Identification and Expression Analysis of C2H2-Type Zinc Finger Protein (C2H2-ZFP) Genes in Bougainvillea in Different Colored Bracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of C2H2-ZFP Gene Family in Bougainvillea
2.2. Phylogenetic Analysis and Sequence Alignment of C2H2-ZFPs
2.3. Conserved Domains, Gene Structure, and Cis-Acting Elements Analysis of C2H2-ZFPs
2.4. Analysis of C2H2-ZFP Gene Expression in Bougainvillea Bracts of Different Colors Using RNA Sequencing (RNA-Seq) Data
2.5. RT-qPCR Validation
2.6. Statistical Analysis
3. Results
3.1. Identification and Physicochemical Properties Analysis of C2H2-ZFP Genes in Bougainvillea
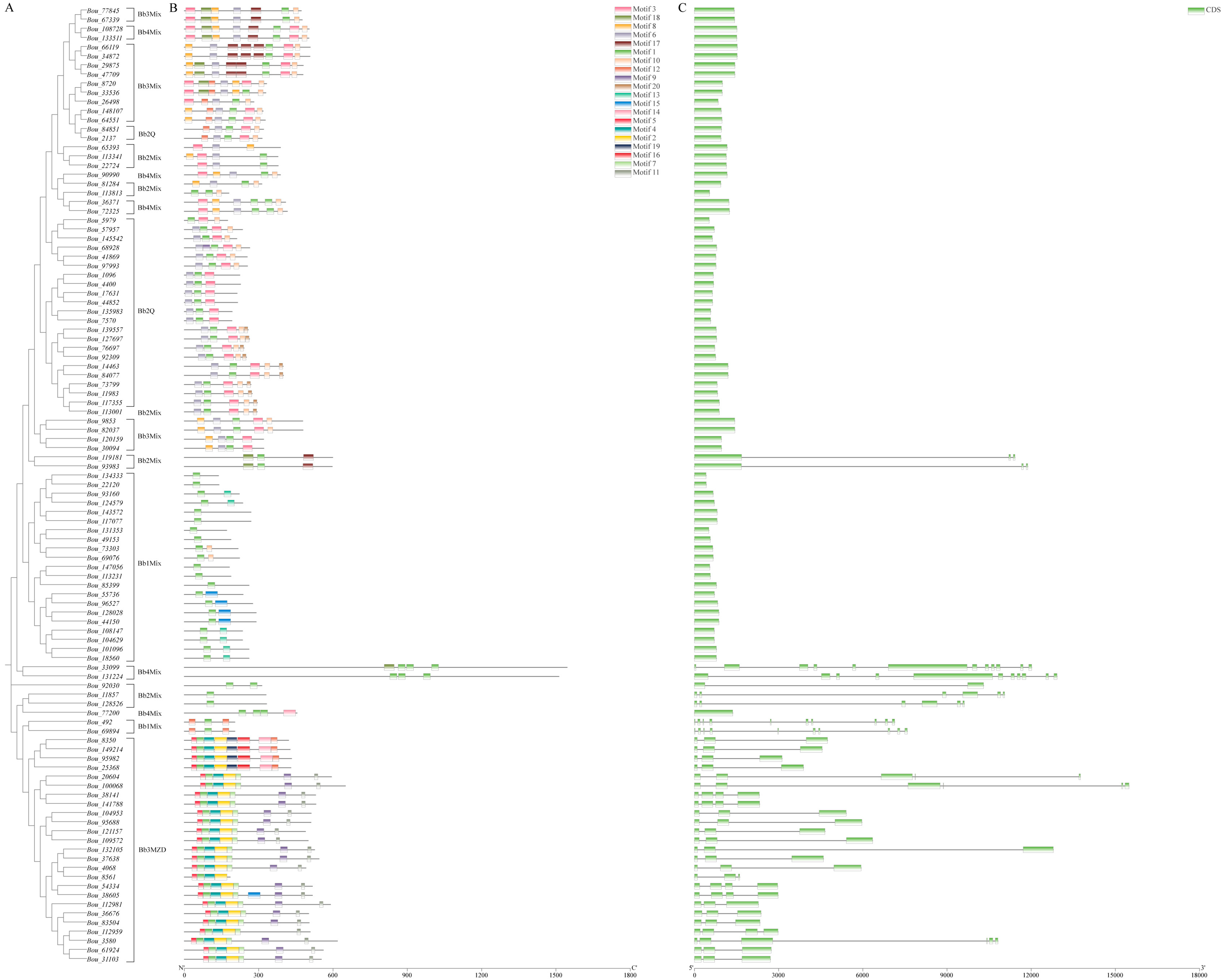
3.2. Phylogenetic Analysis of BbZFP Proteins
3.3. Analysis of Conserved Motifs of BbZFPs
3.4. Gene Structures Analysis of BbZFPs
3.5. Cis-Acting Elements in the BbZFP Gene Promoter
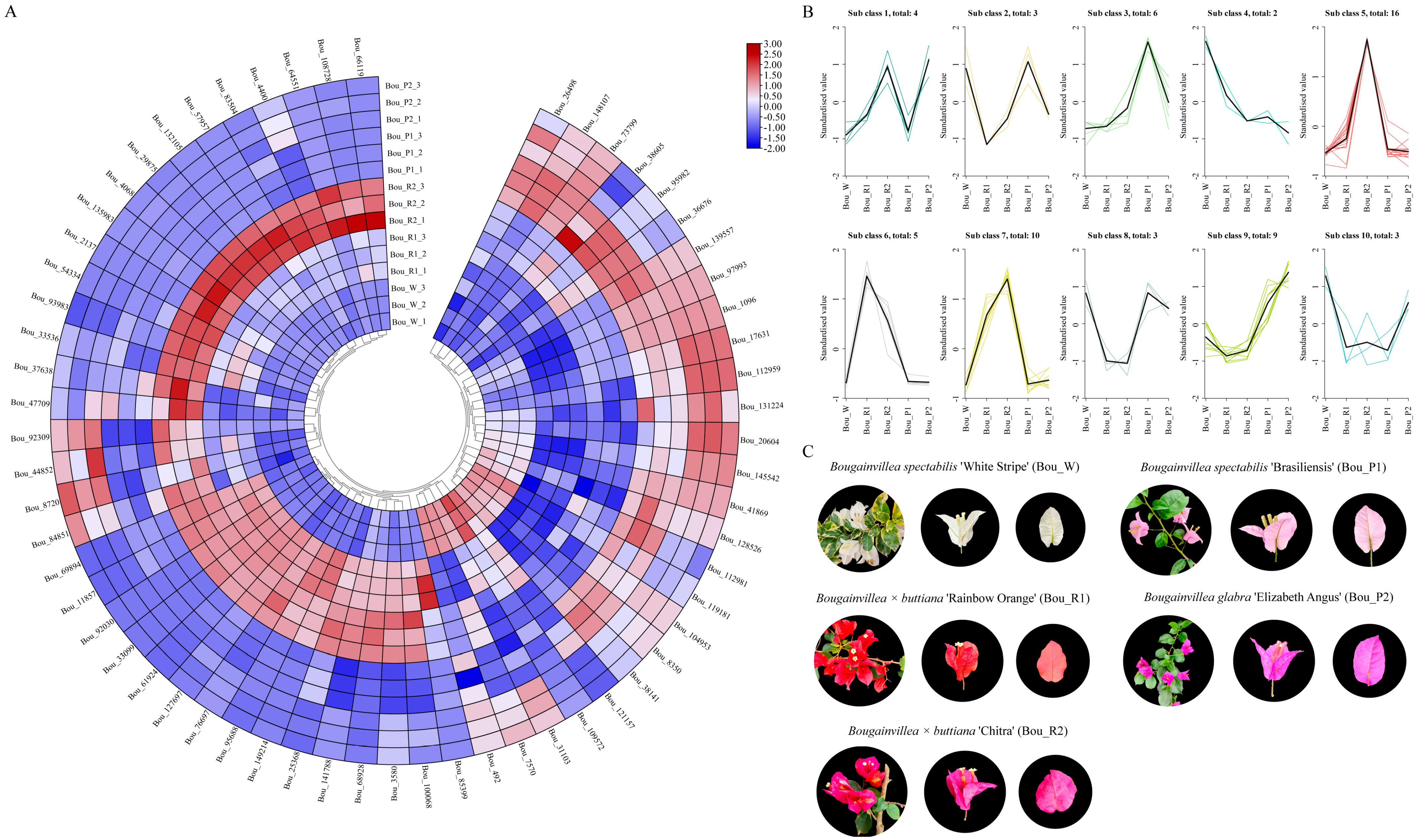
3.6. Expression Analysis of BbZFP Genes in Bracts of Different Colors
3.7. Validation of Differentially Expressed Genes by RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Hu, S.; He, C.; Zhou, J.; Qu, F.; Ai, Z.; Chen, Y.; Ni, D. Chlorophyll metabolism in postharvest tea (Camellia sinensis L.) leaves: Variations in color values, chlorophyll derivatives, and gene expression levels under different withering treatments. J. Agric. Food Chem. 2019, 67, 10624–10636. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, M.; Gan, Q.; Long, J.; Fan, H.; Wang, X.; Guan, Z. The formation and evolution of flower coloration in Brassica crops. Front. Gene. 2024, 15, 1396875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Y.; Long, T.; Wang, S.; Yang, J. Regulation mechanism of plant pigments biosynthesis: Anthocyanins, carotenoids, and betalains. Metabolites 2022, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La Vie en Rose”: Biosynthesis, sources, and applications of betalain pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef]
- Brockington, S.F.; Walker, R.H.; Glover, B.J.; Soltis, P.S.; Soltis, D.E. Complex pigment evolution in the Caryophyllales. New Phytol. 2011, 190, 854–864. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. Botany: Floral fluorescence effect. Nature 2005, 437, 334. [Google Scholar] [CrossRef]
- Felker, P.; Stintzing, F.C.; Müssig, E.; Leitenberger, M.; Carle, R.; Vogt, T.; Bunch, R. Colour inheritance in cactus pear (Opuntia ficus-indica) fruits. Ann. Appl. Biol. 2008, 152, 307–318. [Google Scholar] [CrossRef]
- Takatsuji, H. Zinc-finger proteins: The classical zinc finger emerges in contemporary plant science. Plant Mol. Biol. 1999, 39, 1073–1078. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants. Front. Plant Sci. 2020, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Kielbowicz-Matuk, A. Involvement of plant C2H2-type zinc finger transcription factors in stress responses. Plant Sci. 2012, 185–186, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.; Cao, J. Cys2/His2 zinc-finger proteins in transcriptional regulation of flower development. Int. J. Mol. Sci. 2018, 19, 2589. [Google Scholar] [CrossRef]
- Takatsuji, H.; Mori, M.; Benfey, P.N.; Ren, L.; Chua, N.H. Characterization of a zinc finger DNA-binding protein expressed specifically in Petunia petals and seedlings. EMBO J. 1992, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gamsjaeger, R.; Liew, C.K.; Loughlin, F.E.; Crossley, M.; Mackay, J.P. Sticky Fingers: Zinc-fingers as protein-recognition motifs. Trends Biochem. Sci. 2007, 32, 63–70. [Google Scholar] [CrossRef]
- Englbrecht, C.C.; Schoof, H.; Böhm, S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004, 5, 39. [Google Scholar] [CrossRef]
- Agarwal, P.; Arora, R.; Ray, S.; Singh, A.K.; Singh, V.P.; Takatsuji, H.; Kapoor, S.; Tyagi, A.K. Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol. Biol. 2007, 65, 467–485. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Zhao, J.; Li, Y.; Huang, R.; Jiang, X.; Zhou, X.; Zhu, X.; He, Y.; He, Y.; et al. Genome-wide characterization of the C2H2 zinc-finger genes in Cucumis sativus and functional analyses of four CsZFPs in response to stresses. BMC Plant Biol. 2020, 20, 359. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Xu, X.; Zhang, H.; Li, C. Genome-wide analysis of C2H2 zinc-finger family transcription factors and their responses to abiotic stresses in poplar (Populus trichocarpa). PLoS ONE 2015, 10, e0134753. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Li, R.; Wang, L.; Zhang, C.; Chen, L.; Hao, Q.; Zhang, X.; Chen, H.; Shan, Z.; et al. Genome-wide identification and classification of soybean C2H2 zinc finger proteins and their expression analysis in legume-Rhizobium symbiosis. Front. Microbiol. 2018, 9, 126. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, K.; Ngea, G.L.N.; Godana, E.A.; Ackah, M.; Dhanasekaran, S.; Zhang, Y.; Su, Y.; Yang, Q.; Zhang, H. Recent advances in the multifaceted functions of Cys2/His2-type zinc finger proteins in plant growth, development, and stress responses. J. Exp. Bot. 2024, 75, 5501–5520. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wang, J.P.; Yang, H.F. Identification and functional characterization of the NAC gene promoter from Populus euphratica. Planta 2016, 244, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Li, Y.; Qiao, Z.; Wang, C.; Zhao, Y.; Guo, J.; Chen, M.; Wang, B. Advances in the regulation of epidermal cell development by C2H2 zinc finger proteins in plants. Front. Plant Sci. 2021, 12, 754512. [Google Scholar] [CrossRef] [PubMed]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Liu, Y.; Khan, A.R.; Gan, Y. C2H2 zinc finger proteins response to abiotic stress in plants. Int. J. Mol. Sci. 2022, 23, 2730. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, W.; Yang, L.; Liang, W.; Li, H.; Yang, L.; Chen, M.; Luo, Z.; Huang, G.; Duan, L.; et al. SMALL REPRODUCTIVE ORGANS, a SUPERMAN-like transcription factor, regulates stamen and pistil growth in rice. New Phytol. 2022, 233, 1701–1718. [Google Scholar] [CrossRef]
- Wang, D.-R.; Yang, K.; Wang, X.; Lin, X.-L.; Rui, L.; Liu, H.-F.; Liu, D.-D.; You, C.-X. Overexpression of MdZAT5, an C2H2-type zinc finger protein, regulates anthocyanin accumulation and salt stress response in apple calli and Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1897. [Google Scholar] [CrossRef]
- Sun, Q.; He, Z.; Ye, J.; Wei, R.; Feng, D.; Zhang, Y.; Chai, L.; Cheng, Y.; Xu, Q.; Deng, X. A novel C2H2-type zinc-finger transcription factor, CitZAT4, regulates ethylene-induced orange coloration in Satsuma mandarin flavedo (Citrus unshiu Marc.). J. Int. Plant Biol. 2024, 67, 294–310. [Google Scholar] [CrossRef]
- Saleem, H.; Usman, A.; Mahomoodally, M.F.; Ahemad, N. Bougainvillea glabra (choisy): A comprehensive review on botany, traditional uses, phytochemistry, pharmacology and toxicity. J. Ethnopharmacol. 2021, 266, 113356. [Google Scholar] [CrossRef]
- Wang, F.; Yao, G.; Li, J.; Zhu, W.; Li, Z.; Sun, Z.; Xin, P. Mining and expression analysis of color related genes in Bougainvillea glabra bracts based on transcriptome sequencing. Sci. Rep. 2024, 14, 24491. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, Y.; Zhang, T.; Wang, P.; Liu, W.; Zhang, Z.; Yu, W.; Wang, J.; Wang, J.; Zhou, Y. Integrated metabolome, full-length sequencing, and transcriptome analyses unveil the molecular mechanisms of color formation of the canary yellow and red bracts of Bougainvillea × buttiana ‘Chitra’. Plant J. 2023, 116, 1441–1461. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fu, X.; Chen, Z.; Wang, H.; Wang, J.; Zhu, Z.; Zhu, G. Composition, color stability and antioxidant properties of betalain-based extracts from bracts of Bougainvillea. Molecules 2022, 27, 5120. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Lan, L.; Zhao, H.; Xu, S.; Kan, S.; Zhang, X.; Liu, W.; Liao, X.; Tembrock, L.R.; Ren, Y.; Reeve, W.; et al. A high-quality Bougainvillea genome provides new insights into evolutionary history and pigment biosynthetic pathways in the Caryophyllales. Hortic. Res. 2023, 10, uhad124. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the Expasy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Chou, K.C.; Hong-Bin Shen, H.B. Cell-PLoc 2.0: An improved package of web-servers for predicting subcellular localization of proteins in various organisms. Nat. Sci. 2010, 2, 1090–1103. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Corchete, L.A.; Rojas, E.A.; Alonso-Lopez, D.; Rivas, J.D.L.; Gutierrez, N.C.; Burguillo, F.J. Systematic comparison and assessment of RNA-seq procedures for gene expression quantitative analysis. Sci. Rep. 2020, 10, 19737. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shen, S.; Zhou, S.; Li, Y.; Mao, Y.; Zhou, J.; Shi, Y.; An, L.; Zhou, Q.; Peng, W.; et al. Rice metabolic regulatory network spanning the entire life cycle. Mol. Plant 2022, 15, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, S.; Gao, J. Cloning and expression analysis of flavanone 3-hydroxylase gene from Bougainvillea spectabilis. Biotechnol. Bull. 2022, 38, 122–128. (In Chinese) [Google Scholar]
- Cao, Y.; Liu, S.; Sun, R.; Chen, H.; Xie, Z.; Huang, L.; Chen, Y. Cloning and expression analysis of 4,5-DOPA dioxygenase gene from Bougainvillea glabra Choisy. Mol. Plant Breed. 2018, 16, 54–60. (In Chinese) [Google Scholar]
- Wei, K.; Pan, S.; Li, Y. Functional characterization of maize C2H2 zinc-finger gene family. Plant Mol. Biol. Rep. 2016, 34, 761–776. [Google Scholar] [CrossRef]
- Du, T.; Zhou, Y.; Qin, Z.; Li, A.; Wang, Q.; Li, Z.; Hou, F.; Zhang, L. Genome-wide identification of the C2H2 zinc finger gene family and expression analysis under salt stress in sweet potato. Front. Plant Sci. 2023, 14, 1301848. [Google Scholar] [CrossRef]
- Salih, H.; Odongo, M.R.; Gong, W.; He, S.; Du, X. Genome-wide analysis of cotton C2H2-zinc finger transcription factor family and their expression analysis during fiber development. BMC Plant Biol. 2019, 19, 400. [Google Scholar] [CrossRef]
- Jia, H.H.; Xu, Y.T.; Yin, Z.J.; Qing, M.; Xie, K.D.; Guo, W.W.; Wu, X.M. Genome-wide identification of the C2H2-Zinc finger gene family and functional validation of CsZFP7 in citrus nucellar embryogenesis. Plant Reprod. 2023, 36, 287–300. [Google Scholar] [CrossRef]
- Liu, K.; Hou, Q.; Yu, R.; Deng, H.; Shen, L.; Wang, Q.; Wen, X. Genome-wide analysis of C2H2 zinc finger family and their response to abiotic stresses in apple. Gene 2024, 904, 148164. [Google Scholar] [CrossRef]
- Liu, Z.; Coulter, J.A.; Li, Y.; Zhang, X.; Meng, J.; Zhang, J.; Liu, Y. Genome-wide identification and analysis of the Q-type C2H2 gene family in potato (Solanum tuberosum L.). Int. J. Biol. Macromol. 2020, 153, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Muthamilarasan, M.; Bonthala, V.S.; Mishra, A.K.; Khandelwal, R.; Khan, Y.; Roy, R.; Prasad, M. C2H2 type of zinc finger transcription factors in foxtail millet define response to abiotic stresses. Func. Integr. Genomics 2014, 14, 531–543. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, L.; Du, H.; Wang, Y.; Wang, W.; Liu, J.; Huang, J.; Huang, W.; Ge, L. Genome-wide study of C2H2 zinc finger gene family in Medicago truncatula. BMC Plant Biol. 2020, 20, 401. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Liu, F.; Zeng, L.; Xu, Y.; Jin, Q.; Wang, Y. Genome-wide identification of the Q-type C2H2 zinc finger protein gene family and expression analysis under abiotic stress in lotus (Nelumbo nucifera G.). BMC Genom. 2024, 25, 648. [Google Scholar] [CrossRef]
- Colasanti, J.; Tremblay, R.; Wong, A.Y.; Coneva, V.; Kozaki, A.; Mable, B.K. The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genom. 2006, 7, 158. [Google Scholar] [CrossRef]
- Kumar, M.; Le, D.T.; Hwang, S.; Seo, P.J.; Kim, H.U. Role of the INDETERMINATE DOMAIN genes in plants. Int. J. Mol. Sci. 2019, 20, 2286. [Google Scholar] [CrossRef]
- Coelho, C.P.; Huang, P.; Lee, D.Y.; Brutnell, T.P. Making roots, shoots, and seeds: IDD gene family diversification in plants. Trends Plant Sci. 2018, 23, 66–78. [Google Scholar] [CrossRef]
- Wu, X.; Tang, D.; Li, M.; Wang, K.; Cheng, Z. Loose plant architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Physiol. 2013, 161, 317–329. [Google Scholar] [CrossRef]
- Lyu, T.; Liu, W.; Hu, Z.; Xiang, X.; Liu, T.; Xiong, X.; Cao, J. Molecular characterization and expression analysis reveal the roles of Cys/His zinc-finger transcription factors during flower development of Brassica rapa subsp. chinensis. Plant Mol. Biol. 2020, 102, 123–141. [Google Scholar] [CrossRef]
- Liu, Q.; Liaquat, F.; He, Y.; Munis, M.F.H.; Zhang, C. Functional annotation of a full-length transcriptome and identification of genes associated with flower development in Rhododendron simsii (Ericaceae). Plants 2021, 10, 649. [Google Scholar] [CrossRef]
- Du, H.; Lai, L.; Wang, F.; Sun, W.; Zhang, L.; Li, X.; Wang, L.; Jiang, L.; Zheng, Y. Characterisation of flower colouration in 30 Rhododendron species via anthocyanin and flavonol identification and quantitative traits. Plant Biol. 2018, 20, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.H.; Yang, J.H.; Lee, J.Y.; Lim, S.H. Increased flavonol levels in Tobacco expressing AcFLS affect flower color and root growth. Int. J. Mol. Sci. 2020, 21, 1011. [Google Scholar] [CrossRef]
- Zhao, H.; Basu, U.; Kebede, B.; Qu, C.; Li, J.; Rahman, H. Fine mapping of the major QTL for seed coat color in Brassica rapa var. Yellow Sarson by use of NIL populations and transcriptome sequencing for identification of the candidate genes. PLoS ONE 2019, 14, e0209982. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, S.; Li, J.; Pei, R.; Tian, L.; Qi, J.; Azam, M.; Agyenim-Boateng, K.G.; Shaibu, A.S.; Liu, Y.; et al. Dual-function C2H2-type zinc-finger transcription factor GmZFP7 contributes to isoflavone accumulation in soybean. New Phytol. 2023, 237, 1794–1809. [Google Scholar] [CrossRef]
- Long, O.; Lu, X.; Shi, Y.; Li, G.; Zhang, K.; Wang, J.; Zhou, M. Analysis of C2H2-ZFP family genes and functional characterization of FdC2H2-2 in rutin synthesis and accumulation in golden buckwheat. J. Plant Genet. Res. 2023, 24, 1161–1173. (In Chinese) [Google Scholar]
- Zhao, L.; Li, Y.; Li, Y.; Chen, W.; Yao, J.; Fang, S.; Lv, Y.; Zhang, Y.; Zhu, S. Systematical characterization of the cotton Di19 gene family and the role of GhDi19-3 and GhDi19-4 as two negative regulators in response to salt stress. Antioxidants 2022, 11, 2225. [Google Scholar] [CrossRef]
- DeLoache, W.C.; Russ, Z.N.; Narcross, L.; Gonzales, A.M.; Martin, V.J.; Dueber, J.E. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat. Chem. Biol. 2015, 11, 465–471. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chiu, Y.C.; Tsao, N.W.; Chou, Y.L.; Tan, C.M.; Chiang, Y.H.; Liao, P.C.; Lee, Y.C.; Hsieh, L.C.; Wang, S.Y.; et al. Elucidation of the core betalain biosynthesis pathway in Amaranthus tricolor. Sci. Rep. 2021, 11, 6086. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, X.; Li, S.; Zhang, X.; Yu, S.; Zhao, G.R. Metabolic engineering of Escherichia coli for de novo production of betaxanthins. J. Agric. Food Chem. 2020, 68, 8370–8380. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, X.; Guo, F.; Wang, S.; Liu, Z.; Long, W. Identification, expression analysis of quinoa betalain biosynthesis genes and their role in seed germination and cold stress. Plant Signal. Behav. 2023, 18, 2250891. [Google Scholar] [CrossRef]
- Hatlestad, G.J.; Akhavan, N.A.; Sunnadeniya, R.M.; Elam, L.; Cargile, S.; Hembd, A.; Gonzalez, A.; McGrath, J.M.; Lloyd, A.M. The beet Y locus encodes an anthocyanin MYB-like protein that activates the betalain red pigment pathway. Nat. Genet. 2015, 47, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Chen, C.; Chen, J.; Chen, J.; Hua, Q.; Shah, K.; Zhang, Z.; Zhao, J.; Hu, G.; Chen, J.; et al. Betalain biosynthesis in red pulp pitaya is regulated via HuMYB132: A R-R type MYB transcription factor. BMC Plant Biol. 2023, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.N.; Huang, Z.J.; Hua, Q.Z.; Shan, W.; Kuang, J.F.; Lu, W.J.; Qin, Y.H.; Chen, J.Y. The WRKY transcription factor HpWRKY44 regulates CytP450-like1 expression in red pitaya fruit (Hylocereus polyrhizus). Hortic. Res. 2017, 4, 17039. [Google Scholar] [CrossRef]
- Timoneda, A.; Feng, T.; Sheehan, H.; Walker-Hale, N.; Pucker, B.; Lopez-Nieves, S.; Guo, R.; Brockington, S. The evolution of betalain biosynthesis in Caryophyllales. New Phytol. 2019, 224, 71–85. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, Y.; Liu, W.; Yu, W.; Wang, J.; Zhou, Y. Identification and Expression Analysis of C2H2-Type Zinc Finger Protein (C2H2-ZFP) Genes in Bougainvillea in Different Colored Bracts. Horticulturae 2025, 11, 659. https://doi.org/10.3390/horticulturae11060659
Wang Y, Hu Y, Liu W, Yu W, Wang J, Zhou Y. Identification and Expression Analysis of C2H2-Type Zinc Finger Protein (C2H2-ZFP) Genes in Bougainvillea in Different Colored Bracts. Horticulturae. 2025; 11(6):659. https://doi.org/10.3390/horticulturae11060659
Chicago/Turabian StyleWang, Yushan, Yanping Hu, Wen Liu, Wengang Yu, Jian Wang, and Yang Zhou. 2025. "Identification and Expression Analysis of C2H2-Type Zinc Finger Protein (C2H2-ZFP) Genes in Bougainvillea in Different Colored Bracts" Horticulturae 11, no. 6: 659. https://doi.org/10.3390/horticulturae11060659
APA StyleWang, Y., Hu, Y., Liu, W., Yu, W., Wang, J., & Zhou, Y. (2025). Identification and Expression Analysis of C2H2-Type Zinc Finger Protein (C2H2-ZFP) Genes in Bougainvillea in Different Colored Bracts. Horticulturae, 11(6), 659. https://doi.org/10.3390/horticulturae11060659





