Abstract
Germination ability and seedling development of grape (Vitis vinifera L.) seeds show significant differences depending on cultivar characteristics and germination conditions, and this situation is known to create significant difficulties in grape breeding programs and vegetative propagation. In this study, we explored the effects of different concentrations of sodium nitroprusside (SNP; 500–3000 ppm) and gibberellic acid (GA3) on seed germination and seedling growth in several grape cultivars. Our findings show that cultivar, treatment type, and their interaction had significant effects on both germination and growth. The 5 BB rootstock stood out with consistently high germination rates, reaching up to 95% with 1500 ppm SNP. Overall, SNP treatments outperformed both the control and GA3 applications, although the most effective concentration differed by cultivar. The most beneficial SNP doses ranged between 1000 and 3000 ppm, with 1500 ppm yielding the highest improvement, up to a 21.6% increase compared to the control. Notably, the ‘Çeliksu’ cultivar responded strongly to SNP, while ‘Rizpem’ showed weak germination, regardless of treatment. Seedling growth, as measured by plant height and node number, was also influenced by both treatment and cultivar, with 5 BB again showing the most robust development. Multivariate analyses revealed strong correlations across germination dates and growth traits. Higher SNP concentrations (1500–3000 ppm) consistently promoted better germination and seedling vigor than GA3 and untreated controls. These results highlight the importance of considering cultivar-specific responses and suggest that well-calibrated SNP applications could be a valuable tool for improving seed-based propagation in grape breeding programs.
1. Introduction
More than 90% of the grape cultivars grown in Turkey belong to Vitis vinifera L. and its hybrids, which dominate national viticulture due to their historical, commercial, and agronomic significance [1]. However, V. vinifera cultivars exhibit pronounced susceptibility to foliar diseases such as downy and powdery mildew, especially under the humid summer conditions prevalent in the Black Sea region [2,3]. This vulnerability restricts their cultivation in these ecological zones, necessitating the exploration and adoption of alternative grapevine species better adapted to the climatic conditions of this region. Among these alternatives, Vitis labrusca L. has gained significant attention due to its superior adaptability to humid environments, robust disease resistance, and desirable fruit traits [4]. The species is characterized by thick-skinned berries, a pronounced “foxy” aroma, high seed content, and elevated antioxidant capacity, which make it suitable for both fresh consumption and processing industries [5,6]. Furthermore, its inherent resistance to fungal pathogens like Plasmopara viticola and Uncinula necator provides a distinct advantage in reducing reliance on chemical plant protection agents [7]. As a result of local selection programs, five V. labrusca cultivars (‘Rizessi’, ‘Rizpem’, ‘Ülkemiz’, ‘Çeliksu’, and ‘Rizellim’) have been officially registered in the province of Rize, Turkey [8]. These cultivars represent a valuable genetic pool for future breeding strategies aimed at improving grapevine resilience and fruit quality in challenging climatic regions. Given the importance of these traits for adaptation to various environmental stresses, the ability to propagate these cultivars effectively becomes critical. To utilize this genetic potential effectively in breeding programs, appropriate propagation techniques are essential.
One of these techniques, seed propagation, remains a foundational method in grapevine breeding, particularly for the generation of segregating populations in interspecific hybridization programs [8,9]. However, the success of such breeding efforts is highly dependent on the viability and germinability of seeds, which are often limited by strong physiological dormancy in grapevine seeds [10]. This dormancy is defined as the failure of a viable seed to germinate under optimal environmental conditions, primarily due to seed-coat impermeability, underdeveloped embryos, or imbalances in endogenous hormone levels [11]. To overcome seed dormancy, several physical, chemical, and hormonal pre-treatments have been employed, such as cold stratification, scarification, and the application of plant growth regulators like GA3 [12,13]. GA3, in particular, is widely used for its proven ability to stimulate germination by promoting enzymatic degradation of seed endosperm and enhancing embryo elongation [14,15]. Nevertheless, excessive doses of GA3 may lead to undesirable outcomes, such as abnormal seedling growth or suppressed root development, thereby compromising overall plant quality [16]. In recent years, on the other hand, the role of signaling molecules such as nitric oxide (NO) in plant developmental and stress response mechanisms has garnered increasing interest. NO, a reactive free radical gas, functions as a critical regulator in various physiological processes, including seed dormancy release, germination, root development, and flowering [17,18]. Sodium nitroprusside (SNP), a commonly used NO donor, has been shown to effectively alleviate seed dormancy in several plant species by modulating hormonal balance (e.g., enhancing GA/ABA ratio), weakening seed-coat barriers, and stimulating ethylene biosynthesis [19,20]. Studies also indicate that NO interacts with reactive oxygen species (ROS), enhancing antioxidant responses that further facilitate successful germination [6]. These multifaceted roles of NO underscore its potential application in viticulture to improve seed germination rates and seedling vigor, especially in cultivars exhibiting strong dormancy traits.
Thus, evaluating the effects of SNP at various concentrations on grape seed germination and seedling development can provide essential insights for breeding programs. Such research not only contributes to our understanding of dormancy regulation in grape seeds but also offers practical implications for improving propagation efficiency in Vitis labrusca cultivars selected under the agroecological conditions of the Black Sea region. Given the challenges posed by seed dormancy in grapevine breeding programs, especially under humid, temperate climates, tailored approaches are essential. Therefore, comparative assessments of seed germination across different cultivars and treatments can provide valuable insights for the optimization of propagation strategies. In this regard, the present study aims to evaluate the germination performance of seeds from five Vitis labrusca cultivars (‘Rizessi’, ‘Rizpem’, ‘Ülkemiz’, ‘Çeliksu’, and ‘Rizellim’), Vitis vinifera L. cultivar ‘Alphonse Lavallée’, and the 5 BB rootstock. This study compares the effects of different concentrations of sodium nitroprusside (SNP) on grape seed germination with two conventional methods: 60-day cold stratification and 1000 ppm GA3 application. Our objectives were (I) to evaluate how SNP doses affect the germination rate, viability, emergence, seedling length, and node number; (II) to compare SNP treatments with standard stratification and GA3 application; and (III) to identify cultivar-specific responses to SNP and support selection in breeding efforts.
2. Materials and Methods
Plant Material
This study employed seeds from five Vitis labrusca L. cultivars (‘Rizessi’, ‘Rizpem’, ‘Ülkemiz’, ‘Çeliksu’, and ‘Rizellim’) registered in 2016 [7], one Vitis vinifera L. cultivar (‘Alphonse Lavallèe’), and the 5 BB rootstock (V. berlandieri × V. riparia) (Figure 1).

Figure 1.
Images of the grape cultivars selected for use in the seed germination trial and the processes of manually removing and drying the seeds from the berries, preparing the solution, and applying it to the seeds.
This study employed seeds from five V. labrusca L. cultivars (‘Rizessi’, ‘Rizpem’, ‘Ülkemiz’, ‘Rizellim’, and ‘Çeliksu’), one V. vinifera L. cultivar (‘Alphonse Lavallèe’), and one rootstock (5 BB). The V. labrusca cultivars were harvested from the foundation vineyard of the Department of Horticulture at Ondokuz Mayıs University (OMU), while ‘Alphonse Lavallèe’ and 5 BB rootstock were obtained from the vineyards of the Tokat Gaziosmanpaşa University (TOGU) Application and Research Center. Seeds from all V. labrusca cultivars were harvested simultaneously in the final week of September 2020, while ‘Alphonse Lavallèe’ and 5 BB rootstock were collected in October 2020. The time between harvesting and germination trial initiation was approximately 4 months for V. labrusca cultivars and 3 months for ‘Alphonse Lavallèe’ and 5 BB rootstock. Seed characteristics varied among genotypes: V. labrusca cultivars typically contained 2–3 seeds per berry, ‘Alphonse Lavallèe’ contained 3–4 seeds per berry (with an average berry weight of 6 g), and 5 BB rootstock produced smaller fruits with 1–2 seeds per berry. Seeds were extracted manually from berries, washed thoroughly with tap water, dried at room temperature (22 ± 2 °C) for 48 h, and stored in sealed glass jars at +4 °C until stratification began in January 2021. The ‘Rizessi’ cultivar produces dark bluish–black berries with an average productivity of 10.69 kg per vine. ‘Rizpem’ yields medium-sized, pink to dark-reddish–purple berries at 5.51 kg per vine. ‘Ülkemiz’ features large bluish–black berries with average vine productivity of 4.93 kg. ‘Rizellim’ exhibits a high average yield of 11.43 kg per vine, while ‘Çeliksu’ yields 7.95 kg per vine [7]. The V. vinifera cultivar ‘Alphonse Lavallèe’ forms large (400–600 g), conical clusters with purplish–black berries, typically yielding 1400–1600 kg/ha, with maturity occurring between late August and early September [7]. The 5 BB rootstock (V. berlandieri × V. riparia) is known for vigorous growth, nematode resistance, and tolerance to 20% active lime [7].
Methods
Seed Stratification
The seeds were stored dry at +4 °C in sealed jars immediately after harvest in October 2020. However, the actual stratification process began later, on 25 January 2021, to ensure a defined cold, moist stratification period necessary to break seed dormancy effectively. Moist stratification simulates natural winter conditions by maintaining seeds in a moist medium at low temperature, which enhances germination. Thus, dry cold storage served as temporary preservation, while moist stratification was conducted separately for 60 days at +4 °C, with moisture levels checked and replenished every 15 days as needed [21].
Seed Viability Test
After completing the 60-day moist stratification period on 25 March 2021, seeds were carefully separated from the perlite using a sieve and thoroughly rinsed with tap water. The viability of seeds was then assessed using the flotation method, in which seeds were placed in water and observed for buoyancy: sinking seeds were considered viable, while floating seeds were regarded as non-viable, following the protocol described in [22]. Seeds were immersed in water for 30 min to ensure accurate differentiation between viable and non-viable seeds. This viability test was conducted after stratification to evaluate the proportion of seeds that remained viable following the cold, moist treatment, which is known to affect dormancy release and seed condition. This approach followed protocols established in seed physiology studies, where viability is best assessed post stratification to reflect potential germination capacity. The viability percentage was calculated using the following formula:
Viability (%) = 100 × (Total seeds–Floating seeds)/Total seeds.
This test was conducted using a completely randomized design with three replicates of 200 seeds.
Chemical Treatments
Gibberellic acid (GA3; commercial name: LENAGIBB; 20 g/L GA3) and sodium nitroprusside (SNP) were obtained from the TOGU Horticulture Laboratory and used for treatments. Seven treatment groups were established: a control group with 60-day moist stratification but no chemical treatment, 1000 ppm GA3, and five SNP concentrations (500, 1000, 1500, 2000, and 3000 ppm) [23]. In addition, an unstratified control (no stratification, no chemical treatment) was included in preliminary trials to evaluate the effects of stratification itself; however, it showed negligible germination and was therefore excluded from the main experiment. Stratified seeds were soaked in the respective chemical solutions at room temperature (22 ± 2 °C) for 48 h. Following soaking, seeds were rinsed with distilled water before sowing [23,24]. During soaking the temperature was continuously monitored and maintained at room temperature. For all cultivars, germination was defined as the emergence of a radicle exceeding 2 mm in length, a threshold commonly used in seed germination studies [23,24].
Germination Experiment
Germination was carried out in Petri dishes lined with moistened filter paper placed in a growth chamber maintained at 24 °C and 70% relative humidity, beginning on 31 March 2021. Each treatment included three replicates of 50 seeds, totaling 1050 seeds per cultivar. Fungicide was applied weekly to prevent contamination. Germination was assessed every 5 days for 60 days. The germination rate was calculated as follows:
Germination (%) = 100 × (Germinated seeds/Total seeds)
Emergence Rate, Seedling Height, and Number of Nodes
To evaluate seedling emergence, height, and node count, seeds were sown in 70-cell trays filled with a 1:1 peat–perlite mixture (note corrected spelling: perlite) and covered with vermiculite. Each treatment had three replicates of 70 seeds and was sown on 31 March 2021 in an unheated greenhouse at the TOGU Research Center. The greenhouse temperature was monitored throughout the experiment using data loggers, with average daily temperatures ranging between 18 and 24 °C. Emergence was recorded once shoot tips appeared above the substrate, starting 12 April 2021. The emergence rate was calculated as follows:
Emergence (%) = 100 × (Emerged seeds/Total seeds).
After two months, 15 seedlings from each replicate were randomly selected for measurement of seedling height and node count, using a ruler and manual counting, respectively [21].
Statistical Analysis
Statistical analyses were performed using JMP Pro 13 software (SAS Version 9.4, SAS Institute, Cary, NC, USA). All results are presented as mean ± standard deviation. To better understand the relationships between SNP treatments and measured parameters, correlation analyses were conducted using the SRPLOT online platform (https://www.bioinformatics.com.cn/en, accessed 21 October 2024). A hierarchical clustering heat map was generated to visualize the associations and intensity of responses across treatments and parameters. Additionally, Principal Component Analysis (PCA) was carried out using GraphPad Prism version 9.3.1 (GraphPad Software, LLC, San Diego, CA, USA), and results were interpreted through biplot analysis according to the method described by Evgenidis et al. [25].
3. Results
3.1. Germination Performance in Petri Dishes
Statistical analysis of germination data revealed highly significant effects of cultivar (p < 0.05), treatment (p < 0.05), and cultivar × treatment interaction (p < 0.05) across all measurement dates (Supplementary Material Table S1). The consistently significant interaction effect confirmed that grape cultivars responded differentially to the applied treatments. Initial, on 20 April, the 1500 ppm SNP treatment achieved the highest average germination rate (19.09%), followed by 1000 ppm SNP (16.49%), 2000 ppm SNP (11.74%), and 500 ppm SNP (10.81%). The 3000 ppm SNP treatment resulted in 8.44% germination, while the control group averaged 6.41%. The lowest germination rate was observed under the 1000 ppm GA3 treatment (4.14%). Among cultivars, the highest average germination was observed in ‘5 BB’ (27.57%), followed by ‘Rizellim’ (21.43%) and ‘Çeliksu’ (13.89%). Lower germination rates were recorded for ‘Rizessi’ (8.43%), ‘Ülkemiz’ (7.50%), and ‘Alphonse Lavallée’ (5.50%), while ‘Rizpem’ exhibited the lowest performance (2.21%). Progressive increases in germination rates were observed throughout the experimental period. By 30 April, the highest germination was recorded in 5 BB under 1000 ppm SNP (94.00%), followed by 5 BB under 1500 ppm SNP (90.00%) and 2000 ppm SNP (85.00%). Among the treatments, 1000 ppm SNP (42.26%) was the most effective, while the control (19.73%) showed the lowest performance. The final germination assessment on May 20 demonstrated that the highest rates were achieved in ‘Çeliksu’ and 5 BB under 1000 ppm SNP (92.67%), followed by ‘Rizessi’ under 3000 ppm SNP (92.67%) and ‘Rizellim’ under 1500 ppm SNP (90.33%). The lowest germination was observed in Rizpem with 1500 ppm SNP (16.00%) and 3000 ppm SNP (13.33%). On average, ‘Çeliksu’ (76.67%) achieved the highest final germination rate among varieties, followed by 5 BB (78.67%) and ‘Rizellim’ (66.14%), while ‘Rizpem’ exhibited the lowest performance (22.57%). Among treatments, 3000 ppm SNP (61.00%) and 2000 ppm SNP (58.24%) were most effective, while the control (38.00%) remained least effective (Table 1).

Table 1.
Effects of different SNP and GA3 concentrations on germination rate in various grapevine cultivars in petri dishes.
3.2. Emergence Performance in Seedling Trays
Statistical analysis indicated that the cultivar × treatment interaction was statistically significant (p < 0.05) at all measurement points. Initial emergence rates were substantially lower than those observed in Petri dish conditions, reflecting the more challenging nursery environment. Early assessment showed that SNP treatments yielded the highest emergence rates, with 1500 ppm SNP producing the maximum average emergence (8.78 ± 14.51%), followed by 1000 ppm SNP (7.35 ± 14.15%) and 2000 ppm SNP (5.61 ± 10.40%). Among grape varieties, 5 BB demonstrated significantly superior emergence performance (28.37 ± 14.71%) compared to all other varieties, with the highest individual emergence rate (52.86 ± 2.86%) observed under the 1500 ppm SNP treatment. Progressive improvement in emergence rates was observed throughout the experimental period. By April 30, the 1500 ppm SNP treatment produced the highest average emergence rate (20.51 ± 21.02%), followed by 2000 ppm SNP (19.39 ± 18.34%) and 1000 ppm SNP (19.14 ± 21.73%). Among grape varieties, 5 BB demonstrated substantially superior emergence (53.37 ± 11.27%), with peak performance (65.71 ± 1.43%) under the 1500 ppm SNP treatment. Substantial emergence improvement was observed by May 10, with the 3000 ppm SNP treatment yielding the highest average emergence rate (48.88 ± 24.23%), closely followed by 2000 ppm SNP (47.75 ± 24.81%) and 1500 ppm SNP (45.48 ± 25.10%).
Among varieties, 5 BB exhibited exceptional performance (75.37 ± 8.78%), achieving its highest rate (82.14 ± 7.86%) under the 2000 ppm SNP treatment. The final emergence assessment on June 10 showed that the 1500 ppm SNP treatment achieved the highest average emergence rate (68.33 ± 20.48%), followed by 3000 ppm SNP (67.69 ± 20.40%) and 2000 ppm SNP (67.23 ± 20.03%). These higher SNP concentrations consistently outperformed both the control (61.44 ± 21.02%) and 1000 ppm GA3 (60.99 ± 17.96%) treatments.
Among grape varieties, 5 BB maintained superior emergence performance (85.00 ± 6.62%), reaching its maximum under 2000 ppm SNP (91.43 ± 1.43%). ‘Rizellim’ demonstrated excellent emergence capacity (79.14 ± 4.30%), particularly with 1000 ppm GA3 (84.95 ± 3.33%), while ‘Rizpem’ exhibited the lowest overall emergence (32.89 ± 6.17%) despite showing improvement relative to earlier measurements (Table 2).

Table 2.
Effects of different SNP and GA3 concentrations on the germination rate in various grapevine cultivars in trays.
3.3. Seedling Growth Parameters
Analysis of seedling growth parameters demonstrated significant effects of the cultivar, treatment, and their interaction on both plant length and the number of nodes (p < 0.05). The 3000 ppm SNP treatment produced the tallest plants overall (4.85 ± 1.33 cm), significantly outperforming all other treatments. Among varieties, 5 BB demonstrated superior growth (6.93 ± 1.29 cm) across all treatments, reaching maximum height (9.20 ± 1.17 cm) under control conditions. Treatment-specific responses varied considerably among cultivars. The 1000 ppm GA3 treatment showed the second-best performance (4.52 ± 1.59 cm), followed by 1500 ppm SNP (4.44 ± 1.04 cm) and 2000 ppm SNP (4.33 ± 1.23 cm). Notable variety-specific responses included ‘Rizellim’ displaying excellent growth (4.33 ± 0.66 cm), particularly under 3000 ppm SNP (5.23 ± 1.27 cm), and ‘Alphonse Lavallée’ responding exceptionally well to 1500 ppm SNP (5.28 ± 0.30 cm). ‘Rizpem’ consistently showed the lowest growth potential (2.42 ± 0.25 cm) across all varieties. Node development patterns reflected similar treatment and cultivar effects. The highest node number was observed in 5 BB under control conditions (3.62 ± 0.04), while the lowest was recorded in ‘Rizpem’ with 1000 ppm SNP application (1.30 ± 0.10). Among treatments, 3000 ppm SNP produced relatively high node numbers in most varieties, particularly in ‘Alphonse Lavallée’ (3.96 ± 0.27) and ‘Ülkemiz’ (2.35 ± 0.04). Average node numbers were highest in Alphonse Lavallée (3.26 ± 0.62) and 5 BB (3.10 ± 0.37), while ‘Rizpem’ exhibited the lowest values (1.43 ± 0.16) across all treatment combinations (Table 3).

Table 3.
Effects of SNP and GA3 treatments on plant length and node number in different grapevine cultivars.
3.4. General Evaluation
When the presented PCA graph is analyzed, significant insights are obtained regarding the relationships between various cultivars and measured parameters. PC1 explains 68.95% of the variance, while PC2 accounts for 10.48%, together representing approximately 79.43% of the total variance. A strong positive correlation was observed between the parameters of plant length and the number of nodes. These two parameters also showed a positive relationship with the 5BB rootstock. Notably, the 5BB_CNT sample exhibited the strongest positive correlation with the number of nodes. Regarding germination rates, positive correlations were identified between the germination rates measured at different dates in Petri dishes and seed trays. Germination rates from seed trays on 20 April, 30 April, and 10 May were positively correlated with each other. Similarly, germination rates in Petri dishes on 20 April, 30 April, and 10 May also showed positive intercorrelations. The RZL cultivars, particularly RZL_1500S and RZL_2000S, were positively associated with late-stage germination parameters (20 May and 30 May). In contrast, the RZP cultivars clustered on the negative side of PC1 and showed weak associations with germination parameters. ALP cultivars, especially ALP_3500S and ALP_1000G, were located on the positive side of PC2 but did not exhibit a distinct relationship with germination parameters. ULK cultivars were generally distributed near the center of the plot.
CLK cultivars were mostly clustered on the negative side of PC2 and were moderately associated with germination parameters. Similarly, RZS cultivars were positioned in the negative region of PC2. The strong positive relationship of 5BB cultivars with plant growth parameters (plant length and number of nodes) indicates their superior performance in terms of vegetative development. Overall, the PCA analysis effectively differentiated the cultivars and treatments based on their germination and seedling growth characteristics, revealing distinct grouping patterns. This multivariate approach provided a comprehensive evaluation of the interaction between rootstocks and performance traits, highlighting key genotypic responses across measured variables (Figure 2). Additionally, significant positive correlations were found among germination rates across different dates and conditions. The highest correlation was observed between 20 May and 30 May germination rates in seed trays (r = 0.94), followed by 10 May and 20 May germination rates in Petri dishes (r = 0.93) and 20 May and 30 April germination rates in Petri dishes (r = 0.83). Germination rates in seed trays showed strong correlations with each other (r = 0.73–0.94). Plant length was positively correlated with germination rates, ranging from r = 0.36 (20 April in Petri dishes) to r = 0.76 (10 June in seed trays).
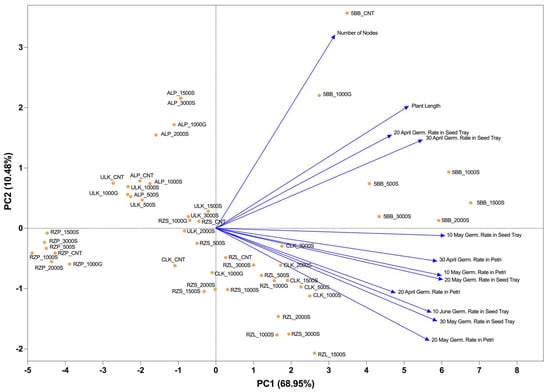
Figure 2.
Biplot analysis of the effects of SNP and GA3 treatments on germination rates.
The number of nodes was moderately correlated with germination parameters (r = 0.12–0.48) and showed the strongest correlation with plant length (r = 0.72) (Figure 3). The heatmap analysis revealed distinct clustering patterns among SNP concentrations, cultivars, and measured parameters. Higher SNP concentrations (3000S) showed negative correlations with germination rates in RZP and ALP cultivars under seed-tray conditions (20–30 May), while moderate concentrations (1500S, 2000S) demonstrated positive associations with plant length and node numbers in ULK and 5BB cultivars. The 5BB samples exhibited consistently high germination performance across all dates and growing media, particularly 5BB_1000S, 5BB_1500S, and 5BB_2000S variants. Conversely, RZP samples displayed generally poor germination rates. ALP variants presented mixed results, with notable strength in 1500S and 3000S concentrations. Temporal patterns revealed that May germination rates were typically higher than April rates, with seed-tray germination outperforming Petri dish results. The 1000G treatments clustered with improved late-stage germination (30 May seed tray) in the ALP and RZP groups. Control groups formed a separate cluster, correlating with lower germination rates and reduced growth metrics. Cultivar-specific responses were observed, with ULK and 5BB demonstrating greater resilience to higher SNP levels than RZP or ALP (Figure 4).
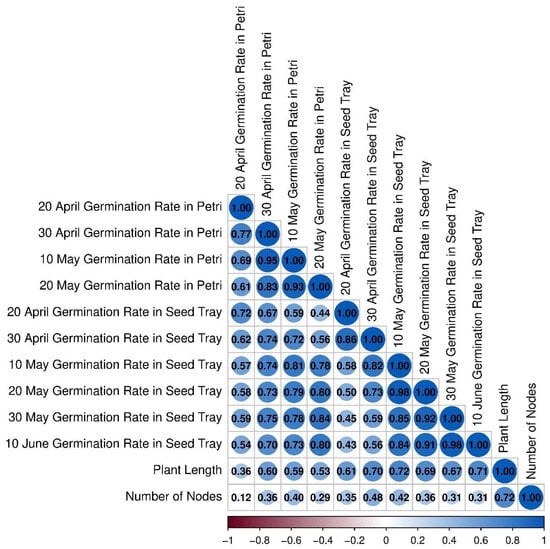
Figure 3.
Correlation matrix of germination rates in response to SNP and GA3 treatments. The intensity and size of circles represent correlation strength, while colors indicate correlation direction (blue for positive and red for negative correlations). Numbers show correlation coefficients (r) ranging from −1.00 to 1.00. Asterisks indicate statistical significance levels: p < 0.05.
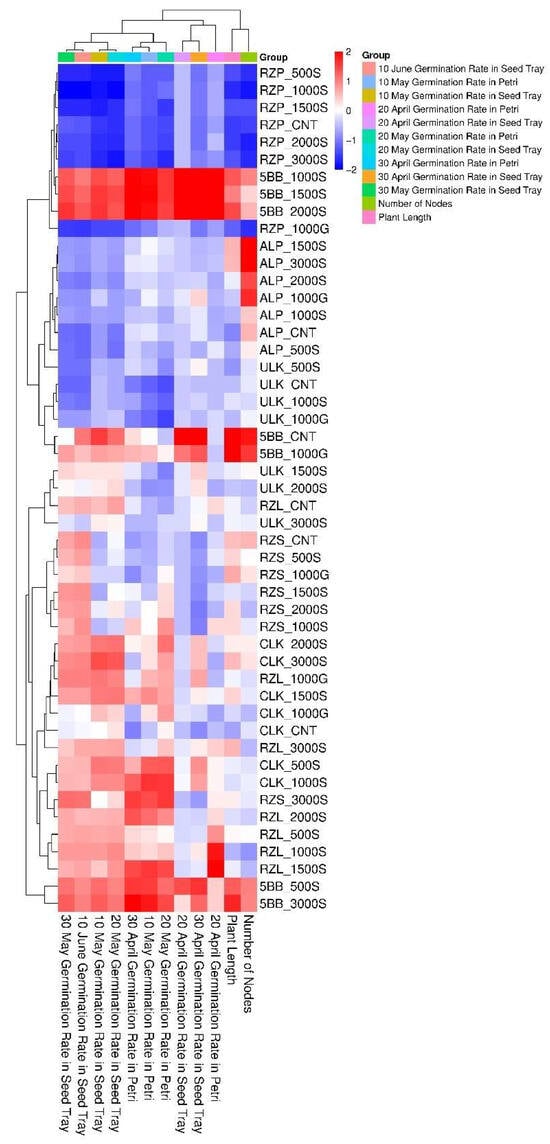
Figure 4.
Hierarchical clustering analysis of germination rates and their responses to SNP and GA3 treatments. The color scale represents parameter values (red for high values and blue for low values). Dendrogram clustering on both axes reveals parameter associations and treatment similarities.
4. Discussion
4.1. Germination Performance in Petri Dishes Across Dates and Treatments
The statistical analysis of germination data across the four measurement periods revealed significant differences among cultivars, treatments, and their interactions (p < 0.05). These findings highlight the complex interplay between genetic factors and exogenous applications of sodium nitroprusside (SNP) and gibberellic acid (GA3) in breaking seed dormancy and promoting germination in grape cultivars. Our results demonstrate a progressive increase in germination rates across all treatments over time, with the most dramatic improvements observed between the first and final measurements. This temporal pattern suggests that all treatments enhance overall germination percentages and accelerate the germination process. Our data reveals particularly striking responses in certain cultivars, with ‘5 BB’ rootstock demonstrating exceptional sensitivity to SNP applications, achieving 90.00% germination with 1500 ppm SNP by 4 weeks after treatment, compared to much lower rates in control conditions. This dramatic response parallels findings from Kara et al. [26], who reported significant germination enhancement in ‘Ekşi Kara’ and ‘Gök Üzüm’ grape cultivars following SNP treatment. The significant cultivar × treatment interaction confirms that grape cultivars respond differentially to exogenous NO application. This genotypic variation in NO response aligns with findings from Bibi et al. [27], who documented similar cultivar-dependent responses in wheat. Our results reveal that the optimal SNP concentration varies considerably among grape cultivars, with 1000–1500 ppm generally proving most effective in the early stages, while higher concentrations (2000–3000 ppm) showed increased effectiveness by the final measurement date. This shift in optimal concentration over time suggests a complex relationship between NO concentration, exposure duration, and dormancy-breaking mechanisms. The concentration dependence we observed parallels findings by Esmail et al. [28], who reported that while lower concentrations of SNP stimulated maximum germination in Lupinus termis, higher concentrations had inhibitory effects. Interestingly, our data showed that ‘Rizessi’ responded exceptionally well to 3000 ppm SNP (92.67% by 6 weeks after treatment), while other cultivars showed optimal responses at lower concentrations, underscoring the cultivar-specific nature of NO signaling pathways. ‘Çeliksu’ showed remarkable improvement, reaching 92.67% germination with 1000 ppm SNP by 6 weeks after treatment, emerging as the cultivar with the highest average germination rate (76.67%) by the end of the experiment. In contrast, ‘Rizpem’ consistently showed minimal responsiveness across all treatments (maximum 22.57% by 6 weeks after treatment), suggesting that this cultivar possesses stronger dormancy mechanisms that may require alternative or combined dormancy-breaking strategies. These findings are consistent with the diverse responses observed in plant species subjected to different dormancy-breaking treatments [11].
A particularly noteworthy finding from our study is the superior performance of SNP treatments compared to traditional GA3 application. By 6 weeks after treatment, SNP treatments at 3000 ppm (61.00%) and 2000 ppm (58.24%) significantly outperformed both GA3 and control treatments, with control conditions consistently yielding the lowest germination rates (38.00% by the final measurement). This suggests that NO-mediated signaling pathways may play a more critical role in dormancy breaking and germination stimulation in some grape cultivars than traditional plant growth regulators, as indicated by Ren et al. [29] in their study on Brassica rapa subsp. chinensis seeds under salt stress conditions. The delayed peak in germination rates suggests that NO likely initiates signaling cascades that gradually overcome dormancy constraints, consistent with observations by Nabaei and Amooaghaie [30] in their work with Catharanthus roseus seeds. The remarkable response of the ‘Çeliksu’ cultivar to SNP application demonstrates the potential of NO-based treatments to overcome dormancy in challenging grape cultivars, similar to findings by Faraji and Sepehri [31], who reported that SNP application significantly improved wheat seed germination under drought stress conditions. The differential response of cultivars to SNP also has important implications for grape breeding programs. As noted by Atak et al. [32], viticulture breeding increasingly focuses on developing seedless cultivars with improved traits, making efficient germination of hybrid seeds crucial. Our findings suggest that SNP treatments could be valuable tools in grape breeding programs to enhance germination rates of valuable hybrid seeds, particularly for crosses involving cultivars with strong dormancy. It is worth noting that the optimal SNP concentration for enhancing germination in grape cultivars is relatively high compared to concentrations reported effective in other species, such as the 150 μM SNP reported by Sepehri and Rouhi [33] for peanut seed germination under drought stress, suggesting that grape seeds may require higher NO concentrations to overcome dormancy, possibly due to their more robust dormancy mechanisms or thicker seed coats that limit NO penetration. The inhibitory effect of higher SNP concentrations observed in some cultivars may be attributed to nitrosative stress, as excessive NO can react with reactive oxygen species to form peroxynitrite, a highly reactive molecule that can damage cellular components [34]. The poor response of the ‘Rizpem’ cultivar across all treatments suggests that this cultivar may possess complex dormancy mechanisms that require additional dormancy-breaking strategies, potentially involving combined approaches, as reported by Bayrak [35] for Hypericum adenotrichum seeds or by Odabaş et al. [36] for Sambucus nigra seeds.
4.2. Germination Performance in Seedling Trays Across Dates and Treatments
The statistical analysis of germination data across all observation dates revealed significant effects of both cultivar and treatment on germination rates, with particularly strong varietal influences, as evidenced by the consistently significant cultivar × treatment interaction (p < 0.05). This interaction underscores the cultivar-dependent nature of responses to SNP treatments, a phenomenon that has been documented in various plant species [27]. Our results demonstrate a progressive increase in germination rates across all treatments from the first observation to the final measurement 12 weeks after treatment, indicating that grape seed dormancy release is a gradual process continuing well after initial treatment application. It should be noted that experiments were conducted in an unheated greenhouse where seasonal environmental changes from April to June, including increasing temperatures and improved light conditions, may have contributed to the temporal improvement in germination rates. This temporal pattern suggests that while SNP can accelerate dormancy breaking, complete release from dormancy in grape seeds occurs over an extended period, similar to the progressive dormancy release reported by Esmail et al. [28] in Lupines termis L. plants. The superior performance of the 5 BB rootstock across all treatments and observation dates (reaching a mean germination rate of 85.00% by 12 weeks after treatment) aligns with findings from Yıldız [37], who similarly reported varietal differences in grape seed germination, with certain cultivars demonstrating inherently higher germination potential than others. The exceptional response of 5 BB to SNP treatments, particularly at 1500–2000 ppm concentrations, reinforces observations by Kara et al. [26], who documented significant improvements in germination rates when grape seeds were treated with SNP. Conversely, the consistently poor performance of the ‘Rizpem’ cultivar (reaching only 32.89% average germination by 12 weeks after treatment) despite all treatments suggests that this cultivar possesses stronger dormancy mechanisms that may require alternative or more aggressive dormancy-breaking strategies. This observation is consistent with Bayrak [35], who found that some seeds with strong dormancy require combination treatments to effectively break dormancy. The concentration-dependent effects of SNP were clearly demonstrated in our results, with germination rates generally increasing with SNP concentrations up to 1500–2000 ppm across most observation dates. By 12 weeks after treatment, the 1500 ppm SNP treatment had achieved the highest average germination rate (68.33%), followed closely by 3000 ppm SNP (67.69%) and 2000 ppm SNP (67.23%). This pattern of response is consistent with the findings of Esmail et al. [28], who reported that moderate concentrations of SNP stimulated maximum germination in Lupinus termis. Similarly, Nabaei and Amooaghaie [30] found that moderate concentrations of SNP enhanced seed germination in Catharanthus roseus under stress conditions, while higher concentrations reduced germination rates. This dose-dependent response pattern suggests the existence of an optimal NO concentration window for effective dormancy breaking and germination stimulation.
The superior performance of SNP treatments over GA3 application across most observation dates and cultivars is particularly noteworthy. By 12 weeks after treatment, all SNP treatments at concentrations between 1500–3000 ppm had consistently produced superior results (67.23–68.33%) compared to GA3 treatment (60.99%). This finding challenges the traditional reliance on GA3 for dormancy breaking in grape seeds, as documented by Uzun et al. [13], who reported 78% germination with GA3 application in ‘Alphonse Lavallèe’ × ‘Regent’ hybrid grape seeds. Our results suggest that SNP may represent a more effective alternative for certain grape cultivars, particularly those that show limited response to conventional GA3 treatment. The mechanism underlying this superior performance may involve NO’s multifaceted roles in cellular signaling, enzyme activation, and oxidative stress regulation, as opposed to GA3’s more specific effects on embryo growth and endosperm weakening [29]. The varietal differences in optimal SNP concentration were clearly evident in our study. While 5 BB rootstock responded optimally to 2000 ppm SNP (91.43% by 12 weeks after treatment), ‘Çeliksu’ demonstrated peak performance at 3000 ppm SNP (84.29%), and ‘Rizesui’ showed the strongest response to 3000 ppm SNP (87.38%). This variability in optimal concentration suggests that different grape cultivars may possess varying sensitivities to NO signaling, possibly due to differences in seed-coat structure, endogenous NO levels, or downstream signaling components. Similar cultivar-dependent optimal concentrations have been reported by Kara et al. [26], who found differential responses to identical SNP treatments between ‘Ekşi Kara’ and ‘Gök Üzüm’ grape cultivars. The remarkable improvement observed in certain cultivars, such as ‘Çeliksu’, reaching 86.43% germination with 3000 ppm SNP by 8 weeks after treatment (representing a substantial improvement from control), demonstrates the potential of NO-based treatments to significantly enhance propagation efficiency in viticulture. This magnitude of improvement exceeds that reported by Kara et al. [26] for their grape cultivars (55–67% germination with SNP treatment) and approaches the levels achieved by Uzun et al. [13] with GA3 treatment under highly controlled humidity conditions. The practical implications of such improvements for grape breeding programs are substantial, as enhanced germination rates can accelerate the development and selection of new cultivars with desired traits, as noted by Atak et al. [32] in their work on grape breeding programs.
The observation that higher SNP concentrations (1500–3000 ppm) consistently outperformed lower concentrations across most cultivars suggests that grape seeds may require relatively high NO levels to overcome dormancy compared to other plant species. This finding contrasts with reports by Ren et al. [29], who found that much lower SNP concentrations (10 μM) were optimal for enhancing Brassica rapa subsp. chinensis seed germination under salt stress. The requirement for higher SNP concentrations in grape seeds may be attributed to their thicker seed coats, more complex dormancy mechanisms, or differences in NO metabolism and signaling pathways compared to herbaceous species [34]. The gradual increase in germination rates for ‘Rizpem’ (from near-zero in early measurements to 32.89% by 12 weeks after treatment) suggests that some cultivars with strong dormancy may require extended periods for dormancy release, even with SNP treatment. This observation parallels findings by Fawzi et al. [38], who reported that some seeds with strong dormancy require multiple treatment combinations to achieve satisfactory germination. Future research might explore combination treatments involving SNP alongside other dormancy-breaking agents for such cultivars with strong dormancy, as suggested by the successful combination approaches reported by Bayrak [35] for Hypericum adenotrichum seeds and by Odabaş et al. [36] for Sambucus nigra seeds.
4.3. Impact of Treatments on Seedling Growth Parameters
The results of our study demonstrated significant effects of cultivar, treatment, and their interaction on seedling growth parameters, namely plant length and the number of nodes (p < 0.05). The clear varietal differences underscore the genetic variability in seedling vigor and growth potential. Notably, the 5 BB rootstock exhibited superior performance, with the highest average plant length (6.93 ± 1.29 cm) and node number (3.10 ± 0.37), whereas ‘Rizpem’ consistently displayed the lowest values for both traits. These results indicate that genetic factors play a crucial role in early seedling development, as previously observed by Wang et al. [39] in V. amurensis cultivars. GA3 treatments also enhanced seedling development, particularly at 1000 ppm, which resulted in an average plant length of 4.52 ± 1.59 cm. ‘Alphonse Lavallée’ showed notable responsiveness to this treatment, consistent with earlier reports of GA3’s efficacy in other species, such as Vaccinium [40] and wheat [41]. However, our findings contrast with those of Çelik [42], who found no significant GA3 effect on germination in ‘Alphonse Lavallée’. This discrepancy may stem from differences in experimental focus, germination vs. post-germination growth. Among the treatments, 3000 ppm SNP resulted in the tallest plants, on average (4.85 ± 1.33 cm), suggesting a strong dose-dependent effect of NO donor application. While 1500 and 2000 ppm SNP also promoted growth in certain cultivars, the responses varied significantly. This aligns with findings by Hayat et al. [43,44], who reported that SNP application at low concentrations improved germination and seedling growth in tomato, but higher doses were less effective or inhibitory. Similar dose-dependent trends were observed in rapeseed by Silva et al. [45]. Additional studies by Sung and Hong [46] in Chinese cabbage further support the beneficial effects of SNP on seedling development. These findings are also consistent with research by Conner [47], who demonstrated the effectiveness of growth regulators in muscadine grape seed germination. Importantly, cultivar × treatment interactions were significant. For example, 5 BB rootstock achieved maximum plant length (9.20 ± 1.17 cm), even under control conditions, suggesting low dormancy or inherent vigor. In contrast, the weak response of ‘Rizpem’ to both SNP and GA3 indicates the need for alternative dormancy-breaking strategies, such as calcium oxide treatment, as suggested by Sabır and Kara [48]. Node development followed similar trends. ‘Alphonse Lavallée’ had the highest node count (3.26 ± 0.62), particularly under 3000 ppm SNP (3.96 ± 0.27), followed by 5 BB and ‘Ülkemiz’. These differences further support the notion of cultivar-specific responses to exogenous regulators. Nayanakantha et al. [49] similarly emphasized the need for species- and cultivar-specific optimization of SNP treatments in rubber seedlings. Overall, our findings highlight that while growth regulators such as GA3 and SNP can enhance seedling development, their effectiveness is highly dependent on cultivar characteristics. For nursery practices, tailoring treatments to each cultivar’s inherent potential will be essential for optimizing propagation efficiency. Cultivars with high intrinsic vigor like 5 BB may not require growth regulators, whereas less responsive types like ‘Rizpem’ may benefit more from alternative or combined approaches.
4.4. General Evaluation
The multivariate analysis revealed complex interactions between cultivars and treatments, with PC1 and PC2 explaining 79.43% of total variance. The strong correlation between plant length and node number confirmed the consistent performance patterns across growth parameters. The 5BB rootstock’s superior performance was validated through positive correlations with growth parameters, particularly for 5BB_CNT, supporting Wang et al.’s [39] observations on inherent vigor in certain grape cultivars. Significant positive relationships between germination measurements across dates and media were identified, with the strongest correlation (r = 0.94) between late-season seed tray measurements, suggesting progressive enhancement of SNP effects similar to findings reported by Nabaei and Amooaghaie [30]. The heatmap analysis highlighted cultivar-specific responses, with 5BB showing consistently high performance at moderate SNP concentrations (1000–2000 ppm), while ‘Rizpem’ remained unresponsive, regardless of treatment. This cultivar-dependent pattern aligns with the findings of Kara et al. [26] and Bibi et al. [27], who documented variable SNP responsiveness among different grape and wheat cultivars. Higher SNP concentrations (3000 ppm) negatively affected germination in some cultivars, while moderate concentrations benefited responsive cultivars, consistent with Esmail et al.’s [28] findings on concentration-dependent inhibitory effects. Growth media significantly influenced treatment effectiveness, with seed trays outperforming Petri dishes, emphasizing the importance of the physical environment in SNP application efficacy for viticulture.
5. Conclusions
This study demonstrates that grapevine seed germination and seedling development are significantly affected by both genetic background and SNP treatment concentrations, with consistent cultivar × treatment interactions across measurement dates. SNP applications at 1500–3000 ppm notably enhanced germination rates in both Petri dishes and seedling trays compared to control and GA3 treatments. The 5 BB rootstock showed the highest germination potential, exceeding 90% in several treatments, while Rizpem consistently exhibited poor germination, regardless of treatment. Germination performance generally improved over time, reaching peak values by late assessment dates under higher SNP concentrations. Cultivars such as ‘Çeliksu’, ‘Rizellim’, and ‘Rizesui’ responded well, whereas ‘Ülkemiz’ and ‘Alphonse Lavallée’ showed moderate and treatment-specific improvements. Despite enhanced germination, the effects of SNP on seedling growth were variable. While the 3000 ppm SNP treatment yielded the tallest seedlings overall, the 5 BB rootstock reached its maximum height under control conditions, indicating that high germination rates do not always correlate with vigorous growth. Variability in plant height and node number across genotypes emphasizes the differential responses to SNP. Overall, the findings highlight the potential of SNP as an effective tool for improving grapevine germination, often outperforming GA3, while also emphasizing the need for cultivar-specific optimization to achieve consistent seedling development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11070754/s1.
Author Contributions
A.Y., Ö.K., H.H.-V., and O.K. collaborated on the study design, methodology, and execution. These individuals contributed to data visualization, statistical analysis, and manuscript preparation. A.Y. and O.K. led the writing, editing, and revision process. All authors have read and agreed to the published version of the manuscript.
Funding
This research was graciously supported by the Scientific Research Fund of Tokat Gaziosmanpaşa University (Project No. 2021/08).
Data Availability Statement
The data supporting the findings of this study are not publicly available, as they were generated and analyzed specifically for the scope of this manuscript.
Acknowledgments
This study also constitutes a part of a master’s thesis. All individuals mentioned in the acknowledgements section have provided their consent to be included.
Conflicts of Interest
The authors declare no conflicts of interest, financial or otherwise, that could have influenced the research outcomes or conclusions presented in this work.
References
- Kunter, B.; Unal, O.B.; Keskin, S.; Hatterman-Valenti, H.; Kaya, O. Comparison of the sugar and organic acid components of seventeen table grape cultivars produced in Ankara (Türkiye): A study over two consecutive seasons. Front. Plant Sci. 2024, 15, 1321210. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, Z.; Atak, A.; Akkurt, M. Determination of downy and powdery mildew resistance of some Vitis spp. Ciência Técnica Vitivinícola 2019, 34, 15–24. [Google Scholar] [CrossRef]
- Keskin, N.; Bilir Ekbic, H.; Kaya, O.; Keskin, S. Antioxidant activity and biochemical compounds of Vitis vinifera L. (cv.‘Katıkara’) and Vitis labrusca L. (cv.‘Isabella’) grown in Black Sea Coast of Turkey. Erwerbs-Obstbau 2021, 63 (Suppl. S1), 115–122. [Google Scholar] [CrossRef]
- Reisch, B.I.; Owens, C.L.; Cousins, P.S. Grape. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: New York, NY, USA, 2012; pp. 225–262. [Google Scholar] [CrossRef]
- Incesu, M.; Karakus, S.; Seyed Hajizadeh, H.; Ates, F.; Turan, M.; Skalicky, M.; Kaya, O. Changes in biogenic amines of two table grapes (cv. Bronx Seedless and Italia) during berry development and ripening. Plants 2022, 11, 2845. [Google Scholar] [CrossRef]
- Kaya, O.; Incesu, M.; Ates, F.; Keskin, N.; Verdugo-Vásquez, N.; Gutiérrez-Gamboa, G. Study of volatile organic compounds of two table grapes (Cv. Italia and Bronx Seedless) along ripening in vines established in the Aegean region (Turkey). Plants 2022, 11, 1935. [Google Scholar] [CrossRef]
- Clippinger, J.I.; Dobry, E.P.; Laffan, I.; Zorbas, N.; Hed, B.; Campbell, M.A. Traditional and emerging approaches for disease management of Plasmopara viticola, causal agent of downy mildew of grape. Agriculture 2024, 14, 406. [Google Scholar] [CrossRef]
- Çelik, H.; Köse, B.; Ateş, S. Karadeniz Bölgesinden Selekte Edilerek Tescillenen Yeni Kokulu Üzüm (Vitis labrusca L.) Çeşitleri. Bahçe Derg. 2018, 47, 299–309. [Google Scholar]
- Magon, G.; De Rosa, V.; Martina, M.; Falchi, R.; Acquadro, A.; Barcaccia, G.; Portis, E.; Vannozzi, A.; De Paoli, E. Boosting grapevine breeding for climate-smart viticulture: From genetic resources to predictive genomics. Front. Plant Sci. 2023, 14, 1293186. [Google Scholar] [CrossRef]
- Boyraz, M.; Korkmaz, H.; Durmaz, A. Dormancy and Germination in Seeds. Black Sea J. Eng. Sci. 2019, 2, 1–2. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Ranpise, S.A.; Patil, B.T. 12 Plant Growth Regulators in Grape. In Plant Growth Regulators in Tropical and Sub-Tropical Fruit Crops; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Uzun, H.İ.; Özer, N.; Akkurt, M.; Özer, C.; Aydın, S.; Aktürk, B. Üzüm Çekirdeklerinin Çimlendirilmesinde Etkili ve Pratik Yöntem: Kutuda Çimlendirme. Bahçe Derg. 2018, 47, 267–272. [Google Scholar]
- Li, S.; Geng, X.; Chen, S.; Liu, K.; Yu, S.; Wang, X.; Zhang, C.; Zhang, J.; Wen, Y.; Luo, Q.; et al. The co-expression of genes involved in seed coat and endosperm development promotes seed abortion in grapevine. Planta 2021, 254, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, Q.; Luo, L.; Yin, C. The endophyte bacillus amyloliquefaciens from picea asperata seeds promotes seed germination and its physiological mechanism. J. Soil Sci. Plant Nutr. 2024, 24, 421–434. [Google Scholar] [CrossRef]
- Algül, B.E.; Tekintaş, F.E.; Günver Dalkılıç, G. Bitki Büyüme Düzenleyicilerinin Kullanımı Ve İçsel Hormonların Biyosentezini Arttırıcı Uygulamalar. Adnan Menderes Üniversitesi Ziraat Fakültesi Derg. 2016, 13, 87. [Google Scholar] [CrossRef]
- Fancy, N.N.; Bahlmann, A.; Loake, G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Morris, P.; Ribeiro, D.; Wilson, I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Nitric oxide in plants: The history is just beginning. Plant Cell Environ. 2001, 24, 267–278. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Dobrzyńska, U.; Babańczyk, T.; Bogatek, R. Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta 2007, 225, 1051–1057. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T. Plant Propagation. Principles and Practices; Prentice-Hall Int. Inc.: Hoboken, NJ, USA, 1990; Volume 647. [Google Scholar]
- Bewley, J.D.; Black, M.; Bewley, J.D.; Black, M. Dormancy and the control of germination. In Seeds: Physiology of Development and Germination, 2nd ed.; Bewley, J.D., Black, M., Eds.; Plenum Press: New York, NY, USA, 1994; pp. 199–271. [Google Scholar]
- Zhao, Y.; Wei, X.; Long, Y.; Ji, X. Transcriptional analysis reveals sodium nitroprusside affects alfalfa in response to PEG-induced osmotic stress at germination stage. Protoplasma 2020, 257, 1345–1358. [Google Scholar] [CrossRef]
- Dokoozlian, N.K.; Peacock, W.L. Gibberellic Acid Applied at Bloom Reduces Fruit Set and Improves Size of “Crimson Seedless” Table Grapes. HortScience 2001, 36, 706–709. [Google Scholar] [CrossRef]
- Evgenidis, G.; Traka-Mavrona, E.; Koutsika-Sotiriou, M. Principal component and cluster analysis as a tool in the as-sessment of tomato hybrids and cultivars. Int. J. Agron. 2011, 9, 1–7. [Google Scholar] [CrossRef]
- Kara, Z.; Yazar, K.; Doğan, O.; Vergili, E. Sodium Nitroprusside and Gibberellin Effects on Seed Germination and Seedling Development of Grapevine (Vitis vinifera L.) Cvs. Ekşi Kara and Gök Üzüm. Erwerbs-Obstbau 2020, 62, 61–68. [Google Scholar] [CrossRef]
- Bibi, A.; Qureshi, S.; Shehzadi, I.; Amjad, M.S.; Azhar, N.; Batool, T.; Firdous, S.; Khan, M.; Shokat, S. Appraisal of nitric oxide priming to improve the physiology of bread wheat. J. Agric. Sci. 2020, 158, 159. [Google Scholar] [CrossRef]
- Esmail, N.Y.; Hashem, H.A.; Hassanein, A.A. Effect of Treatment with Different Concentrations of Sodium Nitroprusside on Survival, Germination, Growth, Photosynthetic Pigments and Endogenous Nitric Oxide Content of Lupines termis L. Plants. Acta Sci. Agric. 2018, 2, 3–8. [Google Scholar]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicol. Environ. Saf. 2020, 187, 109785. [Google Scholar] [CrossRef]
- Nabaei, M.; Amooaghaie, R. Interactive Effect of Melatonin and Sodium Nitroprusside on Seed Germination and Seedling Growth of Catharanthus roseus under Cadmium Stress. Russ. J. Plant Physiol. 2019, 66, 128–139. [Google Scholar] [CrossRef]
- Faraji, J.; Sepehri, A. Ameliorative effects of TiO2 nanoparticles and sodium nitroprusside on seed germination and seedling growth of wheat under PEG-stimulated drought stress. J. Seed Sci. 2019, 41, 309–317. [Google Scholar] [CrossRef]
- Atak, A.; Şen, A.; Doyğacı, Y.; Kandilli, G. Farklı Üzüm Tür ve Çeşitlerinin Melezlenmesi ile Elde Edilen Melez Genotiplerin Canlı Tohum Oranlarının Belirlenmesi. Akad. Ziraat Derg. 2019, 8, 149–156. [Google Scholar] [CrossRef]
- Sepehri, A.; Rouhi, H.R. Enhancement of Seed Vigor Performance in Aged Groundnut (Arachis hypogaea L.) Seeds by Sodium Nitroprusside under Drought Stress. Philipp. Agric. Sci. 2016, 99, 339–347. [Google Scholar]
- Kireçci, O.A.; Yürekli, F. Helianthus annuus L. Yapraklarında Tuz Stresi, Bazı Bitki Hormonları ve SNP Uygulamalarının Sinyal Moleküllerine Etkisi. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2018, 21, 665–671. [Google Scholar] [CrossRef]
- Bayrak, M. Hyperıcum Adenotrıchum Spach. (Hyperıcaceae; Clusıaceae) Türünün Tohum Çimlenmesi Üzerinde Araştırmalar. (Y. Lisans Tezi). Master’s Thesis, Uludağ Üniversitesi Fen Bilimleri Enstitüsü Biyoloji Anabilim Dalı, Bursa, Türkiye, 2016. [Google Scholar]
- Odabaş, S.; Kara, Ş.M.; Özcan, M.M. Bazı Kimyasal Uygulamaların Siyah Mürver (Sambucus nigra L.) Tohumlarında Dormansinin Kırılması ve Çimlenme Üzerine Etkisi. Türk. Tarım Ve Doğa Bilim. Derg. 2020, 7, 920–927. [Google Scholar] [CrossRef]
- Yıldız, V. Bazi Hormon Uygulamalarinin Asma Tohumunda Çïmlenme ve Bïtkï Gelïşïmï Üzerïne Etkïlerï. Master’s Thesis, Bingöl Üniversitesi Fen Bilimleri Enstitüsü Bahçe Bitkileri Anabilim Dalı, Bingöl, Türkiye, 2019. [Google Scholar]
- Fawzi, I.; Atilla, H.M.; Adnan, A. Bazı Uygulamaların Menengiç (Pistacia terebinthus L.) Tohumlarının Çimlenmesi ve Çıkışı Üzerine Etkileri. Ziraat Fakültesi Derg. 2018, 13, 27–39. [Google Scholar]
- Wang, W.Q.; Song, S.Q.; Li, S.H.; Gan, Y.Y.; Wu, J.H.; Cheng, H.Y. Seed dormancy and germination in Vitis amurensis and its variation. Seed Sci. Res. 2011, 21, 255–265. [Google Scholar] [CrossRef]
- Karabulut, B. Karadeniz Bölgesinde Yetişmekte Olan Yüksek Boylu Maviyemiş (Vaccinium Corymbosum L.), Çay Üzümü (Vaccinium Arctostaphylos L.) Ve Çoban Üzümü (Vaccinium Myrtillus L.) Tohumlarında Çıkış Üzerine Bazı Uygulamaların Etkilerinin Saptanması. (Y. Lisans Tezi); Ondokuz Mayıs Üniversitesi Fen Bilimleri Enstitüsü Bahçe Bitkileri Anabilim Dalı: Samsun, Türkiye, 2012. [Google Scholar]
- Li, X.; Jiang, H.; Liu, F.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitric oxide and gibberellin. Plant Growth Regul. 2013, 71, 31–40. [Google Scholar] [CrossRef]
- Çelik, M. The Effects of Stratification Periods and GA3 (Gibberellic acid) Applicatıons on Germination of Seeds of Some Grape Cultivars. Türk. Tarım. Doğa Bilim. Derg. 2014, 6, 1118–1122. [Google Scholar]
- Hayat, S.; Yadav, S.; Alyemeni, M.N.; Ahmad, A. Effect of sodium nitroprusside on the germination and antioxidant activities of tomato (Lycopersicon esculentum mill). Bulg. J. Agric. Sci. 2014, 20, 156–160. [Google Scholar]
- Hayat, S.; Yadav, S.; Wani, A.S.; Irfan, M.; Alyemini, M.N.; Ahmad, A. Impact of sodium nitroprusside on nitrate reductase, proline content, and antioxidant system in tomato under salinity stress. Hortic. Environ. Biotechnol. 2012, 53, 362–367. [Google Scholar] [CrossRef]
- Silva, A.L.D.; Dias, D.C.F.D.S.; Borges, E.E.D.L.E.; Ribeiro, D.M.; Silva, L.J.D. Effect of sodium nitroprusside (SNP) on the germination of Senna macranthera seeds (DC. ex Collad.) HS Irwin & Baneby under salt stress. J. Seed Sci. 2015, 37, 236–243. [Google Scholar]
- Sung, C.H.; Hong, J.K. Sodium nitroprusside mediates seedling development and attenuation of oxidative stresses in Chinese cabbage. Plant Biotechnol. Rep. 2010, 4, 243–251. [Google Scholar] [CrossRef]
- Conner, P.J. Effects of Stratification, Germination Temperature, and Pretreatment with Gibberellic Acid and Hydrogen Peroxide on Germination of ‘Fry’ Muscadine (Vitis rotundifolia) Seed. HortScience 2008, 43, 853–856. [Google Scholar] [CrossRef]
- Sabır, A.; Kara, Z. Giberelik Asit ve Nanoteknolojik Kalsit Uygulamalarının Asma Tohumlarının Çimlenmeleri Üzerine Etkileri, içinde: Türkiye VI. Ulus. Bahçe Bitk. Kongresi. 2011, 135–139. [Google Scholar]
- Nayanakantha, N.M.C.; Hettiarachchi, N.N.; Seneviratne, P.; Wathugala, D.L. Exogenous nitric oxide donor sodium nitroprusside enhanced growth attributes of polybagged rubber (Hevea brasiliensis) seedlings. Trop. Agric. Res. Ext. 2015, 18, 134–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).