0.86 ± 0.35 | 1.71 ± 0.37 | 234.26 ± 20.33 | |
| Average of Number of Days Stored | 0 | 0.00 ± 0.00 D | 36.69 ± 4.67 A | 0.06 ± 0.02 | 3.26 ± 0.05 A | 16.33 ± 1.31 | 0.76 ± 0.04 | 21.83 ± 2.79 | 33.30 ± 0.62 | 0.46 ± 0.33 B | −2.36 ± 0.31 B | 2.55 ± 0.27 A | 276.02 ± 8.04 |
| 25 | 4.40 ± 0.54 C | 25.55 ± 4.27 B | 0.08 ± 0.04 | 3.15 ± 0.10 BC | 16.61 ± 1.29 | 0.70 ± 0.09 | 24.26 ± 4.13 | 34.89 ± 1.43 | 0.43 ± 0.16 B | −2.40 ± 0.32 B | 2.61 ± 0.27 A | 274.34 ± 13.77 | |
| 45 | 7.65 ± 1.04 B | 23.50 ± 5.19 B | 0.09 ± 0.05 | 3.21 ± 0.09 AB | 16.20 ± 1.06 | 0.69 ± 0.11 | 24.13 ± 4.81 | 33.95 ± 1.54 | 0.63 ± 0.23 B | −1.13 ± 0.78 A | 1.67 ± 0.62 B | 242.29 ± 42.15 | |
| 60 | 9.60 ± 1.20 A | 43.05 ± 10.83 A | 0.05 ± 0.02 | 3.12 ± 0.07 C | 16.03 ± 1.09 | 0.70 ± 0.16 | 24.06 ± 5.47 | 33.93 ± 2.20 | 1.00 ± 0.39 A | −1.17 ± 0.38 A | 1.90 ± 0.28 B | 262.70 ± 33.05 | |
| Average of Treatments | CNT | 5.69 ± 4.13 | 34.41 ± 12.57 | 0.07 ± 0.04 | 3.20 ± 0.07 | 16.59 ± 1.29 | 0.73 ± 0.13 | 23.53 ± 4.65 | 33.97 ± 1.54 | 0.65 ± 0.38 | −2.08 ± 0.67 B | 2.44 ± 0.46 A | 263.47 ± 26.80 |
| CMC | 5.44 ± 3.87 | 30.88 ± 8.27 | 0.07 ± 0.05 | 3.18 ± 0.12 | 16.11 ± 1.13 | 0.74 ± 0.08 | 22.09 ± 3.96 | 33.36 ± 1.19 | 0.69 ± 0.35 | −1.73 ± 0.72 A | 2.18 ± 0.46 B | 266.86 ± 35.80 | |
| KB | 5.11 ± 3.63 | 31.31 ± 10.50 | 0.07 ± 0.04 | 3.18 ± 0.09 | 16.18 ± 1.09 | 0.66 ± 0.10 | 25.08 ± 4.24 | 34.72 ± 1.81 | 0.56 ± 0.37 | −1.49 ± 0.88 A | 1.92 ± 0.64 C | 261.18 ± 29.11 | |
| Overall Average | 5.41 ± 3.78 | 32.20 ± 10.41 | 0.07 ± 0.04 | 3.19 ± 0.09 | 16.29 ± 1.16 | 0.71 ± 0.11 | 23.57 ± 4.34 | 34.02 ± 1.59 | 0.63 ± 0.36 | −1.77 ± 0.78 | 2.18 ± 0.56 | 263.84 ± 30.00 | |
| Number of Days Stored | Treatments | Fructose (g/L) | Glucose (g/L) | Glucose/Fructose | Total Sugar (g/L) |
|---|---|---|---|---|---|
| 0 | CNT | 8.66 ± 0.05 | 8.42 ± 0.05 | 0.97 ± 0.00 | 17.08 ± 0.09 |
| CMC | 8.66 ± 0.05 | 8.42 ± 0.05 | 0.97 ± 0.00 | 17.08 ± 0.09 | |
| KB | 8.66 ± 0.05 | 8.42 ± 0.05 | 0.97 ± 0.00 | 17.08 ± 0.09 | |
| 25 | CNT | 11.40 ± 1.92 | 10.62 ± 1.48 | 0.94 ± 0.03 | 22.03 ± 3.39 |
| CMC | 7.91 ± 1.74 | 7.65 ± 1.82 | 0.96 ± 0.02 | 15.56 ± 3.56 | |
| KB | 8.85 ± 0.30 | 8.09 ± 0.18 | 0.92 ± 0.02 | 16.94 ± 0.47 | |
| 45 | CNT | 9.95 ± 0.05 | 9.08 ± 0.24 | 0.91 ± 0.02 | 19.03 ± 0.29 |
| CMC | 9.40 ± 0.97 | 8.37 ± 0.61 | 0.89 ± 0.03 | 17.77 ± 1.58 | |
| KB | 8.85 ± 1.79 | 8.20 ± 1.76 | 0.92 ± 0.02 | 17.06 ± 3.54 | |
| 60 | CNT | 7.23 ± 1.88 | 6.22 ± 1.93 | 0.85 ± 0.05 | 13.46 ± 3.81 |
| CMC | 7.52 ± 0.32 | 6.11 ± 0.20 | 0.81 ± 0.07 | 13.63 ± 0.12 | |
| KB | 5.23 ± 1.98 | 4.57 ± 1.65 | 0.88 ± 0.02 | 9.80 ± 3.63 | |
| Average of Number of Days Stored | 0 | 8.66 ± 0.04 A | 8.42 ± 0.04 A | 0.97 ± 0.00 A | 17.08 ± 0.08 A |
| 25 | 9.39 ± 2.04 A | 8.79 ± 1.82 A | 0.94 ± 0.03 B | 18.18 ± 3.85 A | |
| 45 | 9.40 ± 1.12 A | 8.55 ± 1.02 A | 0.91 ± 0.02 C | 17.95 ± 2.13 A | |
| 60 | 6.66 ± 1.75 B | 5.63 ± 1.50 B | 0.85 ± 0.05 D | 12.30 ± 3.23 B | |
| Average of Treatments | CNT | 9.31 ± 1.98 A | 8.59 ± 1.95 A | 0.92 ± 0.05 | 17.90 ± 3.91 A |
| CMC | 8.37 ± 1.14 AB | 7.64 ± 1.28 AB | 0.91 ± 0.07 | 16.01 ± 2.35 AB | |
| KB | 7.90 ± 1.98 B | 7.32 ± 1.96 B | 0.92 ± 0.04 | 15.22 ± 3.92 B | |
| Overall Average | 8.53 ± 1.79 | 7.85 ± 1.79 | 0.92 ± 0.05 | 16.38 ± 3.56 | |
| Number of Days Stored | Treatments | Total Antioxidants (mg GAE/g) | Total Phenolic (mg GAE/g) |
|---|---|---|---|
| 0 | CNT | 101.19 ± 1.98 a | 376.63 ± 33.75 a |
| CMC | – | – | |
| KB | – | – | |
| 25 | CNT | 46.12 ± 0.13 e | 281.63 ± 47.50 b |
| CMC | 74.81 ± 0.27 b | 261.00 ± 10.63 bc | |
| KB | 39.48 ± 2.19 fg | 244.75 ± 3.13 bc | |
| 45 | CNT | 41.40 ± 4.75 ef | 230.38 ± 1.25 c |
| CMC | 36.91 ± 2.81 fg | 107.25 ± 4.38 e | |
| KB | 62.12 ± 6.85 c | 111.00 ± 8.13 e | |
| 60 | CNT | 34.36 ± 2.49 g | 126.63 ± 26.25 de |
| CMC | 56.49 ± 0.21 d | 153.50 ± 28.13 d | |
| KB | 51.98 ± 0.82 d | 148.50 ± 10.63 de | |
| Average of Number of Days Stored | 0 | 101.19 ± 1.98 | 376.63 ± 33.75 |
| 25 | 53.47 ± 16.30 | 262.46 ± 29.17 | |
| 45 | 46.81 ± 12.45 | 149.55 ± 60.83 | |
| 60 | 47.61 ± 10.21 | 142.88 ± 23.48 | |
| Average of Treatments | CNT | 55.77 ± 27.85 | 253.82 ± 98.13 |
| CMC | 56.07 ± 16.48 | 173.92 ± 69.98 | |
| KB | 51.19 ± 10.46 | 168.09 ± 60.14 | |
| Overall Average | 54.49 ± 20.10 | 204.13 ± 87.80 | |
| Number of Days Stored | Treatments | Tartaric Acid | Malic Acid | Citric Acid | Succinic Acid | Fumaric Acid | Total Acid | Tartaric/Malic Acid |
|---|---|---|---|---|---|---|---|---|
| 0 | CNT | 4.37 ± 1.05 | 1.93 ± 0.28 b–d | 0.70 ± 0.25 ab | 1.27 ± 0.26 | 0.07 ± 0.00 e | 8.35 ± 1.84 | 2.23 ± 0.22 a |
| CMC | – | – | – | – | – | – | – | |
| KB | – | – | – | – | – | – | – | |
| 25 | CNT | 3.67 ± 0.23 | 2.54 ± 0.01 a | 0.89 ± 0.60 ab | 0.75 ± 0.23 | 0.40 ± 0.03 a | 8.25 ± 0.63 | 1.45 ± 0.10 c |
| CMC | 3.59 ± 0.53 | 1.65 ± 0.18 c–e | 0.80 ± 0.00 ab | 0.84 ± 0.45 | 0.18 ± 0.00 c–e | 7.06 ± 0.25 | 2.17 ± 0.09 a | |
| KB | 3.13 ± 0.15 | 1.74 ± 0.05 b–d | 0.70 ± 0.44 ab | 1.00 ± 0.42 | 0.10 ± 0.02 de | 6.67 ± 0.20 | 1.79 ± 0.04 b | |
| 45 | CNT | 3.57 ± 0.07 | 1.97 ± 0.26 bc | 0.97 ± 0.32 ab | 1.08 ± 0.26 | 0.18 ± 0.08 cd | 7.76 ± 0.99 | 1.84 ± 0.21 b |
| CMC | 3.37 ± 0.14 | 2.16 ± 0.09 ab | 0.48 ± 0.10 b | 1.03 ± 0.24 | 0.32 ± 0.07 ab | 7.35 ± 0.25 | 1.56 ± 0.12 ab | |
| KB | 2.73 ± 0.65 | 1.67 ± 0.32 cd | 0.48 ± 0.00 b | 0.49 ± 0.00 | 0.25 ± 0.07 bc | 5.62 ± 1.03 | 1.61 ± 0.09 ab | |
| 60 | CNT | 2.50 ± 0.29 | 1.51 ± 0.25 de | 0.45 ± 0.03 b | 1.11 ± 0.68 | 0.09 ± 0.04 de | 5.66 ± 0.09 | 1.67 ± 0.08 ab |
| CMC | 2.72 ± 0.25 | 1.53 ± 0.14 c–e | 1.20 ± 0.53 a | 1.79 ± 1.17 | 0.16 ± 0.08 c–e | 7.41 ± 2.15 | 1.77 ± 0.01 b | |
| KB | 1.96 ± 0.38 | 1.23 ± 0.43 e | 0.46 ± 0.22 b | 1.64 ± 0.00 | 0.30 ± 0.10 ab | 5.59 ± 1.11 | 1.69 ± 0.28 ab | |
| Average of Number of Days Stored | 0 | 4.37 ± 1.05 A | 1.93 ± 0.28 | 0.70 ± 0.25 | 1.27 ± 0.26 AB | 0.07 ± 0.00 | 8.35 ± 1.84 | 2.23 ± 0.22 |
| 25 | 3.46 ± 0.39 B | 1.98 ± 0.43 | 0.80 ± 0.38 | 0.86 ± 0.35 B | 0.23 ± 0.14 | 7.33 ± 0.80 | 1.80 ± 0.32 | |
| 45 | 3.22 ± 0.50 B | 1.94 ± 0.30 | 0.64 ± 0.30 | 0.87 ± 0.33 B | 0.25 ± 0.09 | 6.91 ± 1.22 | 1.67 ± 0.18 | |
| 60 | 2.39 ± 0.43 C | 1.42 ± 0.29 | 0.70 ± 0.47 | 1.51 ± 0.74 A | 0.18 ± 0.12 | 6.22 ± 1.50 | 1.71 ± 0.15 | |
| Average of Treatments | CNT | 3.53 ± 0.84 A | 1.99 ± 0.43 | 0.75 ± 0.37 | 1.05 ± 0.40 | 0.18 ± 0.14 | 7.51 ± 1.47 A | 1.80 ± 0.33 |
| CMC | 3.23 ± 0.49 A | 1.78 ± 0.31 | 0.83 ± 0.41 | 1.22 ± 0.77 | 0.22 ± 0.09 | 7.27 ± 1.10 A | 1.83 ± 0.28 | |
| KB | 2.60 ± 0.64 B | 1.55 ± 0.36 | 0.55 ± 0.27 | 1.04 ± 0.54 | 0.22 ± 0.11 | 5.96 ± 0.93 B | 1.70 ± 0.16 | |
| Overall Average | 3.16 ± 0.78 | 1.79 ± 0.41 | 0.71 ± 0.36 | 1.10 ± 0.56 | 0.20 ± 0.12 | 6.97 ± 1.36 | 1.78 ± 0.27 | |
| Number of Days Stored | Treatments | Ferulic Acid (µg/g) | Hydroxycinnamic Acid (µg/g) | Gallic Acid (µg/g) | Trans-Caffeic Acid (µg/g) | Chlorogenic Acid (µg/g) | Trans-p-Coumaric Acid (µg/g) | Rutin (µg/g) | Syringic Acid (µg/g) | Vanillic Acid (µg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | CNT | 0.62 ± 0.08 | 0.15 ± 0.00 c | 4.03 ± 0.88 | 0.06 ± 0.01 | 8.60 ± 2.49 | 0.04 ± 0.05 | 1.23 ± 1.13 b | 2.41 ± 0.51 | 0.47 ± 0.43 b |
| CMC | – | – | – | – | – | – | – | – | – | |
| KB | – | – | – | – | – | – | – | – | – | |
| 25 | CNT | 0.68 ± 0.17 | 0.72 ± 0.11 a | 2.41 ± 1.41 | 0.01 ± 0.01 | 2.42 ± 1.57 | 0.57 ± 0.44 | 6.53 ± 5.05 a | 0.74 ± 0.07 | 2.47 ± 1.91a |
| CMC | 0.26 ± 0.11 | 0.28 ± 0.13 bc | 1.98 ± 0.08 | 0.02 ± 0.00 | 2.98 ± 1.39 | 0.20 ± 0.05 | 1.51 ± 0.03 b | 1.23 ± 0.46 | 0.57 ± 0.01 b | |
| KB | 0.23 ± 0.11 | 0.41 ± 0.15 b | 0.90 ± 0.22 | 0.02 ± 0.02 | 2.82 ± 0.85 | 0.10 ± 0.10 | 0.36 ± 0.10 b | 1.00 ± 0.10 | 0.14 ± 0.04 b | |
| 45 | CNT | 0.49 ± 0.20 | 0.19 ± 0.07 bc | 1.41 ± 0.05 | 0.02 ± 0.02 | 7.56 ± 2.06 | 0.31 ± 0.03 | 1.20 ± 0.31 b | 2.67 ± 0.36 | 0.45 ± 0.12 b |
| CMC | 0.35 ± 0.10 | 0.21 ± 0.00 bc | 1.59 ± 0.02 | 0.03 ± 0.01 | 5.96 ± 0.20 | 0.35 ± 0.06 | 0.77 ± 0.39 b | 2.23 ± 0.07 | 0.29 ± 0.15 b | |
| KB | 0.47 ± 0.10 | 0.41 ± 0.23 b | 1.39 ± 0.36 | 0.04 ± 0.01 | 3.66 ± 0.94 | 0.37 ± 0.31 | 0.99 ± 0.13 b | 1.22 ± 0.11 | 0.38 ± 0.05 b | |
| 60 | CNT | 4.74 ± 4.54 | 0.32 ± 0.16 bc | 1.68 ± 0.16 | 0.02 ± 0.02 | 11.16 ± 0.60 | 0.09 ± 0.09 | 0.81 ± 0.38 b | 3.29 ± 0.71 | 0.31 ± 0.15 b |
| CMC | 0.75 ± 0.36 | 0.68 ± 0.12 a | 4.24 ± 2.35 | 0.02 ± 0.02 | 13.55 ± 1.28 | 0.25 ± 0.06 | 0.85 ± 0.18 b | 2.62 ± 1.25 | 0.32 ± 0.07 b | |
| KB | 0.98 ± 0.24 | 0.25 ± 0.00 bc | 2.37 ± 0.09 | 0.05 ± 0.02 | 11.86 ± 3.98 | 0.20 ± 0.10 | 0.98 ± 0.73 b | 3.27 ± 1.59 | 0.37 ± 0.27 b | |
| Average of Number of Days Stored | 0 | 0.62 ± 0.08 | 0.15 ± 0.00 | 4.03 ± 0.88 A | 0.06 ± 0.01 A | 8.60 ± 2.49 B | 0.04 ± 0.05 B | 1.23 ± 1.13 | 2.41 ± 0.51 AB | 0.47 ± 0.43 |
| 25 | 0.39 ± 0.25 | 0.47 ± 0.23 | 1.76 ± 0.98 BC | 0.02 ± 0.01 B | 2.74 ± 1.16 D | 0.29 ± 0.31 A | 2.80 ± 3.80 | 0.99 ± 0.32 C | 1.06 ± 1.44 | |
| 45 | 0.44 ± 0.14 | 0.27 ± 0.16 | 1.46 ± 0.20 C | 0.03 ± 0.01 B | 5.72 ± 2.04 C | 0.34 ± 0.16 A | 0.99 ± 0.31 | 2.04 ± 0.67 B | 0.37 ± 0.12 | |
| 60 | 2.16 ± 2.99 | 0.42 ± 0.22 | 2.76 ± 1.65 B | 0.03 ± 0.02 B | 12.19 ± 2.36 A | 0.18 ± 0.10 AB | 0.88 ± 0.43 | 3.06 ± 1.12 A | 0.33 ± 0.16 | |
| Average of Treatments | CNT | 1.63 ± 2.70 | 0.35 ± 0.25 | 2.38 ± 1.28 | 0.03 ± 0.02 | 7.43 ± 3.66 | 0.25 ± 0.29 | 2.44 ± 3.32 | 2.28 ± 1.07 | 0.92 ± 1.26 |
| CMC | 0.45 ± 0.29 | 0.39 ± 0.24 | 2.60 ± 1.71 | 0.02 ± 0.01 | 7.49 ± 4.82 | 0.27 ± 0.08 | 1.04 ± 0.41 | 2.03 ± 0.91 | 0.39 ± 0.15 | |
| KB | 0.56 ± 0.36 | 0.36 ± 0.16 | 1.55 ± 0.68 | 0.03 ± 0.02 | 6.11 ± 4.80 | 0.22 ± 0.21 | 0.78 ± 0.48 | 1.83 ± 1.34 | 0.29 ± 0.18 | |
| Overall Average | 0.96 ± 1.77 | 0.36 ± 0.22 | 2.20 ± 1.32 | 0.03 ± 0.02 | 7.06 ± 4.27 | 0.25 ± 0.21 | 1.52 ± 2.21 | 2.07 ± 1.09 | 0.58 ± 0.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keskin, N.; Karakus, S.; Hatterman-Valenti, H.; Kaya, O.; Cavusoglu, S.; Tekin, O.; Kunter, B.; Keskin, S.; Kaya, A.Ç.; Karadogan, B. Modulation of Biochemical Traits in Cold-Stored ‘Karaerik’ Grapes by Different Edible Coatings. Horticulturae 2025, 11, 672. https://doi.org/10.3390/horticulturae11060672
Keskin N, Karakus S, Hatterman-Valenti H, Kaya O, Cavusoglu S, Tekin O, Kunter B, Keskin S, Kaya AÇ, Karadogan B. Modulation of Biochemical Traits in Cold-Stored ‘Karaerik’ Grapes by Different Edible Coatings. Horticulturae. 2025; 11(6):672. https://doi.org/10.3390/horticulturae11060672
Chicago/Turabian StyleKeskin, Nurhan, Sinem Karakus, Harlene Hatterman-Valenti, Ozkan Kaya, Seyda Cavusoglu, Onur Tekin, Birhan Kunter, Sıddık Keskin, Ahmet Çağlar Kaya, and Birol Karadogan. 2025. "Modulation of Biochemical Traits in Cold-Stored ‘Karaerik’ Grapes by Different Edible Coatings" Horticulturae 11, no. 6: 672. https://doi.org/10.3390/horticulturae11060672
APA StyleKeskin, N., Karakus, S., Hatterman-Valenti, H., Kaya, O., Cavusoglu, S., Tekin, O., Kunter, B., Keskin, S., Kaya, A. Ç., & Karadogan, B. (2025). Modulation of Biochemical Traits in Cold-Stored ‘Karaerik’ Grapes by Different Edible Coatings. Horticulturae, 11(6), 672. https://doi.org/10.3390/horticulturae11060672








