Abstract
This study aimed to investigate the cracking characteristics of various sweet potato germplasm resources, explore their genetic associations, and identify crack-resistant varieties. Using 40 sweet potato varieties as experimental materials, we systematically analyzed their cracking traits and assessed 24 parameters. The results indicated that genotypic differences significantly influenced sweet potato cracking (p = 1.11 × 10−16). Correlation analyses revealed that skin thickness (r = −0.81, p < 0.01), skin hardness (r = −0.50, p < 0.01), and starch content (r = −0.51, p < 0.01) were highly significantly negatively correlated with cracking incidence. Microscopic observations of the cell structure revealed that the development quality of the cork cambium and vascular cambium during the secondary growth stage plays a crucial role in maintaining the structural stability of the tuber skin, whereas the internal expansion force during the rapid growth phase is a direct factor that induces cracking. A multiple regression prediction model (R2 = 0.85) was established based on ten core indices. Furthermore, a comprehensive evaluation system for sweet potato cracking resistance was developed by integrating principal component analysis and the entropy-weighted TOPSIS model (kappa = 0.752, p = 5 × 10−6), identifying seven extremely crack-resistant and nine crack-resistant varieties. This study is the first to construct a multidimensional evaluation system for cracking traits in sweet potato, offering a reference for breeding crack-resistant varieties and developing cultivation, prevention, and management strategies.
1. Introduction
As the sixth-largest food crop in the world, sweet potato (Ipomoea batatas (L.) Lam.) holds significant economic and social value in the fields of food security, industrial raw materials, and feed supply [1]. In China, sweet potato has become a vital cash crop in certain regions, contributing to improved agricultural efficiency and increased farmer income [2]. This is primarily attributable to its strong adaptability and ease of management. However, in the context of intensifying global climate change, sweet potato crops are increasingly vulnerable to extreme weather events, characterized by alternating periods of high temperature and drought interspersed with heavy rainfall during their reproductive phase [3,4]. These conditions cause substantial fluctuations in soil moisture levels and significantly increase the incidence of tuber cracking. The severity of cracking ranges from 30% to 40% [5,6], which heightens the vulnerability of sweet potatoes to pathogens while simultaneously compromising quality, marketability, and storage performance [7]. This phenomenon has led to considerable economic losses in agricultural production.
Sweet potato cracking is a physiological disorder influenced by multiple factors, including genetic traits, morphological characteristics, environmental conditions, and physiological and biochemical properties [8]. The skin tissues of sweet potato play crucial roles in mechanical protection against biotic and abiotic stresses. When intense physiological and biochemical changes occur internally, resulting in sustained high expansion pressure [9], the sweet potato skin may crack. This issue is further exacerbated by adverse external factors such as climate, cultivation practices, pests, and diseases [3,10,11]. The lack of in-depth studies on sweet potato cracking is evident, with most research in China focusing on empirical summaries of external causes and control measures [8,12]. There is a noticeable gap in systematic studies on its physiological and molecular mechanisms. In terms of agronomic regulation, the excessive application of nitrogen and potassium adversely affects the development of the tuber epidermis [6]. Rapid expansion during the early stage of tuber formation contributes to cracking, although the healing process is more effective in later growth stages. However, under low-temperature or arid conditions, the healing capacity is significantly reduced, leading to a higher incidence of cracking [13]. Growers and researchers widely acknowledge that the epidermis of expanded root systems tends to harden under drought conditions. In such environments, cells rapidly absorb water and subsequently undergo division and expansion when exposed to high humidity. This imbalance between internal and external tissue growth predisposes sweet potato skin to cracking [14]. Additionally, variety susceptibility, the tuber expansion rate, and tissue expansion balance are closely related to cracking. At present, there is a scarcity of experimental methods capable of effectively inducing cracking, making it difficult to stably replicate cracking in sweet potato even under controlled conditions.
Currently, two major bottlenecks exist in the screening of cracking-resistant germplasm. First, the identification of key resistance indices relies heavily on empirical judgment and lacks quantitative model support. Second, there is no standardized phenotypic evaluation system, and traditional field observations are highly susceptible to environmental interference. To address these issues, this study proposes an innovative research framework that integrates phenotyping, correlation analysis, model prediction, and comprehensive evaluation. This framework utilizes multimodel linkages to analyze the genetic basis of cracking traits and establish a cracking resistance evaluation system based on physical and biochemical indices. The framework aims to identify core resistance indices, construct a predictive model, and screen for superior cracking-resistant germplasms. Ultimately, this research provides theoretical guidance and material support for the breeding of cracking-resistant varieties. The findings of this study will be valuable for improving sweet potato cultivation techniques and reducing economic losses caused by tuber cracking.
2. Materials and Methods
2.1. Experimental Setup
The experiment was conducted from June to October in both 2023 and 2024 in the same plot at the Chongzhou Modern Research and Development Base of Sichuan Agricultural University (103°65′ E, 30°56′ N). The field had undergone crop rotation with one season of barley between the two experimental years. Detoxified seedlings of 40 sweet potato varieties (Table 1) were used as materials. These varieties were provided by the Sweet Potato Innovation Team of Sichuan Agricultural University (The sources of the varieties are listed in Table S1). The experiment adopted a randomized block design with 150 cuttings per variety and three replications. The plants were cultivated in two rows per ridge, with a ridge height of 25 cm, a row spacing of 100 cm, and a planting distance of 30 cm. Before planting, a base fertilizer application of 600 kg/hm2 compound fertilizer (N:P2O5:K2O = 16-6-18) was applied. Harvesting occurred after a 120-day growth period. In each replication, 100 tubers were randomly sampled, and their morphological characteristics—including skin color, flesh color, tuber shape, number of tubers per plant (N, g), single-tuber weight (G, g), and degree of skin separation (S)—were recorded. Sweet potato skin refers to the outer protective tissue of the tuber, which is located outside the vascular cambium and partially extends into the secondary phloem. The cracking rate (CR, %) and cracking type were also assessed and classified into cracking grades via cluster analysis. The physical characteristics of each variety were measured, including longitudinal and transverse diameters (L/W, cm), skin and flesh hardness (CH/MH, N), thickness (CT/MT, cm), moisture content (CW/MW, %), and biochemical indices such as the dry matter ratio (CD/MD, %), starch content (CS/MS, mg/g), soluble sugar content (CSS/MSS, mg/g), tuber shape index (SI = L:W), coefficient of variation for hardness and thickness (CV, %), percentage of tuber skin thickness (ΔT, %), and difference in internal and external water content (ΔW, %). Furthermore, six varieties with different cracking types were selected. During peak cracking periods, samples were collected and subjected to paraffin sectioning to observe the cellular structure of the tuber skin.

Table 1.
Names and abbreviations of 40 sweet potato varieties.
2.2. Measurements
2.2.1. Determination of the Cracking Rate
The presence of visible cracks on the root surface of sweet potato tubers (with a minimum length of 1 cm) was used as the primary criterion for evaluating the severity of cracking. The cracking rate (%) was thus determined as the number of cracked tubers divided by the total number of tubers inspected × 100 [15].
2.2.2. Hardness and Water Content
A hardness tester (WDGY-4, Weidu Electronics Co., Ltd. Wenzhou, Zhejiang, China) was used to apply uniform pressure perpendicular to the tuber skin or flesh surface. The largest sweet potato from each of the nine individual plants was selected, and six different points on the same cross-section of each tuber were measured. The average value was taken as the hardness of the sweet potato, and this process was repeated three times.
For water content determination, the largest tubers from three individual plants were selected. The skin and flesh were separated, chopped, mixed, and weighed to determine the fresh weight. The samples were then oven-dried until a constant weight was reached, and the dry weight was recorded. The moisture content (%) was obtained by dividing the difference between the fresh weight and dry weight by the fresh weight. This procedure was repeated three times, and the mean value was obtained.
2.2.3. Starch and Total Soluble Sugars
Fresh samples (1.00 g) of the skin or flesh were collected and analyzed using the anthrone sulfate method, following the approach of a previous study [16].
2.2.4. Tissue Sectioning
Sweet potato pieces approximately 5 mm in thickness were cut from the upper-middle parts of the tubers. These sections were immediately placed in FAA fixative for preservation and sent to Sevier Biotechnology Limited for tissue sectioning. Imaging was conducted using a high-magnification microscope (OLYMPUS, Olympus Corporation, Tokyo, Japan) to measure the thickness of the periderm and skin. For each section, thickness was measured in 15 different areas, and the process was repeated three times for accuracy.
2.2.5. The Entropy-Weighted TOPSIS Model
The entropy-weighted TOPSIS model was applied based on the approach of Tang et al. [17] with slight modifications. Initially, the correlation between each indicator and the cracking rate was determined, categorizing indicators as either positive or negative. Standardization and nonnegative normalization were then applied to compute the indicator weights. The highest value in each column of the weighted normalization matrix was defined as the positive ideal solution, whereas the lowest value was defined as the negative ideal solution. Using the Euclidean distance method, the distance of each object from the positive and negative ideal solutions was calculated. The relative closeness to the negative ideal solution was then determined. Higher values indicated a greater similarity to the negative ideal solution and thus a greater susceptibility to cracking.
2.2.6. Others
The length, width, and thickness of the sweet potato skin were measured using Vernier calipers. The largest tuber from nine individual plants was selected, and the same cross-section of each tuber was measured three times, with the process repeated three times. The ease of separation between the skin and flesh was assessed in three tubers. Three different parts of each tuber were cut into cubes of uniform size, and the ease and completeness of peeling were evaluated as criteria for determining the difficulty of peeling. The coefficient of variation was used to assess the variability in hardness and thickness across all samples, providing a measure of uniformity.
2.3. Statistical Analysis
A multimodel fusion strategy was employed for data analysis, incorporating the following steps: (1) the screening of key indicators through correlation analysis, (2) the construction of a prediction model using univariate and multiple regression analyses, (3) comprehensive evaluation through a combination of principal component analysis (PCA) and the entropy-weighted TOPSIS model, and (4) the validation of model reliability using the kappa consistency test. Finally, the cracking grades were classified using cluster analysis. The screening of cracking-resistant germplasm resources was conducted, and the associations between genetic characteristics and cracking were examined.
Excel 2021 was used for data statistical analysis and entropy-weighted TOPSIS model analysis. SPSS Statistics 26 was employed for ANOVA, PCA, and significance testing. Origin 2021 was used for Pearson correlation analysis, cluster analysis, regression analysis, and graphical visualization. The Duncan test was applied to evaluate statistical significance, with multiple comparisons performed using the least significant difference (LSD) method. ImageJ v1.8.0 and IciMapping4.2 were utilized for additional analysis.
3. Results
3.1. Investigation of the Cracking of 40 Sweet Potato Varieties over Two Years
Field observations conducted over two consecutive years revealed that 19 sweet potato varieties (47.5% of the tested varieties) exhibited varying degrees of cracking (Table 2). Cracking occurred throughout the later stages of growth, from the tuber expansion period to the harvest period. It initially manifested as mechanical ruptures in the tuber skin, followed by the exposed expansion of the flesh tissue, often accompanied by further cracking. Among the tested varieties, ‘N008’ and ‘S524’ presented the most severe cracking, with average cracking rates of 35.84% and 43.84%, respectively, over the two years. Additionally, the cracking rates of varieties ‘B553’ and ‘W7’ exceeded 20%. Genotypic differences among sweet potato varieties significantly affected cracking rates. Notably, varieties such as ‘N008’, ‘S524’, ‘B553’, and ‘L9’ presented highly significant variations in cracking rates across different years. Furthermore, ‘Y8’ and ‘M25’ presented cracking in 2024 but not in 2023, indicating a significant interaction between genotype and environmental factors. Broad-sense heritability analysis indicated that genotype effects accounted for 78% of the variation in cracking rates. However, the genotype × environment interaction effect was also significant, underscoring the influence of environmental conditions on sweet potato cracking.

Table 2.
Incidence of sweet potato cracking in different varieties.
A comparison of climatic differences between the two years highlighted variations in temperature and precipitation that may have contributed to cracking disparities. During the early rapid expansion stage of sweet potato tubers (June to July), the maximum temperature and diurnal temperature differences were greater in 2023 than in 2024. In contrast, in 2024, high temperatures were more pronounced in the later reproductive stage, with both the maximum and minimum temperatures surpassing those of 2023. Furthermore, the precipitation levels were significantly higher in 2023 than in 2024. Both years experienced alternating periods of high temperatures, drought, and rainfall following the onset of the rapid expansion phase, with particularly pronounced fluctuations in 2024 (Figure 1).

Figure 1.
Comparison of temperature and rainfall between the two years. The values shown below the figure represent the monthly differences in rainfall between the two years.
3.2. Basic Characteristics of 40 Sweet Potato Varieties
The determination of single tuber weight (Table S2) revealed that in 2023, the average weight of cracked tubers from the ‘S524’, ‘HJ’, and ‘J25’ varieties was significantly lower. Similarly, in 2024, the single tuber weight of cracked tubers from ‘S524’ and ‘W7’ significantly decreased. Conversely, the average weight of cracked tubers in the ‘Y10’, ‘L9’, and ‘Y46’ varieties exceeded that of intact tubers. This trend suggests that cracking predominantly occurs in larger tubers after the reproductive period.
Additionally, orange- and yellow-fleshed varieties presented a greater tendency for cracking than did white- and purple-fleshed varieties. Most white- and purple-fleshed varieties also presented higher starch contents than those of their orange and yellow counterparts. These results further indicated that varieties with lower starch contents were more prone to cracking. Tuber morphology was also a key factor influencing cracking rates. The round tuber varieties presented relatively high cracking rates, likely due to the increased expansion force exerted on the tuber skin as it grows in all directions. Furthermore, varieties with a weak bond between the skin and flesh were more susceptible to cracking. For example, among the eight purple-fleshed varieties tested, ‘N008’ and ‘Y10’ had significantly higher cracking rates. This may be associated with their rounder shape, looser skin attachment, and lower starch content. However, not all varieties followed these trends. For example, the varieties ‘S19’ and ‘X18’, as well as ‘M22’ and ‘Y98’, did not exhibit a clear pattern of cracking or trends contrary to expectations. These findings suggest that, beyond genotype and tuber morphology, other physiological or environmental factors may also contribute to the cracking susceptibility of sweet potato.
3.3. Cracking Types and Classification of Sweet Potato Varieties
Sweet potato cracking can be categorized into three distinct types based on the direction of cracking (Figure 2a): ring cracking, transverse cracking, and longitudinal cracking. Ring cracking was predominantly observed in varieties such as ‘N008’ and ‘W7’, whereas transverse cracking was identified in varieties such as ‘Y10’ and ‘W7’. However, most cracked varieties exhibited longitudinal cracking. Except for transverse cracking, most cracks initiated near the stem end of the tuber. Among the longitudinal cracking types, cracks could be further categorized into end-cracking and side-cracking, with end-cracking being the most common. The study concluded that different types of cracks are influenced by different factors and that the cracking type is strongly correlated with genetic characteristics. In terms of tuber morphology, ring and transverse cracks were more common in thicker, larger tubers, and their occurrence was closely linked to an extended growing season. Consequently, the mean weight of tubers exhibiting these types of cracking was frequently greater than that of uncracked tubers (Table S2 and Table 3), as observed in varieties such as ‘W7’, ‘Y10’, and ‘L9’. Additionally, hierarchical clustering analysis was conducted to classify sweet potato varieties based on cracking rates (Figure 2b). The results grouped ‘N008’, ‘S524’, ‘B553’, and ‘W7’ as extremely fragile varieties, whereas ‘S19’, ‘Y10’, ‘M22’, ‘HJ’, ‘L9’, and ‘Y8’ were categorized as fragile varieties. The remaining cracked and uncracked varieties were grouped separately in the cluster analysis. This suggests that their cracking performance was either relatively unstable at the genetic level or strongly influenced by environmental factors.
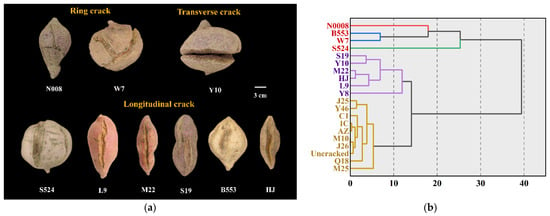
Figure 2.
Types (a) and grades (b) of sweet potato root cracking. In grades (b), red-labeled varieties represent extremely fragile varieties, blue-labeled represent fragile varieties, and orange-labeled indicate other types.

Table 3.
Characteristics of cracking varieties.
3.4. Correlation Analysis Between Variety Characteristics and Cracking Rate
A visual analysis of the distribution map (Figure 3) revealed that highly susceptible varieties share several key characteristics, including a tuber shape index of less than 1.7, a tuber skin hardness of less than 40 N, a tuber skin moisture content greater than 78.5%, a tuber skin thickness of less than 0.22 cm, and a tuber skin starch content of less than 115 mg/g (FW). These characteristics suggest that the physical strength and biochemical properties of the tuber skin play crucial roles in determining the susceptibility of sweet potato skin to cracking. This hypothesis was confirmed by Spearman’s correlation analysis (Figure 4). The cracking rate of sweet potato tubers was significantly correlated with both their morphological and physicochemical characteristics. Specifically, the cracking rate was highly significantly negatively correlated with the tuber length, shape index, skin hardness, flesh hardness, dry matter content of the skin, skin thickness, skin percentage, and starch content of the skin (p < 0.01). Conversely, there was a highly significant positive correlation with the coefficients of variation for skin thickness, skin moisture content, tuber width, and flesh thickness (p < 0.01). The coefficients of variation for flesh hardness, flesh moisture content, and single potato weight were significantly positively correlated, whereas those for flesh dryness, the number of tubers per plant, and flesh starch content were significantly negatively correlated (p < 0.05). Since the S index did not follow a normal distribution, it was excluded from subsequent analyses. Moreover, starch content in sweet potato showed a highly significant positive correlation with dry matter rate (p < 0.01). Specifically, the starch content in the tuber skin was significantly positively correlated with skin hardness (p < 0.05), whereas the starch content in the tuber flesh was highly significantly positively correlated with flesh hardness (p < 0.01). These findings further underscore the critical role of starch in maintaining the structural stability of tuber tissues.
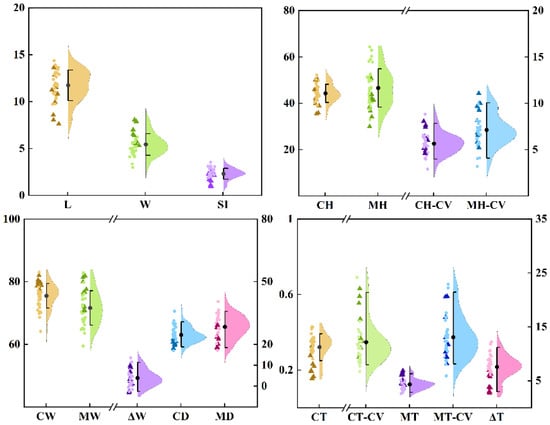
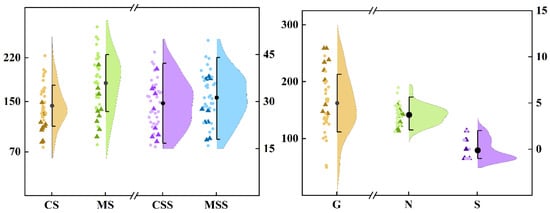
Figure 3.
Performance of 24 traits in 40 germplasm resources. The triangles in the figure represent extremely fragile and fragile varieties, whereas the dots represent other varieties. The index to the left of the abscissa breakpoint uses the left ordinate as the reference line, and vice versa.
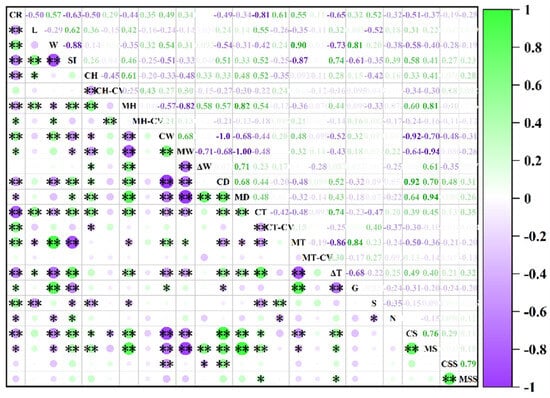
Figure 4.
Correlation analysis of 25 traits. * denotes a significant difference at 0.01 < p < 0.05, ** indicates p < 0.01.
3.5. Cell Structure Characteristics of the Skins of Different Cracked Sweet Potato Varieties
Correlation analyses revealed that the morphological and physiological characteristics of the skins significantly affect the cracking characteristics of sweet potato. To further investigate the underlying mechanisms, three extremely fragile varieties (‘S524’, ‘W7’, and ‘B553’), one fragile variety (‘S19’), and two relatively crack-resistant varieties (‘P32’ and ‘Y198’) were selected for field planting. Their cracking characteristics and skin histological structures were analyzed. Field observations revealed that varieties prone to cracking primarily exhibited noticeable cracking 70–80 days after entering the rapid expansion period, with a continuous increase in cracking (Table 4). Minimal cracking was observed at 60 days, indicating that the cracking of sweet potato tubers is caused predominantly by internal expansion forces generated during tuber growth. To investigate the organizational characteristics of different varieties, this study performed upper-middle section observations on tubers planted in the field for 80 days. The results revealed highly significant differences in skin and periderm thickness between different cracking varieties (p < 0.001). Varieties with poorer cracking resistance had thinner skins and periderms (Figure 5). The periderm, which is the outer protective tissue of the sweet potato, consists of the cork layer, cork cambium, and phelloderm, all of which influence its resistance and storage characteristics. Additionally, more intercellular spaces were observed near the vascular cambium in the secondary phloem of the highly susceptible varieties ‘S524’ and ‘B553’. This could lead to a lower skin hardness and weaker resistance to mechanical impacts, while also reducing the bond strength between the skin and underlying tissues (Table S2). As a result, the skin is more easily separated from the flesh under stress, increasing the risk of cracking. In general, as the sweet potato enters the secondary growth stage, the skin layer (including the endothelium) adjacent to the mid-column sheath, corresponding to the primary bast sieve tube, gradually dissolves and falls off, forming a large solubilized gap. This gap flattens and disappears with the expansion of the middle column. The expansion process is influenced primarily by the activity of the vascular cambium, which undergoes periclinal division in both the inward and outward directions. However, in the ‘S524’ and ‘B553’ varieties, the development of the vascular cambium was slower and incomplete compared to that in the other varieties. In some areas, a complete ring of the formation layer did not form (Figure 5a), which affected the subsequent development of the sweet potato skin. Consequently, the quality of cork cambium and vascular cambium development during the secondary growth stage is crucial in determining the structural stability of the skin. The internal expansion force during the rapid expansion period directly contributes to cracking.

Table 4.
Occurrence of cracking in each period for the six varieties.
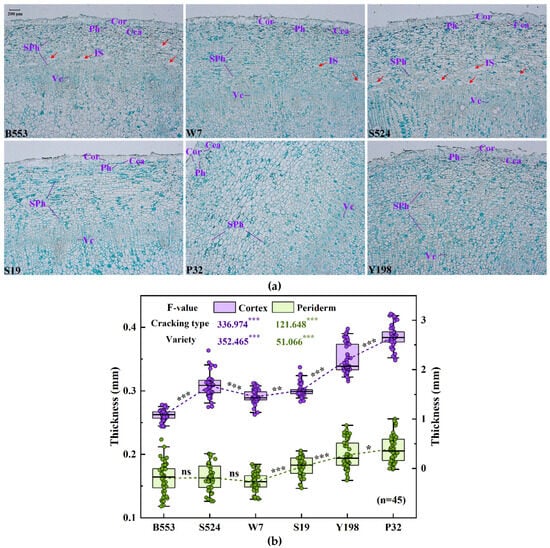
Figure 5.
Cell structure characteristics of the skins of six different sweet potato varieties. (a) A paraffin section of the root, with the following labels: Cor, Cork layer. Cca, Cork cambium. Ph, Phelloderm. SPh, Secondary phloem. Vc, Vascular cambium. IS, Intercellular space. (b) The thickness of the periderm and tuber skin across the six varieties. The asterisks on the mean connection line indicate the significance level of differences between adjacent varieties: * denotes a significant difference at 0.01 < p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, and ns means no significant difference.
3.6. Linear Regression Analysis of the Characteristics and Cracking Rates of Different Varieties
A one-dimensional linear model was fitted to the indicators that exhibited a very significant correlation (p < 0.01) with the cracking rate (Figure 6a). The results indicated that the skin thickness, the coefficient of variation in skin thickness, skin percentage, and tuber shape index had the most significant effects on sweet potato cracking (standardized regression coefficient β > 0.6, p < 0.01). Additionally, multiple linear regression analysis was performed on all indicators with significant correlations (p < 0.05), and the final independent variables were selected based on the adjusted R2 value. The regression coefficients of the fitted model are presented in Table 5. To assess the model’s fit, the cracking rates predicted by the regression equations were compared with the actual values. The results revealed that the P-values of the intercepts (b) and the slopes of the linear (a) fitted equations y’ were greater than 0.05 (Figure 6b), indicating no significant differences. This finding suggests that the fitted model has high accuracy. Therefore, this regression equation can be used to identify key traits influencing sweet potato cracking and serve as a reference for predicting the cracking susceptibility of different varieties. Furthermore, the indicators in the model were standardized, revealing that tuber skin thickness (standardized regression coefficient = −0.8, p = 0.0001) had a very significant negative effect on the cracking rate. In contrast, the coefficient of variation in tuber skin thickness (standardized regression coefficient = 0.243, p = 0.011) had a very significant positive effect. The model’s reliability was further validated via bootstrap resampling with 1000 iterations. The standardized regression coefficient of skin thickness had a 95% confidence interval of [−0.363, −0.273], confirming that this variable consistently exerted a stable influence on the cracking rate.
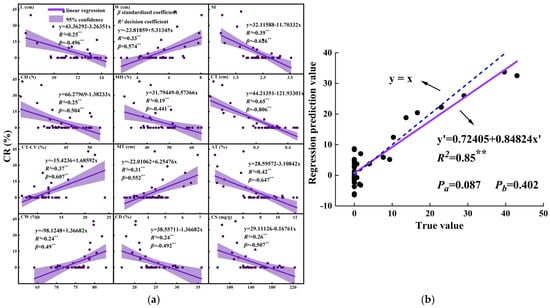
Figure 6.
One-way (a) and multiple (b) linear regression model fitting. ** indicates p < 0.01.

Table 5.
Multiple regression equation parameters and bootstrap test results.
3.7. Construction of a Multimodel Comprehensive Evaluation System for Crack Resistance in Sweet Potato
In this study, 40 sweet potato germplasm samples were assigned scores based on 18 primary screening indicators that were significantly related to sweet potato cracking (Figure 7a) and 10 core indicators identified through multiple linear regression analysis (Figure 7b). The samples were then comprehensively evaluated for their cracking characteristics, and cluster analysis was performed using the predicted results from each model, including those from PCA and the entropy-weighted TOPSIS model (Figure 7). To assess the accuracy of each model’s evaluation, the kappa consistency test was employed. The closer the kappa value is to 1, the greater the agreement between the model predictions and real observations. The results showed that Model A (PCA cumulative variance contribution rate: 79.42%) exhibited moderate consistency with real observations (0.4 < kappa = 0.466 < 0.6), whereas Model B (PCA cumulative variance contribution rate: 70.66%) demonstrated high consistency (0.6 < kappa = 0.752 < 0.8). Moreover, the kappa value of the B integrated evaluation model was higher than that of any single model (Table 6). Consequently, key traits influencing cracking were first identified through multiple linear regression analysis. These traits were then combined with PCA to extract the main feature information. Then, the entropy-weighted TOPSIS model was applied to assign objective weights and support multi-attribute decision-making. This combined approach improved the accuracy of the cracking susceptibility assessment. Furthermore, the predictive performance of the three individual models was compared. The multiple regression model demonstrated the highest predictive accuracy, followed by PCA, and then the entropy-weighted TOPSIS model. Based on the final comprehensive evaluation system (Figure 7b), 40 germplasm samples were classified, and the ‘P32’, ‘Y98’, ‘HY’, ‘ZL’, ‘X37’, ‘X8’, and ‘Q5’ varieties were classified as extremely crack-resistant varieties, while the ‘Z75’, ‘N88’, ‘M9’, ‘H8’, ‘Y25’, ‘G87’, ‘M23’, ‘HM’, and ‘Y198’ varieties are categorized as crack-resistant varieties. Additionally, four varieties, ‘Y46’, ‘IC’, ‘M25’, and ‘AZ’, were grouped within the crack-resistant and extremely crack-resistant categories. However, they were not included in the final crack-resistant classification due to the occurrence of cracked tubers in a two-year cracking survey. However, this does not negate their intrinsic crack resistance characteristics. Since cracking is influenced by numerous factors, even identified crack-resistant varieties may occasionally exhibit cracking behavior. Therefore, this study considers a cracking rate below 5% under normal fertility conditions as an incidental phenomenon rather than an inherent trait. This classification approach enables a more precise evaluation of the cracking tendency of each variety. It also provides a scientific basis for selecting and promoting sweet potato varieties with improved crack resistance.
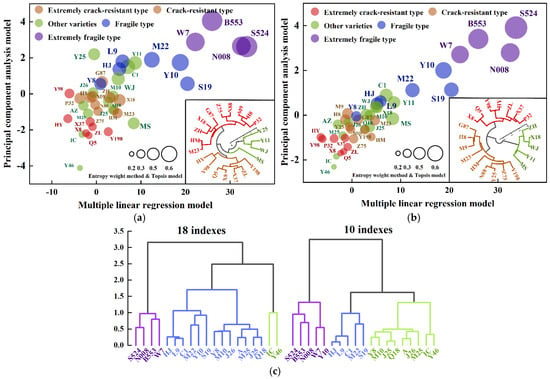
Figure 7.
Comprehensive evaluation of crack-resistant varieties. (a,b) The scoring results of 40 sweet potato varieties for cracking susceptibility, as evaluated using three models; (a) is based on 18 traits significantly correlated with cracking, while (b) uses 10 core indicators selected through multiple linear regression. The mosaic diagram represents a cluster analysis of crack-resistant varieties. (c) The clustering of crack-susceptible varieties using two different models.

Table 6.
Prediction of cracking varieties by each model and the kappa consistency test.
4. Discussion
4.1. Self-Healing and Cracking Characteristics of Sweet Potato
Cracking in sweet potato tubers significantly affects their commercial value, especially in edible varieties. Consumers often reject cracked sweet potatoes due to their compromised appearance, relegating these tubers to use as raw materials for feed or starch processing and thereby reducing economic returns. These findings underscore the importance of investigating the physiological mechanisms and genetic basis of sweet potato cracking. Identifying crack-resistant germplasms is also essential to improve the quality and efficiency of sweet potato production. Sweet potato tubers possess a natural ability to self-repair after wounding through a series of complex physiological and biochemical responses. These include phenylpropanoid metabolism, reactive oxygen species metabolism, and fatty acid metabolism. The healing process involves wound recognition and signal transduction, cell wall repair, cell division, tissue regeneration, antioxidant defense mechanisms, and hormonal regulation [18,19,20]. Following cracking, the healing of sweet potato tissues is marked by the formation of a cork layer and a new periderm, accompanied by the gradual deposition of suberin, lignin, and polyphenolic compounds, which together establish a protective barrier [21]. However, considerable variation in healing capacity exists among different sweet potato varieties [22], which may be a key factor influencing the extent and type of cracking observed. If a variety’s healing ability is insufficient to keep pace with tuber expansion, cracking may worsen or become more pronounced. Furthermore, the formation of wound-healing tissues demands substantial energy while simultaneously maintaining internal metabolic homeostasis to counteract external stressors. In the present study, highly crack-susceptible varieties (e.g., ‘S524’) presented a reduced accumulation of assimilates in their tubers after cracking. This reduction ultimately led to a significant reduction in both individual tuber weight and overall yield (Table S2).
This study identified three primary types of cracking, with longitudinal cracking being the most prevalent. Different types of cracking may result from distinct internal and external factors, a pattern also observed in both fruit crops [23,24] and tuberous crops [25,26]. In fruit, cracking types do not represent sequential stages of a single process but rather arise from a combination of factors such as variations in water uptake pathways, spatial heterogeneity in mechanical properties, and other physiological and environmental influences [27,28]. In sweet potato tubers, longitudinal cracking typically initiates near the stem end, a region that is more actively involved in water uptake and assimilate transport. This increased activity may lead to localized stress concentration, making the area more prone to cracking. In contrast, the middle section of the tuber functions primarily as a storage organ. Studies on fruits have shown that the apical and petiole regions, which contain a high density of vascular bundles, serve as preferential water uptake sites. These regions are more susceptible to expansion-induced stress when soil moisture levels fluctuate drastically, thereby increasing the risk of cracking [29,30]. Additionally, significant variations in the mechanical properties of the pericarp across different regions have been reported [31]. Similarly, in sweet potato, the stem end and middle sections of the tuber present significantly greater hardness and cohesion than the lower portion [32]. The vascular bundles in the tuber are predominantly distributed longitudinally. This structural arrangement, along with mechanical differences, may explain why longitudinal cracking occurs more frequently in these regions. During tuber expansion, sweet potato tubers of different sizes from the same plant expand sequentially. In cracking-prone varieties, cracks often appear in the early stages of expansion and continue to develop as the tuber grows. Ultimately, the largest tubers present the highest incidence of cracking. Sweet potato tubers continue to grow even during the harvest period, unlike many other crops [33]. However, their skin may be partially or fully senesced by this time, increasing susceptibility to cracking. For example, the early-maturing variety ‘L9’ is especially prone to cracking in the late growth stage. Therefore, delayed harvesting and an extended growing period can increase cracking risk, regardless of variety. Future research should focus on uncovering the internal physiological and genetic factors that cause different cracking types. Identifying related varieties and minimizing environmental variability will also be important to reduce cracking.
4.2. Effects of Genetic Factors on Sweet Potato Cracking and Breeding Strategies for Crack Resistance
Cracking in various economically important plant organs is generally considered a quantitative trait controlled by multiple genes and influenced by genetic characteristics [34,35,36]. Genetic analyses have shown that sweet potato cracking is highly heritable (h2 = 0.78), indicating that genetic factors play a predominant role in determining the cracking rate. Similarly, high heritability has been observed in fruit-cracking traits of other plant species [37,38,39]. Furthermore, substantial variations have been identified among different types of cracking [40]. To increase the efficiency of breeding for crack resistance, identifying key genes or quantitative trait loci associated with cracking in sweet potato and elucidating their genetic mechanisms at the molecular level are essential. Correlation and linear regression analyses in this study demonstrated that the skin plays a critical role in crack resistance, whereas the physicochemical properties of the flesh determine the magnitude of expansion forces exerted on the skin. Specifically, skin thickness, hardness, and starch content showed a highly significant negative correlation with the cracking rate, whereas the coefficients of variation (CV) for skin thickness and water content exhibited a highly significant positive correlation. Notably, significant differences in the mechanical strength of the pericarp were observed among varieties with varying degrees of cracking resistance. Crack-resistant varieties exhibit superior tensile strength and ductility [41,42]. Additionally, the thickness and structural stability of the periderm and secondary phloem are influenced by the development of the cork cambium and vascular cambium. In varieties such as ‘S524’ and ‘B553’, incomplete vascular cambium development resulted in thin and uneven skin thickness, with a high coefficient of variation (CV > 7.4%). This structural irregularity led to an uneven internal pressure distribution on the skin, thereby increasing the risk of tuber cracking. Furthermore, vascular cambium developmental defects contributed to the formation of numerous intercellular spaces, weakening the mechanical strength of the tissue (CH < 40 N) and increasing the skin water content (CW > 78.5%). Consequently, an elevated water content increased the cell expansion pressure and induced significant fluctuations in the osmotic potential during water uptake or loss, leading to uneven tissue stretching and an increased risk of cracking. Moreover, previous studies have demonstrated that the application of a high concentration of ethylene (2 g/L) on the surface of sweet potato enhances the activity of tuber cell wall hydrolases. This results in a reduction in the protopectin content and decreased cell wall ductility. These biochemical changes increase the susceptibility of tubers to cracking [15].
In potato tubers, dry matter and starch contents are closely associated with rheological properties. As the starch content increases, the resistance of tubers to cracking and mechanical shock also improves [43]. In contrast, sweet potato tubers are generally more prone to cracking, which may be attributed to differences in tissue structure and water metabolism. Higher starch accumulation has been found to reduce the proportion of free water in cells (correlation coefficient r = −0.94), thereby mitigating turgor pressure fluctuations and osmotic potential changes in response to soil moisture variation [44]. Based on this, it is hypothesized that increasing the starch content of sweet potato flesh to above 15% (FW) could help stabilize internal osmotic conditions, reduce abrupt expansion pressure, and ultimately lower the risk of cracking. This study further revealed that round-shaped varieties were more susceptible to cracking. A significantly positive correlation was observed between the cracking rate and flesh thickness, whereas a very significant negative correlation was detected with both the tuber length and the shape index. This may be explained by the accumulation of circumferential stresses due to restricted longitudinal growth, which accelerates lateral expansion and increases the likelihood of cracking. In contrast, long-shaped varieties (shape index > 1.7) appeared to mitigate cracking risk by dispersing axial stress more effectively. Based on these findings, cultivation practices should prioritize the selection of loose soil conditions that promote longitudinal tuber growth and favor the cultivation of stable, long fusiform varieties. In addition, enhancing the toughness of sweet potato skin and regulating the expansion rate during the rapid growth phase are key to improving crack resistance. Calcium has been identified as a critical factor in enhancing a plant’s resistance to cracking [45,46,47]. Calcium ions, particularly during cell wall formation, contribute to increased mechanical strength by reinforcing cell wall structure and regulating the activity of cell wall metabolic enzymes [48,49]. Chen et al. [12] conducted a multisite demonstration experiment that revealed that the application of calcium sulfate could enhance the toughness of sweet potato stems and skins. This treatment significantly reduced the incidence of cracking and simultaneously improved yield and marketability.
In this study, key traits influencing sweet potato cracking were identified using a multiple regression model. A comprehensive evaluation system for cracking resistance was subsequently developed by integrating principal component analysis with the entropy-weighted TOPSIS model. The model demonstrated a high level of accuracy (kappa = 0.752) and ultimately identified seven genotypes with extreme cracking resistance and nine with moderate resistance. These findings provide a solid foundation for future research aimed at uncovering the physiological and genetic mechanisms underlying sweet potato cracking and improving resistant cultivars. The model will be further optimized to predict the cracking sensitivity of diverse sweet potato germplasm resources. This will be achieved by expanding the sample size and trait parameters. Additionally, multilocation field trials and cross-validation techniques will be conducted to enhance the model’s robustness and predictive accuracy.
5. Conclusions
In this study, we systematically analyzed the genetic characteristics, anatomical structure, and key influencing factors associated with sweet potato cracking using 40 varieties and 24 measured traits. Our results indicate that cracking is a highly heritable trait (h2 = 0.78), which is influenced primarily by skin thickness and uniformity, mechanical strength, the physicochemical properties of the flesh, and water metabolism in tuberous roots. Among these factors, skin thickness (standardized regression coefficient = −0.8) and consistency (standardized regression coefficient = 0.243) were found to be particularly critical, showing a strong negative correlation with the cracking rate. Histological analysis further revealed that developmental defects in the vascular cambium and cork cambium during early growth stages led to uneven skin structure and reduced mechanical strength, thereby increasing cracking susceptibility. Additionally, increased starch content in the flesh (>15%) and an elongated tuber morphology (shape index > 1.7) may mitigate cracking risk by stabilizing internal expansion pressure and promoting uniform stress distribution. To evaluate cracking resistance, a comprehensive assessment model was developed using multiple regression analysis, PCA, and the entropy-weighted TOPSIS method, which identified seven extremely crack-resistant and nine moderately crack-resistant genotypes. The model exhibited robust stability and predictive accuracy (kappa = 0.752, p = 5 × 10−6), as validated by actual cracking data collected over two consecutive years at the same location. From a cultivation management perspective, the findings highlight the importance of selecting cracking-resistant varieties and optimizing soil conditions, such as promoting loose, well-aerated soil layers, to support the stable expansion of tuberous roots. Furthermore, regulating tuber expansion rates, improving water and fertilizer management, and enhancing skin mechanical strength are key strategies for mitigating cracking risk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11060674/s1, Table S1. Sources of 40 sweet potato varieties. Table S2. Characteristics of 40 sweet potato varieties.
Author Contributions
Conceptualization, J.L., X.Z., S.Z., Z.R. and C.Y.; methodology, J.L., F.D., X.Z., S.Z., Z.R., and C.Y.; investigation, J.L.; data curation, J.L., X.Z., Q.W. and C.Y.; writing—original draft preparation, J.L.; writing—review and editing, J.L., F.D., X.Z., Y.Q., S.Z., Z.R., Q.W. and C.Y.; supervision, F.D., X.Z., Y.Q., S.Z., Z.R., Q.W. and C.Y.; funding acquisition, F.D., X.Z., Q.W., and C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Sichuan Potato Innovation Team Project of the Chinese Modern Agricultural Industrial Technology System (SCCXTD-2024-09). Additional support was provided by the earmarked fund for CARS-10-Sweetpotato.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas (L.) Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Mu, T.H.; Li, P.G. Sweet potato: Origin and production. In Sweet Potato; Academic Press: Cambridge, MA, USA, 2019; pp. 5–25. [Google Scholar] [CrossRef]
- Manzoor, M.A.; Xu, Y.; Xu, J.; Shah, I.H.; Sabir, I.A.; Wang, Y.; Zhang, C. Horticulture crop under pressure: Unraveling the impact of climate change on nutrition and fruit cracking. J. Environ. Manag. 2024, 357, 120759. [Google Scholar] [CrossRef] [PubMed]
- Butani, A.; Purohit, H.P.; Solanki, R.; Mishra, P.; Dadhaniya, D. A chronic problem of fruit cracking in fruit crops: A review. Acta Sci. Agric. 2019, 3, 270–274. [Google Scholar]
- Hammett, L.K. Effects of late-season nitrogen and foliar calcium applications on sweet potatoes. HortScience 1981, 16, 336–337. [Google Scholar] [CrossRef]
- Du, W.J.; Yang, H.L. Cause analysis of sweet potato cracking. Mod. Rural. Sci. Technol. 2019, 5, 21–22. [Google Scholar] [CrossRef]
- Onunka, N.A.; Njoku, J.C.; Ehisianya, C.N. Integrating agronomic practices for the mitigation of crack formation on sweet potato roots at Umudike, south eastern Nigeria. Univers. J. Agric. Res. 2017, 5, 213–218. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Ao, L.L.; Rao, W.H.; Yu, Z.G. Causes of sweet potato cracking and comprehensive prevention and control measures. Sci. Breed. 2019, 10, 20–21. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Clark, C.A.; Wright, V.L. Effect and reproduction of Rotylenchulus reniformis on sweet potato selections. J. Nematol. 1983, 15, 197. [Google Scholar] [PubMed Central]
- Lockley, R.A.; Beacham, A.M.; Grove, I.G.; Monaghan, J.M. Postharvest temperature and water status influence postharvest splitting susceptibility in summer radish (Raphanus sativus L.). J. Sci. Food Agric. 2021, 101, 536–541. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.J.; Wu, Q.W.; Zhu, Y.Y.; Li, Z.F. Causes and prevention of cracks in sweet potato tubers. Bull Agric. Sci. Tech. 2012, 10, 88–89. [Google Scholar] [CrossRef]
- Koyanagi, A.; Nakatani, M.; Watanabe, Y. Studies on the causes of sweet potato root cracking. J. Crop Sci Soc. Jpn. 1987, 56, 190–197. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Jia, H. Analysis of the causes of the formation of malformed tuber roots of sweet potato and the countermeasures for prevention and control. Mod. Rural. Sci. Technol. 2022, 29, 10. [Google Scholar] [CrossRef]
- Cheng, S.; Jin, M.M.; He, X.Y.; Liu, J.; Zhang, M. Effects of exogenous ethylene treatment on sweet potato fruit cracking and cell wall metabolism. Food Ferment. Ind. 2020, 46, 161–166. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, R.; Yao, S.; Wang, Y.; Wang, J.; Wan, S.; Hu, W.; Chen, T.; Zhang, L. Magnesium fertilizer application increases peanut growth and pod yield under reduced nitrogen application in southern China. Crop J. 2024, 12, 915–926. [Google Scholar] [CrossRef]
- Tang, C.; Xu, Y.; Zhang, R.; Mo, X.; Jiang, B.; Wang, Z. Comprehensive quality assessment of 296 sweetpotato core germplasm in China: A quantitative and qualitative analysis. Food Chem. X 2024, 24, 102009. [Google Scholar] [CrossRef]
- Xin, Q.; Sun, J.; Feng, X.X.; Zhao, Z.Z.; Liu, B.D.; Jiang, L.H.; Hao, G.F. Effect of rapid heat treatment on wound healing and metabolic mechanism in sweet potato. Food Sci. 2022, 43, 228–238. [Google Scholar] [CrossRef]
- Panda, V.; Sonkamble, M.; Patil, S. Wound healing activity of Ipomoea batatas tubers (sweet potato). Funct. Foods Health Dis. 2011, 1, 403. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Peng, C.; Shang, X.; Lv, X.; Sun, J.; Li, C.; Wei, L.; Liu, X. Postharvest benzothiazole treatment enhances healing in mechanically damaged sweet potato by activating the phenylpropanoid metabolism. J. Sci. Food Agric. 2020, 100, 3394–3400. [Google Scholar] [CrossRef]
- Xuan, H.; Cheng, J.; Pang, L.; Yin, L.; Guan, Y.; Cheng, J.; Lu, X.; Lu, G. Physiological–biochemical characteristics and a transcriptomic profiling analysis reveal the postharvest wound healing mechanisms of sweet potatoes under ascorbic acid treatment. Foods 2024, 13, 2569. [Google Scholar] [CrossRef]
- Van Oirschot, Q.E.A.; Rees, D.; Aked, J.; Kihurani, A. Sweetpotato cultivars differ in efficiency of wound healing. Postharvest Biol. Technol. 2006, 42, 65–74. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Zhao, X.; Zhao, Y.; Hao, Z.; Luo, H.; Yuan, Z. Advances in mechanisms and omics pertaining to fruit cracking in horticultural plants. Agronomy 2021, 11, 1045. [Google Scholar] [CrossRef]
- Balbontín, C.; Ayala, H.; Bastías, R.M.; Tapia, G.; Ellena, M.; Torres, C.; Yuri, J.A.; Quero-García, J.; Ríos, J.C.; Silva, H. Cracking in sweet cherries: A comprehensive review from a physiological, molecular, and genomic perspective. Chil. J. Agric. Res. 2013, 73, 66–72. [Google Scholar] [CrossRef]
- Manzoor, A.; Bashir, M.A.; Naveed, M.S.; Cheema, K.L.; Cardarelli, M. Role of different abiotic factors in inducing pre-harvest physiological disorders in radish (Raphanus sativus). Plants 2021, 10, 2003. [Google Scholar] [CrossRef]
- Lippert, F. Cracking symptoms of kohlrabi tubers. J. Plant Dis. Prot. 1999, 106, 512–516. Available online: https://www.jstor.org/stable/43215324 (accessed on 5 June 2025).
- Measham, P.F.; Bound, S.A.; Gracie, A.J.; Wilson, S.J. Incidence and type of cracking in sweet cherry (Prunus avium L.) are affected by genotype and season. Crop Pasture Sci. 2009, 60, 1002–1008. [Google Scholar] [CrossRef]
- Measham, P.F.; Gracie, A.J.; Wilson, S.J.; Bound, S.A. Vascular flow of water induces side cracking in sweet cherry (Prunus avium L.). Adv. Hortic. Sci. 2010, 24, 243–248. [Google Scholar] [CrossRef]
- Measham, P.F.; Wilson, S.J.; Gracie, A.J.; Bound, S.A. Tree water relations: Flow and fruit. Agric. Water Manag. 2014, 137, 59–67. [Google Scholar] [CrossRef]
- Peschel, S.; Knoche, M. Characterization of microcracks in the cuticle of developing sweet cherry fruit. J. Am. Soc. Hortic. Sci. 2005, 130, 487–495. [Google Scholar] [CrossRef]
- Yu, J. Study on Grapefruit Cracking and Its Regulation Mechanism by Exogenous Calcium. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2020. [Google Scholar] [CrossRef]
- Chen, L. Evaluation of Texture Properties of Sweet Potato Tubers. Master’s Thesis, Zhejiang Agriculture and Forestry University, Hangzhou, China, 2013. [Google Scholar] [CrossRef]
- Han, Y.; Qu, X.; Huang, D.; Wu, L.P. Calculation of loss rate and analysis of influencing factors in sweet potato harvest. Southwest China J. Agric. Sci. 2019, 32. [Google Scholar] [CrossRef]
- Yu, X.; Choi, S.R.; Chhapekar, S.S.; Lu, L.; Ma, Y.; Lee, J.Y.; Hong, S.; Kin, Y.Y.; Oh, S.H.; Lim, Y.P. Genetic and physiological analyses of root cracking in radish (Raphanus sativus L.). Theor. Appl. Genet. 2019, 132, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Capel, C.; Yuste-Lisbona, F.J.; López-Casado, G.; Angosto, T.; Cuartero, J.; Lozano, R.; Capel, J. Multi-environment QTL mapping reveals genetic architecture of fruit cracking in a tomato RIL Solanum lycopersicum × S. pimpinellifolium population. Theor. Appl. Genet. 2017, 130, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 2015, 37, 1718. [Google Scholar] [CrossRef]
- Crump, W.W.; Peace, C.; Zhang, Z.; McCord, P. Detection of breeding-relevant fruit cracking and fruit firmness quantitative trait loci in sweet cherry via pedigree-based and genome-wide association approaches. Front. Plant Sci. 2022, 13, 823250. [Google Scholar] [CrossRef]
- Mustafa, M.; Syukur, M.; Sutjahjo, S.H. Inheritance of radial fruit cracking resistance in tomatoes (Solanum lycopersicum L.). IOP Conf. Ser. Earth Environ. Sci. 2019, 270, 012032. [Google Scholar] [CrossRef]
- Qi, Z.; Li, J.; Raza, M.A.; Zou, X.; Cao, L.; Rao, L.; Chen, L. Inheritance of fruit cracking resistance of melon (Cucumis melo L.) fitting E-0 genetic model using major gene plus polygene inheritance analysis. Sci. Hortic. 2015, 189, 168–174. [Google Scholar] [CrossRef]
- Quero-García, J.; Letourmy, P.; Campoy, J.A.; Branchereau, C.; Malchev, S.; Barreneche, T.; Dirlewanger, T. Multi-year analyses on three populations reveal the first stable QTLs for tolerance to rain-induced fruit cracking in sweet cherry (Prunus avium L.). Hortic. Res. 2021, 8, 136. [Google Scholar] [CrossRef]
- Brüggenwirth, M.; Knoche, M. Mechanical properties of skins of sweet cherry fruit of differing susceptibilities to cracking. J. Amer. Soc. Hort. Sci. 2016, 141, 162–168. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Z.; Zhang, C.; Hu, E.; Zhou, R.; Jiang, F. The composition of pericarp, cell aging, and changes in water absorption in two tomato genotypes: Mechanism, factors, and potential role in fruit cracking. Acta Physiol. Plant. 2016, 38, 1–16. [Google Scholar] [CrossRef]
- Koch, M.; Naumann, M.; Pawelzik, E. Cracking and fracture properties of potato (Solanum tuberosum L.) tubers and their relation to dry matter, starch, and mineral distribution. J. Sci. Food Agric. 2019, 99, 3149–3156. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, B.; Gu, M.; Lee, U.Y.; Kim, M.S.; Jung, S.K.; Choi, H.S. Course of fruit cracking in ‘Whansan’ pears. Hortic. Environ. Biotechnol. 2020, 61, 51–59. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Wang, R.; Zeng, Y.; Kang, L.; Chen, Z. Nano-calcium alleviates the cracking of nectarine fruit and improves fruit quality. Plant Physiol. Biochem. 2023, 196, 370–380. [Google Scholar] [CrossRef]
- Bakeer, S.M. Effect of ammonium nitrate fertilizer and calcium chloride foliar spray on fruit cracking and sunburn of Manfalouty pomegranate trees. Sci. Hortic. 2016, 209, 300–308. [Google Scholar] [CrossRef]
- Kafle, G.K.; Khot, L.R.; Zhou, J.; Bahlol, H.Y.; Si, Y. Towards precision spray applications to prevent rain-induced sweet cherry cracking: Understanding calcium washout due to rain and fruit cracking susceptibility. Sci. Hortic. 2016, 203, 152–157. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, M.; Bai, M.; Xu, Y.; Fan, S.; Yang, G. Effect of calcium on relieving berry cracking in grape (Vitis vinifera L.) ‘Xiangfei’. Peer J. 2020, 8, e9896. [Google Scholar] [CrossRef]
- White, P.J. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).