Isolation and Functional Analysis of the DhMYB2 and DhbHLH1 Promoters from Phalaenopsis-Type Dendrobium Involved in Stress Responses and Tissue-Specific Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Isolation and Bioinformatics Analysis of DhMYB2 and DhbHLH1 Promoters
2.3. Vector Construction
2.4. Transient Expression Analysis in Tobacco Leaves
2.5. Transformation of Arabidopsis thaliana
2.6. Abiotic Stress and Phytohormone Treatments
2.7. GUS Staining and Activity Analysis
2.8. Statistical Analysis
3. Results
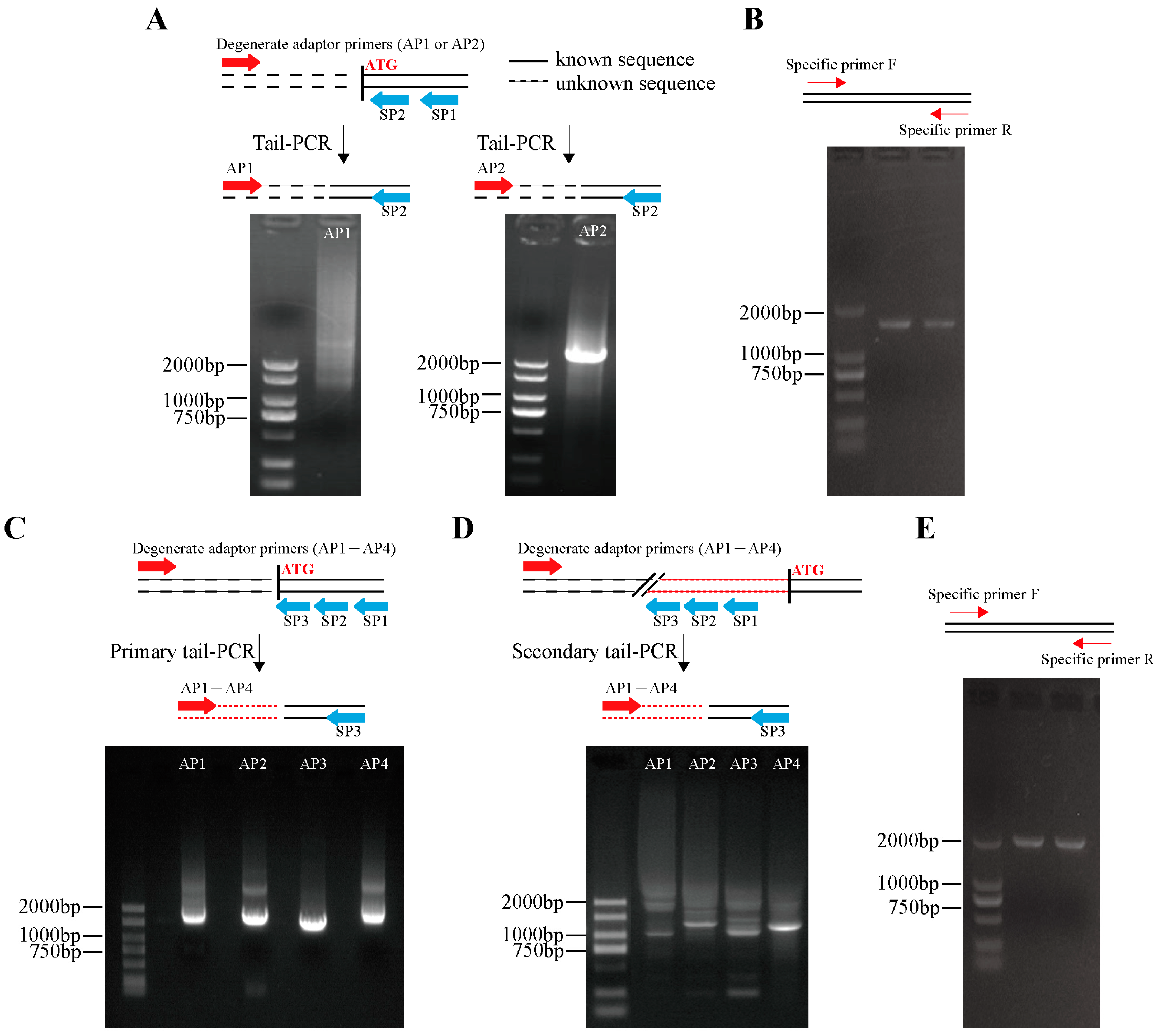
3.1. Sequence Isolation of the DhMYB2 and DhbHLH1 Promoters
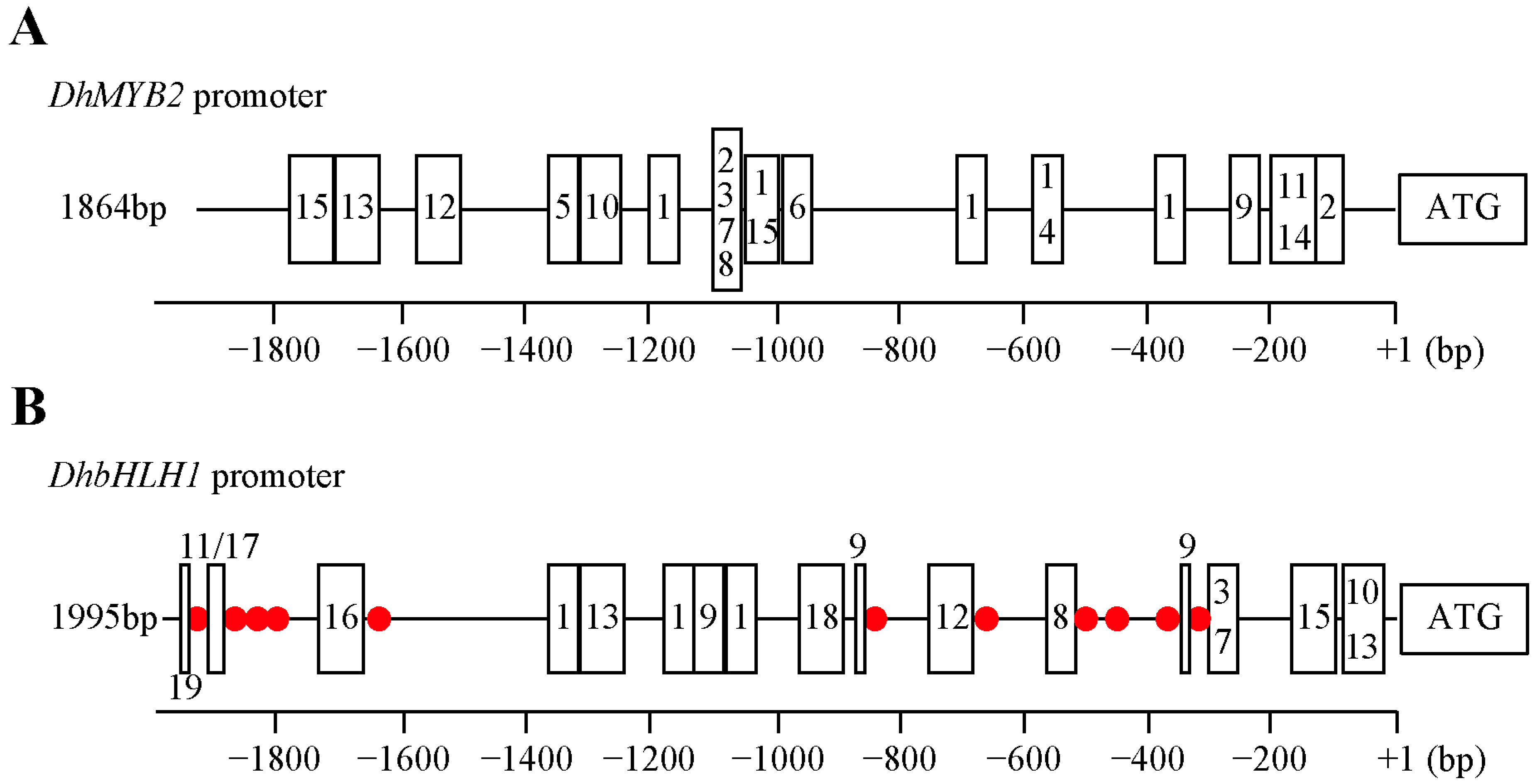
3.2. Characterization of cis-Acting Elements in DhMYB2 and DhbHLH1 Promoters
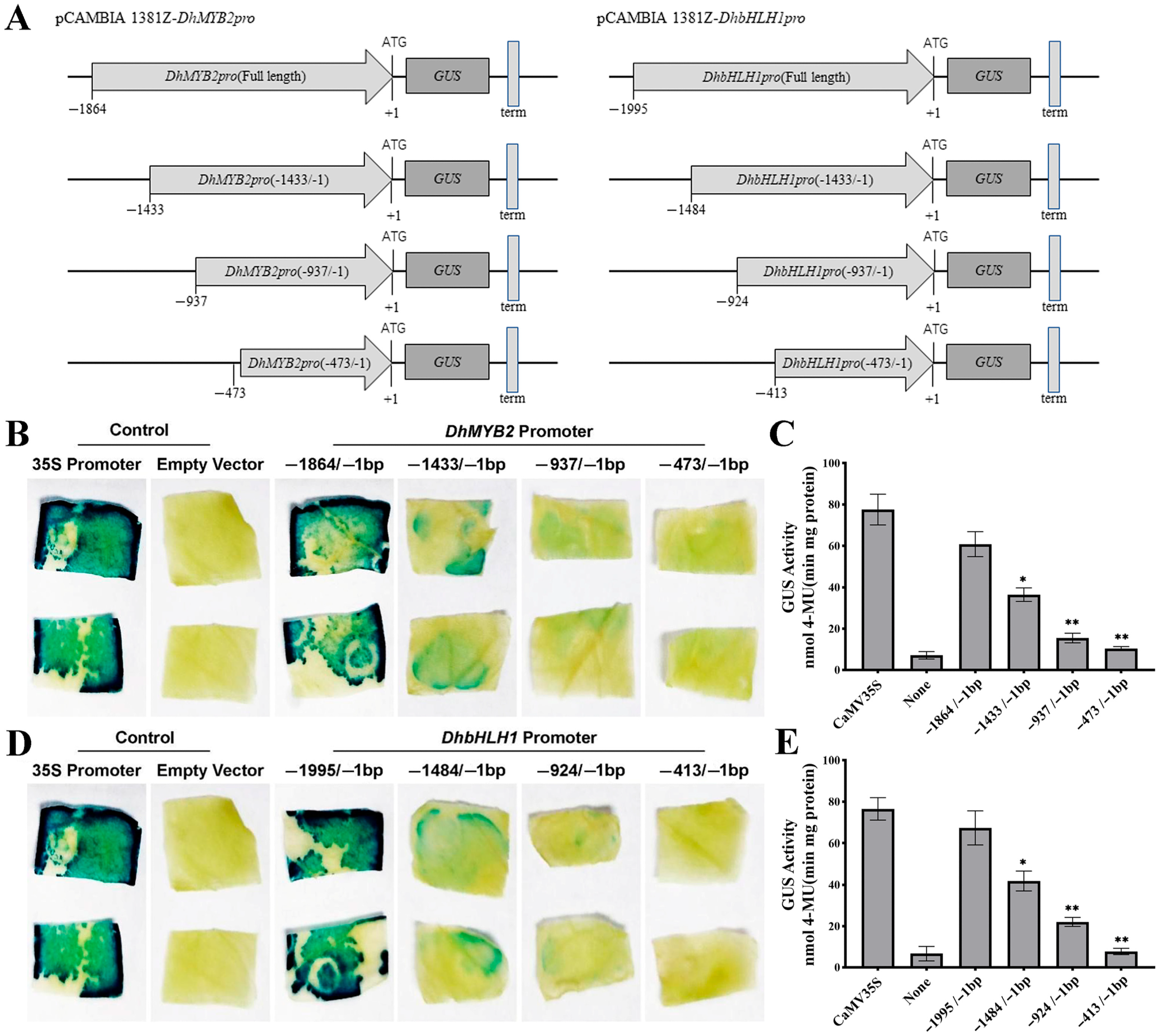
3.3. 5′-Deletion Analysis of Promoter Activity
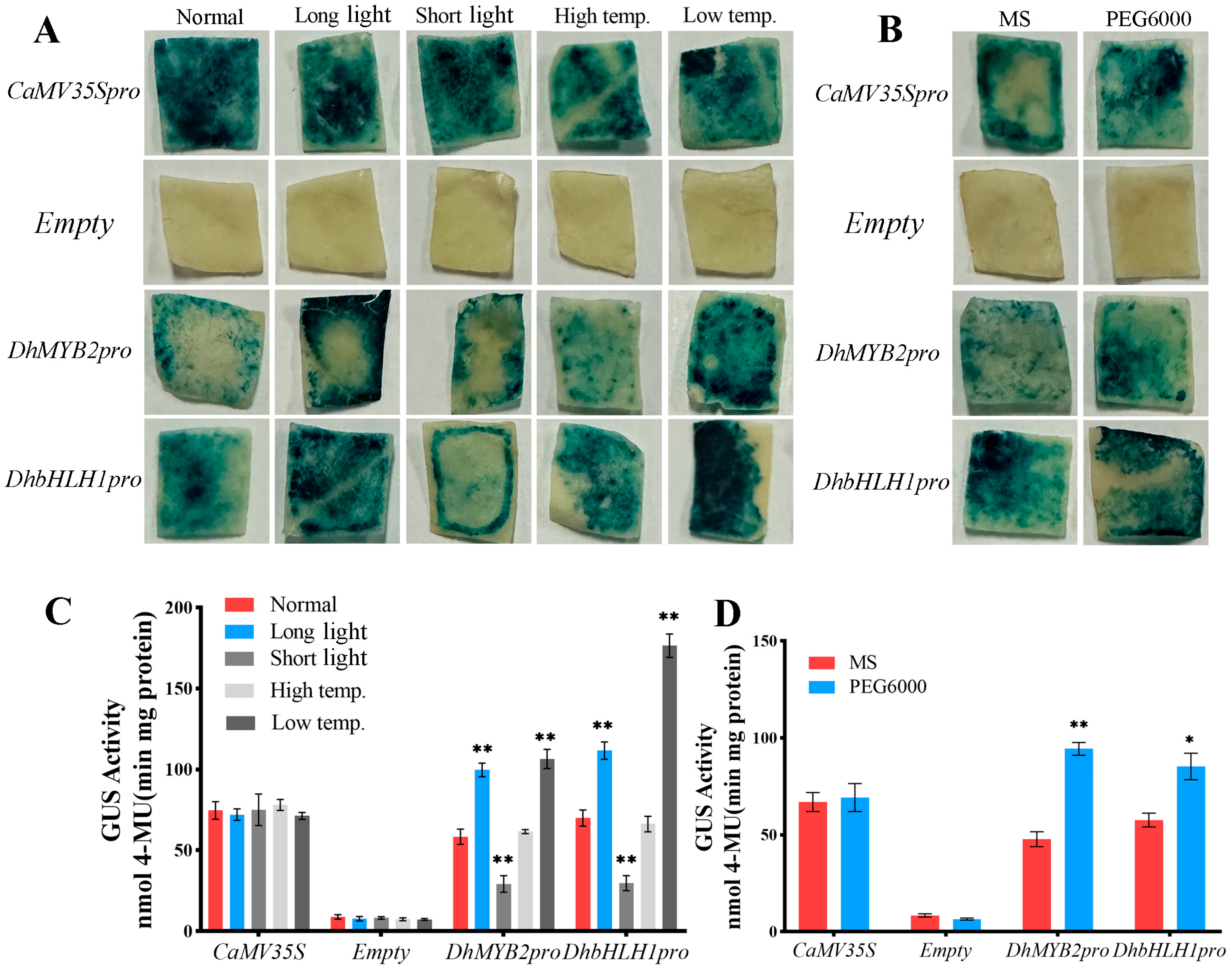
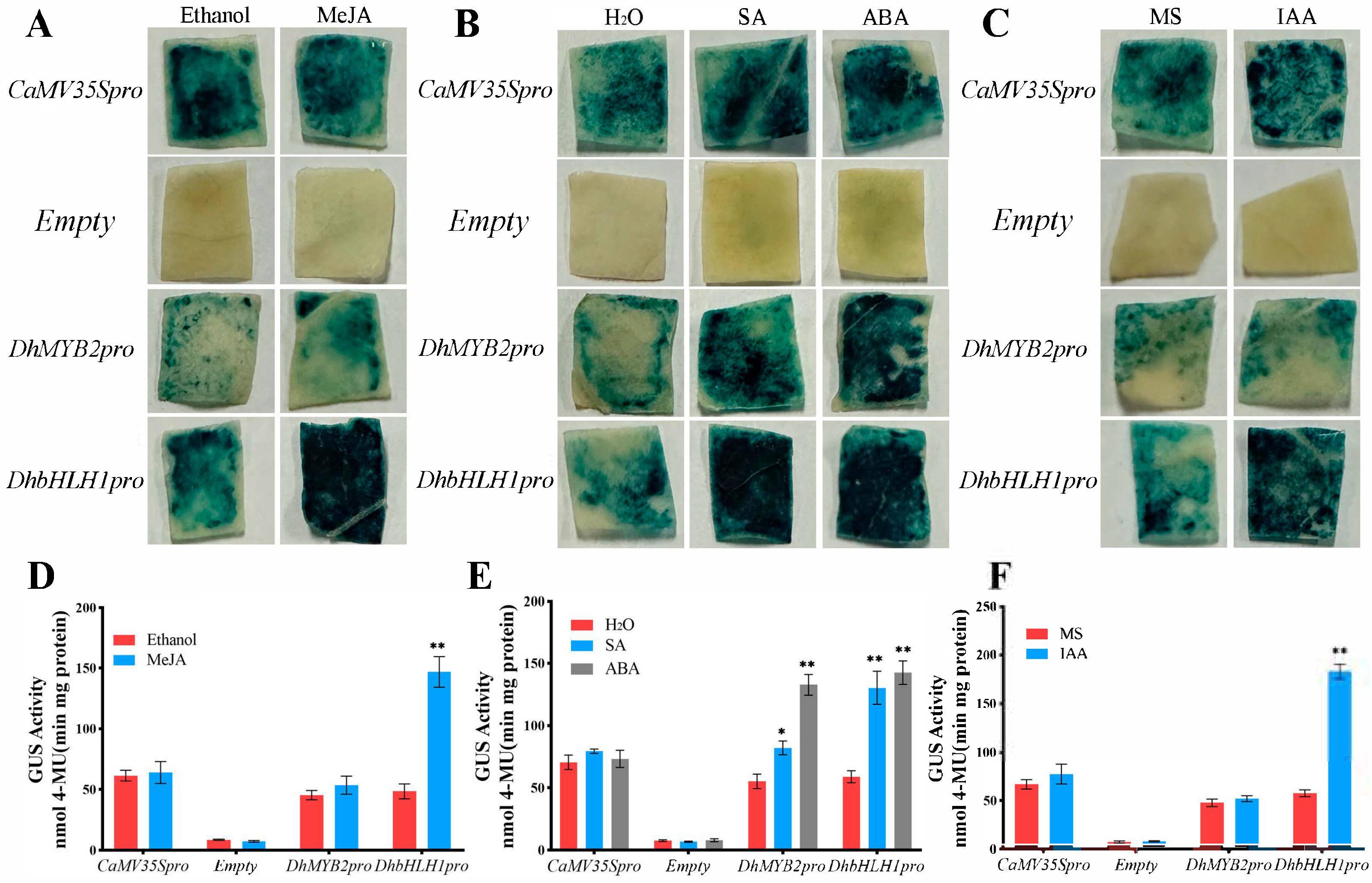
3.4. DhMYB2 and DhbHLH1 Promoter Responses to Abiotic Stress and Phytohormones
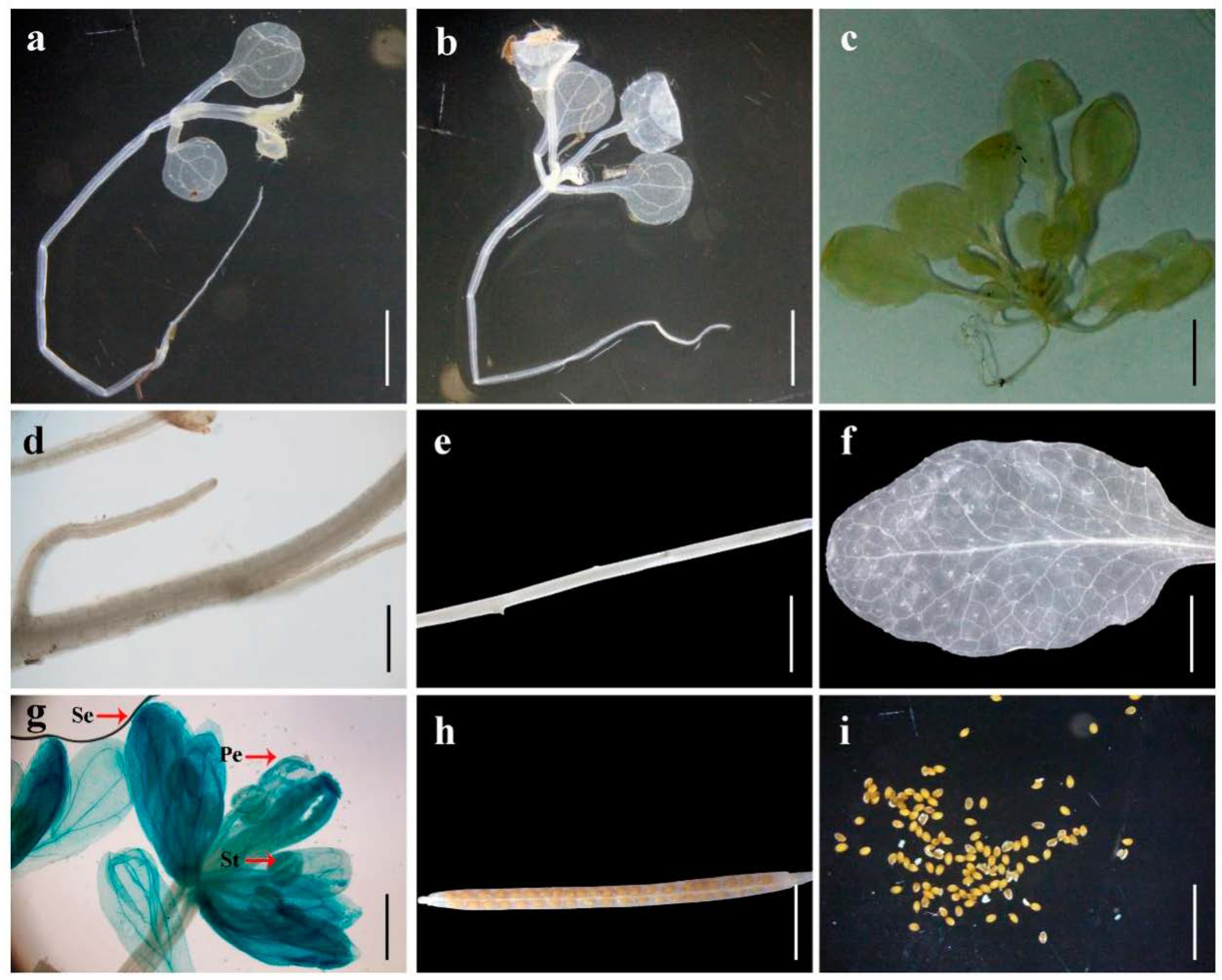
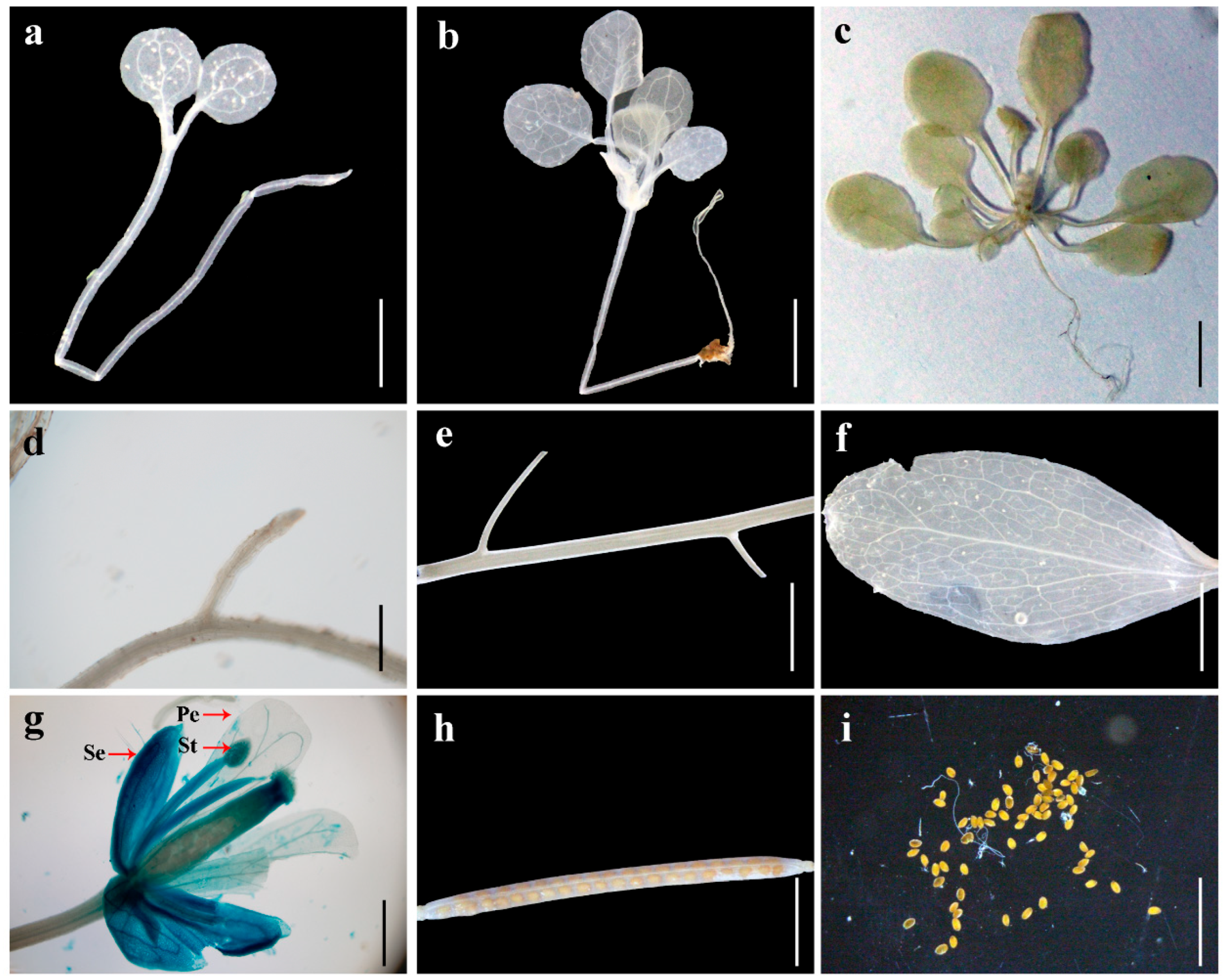
3.5. Tissue-Specific Expression Analysis of the Promoters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Qiu, J.; Ding, L.; Huang, M.; Huang, S.; Yang, G.; Yin, J. Anthocyanin biosynthesis regulation of DhMYB2 and DhbHLH1 in Dendrobium hybrids petals. Plant Physiol. Biochem. 2017, 112, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Mekapogu, M.; Vasamsetti, B.M.K.; Kwon, O.K.; Ahn, M.S.; Lim, S.H.; Jung, J.A. Anthocyanins in Floral Colors: Biosynthesis and Regulation in Chrysanthemum Flowers. Int. J. Mol. Sci. 2020, 21, 6537. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Navas, M.; Jiménez-Moreno, A.; Garcia Asuero, A. Anthocyanin Pigments: Importance, Sample Preparation and Extraction. In Phenolic Compounds: Natural Sources, Importance and Applications; BoD—Books on Demand: Hamburg, Germany, 2017. [Google Scholar] [CrossRef]
- Zhou, Z.; Ying, Z.; Wu, Z.; Yang, Y.; Fu, S.; Xu, W.; Yao, L.; Zeng, A.; Huang, J.; Lan, S.; et al. Anthocyanin Genes Involved in the Flower Coloration Mechanisms of Cymbidium kanran. Front. Plant Sci. 2021, 12, 737815. [Google Scholar] [CrossRef]
- Cui, X.; Deng, J.; Huang, C.; Tang, X.; Li, X.; Li, X.; Lu, J.; Zhang, Z. Transcriptomic Analysis of the Anthocyanin Biosynthetic Pathway Reveals the Molecular Mechanism Associated with Purple Color Formation in Dendrobium Nestor. Life 2021, 11, 113. [Google Scholar] [CrossRef]
- Deng, X.; Hu, C.; Xie, C.; Lu, A.; Luo, Y.; Peng, T.; Huang, W. Metabolomic and Transcriptomic Analysis Reveal the Role of Metabolites and Genes in Modulating Flower Color of Paphiopedilum micranthum. Plants 2023, 12, 2058. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chen, Y.Y.; Tsai, W.C.; Chen, W.H.; Chen, H.H. Three R2R3-MYB transcription factors regulate distinct floral pigmentation patterning in Phalaenopsis spp. Plant Physiol. 2015, 168, 175–191. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Suzuki, T.; Harada, K.; Kobayashi, Y.; Dohra, H.; Ohno, H. Floral organ- and temperature-dependent regulation of anthocyanin biosynthesis in Cymbidium hybrid flowers. Plant Sci. Int. J. Exp. Plant Biol. 2019, 287, 110173. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.J.; Zheng, Q.D.; Yao, Y.H.; Ou, Y.; Chen, J.Y.; Wang, M.J.; Lai, H.P.; Yan, L.; Liu, Z.J.; Ai, Y. Genome-Wide Identification of the MYB Gene Family in Cymbidiumensifolium and Its Expression Analysis in Different Flower Colors. Int. J. Mol. Sci. 2021, 22, 13245. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, Q.; Xu, Q.; Yu, X.; Wang, W.; Gai, R.; Ming, F. PhCHS5 and PhF3’5’H Genes Over-Expression in Petunia (Petunia hybrida) and Phalaenopsis (Phalaenopsis aphrodite) Regulate Flower Color and Branch Number. Plants 2023, 12, 2204. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhou, L.-J.; Peng, J.; Chen, C.; Liu, S.; Song, A.; Jiang, J.; Chen, S.; Chen, F. CmNAC25 targets CmMYB6 to positively regulate anthocyanin biosynthesis during the post-flowering stage in chrysanthemum. BMC Biol. 2023, 21, 211. [Google Scholar] [CrossRef]
- Ruokolainen, S.; Ng, Y.P.; Albert, V.A.; Elomaa, P.; Teeri, T.H. Over-expression of the Gerbera hybrida At-SOC1-like1 gene Gh-SOC1 leads to floral organ identity deterioration. Ann. Bot. 2011, 107, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Peremarti, A.; Twyman, R.M.; Gómez-Galera, S.; Naqvi, S.; Farré, G.; Sabalza, M.; Miralpeix, B.; Dashevskaya, S.; Yuan, D.; Ramessar, K.; et al. Promoter diversity in multigene transformation. Plant Mol. Biol. 2010, 73, 363–378. [Google Scholar] [CrossRef]
- Wang, L.W.; He, M.W.; Guo, S.R.; Zhong, M.; Shu, S.; Sun, J. NaCl stress induces CsSAMs gene expression in Cucumis sativus by mediating the binding of CsGT-3b to the GT-1 element within the CsSAMs promoter. Planta 2017, 245, 889–908. [Google Scholar] [CrossRef]
- Xue, M.; Long, Y.; Zhao, Z.; Huang, G.; Huang, K.; Zhang, T.; Jiang, Y.; Yuan, Q.; Pei, X. Isolation and Characterization of a Green-Tissue Promoter from Common Wild Rice (Oryza rufipogon Griff.). Int. J. Mol. Sci. 2018, 19, 2009. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. Int. J. Exp. Plant Biol. 2014, 217–218, 109–119. [Google Scholar] [CrossRef]
- Villao-Uzho, L.; Chávez-Navarrete, T.; Pacheco-Coello, R.; Sánchez-Timm, E.; Santos-Ordóñez, E. Plant Promoters: Their Identification, Characterization, and Role in Gene Regulation. Genes 2023, 14, 1226. [Google Scholar] [CrossRef]
- Kong, K.; Ntui, V.O.; Makabe, S.; Khan, R.S.; Mii, M.; Nakamura, I. Transgenic tobacco and tomato plants expressing Wasabi defensin genes driven by root-specific LjNRT2 and AtNRT2.1 promoters confer resistance against Fusarium oxysporum. Plant Biotechnol. 2014, 31, 89–96. [Google Scholar] [CrossRef][Green Version]
- Li, A.X.; Han, Y.Y.; Wang, X.; Chen, Y.H.; Zhao, M.R.; Zhou, S.-M.; Wang, W. Root-specific expression of wheat expansin gene TaEXPB23 enhances root growth and water stress tolerance in tobacco. Environ. Exp. Bot. 2015, 110, 73–84. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Y.; Shi, Y.; Liang, Q.; Lu, X.; Su, D.; Xu, X.; Pirrello, J.; Gao, Y.; Huang, B.; et al. Identification of SRS transcription factor family in Solanum lycopersicum, and functional characterization of their responses to hormones and abiotic stresses. BMC Plant Biol. 2023, 23, 495. [Google Scholar] [CrossRef]
- Xu, X.; Mo, Q.; Cai, Z.; Jiang, Q.; Zhou, D.; Yi, J. Promoters, Key Cis-Regulatory Elements, and Their Potential Applications in Regulation of Cadmium (Cd) in Rice. Int. J. Mol. Sci. 2024, 25, 13237. [Google Scholar] [CrossRef]
- Tran, L.S.; Nakashima, K.; Sakuma, Y.; Osakabe, Y.; Qin, F.; Simpson, S.D.; Maruyama, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Co-expression of the stress-inducible zinc finger homeodomain ZFHD1 and NAC transcription factors enhances expression of the ERD1 gene in Arabidopsis. Plant J. Cell Mol. Biol. 2007, 49, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Riedl, K.; Otegui, M.S.; Grotewold, E. Not all anthocyanins are born equal: Distinct patterns induced by stress in Arabidopsis. Planta 2014, 240, 931–940. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Zhang, X.W.; Liu, Y.J.; Wang, X.F.; You, C.X.; Hao, Y.J. ABI5 regulates ABA-induced anthocyanin biosynthesis by modulating the MYB1-bHLH3 complex in apple. J. Exp. Bot. 2021, 72, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Li, T.; Chen, Y.; Zhang, L.; Zhao, G.; Zhuang, J.; Zhao, W.; Gao, L.; Xia, T. Airborne fungus-induced biosynthesis of anthocyanins in Arabidopsis thaliana via jasmonic acid and salicylic acid signaling. Plant Sci. 2020, 300, 110635. [Google Scholar] [CrossRef]
- Li, C.; Shi, L.; Wang, Y.; Li, W.; Chen, B.; Zhu, L.; Fu, Y. Arabidopsis ECAP Is a New Adaptor Protein that Connects JAZ Repressors with the TPR2 Co-repressor to Suppress Jasmonate-Responsive Anthocyanin Accumulation. Mol. Plant 2020, 13, 246–265. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H.; et al. Development of Purple Endosperm Rice by Engineering Anthocyanin Biosynthesis in the Endosperm with a High-Efficiency Transgene Stacking System. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Mu, B.; Li, S.; Li, Y.; Zhou, X.; Zhang, C.; Fan, Y.; Chen, R. Engineering of Purple Embryo Maize with a multigene expression system derived from a bidirectional promoter and self-cleaving 2A peptides. Plant Biotechnol. J. 2018, 16, 1107–1109. [Google Scholar] [CrossRef]
- Naing, A.H.; Kang, H.; Jeong, H.; Soe, M.; Xu, J.; Kim, C. Overexpression of the Raphanus sativus RsMYB1 Using the Flower-specific Promoter (InMYB1) Enhances Anthocyanin Accumulation in Flowers of Transgenic Petunia and Their Hybrids. Mol. Breed. 2020, 40, 97. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, H.; Long, Z.; Zhu, W.; Yin, J.; Song, X.; Li, C. DhMYB2 and DhbHLH1 regulates anthocyanin accumulation via activation of late biosynthesis genes in Phalaenopsis-type Dendrobium. Front. Plant Sci. 2022, 13, 1046134. [Google Scholar] [CrossRef]
- Rogers, H.J.; Bate, N.; Combe, J.; Sullivan, J.; Sweetman, J.; Swan, C.; Lonsdale, D.M.; Twell, D. Functional analysis of cis-regulatory elements within the promoter of the tobacco late pollen gene g10. Plant Mol. Biol. 2001, 45, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Filichkin, S.A.; Leonard, J.M.; Monteros, A.; Liu, P.P.; Nonogaki, H. A novel endo-beta-mannanase gene in tomato LeMAN5 is associated with anther and pollen development. Plant Physiol. 2004, 134, 1080–1087. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, K.; Tan, C.; Zhang, J.; Zhou, J.; Jin, L.; Ma, G.; Zou, Q. Identification and characterization of PsDREB2 promoter involved in tissue-specific expression and abiotic stress response from Paeonia suffruticosa. PeerJ 2019, 7, e7052. [Google Scholar] [CrossRef]
- Zhong, Y.; Lu, X.; Deng, Z.; Lu, Z.; Fu, M. A 1232 bp upstream sequence of glutamine synthetase 1b from Eichhornia crassipes is a root-preferential promoter sequence. BMC Plant Biol. 2021, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H.; Han, Y.N.; Xiao, X.G. A PPO Promoter from Betalain-Producing Red Swiss Chard, Directs Petiole- and Root-Preferential Expression of Foreign Gene in Anthocyanins-Producing Plants. Int. J. Mol. Sci. 2015, 16, 27032–27043. [Google Scholar] [CrossRef] [PubMed]
- Lotkowska, M.E.; Tohge, T.; Fernie, A.R.; Xue, G.P.; Balazadeh, S.; Mueller-Roeber, B. The Arabidopsis Transcription Factor MYB112 Promotes Anthocyanin Formation during Salinity and under High Light Stress. Plant Physiol. 2015, 169, 1862–1880. [Google Scholar] [CrossRef]
- Tao, R.; Yu, W.; Gao, Y.; Ni, J.; Yin, L.; Zhang, X.; Li, H.; Wang, D.; Bai, S.; Teng, Y. Light-Induced Basic/Helix-Loop-Helix64 Enhances Anthocyanin Biosynthesis and Undergoes CONSTITUTIVELY PHOTOMORPHOGENIC1-Mediated Degradation in Pear. Plant Physiol. 2020, 184, 1684–1701. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.-J.; Zhang, F.-J.; Zhang, G.-Z.; Jiang, X.-Y.; Yu, H.-M.; Hou, B.-K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef]
- He, Q.; Ren, Y.; Zhao, W.; Li, R.; Zhang, L. Low Temperature Promotes Anthocyanin Biosynthesis and Related Gene Expression in the Seedlings of Purple Head Chinese Cabbage (Brassica rapa L.). Genes 2020, 11, 81. [Google Scholar] [CrossRef]
- Sumbur, B.; Gao, F.; Liu, Q.; Feng, D.; Bing, J.; Dorjee, T.; Li, X.; Sun, H.; Zhou, Y. The Characterization of R2R3-MYB Genes in Ammopiptanthus nanus Uncovers That the miR858-AnaMYB87 Module Mediates the Accumulation of Anthocyanin under Osmotic Stress. Biomolecules 2023, 13, 1721. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Liang, X.; Zhang, Y.; Fernie, A.R. Mass spectrometric exploration of phytohormone profiles and signaling networks. Trends Plant Sci. 2023, 28, 399–414. [Google Scholar] [CrossRef]
- Lama, K.; Harlev, G.; Shafran, H.; Peer, R.; Flaishman, M.A. Anthocyanin accumulation is initiated by abscisic acid to enhance fruit color during fig (Ficus carica L.) ripening. J. Plant Physiol. 2020, 251, 153192. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, G.; Cao, S.; Xie, Q.; Qi, H. Isolation and functional validation of the CmLOX08 promoter associated with signalling molecule and abiotic stress responses in oriental melon, Cucumis melo var. makuwa Makino. BMC Plant Biol. 2019, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, M.-Z.; Xie, D.-Y. Regulation of anthocyanin biosynthesis in Arabidopsis thaliana red pap1-D cells metabolically programmed by auxins. Planta 2014, 239, 765–781. [Google Scholar] [CrossRef]
- Jia, H.; Xie, Z.; Wang, C.; Shangguan, L.; Qian, N.; Cui, M.; Liu, Z.; Zheng, T.; Wang, M.; Fang, J. Abscisic acid, sucrose, and auxin coordinately regulate berry ripening process of the Fujiminori grape. Funct. Integr. Genom. 2017, 17, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Wang, N.; Xu, H.F.; Jiang, S.H.; Fang, H.C.; Su, M.Y.; Zhang, Z.Y.; Zhang, T.L.; Chen, X.S. Auxin regulates anthocyanin biosynthesis through the Aux/IAA-ARF signaling pathway in apple. Hortic. Res. 2018, 5, 59. [Google Scholar] [CrossRef]
- Wang, C.K.; Han, P.L.; Zhao, Y.W.; Ji, X.L.; Yu, J.Q.; You, C.X.; Hu, D.G.; Hao, Y.J. Auxin regulates anthocyanin biosynthesis through the auxin repressor protein MdIAA26. Biochem. Biophys. Res. Commun. 2020, 533, 717–722. [Google Scholar] [CrossRef]
- Clayton-Cuch, D.; Yu, L.; Shirley, N.; Bradley, D.; Bulone, V.; Böttcher, C. Auxin Treatment Enhances Anthocyanin Production in the Non-Climacteric Sweet Cherry (Prunus avium L.). Int. J. Mol. Sci. 2021, 22, 10760. [Google Scholar] [CrossRef]
- Liu, N. Effects of IAA and ABA on the Immature Peach Fruit Development Process. Hortic. Plant J. 2019, 5, 145–154. [Google Scholar] [CrossRef]
- Li, L.; Yang, G.; Ren, M.; Wang, Z.; Peng, Y.; Xu, R. Co-regulation of Auxin and Cytokinin in Anthocyanin Accumulation During Natural Development of Purple Wheat Grains. J. Plant Growth Regul. 2021, 40, 1881–1893. [Google Scholar] [CrossRef]
- Wang, J.X.; Ming, X.; Tao, Y.B.; Xu, Z.F. Jatropha curcas ortholog of tomato MADS-box gene 6 (JcTM6) promoter exhibits floral-specific activity in Arabidopsis thaliana. PeerJ 2020, 8, e9827. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Aljabali, A.A.A.; Takova, K.; Minkov, G.; Tambuwala, M.M.; Minkov, I.; Lomonossoff, G.P. Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals. Int. J. Mol. Sci. 2023, 24, 17575. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, M.; Osmani, Z.; Frébortová, J.; Frébort, I. Recent advances in molecular farming using monocot plants. Biotechnol. Adv. 2022, 58, 107913. [Google Scholar] [CrossRef]
- Frusciante, S.; Demurtas, O.C.; Sulli, M.; Mini, P.; Aprea, G.; Diretto, G.; Karcher, D.; Bock, R.; Giuliano, G. Heterologous expression of Bixa orellana cleavage dioxygenase 4-3 drives crocin but not bixin biosynthesis. Plant Physiol. 2022, 188, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, C.; Zhu, W.; Li, Y.; Song, X.; Yin, J. Isolation and Functional Analysis of the DhMYB2 and DhbHLH1 Promoters from Phalaenopsis-Type Dendrobium Involved in Stress Responses and Tissue-Specific Expression. Horticulturae 2025, 11, 550. https://doi.org/10.3390/horticulturae11050550
Wang Y, Li C, Zhu W, Li Y, Song X, Yin J. Isolation and Functional Analysis of the DhMYB2 and DhbHLH1 Promoters from Phalaenopsis-Type Dendrobium Involved in Stress Responses and Tissue-Specific Expression. Horticulturae. 2025; 11(5):550. https://doi.org/10.3390/horticulturae11050550
Chicago/Turabian StyleWang, Yachen, Chonghui Li, Wenjuan Zhu, Yamei Li, Xiqiang Song, and Junmei Yin. 2025. "Isolation and Functional Analysis of the DhMYB2 and DhbHLH1 Promoters from Phalaenopsis-Type Dendrobium Involved in Stress Responses and Tissue-Specific Expression" Horticulturae 11, no. 5: 550. https://doi.org/10.3390/horticulturae11050550
APA StyleWang, Y., Li, C., Zhu, W., Li, Y., Song, X., & Yin, J. (2025). Isolation and Functional Analysis of the DhMYB2 and DhbHLH1 Promoters from Phalaenopsis-Type Dendrobium Involved in Stress Responses and Tissue-Specific Expression. Horticulturae, 11(5), 550. https://doi.org/10.3390/horticulturae11050550






